Abstract
In rodents, embryo implantation is an invasive process which begins with its attachment to the uterine wall and culminates in the formation of the definitive placenta several days later. It is critical that the endometrium provide a supportive environment for the implanting embryo during this process as the placenta is not yet established. The concept of changing permeability barriers to macromolecules between different extracellular compartments in the rodent uterus at the onset of implantation has been established. This chapter provides protocols that can be used to assess this changing permeability barrier and the associated redistribution of macromolecules during the early phases of implantation in rodents. An increased permeability of the endometrial vasculature to plasma proteins occurs in areas adjacent to the implanting blastocyst. In addition, alterations in the extracellular matrix enhance the accumulation of fluid and extravasated macromolecules. We describe several protocols proven to be effective in studying and quantifying early vascular and extravascular responses to natural and artificial “implantation stimuli”. The first three protocols represent qualitative and quantitative methods to assess the early endometrial “vascular permeability” response. On the other hand, the fourth protocol addresses the onset of decidualization and the arising permeability barrier which restricts the movement of macromolecules through the extracellular space. This barrier is believed to provide transient protection for the implanting embryo against potential harmful maternal serum proteins. This protocol describes assessment of resistance of the primary decidual zone to the movement of macromolecules among the compartments of the extracellular space.
Keywords: uterus, endometrium, decidualization, embryo implantation, vascular permeability
1. Introduction
Highly restrictive barriers to the passage of macromolecules have long been accepted for brain and testis. These barriers involve various intercellular junctions involving endothelial cells, astrocytes and sertoli cells (1, 2). Ordinarily, the permeability of these barriers is only increased as a result of pathological conditions. In contrast, the uterus of many mammals contains a barrier to macromolecules which is influenced by physiological processes including embryo implantation. Undoubtedly, the most studied permeability barrier to macromolecules in the rodent uterus during implantation is that between the vascular blood space and the extracellular tissue. At the onset of implantation, there is a dramatic increase in the endometrial vascular permeability barrier in areas immediately adjacent to the implanting blastocyst. This was first described by the work of Psychoyos over 50 years ago (3), and is the earliest macroscopically observable sign of the onset of implantation in rodents (4, 5) as well as some non-rodent species (6–10). A similar response may also be elicited by applying a suitable artificial “deciduogenic” stimulus to the uterine lumen in place of an implanting embryo. These artificial stimuli require specific temporal and concentration-dependent exposure to hormonal fluctuations (“sensitization”) very similar to those which precede embryo implantation (11–14). Previous studies have indicated that the “permeability response” comprises a number of vascular and extravascular responses including: increased permeability of the endometrial vessels, increased uterine blood flow, and increased extracellular fluid volume (15–22). The permeability of the endometrial vessels can be assessed qualitatively through the accumulation of albumin-dye (Evans blue) complexes in the uterine wall after intravenous injection of the dye (see Fig. 1). The dye complexes cannot leave the uterine vessels in areas which have not exhibited the “vascular permeability” response. By the same mechanism the extent of extravasation can be quantitatively evaluated using 125I-labeled albumin or other proteins (19). Finally, tissue distribution of iv-injected 51Cr-EDTA has also been used to calculate changes in the size of the extravascular space (15, 16). Much like the use of Evans blue dye, MRI has been used to demonstrate sites of embryo implantation and enhanced endometrial fluid accumulation (17, 18, 23). The first three protocols described in this chapter provide the basis for qualitative and quantitative assessment uterine vascular permeability (Vp) and extracellular fluid volume (ECFV) changes observed in rodents at the onset of implantation or after artificial deciduogenic stimulation.
Fig. 1.

Increased uterine vascular permeability in the mouse uterus as determined by the Evans blue dye method. A. Representative pregnant mouse uterus on Day 4.5 of pregnancy showing areas of bluing in segments of the uterus containing an implanting conceptus. B. Representative mouse uterus where one horn was exposed to an artificial deciduogenic stimulus while to contralateral horn served as a control. Ovariectomized mice were hormonally sensitized and artificially induced (intraluminal injection of sesame oil) to undergo decidualization as previously described (27). The Evans blue bluing reaction was determined 6 h after application of the artificial deciduogenic stimulus (sesame oil).
