Abstract
Objective
To facilitate clinical use of central PP, we sought to determine a value that might predict adverse outcome and thereby provide a target for assessment of intervention strategies.
Background
We previously documented that central pulse pressure (PP) more strongly relates to carotid hypertrophy and extent of atherosclerosis and, more importantly, better predicts incident cardiovascular disease (CVD) than brachial PP.
Methods
Radial applanation tonometry was performed in the 3rd Strong Heart Study exam to determine central blood pressure. Cox regression analyses were performed using pre-specified covariates and quartiles of central and brachial PP.
Results
Among 2,405 participants without prevalent CVD, 344 suffered CVD events during 5.6±1.7 years. Quartiles of central PP (p<0.001) predicted outcome more strongly than quartiles of brachial PP (p=0.052). With adjustment for covariates, only the event rate in the 4th quartile of central PP (≥50 mmHg) was significantly higher than that in the first quartile (HR 1.69, 95% CI: 1.20–2.39, p=0.003). Central PP ≥50 mmHg was related to outcome in both men (HR 2.06, 95% CI: 1.39–3.04, p<0.001) and women (HR 2.03, 95% CI: 1.55–2.65, p<0.001); in participants with (HR 1.84, 95% CI: 1.41–2.39, p<0.001) and without diabetes (HR 1.91, 95% CI: 1.29–2.83, p=0.001); and in individuals below (HR 2.51, 95% CI: 1.59–3.95, p<0.001) and above (HR 1.53, 95% CI: 1.19–1.97, p=0.001) the age of 60.
Conclusions
Central PP ≥50 mmHg predicts adverse CVD outcome and may serve as a target in intervention strategies, if confirmed in other populations and in prospective studies.
Keywords: blood pressure determination, elasticity, hypertension, detection and control, vasculature
INTRODUCTION
Central (aortic) and brachial (peripheral) systolic and pulse pressures differ due to pulse wave amplification, a function of vascular compliance and wave reflection (1). The difference between central and brachial systolic and pulse pressures decreases with age and other cardiovascular disease (CVD) risk factors that cause vascular stiffening (2,3). Central arterial pressure more closely reflects the load placed on the left ventricle and the coronary and cerebral vasculature. Thus, central blood pressure (BP) should be a more accurate marker of risk and an appropriate target for assessment of efficacy of intervention strategies.
In the population-based Strong Heart Study (SHS), we demonstrated that pulse pressure (PP) was more strongly related to vascular hypertrophy and extent of atherosclerosis than was systolic pressure and that central PP was more strongly related to these subclinical manifestations of cardiovascular disease than was brachial PP (4). More importantly, central PP as a continuous variable better predicted incident cardiovascular disease than did brachial PP. We subsequently reported similar findings in a separate population-based study of elderly community-dwelling individuals living in Dicomano, Italy (5). However, to facilitate use of central PP in intervention strategies and clinical practice, a value that may be of clinical utility in predicting adverse clinical outcome is needed. To this end, we have extended follow-up of SHS participants for an additional year and examined the relation of quartiles of brachial and central PPs to cardiovascular outcomes.
METHODS
Study Population
The SHS is a population-based, longitudinal study of prevalent and incident CVD in American Indians which began in 1989. Details of the study design have been previously published (6). At the 3rd examination in 1997–1999, radial artery applanation tonometry to estimate central blood pressure was added to the study protocol.
Blood was drawn following a 12-hour fast to determine lipids, fasting plasma glucose, creatinine, and fibrinogen. Diabetes was defined by the American Diabetes Association criteria (7) as fasting plasma glucose ≥7.0 mmol/L (126 mg/dl) or by use of hypoglycemic treatment. Brachial BP was measured in triplicate in the right arm by cuff and mercury sphygmomanometer after the participant had rested in a seated position for 5 minutes; the average of the last two measurements was used as brachial BP. PP was calculated as the difference between systolic and diastolic pressures. Hypertension was defined by Joint National Committee 7 criteria (8) as systolic pressure ≥140 mmHg, diastolic pressure ≥90 mmHg or current use of antihypertensive medication.