During the early phase of implantation, another permeability barrier comes into play. The endometrial tissue immediately surrounding the implanting embryo begins to differentiate into decidual tissue just after the onset of implantation. This results in the formation of a distinct zone of tissue within 24 h after the onset of implantation in rodents. The tissue, called the primary decidual zone, has the unique characteristic of being quite avascular and the extracellular space is highly impermeable to macromolecules (24–28). This impermeability is due to the presence of numerous cell-cell junctions such as adherens, tight and gap junctions (26, 28–30) and may also be related to changes in the extracellular matrix. The fourth protocol described in this chapter is used to visualize this relative impermeability of the primary decidual zone to macromolecules. Although some models of artificially inducing decidualization do not result in the formation of a similar primary decidual zone (28), we have recently shown that some models do (27) using similar methods.
2. Materials (see Notes 1,2 and 3)
2.1 General Solutions and Materials
2.2 Animals
Animals: Keep mice or rats under controlled light conditions (lights on 6 a.m. to 8 p.m.) with free access to food and water. Obtain pregnancies by placing females with males and the day a vaginal plug (mouse) or sperm in vaginal smears (rats) is found at 9 a.m. is considered Day 0.5 of pregnancy. By this convention, implantation in mice begins between approximately 12 and 5 a.m. in the dark phase between Days 3.5 and 4.5 of pregnancy (4) while in rats begins later on day 4.5 of pregnancy (3).
Ketamine and xylazine: use at a dose of 80 mg/g and 15 mg/g, respectively (Henry Schein, Melville, NY).
Dissection instruments: forceps, fine plus coarse scissors, and scalpel handle with blades, surgical silk sutures.
2.3 Qualitative Assessment of Vp
Evans Blue dye solution (1% w/v) is made by dissolving 0.5 gram of Evans Blue dye powder in 50 ml of normal saline. The solution is filter sterilized using a syringe and 0.45μm filters (Millipore) into sterile 1.5 ml tubes (see Note 5).
1 ml syringe (Fisher Scientific).
21 gauge needles (Fisher Scientific).
2.4 Quantitative Assessment of Vp
125I-labeled albumin: Iodinate 50 ug of bovine albumin (Roche) using IODO-GEN as recommended by the manufacturer (Thermo Scientific) using 0.7 mCi Na125I (Perken Elmer, Waltham, MA). Subject the reaction mixture using Biogel P6 (Bio-RAD) gel exclusion chromatography (10 ml) to separate the labeled protein from free 125I (see Note 6).
Radioactivity waste bags and containers.
Freshly heparinized 1 ml syringe with 21 gauge needles, 1.5 ml microtubes, centrifuge capable of 10,000g with microtube rotor.
Pipettes and tips.
1.5 ml microtubes.
Gamma counter and sample vials.
2.5 Quantitative Assessment of ECFV
51Cr-EDTA (PerkinElmer)
0.9% NaCl
1 ml syringe (Fisher Scientific).
21 gauge needles (Fisher Scientific).
1.5 ml microtubes.
Pipettes and tips.
Gamma counter and sample vials.
Radioactivity waste bags and containers
2.6 Visualizing PDZ Impermeability to Macromolecules
Biotinylated albumin solution: Prepare a 5 mg/ml solution of biotinylated albumin (A8549, Sigma) in sterile PBS (see Note 7).
Fixative solution: 4% (w/v) paraformaldehyde in PBS (see Note 8).
Glass slides and coverslips (Fisher Scientific)
TBS-tween solution (TBST): 0.1% Tween-20 in TBS. Store at room temperature.
PAP Pen (ScyTek Laboratories, Logan, UT).
Humid chamber.
Blocking buffer: 2% (v/v) of equine serum (Equitech-Bio Inc., Kerrville, TX) in TBST (see Note 8).
Streptavidin-alkaline phosphatase conjugate: Prepare at a concentration of 0.5 μg/ml (Jackson Immunoresearch, West Grove, PA) in TBST (see Note 8).
Alkaline phosphatase buffer (AB): 100mM Tris-HCl, 100mM NaCl, 5mM MgCl2, 0.05% Tween 20, 5 mM levamisole, pH 9.5 (see Note 8).
BCIP (5-Bromo-4-Chloro-3-Indolyl-Phospate-P-Toluidine Salt) stock solution (50 mg/ml): dissolve BCIP (Research Products International Corp., Mt. Prospect, IL) in 100% dimethylformamide (DMF)(see Note 9).
NBT (2,2′-Di-p-nitrophenyl-5, 5′-diphenyl-3, 3′-(3,3′-dimethoxy-4,4′-diphenylene)-ditetrazolium chloride) stock solution (50 mg/ml): dissolve NBT (Research Products International Corp.) in 70% DMF (see Note 9).
Color development solution (CDS): For each ml of AB, add 6.7 μl and 5.2μl of BCIP and NBT stock solutions, respectively (see Note 8).
Coplin jars.
Nuclear fast red staining solution (ScyTek Laboratories).
Citramount (Polysciences Inc., Warrington, PA).
3. Methods
3.1 Qualitative Assessment Vp using Evans Blue Dye
Inject Evans Blue solution (mice, 0.1 ml; hamster, 0.25 ml; rat, 0.5 ml) into a lateral tail vein while under ketamine anesthetic. Evans Blue is a diazo dye which has a very high affinity for serum albumin (31).
After allowing the dye to circulate for 15 minutes, kill the animal and dissect uteri.
Areas of “bluing” represent implantation site areas of increased vascular permeability to Evans Blue-albumin complexes (see Fig. 1).
3.2 Quantitative Assessment of Vp
-
Before autopsy on Day 4.5 (mice) or 5.5 (rats) of pregnancy, animals are given two intravenous injections (tail vein) while under ketamine anesthetic:
Inject 125I-albumin (2–3 μCi) into the tail vein. For experiment 1 this is at 5, 10, 20, 40, 80 or 160 minutes before autopsy on Day 4.5 of pregnancy. For experiment 2 the injections are given at 6, 12 or 24 h (see Note 10).
Inject Evans blue dye solution at 15 minutes prior to autopsy as described above.
Plasma collection: Upon autopsy, inject animal with ketamine/xylazine mixture intraperitoneally and collect blood slowly from the heart or abdominal vein into heparinized syringes. Place blood into 1.5 ml centrifuge tubes and centrifuge at 10,000 g for 2 minutes. Collect plasma sample (top clear phase) into another 1.5 ml tube and prepare duplicate 0.1 ml plasma samples for radioactivity determination.
Tissue collection: Dissect uterine horns and trim of mesentery. After washing quickly in PBS the implantation (as revealed by the intense bluing by Evans blue) and non-implantation sites need to be separated using a scalpel and pooled into pre-weighed tubes. Weigh the tube containing the tissues to obtain tissue weight.
Measure radioactivity levels of the plasma and tissue samples using a gamma counter.
-
Calculations: To assess differences in vascular permeability in the implantation verses non-implantation segments of the uterus:
Calculate the tissue volumes of distribution (VD) of 125I-albumin for both samples from each mouse by dividing tissue radioactivity (cpm/g) by plasma radioactivity (cpm/ml).
Steady-state volume of distribution (VDss) for 125I-albumin is an estimate of the extracellular fluid space of the tissue and is calculated for implantation (VDssI) and non-implantation segment (VDssN) tissue. This is achieved by calculating the overall average volume of distribution for the 6, 12 and 24 h time points (experiment 2).
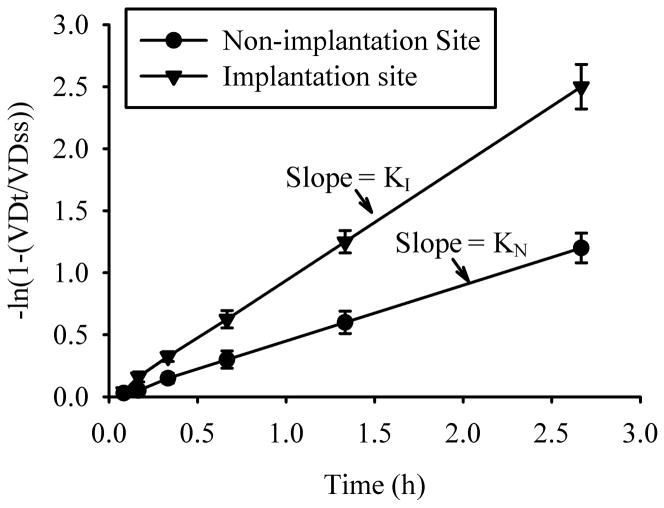
Calculate the slopes and coefficients of determination of the line defined by –ln(1-(VDt/VDss)) as a function of time (hours) for each tissue type of Experiment 1 by the least-squares method of linear regression. For pregnant uteri the slopes are equal to the rate constant K for implantation (KI) and non-implantation (KN) tissue (Fig. 2)(see Note 11).