Participants free of clinically overt CVD, including atrial fibrillation, at the 3rd SHS exam were included in analyses. The occurrence of fatal and non-fatal CVD events (myocardial infarction, coronary heart disease, sudden death, congestive heart failure, and stroke) was tabulated during follow-up, as previously described (9,10). CVD events were determined from medical records, autopsy reports, and informant interviews; all materials were independently reviewed by physician members of the SHS morbidity and mortality committees. Follow-up through December 2005 was 99.8% complete for mortality and 99.2% complete for morbid events. The Indian Health Service Institutional Review Board, institutional review boards of the participating institutions and participating tribes approved the study; informed consent was obtained from all participants.
Applanation Tonometry
As previously described (4), radial arterial pressure waveforms were obtained by applanation tonometry using the SphygmoCor device (AtCor Medical, Sydney, Australia). Applanation tonometry has been validated to yield accurate estimates of intra-arterial pulse pressure by comparison with simultaneous invasive pressure recordings (11,12).
Statistical Analyses
Data are presented as mean±standard deviation. Means of continuous variables were compared using the Student t-test for independent samples. Categorical variables were compared by chi square analysis. Relations of quartiles of central and brachial PP to cardiovascular events were determined in Cox regression analyses. Logistic regression analysis was performed to determine the independent correlates of central PP ≥50 mmHg. Differences in systolic and diastolic pressures across PP quartiles were assessed by ANOVA. Two-tailed p<0.05 was considered significant. Statistical analyses were performed with SPSS, version 12.0 (SPSS Inc., Chicago, Illinois). The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
RESULTS
Population Characteristics and CVD Outcomes
Among the 2405 participants free of prevalent CVD at the time of examination, 344 suffered fatal and non-fatal CVD events (61 myocardial infarction, 163 definite coronary heart disease, 49 stroke, 71 congestive heart failure) during a mean follow-up of 5.6±1.7 years. Mean age was 63±8 years (range 51 to 84 years); 65% were women; body mass index was 31.3±6.6 kg/m2. Hypertension was present in 52% of the population, of whom 68% were taking antihypertensive medications. Diabetes was present in 47% of the population, and 28% were active smokers.
Quartiles of PP and CVD Outcomes
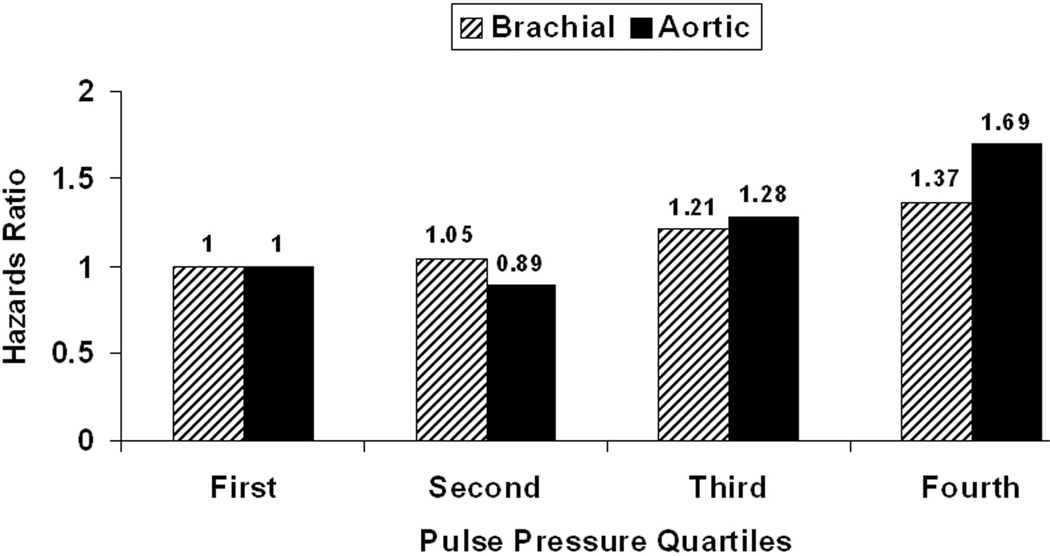
Cox regression models of traditional CVD risk factors and quartiles of brachial and central PP are presented in Table 1. Quartiles of central PP (p<0.001) were much more predictive of outcome than quartiles of brachial PP (p=0.052). Event rates in the 1st to 4th quartile of central PP were 11.0%, 9.9%, 15.0%, and 21.3%, p<0.001 for trend. With adjustment for covariates, only the hazard rate in the 4th quartile (central PP ≥50 mmHg) was significantly higher than that in the 1st quartile (HR 1.69, 95% CI: 1.20–2.39, p=0.003). Event rates in the 4th quartile were likewise significantly higher than in the 2nd quartile (p<0.001) and tended to be higher than in the 3rd quartile (p=0.066). Furthermore, the hazard rate in the 4th quartile was significantly higher than that of the other quartiles combined (1.57 [1.22–2.02], p<0.001). Hazards ratios for quartiles of brachial and central PPs are depicted in Figure 1. Addition of use of anti-hypertensive medications or substitution of HDL and LDL cholesterol for the ratio in secondary analyses did not alter results. Furthermore addition of indicator variables for use of either beta blocking agents or statins did not alter results. Across both central and brachial PP quartiles, there were significant stepwise increases in systolic (p<0.001) but not diastolic pressures (p>0.20, data not shown).