Fig. 2.
Determination of KI and KN by linear regression. The slopes of the lines (K) defined by ln(1-(VDt/VDss)) verses time for implantation (I) compared to non-implantation (N) segments is a measure of the differences in vascular permeability to 125I-albumin. A similar difference in vascular permeability constants between artificially-induced compared to contralateral control uterine horns can be made in animals undergoing artificially-induced decidualization.
3.3 Quantitative Assessment of ECFV
While under ketamine plus xylazine, a midventral incision is made in the abdomen. After carefully exposing each kidney, the renal pedicles are ligated with surgical silk and the kidneys are removed with scissor cuts made distal to the ligation (see Note 12).
Using a 1 ml syringe and a 25 gauge needle, 5μCi 51Cr-EDTA in saline (0.2 ml mouse; 0.5 ml rat) as well as Evans blue dye (as described above) are injected into a lateral tail vein.
After 15 minutes, plasma samples are collected as above and the animals are killed. Prepare duplicate 0.1 ml plasma samples for radioactivity determination.
Tissue collection: Dissect uterine horns and trim of mesentery. After washing quickly in PBS the implantation (as revealed by the intense bluing by Evans blue) and non-implantation sites need to be separated using a scalpel and pooled into pre-weighed tubes. Weigh the tube containing the tissues to obtain tissue weight.
Measure radioactivity levels of the plasma and tissue samples using a gamma counter.
The tissue volume of distribution of 51Cr-EDTA represents the ECFV. This is determined by dividing the uterine tissue radioactivity (cpm/mg) by the plasma radioactivity (cpm/μl). The ECFV (in μl) can be compared in implantation sites versus non implantation sites or in uterine horns subjected to artificial deciduogenic stimulation versus unstimulated horns (see Note 13).
3.4 Visualizing PDZ Impermeability to Macromolecules
While under anesthesia, inject 1 mg of biotinylated albumin into a lateral tail vein (see Note 14).
After 1–2 h, collect the uteri and place in fixative solution.
Fix tissue in fixative solution for 24 h at 4 °C with gentle shaking.
Wash in 70% ethanol twice.
Place in 70% ethanol for 24 h with shaking at 4 °C. Replace the 70% ethanol. You can store the tissue at 4 °C indefinitely.
Process the tissues into paraffin blocks then prepare cross sections (4–10 μm) and mount onto glass slides using standard techniques.
-
De-paraffinize and hydrate sections:
Heat up slides to 80 °C for 5 minutes to melt wax
Place in xylene for 10 minutes. Repeat twice.
Place in 100% ethanol for 5 minutes. Repeat twice.
Place in 95% ethanol for 5 minutes. Repeat once.
Place in 70% ethanol for 5 minutes. Repeat once.
Place in water for 5 minutes. Repeat once.
Place in TBST for 5 minutes. Repeat once.
Encircle the section(s) with a hydrophobic barrier using a PAP Pen and incubate sections in an appropriate volume of blocking buffer for 1 hour at room temperature in a humid chamber.
Wash sections in TBST for 5 minutes. Repeat once.
Incubate sections in humid chamber in a 1:500 dilution of streptavidin-alkaline phosphatase solution for 30 minutes (see Note 15).
Wash for 15 minutes in TBST. Repeat twice.
Incubate for 5 minutes in AB buffer. Repeat once.
Incubate sections with CDS until desired color precipitate develops.
Wash in TBST for 10 minutes
Wash twice in distilled water for 10 minutes
Immerse slides in nuclear fast red for 1–5 minutes to stain the nuclei a reddish color.
Place two changes of tap water for 30 seconds
-
Dehydrate slides in alcohol series:
Place in 70% ethanol for 5 minutes
Plave in 95% ethanol for 5 minutes. Repeat once.
Place in 100% ethanol for 5 minutes. Repeat once.
Place slides in xylene or appropriate xylene replacement for 5 minutes. Repeat once.
Place coverslip over section using Citramount mounting medium.
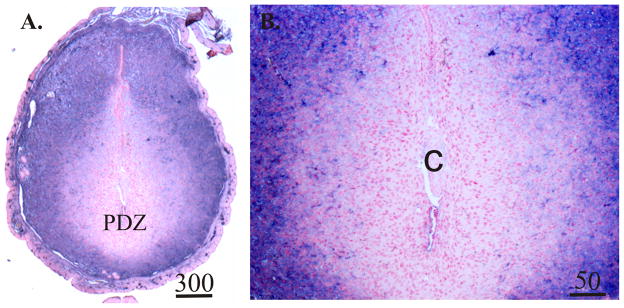
Examine slides under a light microscope. Nuclei are stained red and a blue/purple precipitate is localized to the intravenously injected biotinylated albumin (see Fig. 2).
Fig. 3.
Representative photomicrographs of biotinylated-albumin histochemistry of sections prepared from Day 5.5 pregnant mice. Tissues were collected 1 hour after intravenous injection of biotinylated albumin. Values above scale bars represent microns. Sections from the uteri of mice that were not injected with biotinylated albumin showed no purple staining (not shown). PDZ, primary decidual zone of the endometrium; C, implanting conceptus.
Acknowledgments
This work was supported, in part, by RO1 HD049010 from NIH (Eunice Shriver Kennedy National Institute of Child Health and Development) to B.B..
Footnotes
Unless otherwise stated, materials were purchased from Sigma-Aldrich (St. Louis, MO).
Users need to inform themselves of the dangers of the reagents, especially the radioactive materials, and potential or known carcinogens used in these protocols. Ensure proper personal protective gear and methods of waste disposal are used as recommended by Federal and local regulatory agencies.
All solutions should be prepared in water that has a resistivity of 18.2 MOhm-cm and total organic content of less than five parts per billion unless otherwise stated.
Can be stored at room temperature.
These tubes can be stored indefinably but over time a precipitate may form which needs to be centrifuged out.
This can be used immediately or store at 4 °C. Do not store for more than 48 h.
Store in usable aliquots indefinitely at −20 °C but avoid repeated freeze-thaw cycles.
Prepare fresh on the day of use.
Store in aliquots at −20 °C protected from light.
This is carried out in 2 separate experiments using at least 5–6 animals per time point. The shorter time points (5–160 minutes) are before the volume of distribution for 125I-albumin reaches a steady-state while those of experiment 2 (6–24 hours) are after a steady-state is reached.
To determine if the slopes of the lines between implantation and non-implantation segments differ, use statistical methods described by Sokal and Rohlf (32).
When using 51Cr-EDTA for assessment of ECFV, animals must be nephrectomized to allow equilibration of the tracer among fluid compartments. In intact animals, the kidney eliminates the EDTA fairly rapidly.
Perform a statistical analysis using repeated-measures analysis of variance methods to determine differences between implantation verses non-implantation segments or stimulated verses control uterine horns for pregnant mice or mice undergoing artificially induced decidualization, respectively.
For negative controls, refrain from injecting the animals with the biotinylated albumin. Alternatively, these control animals can be injected with the same amount of non-biotinylated albumin.
Make sure not to have serum in this incubation as serum can have appreciable amounts of biotin or biotin-like molecules that can bind the streptavidin.