Table 1.
Multivariable Cox Regression Models of Relation of Traditional Risk Factors and Central and Brachial Pulse Pressure Quartiles to Cardiovascular Outcome
| Variable | HR (95% CIs) | HR (95% CIs) | |
|---|---|---|---|
| Age, years | 1.054 (1.037–1.070)* | Age, years | 1.051 (1.035–1.067)* |
| Male gender (%) | 1.212 (0.948–1.54) | Male gender (%) | 1.266 (0.990–1.620) |
| BMI, kg/m2 | 0.987 (0.968–1.006) | BMI, kg/m2 | 0.990 (0.971–1.009) |
| Current smoking (%) | 1.372 (1.052–1.788)† | Current smoking (%) | 1.355 (1.041–1.763)† |
| Cholesterol:HDL | 1.058 (0.988–1.133) | Cholesterol:HDL | 1.062 (0.991–1.138) |
| Creatinine, mg/dl | 1.172 (1.094–1.256)* | Creatinine, mg/dl | 1.164 (1.086–1.247)* |
| Fibrinogen, mg/dl | 1.001 (1.000–1.002)§ | Fibrinogen, mg/dl | 1.001 (1.000–1.002)† |
| Diabetes mellitus (%) | 2.536 (1.974–3.258)* | Diabetes mellitus (%) | 2.48 (1.931–3.186)* |
| Heart rate, bpm | 1.014 (1.004–1.024)§ | Heart rate, bpm | 1.018 (1.008–1.029)‡ |
| Brachial PP Quartiles | 1.117 (0.999–1.248) | Central PP Quartiles | 1.229 (1.098–1.376)* |
| First Quartile (≤45 mmHg) | First Quartile (≤31 mmHg) | ||
| Second Quartile (46–54 mmHg) | 1.052 (0.738–1.499) | Second Quartile (32–39 mmHg) | 0.89 (0.62–1.29) |
| Third Quartile (55–66 mmHg) | 1.210 (0.860–1.704) | Third Quartile (40–49 mmHg) | 1.28 (0.91–1.82 |
| Fourth Quartile (≥67 mmHg) | 1.370 (0.967–1.942) | Fourth Quartile (≥50 mmHg) | 1.69 (1.20–2.39)‡ |
Abbreviations: BMI=body mass index; PP=pulse pressure;
p<0.001;
p<0.05;
p<0.005;
P<0.01
Figure 1. Hazards Ratios for Incident Cardiovascular Event.
Hazards ratios for incident cardiovascular events in 2,405 individuals initially free of clinical cardiovascular disease are stratified by quartiles of brachial (hatched bars) and central aortic (solid bars) PPs. Quartiles of central PP (p<0.001) predicted outcome more strongly than quartiles of brachial PP (p=0.052). Only the event rate in the fourth central PP quartile (PP ≥50 mmHg) was significantly higher than in the first quartile (p=0.003).
Correlates of Central PP ≥50 mmHg
Significant differences between the 4th quartile and the other quartiles led us to perform additional analyses focusing on this quartile (Table 2). In multivariable analysis, central PP ≥50 mmHg was independently related to female gender, age, plasma creatinine, the presence of diabetes and hypertension (or brachial systolic pressure), and lower body mass index and heart rate but not to current smoking, fibrinogen or cholesterol:HDL ratio. Central PP ≥50 mmHg (compared to <50 mmHg) was significantly related to outcome in both men and women, in participants with and without diabetes, and in individuals below and above the ages of both 60 and 65 (Table 3).