References
- 1.Maddocks S, Setchell BP. The physiology of the endocrine testis. Oxf Rev Reprod Biol. 1988;10:53–123. [PubMed] [Google Scholar]
- 2.Ueno M. Mechanisms of the penetration of blood-borne substances into the brain. Curr Neuropharmacol. 2009;7:142–149. doi: 10.2174/157015909788848901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Psychoyos A. La reaction deciduale est precede de modifications prcoces de la permeabilite capillaire de l’uterus. C R Seances Soc Biol Fil. 1960;154:1384–1387. [PubMed] [Google Scholar]
- 4.Finn CA, McLaren A. A study of the early stages of implantation in mice. J Reprod Fertil. 1967;13:259–267. doi: 10.1530/jrf.0.0130259. [DOI] [PubMed] [Google Scholar]
- 5.Orsini MW, Donovan BT. Implantation and induced decidualization of the uterus in the guinea pig, as indicated by Pontamine blue. Biol Reprod. 1971;5:270–281. doi: 10.1093/biolreprod/5.3.270. [DOI] [PubMed] [Google Scholar]
- 6.Hoffman LH, DiPietro DL, McKenna TJ. Effects of indomethacin on uterine capillary permeability and blastocyst development in rabbits. Prostaglandins. 1978;15:823–828. doi: 10.1016/0090-6980(78)90148-x. [DOI] [PubMed] [Google Scholar]
- 7.Boshier DP. The pontamine blue reaction in pregnant sheep uteri. J Reprod Fertil. 1970;22:595–596. doi: 10.1530/jrf.0.0220595. [DOI] [PubMed] [Google Scholar]
- 8.Guillomot M, Flechon JE, Wintenberger-Torres S. Conceptus attachment in the ewe: an ultrastructural study. Placenta. 1981;2:169–182. doi: 10.1016/s0143-4004(81)80021-5. [DOI] [PubMed] [Google Scholar]
- 9.Keys JL, King GJ, Kennedy TG. Increased uterine vascular permeability at the time of embryonic attachment in the pig. Biol Reprod. 1986;34:405–411. doi: 10.1095/biolreprod34.2.405. [DOI] [PubMed] [Google Scholar]
- 10.Mead RA, Bremner S, Murphy BD. Changes in endometrial vascular permeability during the periimplantation period in the ferret (Mustela putorius) J Reprod Fertil. 1988;82:293–298. doi: 10.1530/jrf.0.0820293. [DOI] [PubMed] [Google Scholar]
- 11.Kennedy TG. Prostaglandins and increased endometrial vascular permeabiltiy resulting from the application of artificial stimulus to the uterus of the rat sensitized for the decidual cell reaction. Biol Reprod. 1979;20:560–566. doi: 10.1095/biolreprod20.3.560. [DOI] [PubMed] [Google Scholar]
- 12.Psychoyos A. Capillary permeability and uterine deciduation. C R Hebd Seances Acad Sci. 1961;252:1515–1517. [PubMed] [Google Scholar]
- 13.Lundkvist O, Nilsson BO. Endometrial ultrastructure in the early uterine response to blastocysts and artificial deciduogenic stimuli in rats. Cell Tissue Res. 1982;225:355–364. doi: 10.1007/BF00214688. [DOI] [PubMed] [Google Scholar]
- 14.Milligan SR, Mirembe FM. Time course of the changes in uterine vascular permeability associated with the development of the decidual cell reaction in ovariectomized steroid-treated rats. J Reprod Fertil. 1984;70:1–6. doi: 10.1530/jrf.0.0700001. [DOI] [PubMed] [Google Scholar]
- 15.McRae AC, Heap RB. Uterine vascular permeability, blood flow and extracellular fluid space during implantation in rats. J Reprod Fertil. 1988;82:617–625. doi: 10.1530/jrf.0.0820617. [DOI] [PubMed] [Google Scholar]
- 16.Hamilton GS, Kennedy TG. Uterine extracellular fluid volume and blood flow after artificial uterine stimulation to rats differentially sensitized for the decidual cell reaction. Biol Reprod. 1993;48:910–915. doi: 10.1095/biolreprod48.4.910. [DOI] [PubMed] [Google Scholar]