Table 2.
Comparison of Demographic Variables and Cardiovascular Disease Risk Factors in Participants with Central Pulse Pressure <50 mmHg vs. ≥50 mmHg
| Variable | PP <50 mmHg (n=1791) |
PP ≥50 mmHg (n=614) |
P value |
|---|---|---|---|
| Age (years) | 61.6±7.0 | 66.6±8.0 | <0.001 |
| Male gender (%) | 38.7 | 23.7 | <0.001 |
| Body mass index (kg/m2) | 31.5±6.8 | 30.9±6.1 | 0.049 |
| Hypertension (%) | 43.3 | 77.2 | <0.001 |
| Diabetes mellitus (%) | 44.9 | 53.5 | <0.001 |
| Current smoking (%) | 29.5 | 22.1 | <0.001 |
| Brachial SBP (mmHg) | 126±16 | 146±21 | <0.001 |
| Brachial DBP (mmHg) | 75±10 | 74±11 | 0.126 |
| Brachial PP (mmHg) | 51±13 | 73±18 | <0.001 |
| Heart rate (bpm) | 70±11 | 66±10 | <0.001 |
| Total cholesterol/HDL cholesterol | 4.8±1.5 | 4.6±1.5 | 0.016 |
| Creatinine (mg/dl) | 0.8 (0.2) | 0.8 (0.3) | 0.004* |
| Fibrinogen (mg/dl) | 380±121 | 396±126 | 0.007 |
Abbreviations: DBP=diastolic blood pressure; HDL=high density lipoprotein; PP=pulse pressure; SBP=systolic blood pressure
Compared by Mann-Whitney test and reported as median (inter-quartile range).
Table 3.
Performance of Central Pulse Pressure ≥50 mm Hg for Prediction of Cardiovascular Outcome in Population Subsets
| Variable | n | HR (95% CIs) | P Value | Interaction P value |
|---|---|---|---|---|
| Sex | 0.94 | |||
| Men | 838 | 2.06 (1.39–3.04) | <0.001 | |
| Women | 1567 | 2.03 (1.55–2.65) | <0.001 | |
| Diabetes mellitus | 0.98 | |||
| Absent | 1259 | 1.91 (1.29–2.83) | 0.001 | |
| Present | 1122 | 1.84 (1.41–2.39) | <0.001 | |
| Age | 0.079 | |||
| <60 years | 994 | 2.51 (1.59–3.95) | <0.001 | |
| ≥60 years | 1411 | 1.53 (1.19–1.97) | 0.001 | |
| Age | 0.47 | |||
| <65 years | 1559 | 1.91 (1.39–2.64) | <0.001 | |
| ≥65 years | 846 | 1.64 (1.20–2.22) | 0.002 |
Comparison of Brachial and Central PP Quartiles
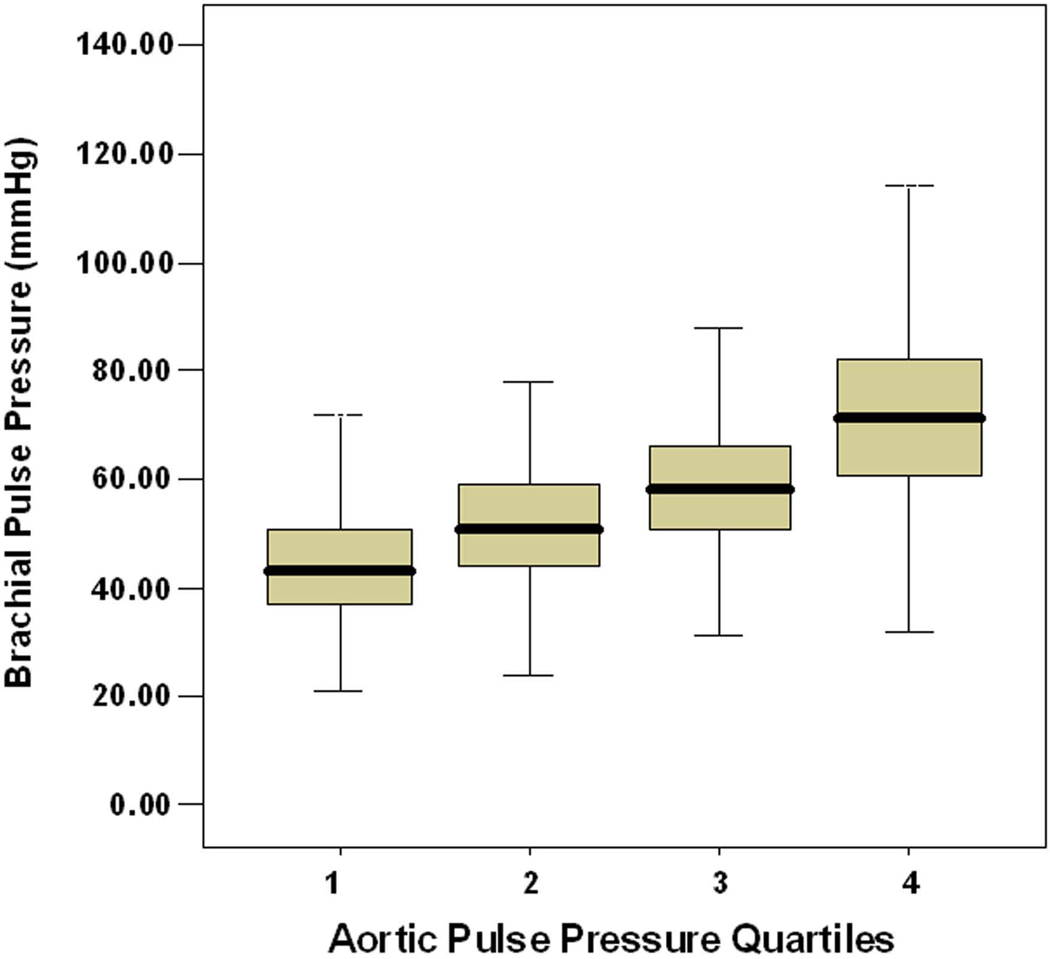
As can be seen in Table 1, both brachial and central PPs increased by roughly 10 mmHg per quartile. In addition, there was a strong correlation between brachial and central PP (r=0.67, p<0.001). However, as can be seen in the box plots in Figure 2, there was substantial overlap of brachial PPs between quartiles of central PP. Furthermore only 61% of individuals in the highest brachial PP quartile fell within the highest central PP quartile and only 58% of individuals in the lowest brachial PP quartile fell within the lowest central PP quartile.
Figure 2. Box Plots of Brachial PP per Quartile of Central Aortic PP.
Box plots (median, quartiles and range) of brachial PP stratified by quartile of central aortic PP demonstrate substantial overlap of brachial PP values across quartiles and highlight the inability to accurately estimate central pressure from brachial pressure.
DISCUSSION
The present study documents the superiority of central over brachial PP for prediction of cardiovascular events in the SHS population and suggests a value that might be of clinical utility if confirmed in other populations and in prospective studies. Importantly, this value, derived from the distribution within our study population rather than from a formal, adequately-powered analysis to determine a threshold of increased risk, performed well in clinically relevant subsets of the SHS population suggesting that it is robust and not based on skewed distribution.
From a pathophysiologic perspective, it is not surprising that central BP better correlates with target organ damage and cardiovascular outcomes than brachial BP since it more accurately reflects vascular load on the left ventricle and cerebral and coronary vasculature. This concept has only recently been possible to test with the development of accurate non-invasive techniques permitting pulse wave analysis and determination of central blood pressure (11–13). Thus, several small studies in select populations have documented stronger relations of central than brachial BP to carotid artery intimal-medial thickness (14), severity of coronary artery disease (15), and all-cause mortality in patients with end-stage renal disease (16). In the large, population-based SHS, central pressure, particularly PP, was more strongly related to vascular hypertrophy and extent of atherosclerosis, as well as to incident CVD, than was brachial pressure (4). This observation has been confirmed in another population-based study (The ICARe Dicomano Study) of elderly individuals (5), despite the decrease in pressure amplification with age and associated lesser difference, on average, between central and brachial pressures.