- 17.Hamilton GS, Kennedy TG, Karlik SJ. Early identification of sites of embryo implantation in rats by means of gadolinium-enhanced MR imaging. J Magn Reson Imaging. 1994;4:481–484. doi: 10.1002/jmri.1880040340. [DOI] [PubMed] [Google Scholar]
- 18.Hamilton GS, Kennedy TG, Norley CJ, Karlik SJ. Gadolinium-DTPA enhanced MRI demonstrates uterine vascular changes associated with artificially induced decidualization and ovoimplantation in rats. Magn Reson Med. 1993;29:817–821. doi: 10.1002/mrm.1910290615. [DOI] [PubMed] [Google Scholar]
- 19.Bany BM, McRae AC. Uterine uptake of alpha 2-macroglobulin and alpha 1-proteinase inhibitor from the blood during early implantation in the mouse. Biol Reprod. 1992;47:514–519. doi: 10.1095/biolreprod47.4.514. [DOI] [PubMed] [Google Scholar]
- 20.McRae AC, Kennedy TG. Evidence for a blood - uterine lumen permeability barrier in rats treated with hormones to mimic early pseudopregnancy. Can J Physiol Pharmacol. 1982;60:1630–1635. doi: 10.1139/y82-241. [DOI] [PubMed] [Google Scholar]
- 21.McRae AC, Kennedy TG. Selective permeability of the blood-uterine lumen barrier in rats: importance of lipid solubility. Biol Reprod. 1983;29:886–894. doi: 10.1095/biolreprod29.4.886. [DOI] [PubMed] [Google Scholar]
- 22.McRae AC, Kennedy TG. Selective permeability of the blood-uterine lumen barrier in rats: importance of molecular size. Biol Reprod. 1983;29:879–885. doi: 10.1095/biolreprod29.4.879. [DOI] [PubMed] [Google Scholar]
- 23.Plaks V, Kalchenko V, Dekel N, Neeman M. MRI analysis of angiogenesis during mouse embryo implantation. Magn Reson Med. 2006;55:1013–1022. doi: 10.1002/mrm.20881. [DOI] [PubMed] [Google Scholar]
- 24.Rogers PA, Murphy CR, Rogers AW, Gannon BJ. Capillary patency and permeability in the endometrium surrounding the implanting rat blastocyst. Int J Microcirc Clin Exp. 1983;2:241–249. [PubMed] [Google Scholar]
- 25.Parr MB, Parr EL. Permeability of the primary decidual zone in the rat uterus: studies using fluorescein-labeled proteins and dextrans. Biol Reprod. 1986;34:393–403. doi: 10.1095/biolreprod34.2.393. [DOI] [PubMed] [Google Scholar]
- 26.Parr MB, Tung HN, Parr EL. The ultrastructure of the rat primary decidual zone. Am J Anat. 1986;176:423–436. doi: 10.1002/aja.1001760405. [DOI] [PubMed] [Google Scholar]
- 27.Herington JL, Underwood T, McConaha M, Bany BM. Paracrine signals from the mouse conceptus are not required for the normal progression of decidualization. Endocrinology. 2009;150:4404–4413. doi: 10.1210/en.2009-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang X, Matsumoto H, Zhao X, Das SK, Paria BC. Embryonic signals direct the formation of tight junctional permeability barrier in the decidualizing stroma during embryo implantation. J Cell Sci. 2004;117:53–62. doi: 10.1242/jcs.00826. [DOI] [PubMed] [Google Scholar]
- 29.Tung HN, Parr MB, Parr EL. The permeability of the primary decidual zone in the rat uterus: an ultrastructural tracer and freeze-fracture study. Biol Reprod. 1986;35:1045–1058. doi: 10.1095/biolreprod35.4.1045. [DOI] [PubMed] [Google Scholar]
- 30.Paria BC, Zhao X, Das SK, Dey SK, Yoshinaga K. Zonula occludens-1 and E-cadherin are coordinately expressed in the mouse uterus with the initiation of implantation and decidualization. Dev Biol. 1999;208:488–501. doi: 10.1006/dbio.1999.9206. [DOI] [PubMed] [Google Scholar]
- 31.Freedman FB, Johnson JA. Equilibrium and kinetic properties of the Evans blue-albumin system. Am J Physiol. 1969;216:675–681. doi: 10.1152/ajplegacy.1969.216.3.675. [DOI] [PubMed] [Google Scholar]
- 32.Sokal RR, Rohlf FJ. Biometry. 2. New York: W.H. Freeman and Company; 1981. [Google Scholar]