Furthermore, reduction of central pressure may add to reduction of brachial pressure in improving clinical outcome in the treatment of hypertension. In the CAFE substudy of the ASCOT hypertension trial (17), brachial BP was reduced to a similar extent in both the atenolol±thiazide and amlodipine±perinopril arms, whereas central systolic and pulse pressures were reduced significantly more by amlodipine-based treatment. Both brachial and central PPs were similarly related (χ2=4.1 for both) to a post hoc-defined composite outcome (new cardiovascular events, cardiovascular procedures, renal impairment) independent of other risk factors (17). It is uncertain whether the more favorable outcome associated with the amlodipine-based arm in the overall ASCOT trial (18) was related to the greater central BP lowering with this regimen. This possibility, however, is supported by observations that beneficial effects of regimens based on calcium-channel and angiotensin receptor blockade therapy on outcome were independent of lowering of brachial blood pressure (19,20).
The findings of the current study complement the recent report from the Anglo-Cardiff Collaborative Trial II (2) wherein levels of brachial systolic pressure based on blood pressure classifications were compared to aortic systolic pressures in 6779 healthy normotensive or untreated hypertensive individuals. There was substantial overlap of aortic systolic pressures between individuals with normal or high normal pressures and those with Stage 1 hypertension based on brachial systolic pressure, indicating that central systolic pressure cannot be inferred from brachial systolic pressure. These data also indicate the potential for under- or over-treatment of hypertension based on brachial BP targets, if indeed central BP is a more accurate marker of risk. Our data provide further confirmation of the inability to accurately estimate central pressure from brachial pressure.
The independent prognostic utility of central PP needs confirmation in larger studies with more outcome events in which it will be possible to apply more formal methods for threshold estimation, and to assess formally the costs and benefits of treatment based on such cut-points. While our study population is limited to American Indians, our findings are likely to be highly applicable to the general U.S. population given its increasing prevalences of obesity and diabetes. Furthermore the same traditional risk factors for cardiovascular disease in the general U.S. population have been shown to be operative in the SHS population (9).
In conclusion, this and other recent studies provide strong evidence for the superiority of central BP, particularly PP, to brachial BP in correlation with subclinical vascular disease and association with CVD events. Furthermore preliminary evidence suggests that achievement of a lower central BP for a given level of brachial BP may be more effective in reducing CVD target organ damage and morbidity and mortality. These findings lend strong support for prospective examination of central blood pressure thresholds for prediction of CVD events and potential treatment targets in future trials (21).
Acknowledgments
Funding Source: Supported by grants HL41642, HL41652, HL41654, and HL65521 from the National Heart, Lung, and Blood Institute.
ABBREVIATIONS
- BP
blood pressure
- CVD
cardiovascular disease
- HDL
high-density lipoprotein
- LDL
low-density lipoprotein
- PP
pulse pressure
- SHS
Strong Heart Study
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Industry Relations: Dr. Kizer reports having received grant support from DiaDexus, Inc. and serving on the speakers bureau for Merck & Co. No disclosures for other authors.
REFERENCES
- 1.O’Rourke MF. Principles and definitions of arterial stiffness, wave reflections and pulse pressure amplifications. In: Safar ME, O’Rourke MF, editors. Arterial Stiffness in Hypertension (Handbook of Hypertension, Volume 23) Elsevier; 2006. pp. 3–19. [Google Scholar]
- 2.McEniery CM, Yasmin, McDonnell B, et al. on Behalf of the Anglo-Cardiff Collaborative Trial Investigators. Central pressure: variability and impact of cardiovascular risk factors: The Anglo-Cardiff Collaborative Trial II. Hypertension. 2008;51:1476–1482. doi: 10.1161/HYPERTENSIONAHA.107.105445. [DOI] [PubMed] [Google Scholar]
- 3.O’Rourke MF, Adji A. Basis for use of central blood pressure measurement in office clinical practice. J Am Soc Hypertens. 2008;2:28–38. doi: 10.1016/j.jash.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Roman MJ, Devereux RB, Kizer JR, et al. Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: The Strong Heart Study. Hypertension. 2007;50:197–203. doi: 10.1161/HYPERTENSIONAHA.107.089078. [DOI] [PubMed] [Google Scholar]
- 5.Pini R, Cavallini MC, Palmieri V, et al. Central but not brachial blood pressure predicts cardiovascular events in an unselected geriatric population: The ICARe Dicomano Study. J Am Coll Cardiol. 2008;51:2432–2439. doi: 10.1016/j.jacc.2008.03.031. [DOI] [PubMed] [Google Scholar]
- 6.Lee ET, Welty TK, Fabsitz RR, et al. The Strong Heart Study: a study of cardiovascular disease in American Indians: design and methods. Am J Epidemiol. 1990;132:1141–1155. doi: 10.1093/oxfordjournals.aje.a115757. [DOI] [PubMed] [Google Scholar]
- 7.The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus: follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–3167. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 8.Chobanian AV, Bakris GL, Black HR, et al. the National High Blood Pressure Education Program Coordinating Committee. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 9.Howard BV, Lee ET, Cowan LD, et al. Rising tide of cardiovascular disease in American Indians: The Strong Heart Study. Circulation. 1999;99:2389–2395. doi: 10.1161/01.cir.99.18.2389. [DOI] [PubMed] [Google Scholar]
- 10.Lee ET, Cowan LD, Welty TK, et al. All-cause mortality and cardiovascular disease mortality in three American Indian populations, aged 45–74 years, 1984–1988: The Strong Heart Study. Am J Epidemiol. 1998;147:995–1008. doi: 10.1093/oxfordjournals.aje.a009406. [DOI] [PubMed] [Google Scholar]
- 11.Kelly R, Hayward C, Ganis J, Daley J, Avolio A, O'Rourke M. Noninvasive registration of the arterial pressure waveform using high-fidelity applanation tonometry. J Vasc Med Biol. 1989;1:142–149. [Google Scholar]
- 12.Pauca AL, O’Rourke MF, Kon ND. Prospective evaluation of a method for estimating ascending aortic pressure from the radial artery pressure waveform. Hypertension. 2001;38:932–937. doi: 10.1161/hy1001.096106. [DOI] [PubMed] [Google Scholar]
- 13.Oliver JJ, Webb DJ. Noninvasive assessment of arterial stiffness and risk of atherosclerotic events. Arterioscler Thromb Vasc Biol. 2003;23:554–566. doi: 10.1161/01.ATV.0000060460.52916.D6. [DOI] [PubMed] [Google Scholar]
- 14.Boutouyrie P, Bussy C, Lacolley P, Girerd X, Laloux B, Laurent S. Association between local pulse pressure, mean blood pressure, and large-artery remodelling. Circulation. 1999;100:1387–1393. doi: 10.1161/01.cir.100.13.1387. [DOI] [PubMed] [Google Scholar]
- 15.Waddell TK, Dart AM, Medley TL, Cameron JD, Kingwell BA. Carotid pressure is a better predictor of coronary artery disease severity than brachial pressure. Hypertension. 2001;38:927–931. doi: 10.1161/hy1001.096107. [DOI] [PubMed] [Google Scholar]
- 16.Safar ME, Blacher J, Pannier B, et al. Central pulse pressure and mortality in end-stage renal disease. Hypertension. 2002;39:735–738. doi: 10.1161/hy0202.098325. [DOI] [PubMed] [Google Scholar]
- 17.The CAFÉ Investigators, for the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT) Investigators. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes. Principal results of the Conduit Artery Function Evaluation (CAFÉ) Study. Circulation. 2006;113:1213–1225. doi: 10.1161/CIRCULATIONAHA.105.595496. [DOI] [PubMed] [Google Scholar]
- 18.Dahlöf B, Sever PS, Poulter NR, et al. for the ASCOT investigators. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomized controlled trial. Lancet. 2005;366:895–906. doi: 10.1016/S0140-6736(05)67185-1. [DOI] [PubMed] [Google Scholar]
- 19.Dahlöf B, Devereux RB, Kjeldsen S, et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomized trial against atenolol. Lancet. 2002;359:995–1003. doi: 10.1016/S0140-6736(02)08089-3. [DOI] [PubMed] [Google Scholar]
- 20.Poulter NR, Wedel H, Dahlöf B, et al. for the ASCOT investigators. Role of blood pressure and other variables in the differential cardiovascular rates noted in the Anglo-Scandinavian Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA) Lancet. 2005;366:907–913. doi: 10.1016/S0140-6736(05)67186-3. [DOI] [PubMed] [Google Scholar]
- 21.Agabiti-Rosei E, Mancia G, O’Rourke MF, et al. Central blood pressure measurements and antihypertensive therapy: a consensus document. Hypertension. 2007;50:154–160. doi: 10.1161/HYPERTENSIONAHA.107.090068. [DOI] [PubMed] [Google Scholar]