Abstract
Community home-based care (CHBC) plays an integral role in the care of HIV-infected patients living in resource-limited regions. A longitudinal cohort study has recently been conducted, in the Kilimanjaro Region of northern Tanzania, in order to identify the components of an effective CHBC programme. Structured questionnaires were administered to clients over two census rounds, one in October 2003–February 2004 and the other in January 2005–October 2005. In the second round, follow-up interviews were completed for 226 (87.9%) of the 257 clients included in the first round. The clients included in the first round had a median (range) age of 38 (20–66) years and 182 (75.2%) of them were female. Although only 27 (12.9%) of them were using antiretroviral therapy (ART) when first interviewed, 108 (44.6%) were taking trimethoprim–sulfamethoxazole (SXT) prophylaxis. By the time of the follow-up interviews, 102 (45.1%) of the clients included in the first round had died, giving a mortality of 51/100 person-years of observation. The primary cause of death for 87 (85.3%) of the clients who had died was respiratory and/or gastro-intestinal infection, and the most common contributory causes of death were malnutrition (81.4%) and anaemia (42.2%). On bivariable analysis, the following first-round conditions were found to be significantly associated with death by the second census round: weakness for >1 month [odds ratio (OR)=2.64; P=0.008]; oral thrush (OR=2.31; P=0.015); painful swallowing (OR=2.02; P=0.036); staying in bed for part of the day over most of the previous month (OR=1.94; P=0.017); fever for >1 month (OR=1.95; P=0.016); and severe bacterial infections (OR=1.80; P=0.036). The high mortality was associated with advanced, symptomatic HIV disease for which antiretroviral therapy was indicated. Clients who were in the advanced stages of HIV disease (as defined by the World Health Organization's criteria) in the first census round were significantly more likely to have died by the time of the second round than the other clients investigated (log-rank χ2=8.115; P=0.044).
The high level of morbidity observed in this study, and the causes of mortality that were identified, emphasise the need for CHBC programmes to provide HIV-infected patients with improved access to basic resources such as SXT and isoniazid prophylaxis, clean water, oral rehydration therapy, and micronutrient supplementation, in addition to increased access to ART.
The current burden posed by HIV/AIDS is estimated at 33 million infected people, with two-thirds of those infected living in sub-Saharan Africa. In Tanzania, the prevalence of HIV/AIDS among adults aged 15–49 years was estimated at 6.2% in 2007, indicating that about 1.3 million adults in the country were HIV-infected (Anon., 2008).
The current capacity of Tanzania's health services to care for the country's HIV-infected individuals is limited. A survey of healthcare facilities in the Northern Zone of Tanzania in 2004 (Landman et al., 2006) revealed a need for additional trained personnel, medications and laboratory capacity to care for the region's HIV-infected patients — a challenge also illustrated by the results of a nation-wide survey in 2006 (Anon., 2007a). In government hospitals in the Kilimanjaro and Kagera Regions, 21.6% and 32.8% of inpatient beds, respectively, may already be occupied by patients living with HIV/AIDS (Kwesigabo et al., 1999; Ole-Nguyaine et al., 2004). It has been estimated that delivering antiretroviral therapy (ART) to all those in need of such treatment in Tanzania would require the full-time services of almost half of the country's current healthcare workforce (Beckmann and Rai, 2004). With formal healthcare services overwhelmed, alternative models — such as community home-based care (CHBC), a service in which basic care is delivered to a patient's home by trained members of the community — will become increasingly important in delivering healthcare to Tanzania's HIV-infected population.
CHBC has already been recognised as a vital component of the care of HIV-infected populations by both the World Health Organization (WHO) and the Tanzanian government (Anon., 2001; WHO, 2002). It has the potential to provide care for large numbers of HIV-infected patients, especially in rural areas, where the majority of Tanzanians live and where prevention and care services are often lacking (Somi et al., 2006). Unfortunately, although such data are needed to determine the essential components of successful CHBC programmes, and to help structure such programmes to deliver effective care, few data exist on the current needs of the HIV-infected clients whose main care is community home-based (Bowie et al., 2006). To address this issue, a longitudinal cohort study among HIV-infected adults receiving CHBC in the Kilimanjaro Region of northern Tanzania was recently conducted. The methodology, results and conclusions of this study are detailed below.
Subjects and Methods
Location
The Kilimanjaro Region of northern Tanzania has an estimated population of 1.4 million (Anon., 2003). At the time of the present study (2003–2005), the region was divided into the six districts of Hai, Moshi Rural, Moshi Urban, Mwanga, Rombo and Same. The subjects of the present study, who were all HIV-infected individuals (‘clients’) receiving CHBC, were surveyed through an existing AIDS-service organization in Moshi, known as Women Against AIDS in Kilimanjaro or, in the local Swahili, Kikundi cha Wanawake Kilimanjaro Kupambana na UKIMWI (KIWAKKUKI). This organization is a community-based women's organization that provides HIV voluntary counselling and testing (VCT), CHBC for HIV-infected individuals, and education and support for people living with HIV/AIDS.
Home-based Care
Clients presenting to KIWAKKUKI are each checked for anti-HIV antibodies (Chu et al., 2005) using two commercial rapid tests: the CapillusTM HIV-1/HIV-2 (Trinity Biotech, Bray, Ireland) and the Determine™ HIV-1/2 (Abbott Laboratories, Abbott Park, IL). All clients found seropositive are offered enrollment in KIWAKKUKI's CHBC programme. At the start of the present study, this programme provided care for approximately 800 of the estimated 98,000 HIV-infected individuals in the Kilimanjaro Region. It was maintained by 180 ‘providers’ who each visited a median of five clients/month. A typical home visit included basic nursing care, health education, nutritional and spiritual counselling, and the provision of food and basic medications. The providers were community members who volunteered their services, and who had received training according to a Tanzania Ministry of Health curriculum. The training included an intensive, 5-day course, covering topics such as social and emotional support, basic medical care and essential medications, networking with community members, and assessing the need for referrals to formal healthcare services.
Questionnaire Administration
Those HIV-infected clients enrolled in the KIWAKKUKI's CHBC programme who received home visits during the period of the organization's first census of its clients, which ran from October 2003 to February 2004, were recruited for participation in the present study. The aim of the first census round was to improve the quality of the CHBC programme, by improving understanding of the number and health status of the clients and by providing the information needed to advocate for increased resources for rural clients who could benefit from CHBC. The programme's providers administered structured questionnaires during home visits in both this first census and, as follow-up for the present study, in a second census round that ran from the January to the October of 2005. All those clients for whom identifying information was available and who were aged ≥18 years at the time of the first census round were sought for follow-up in the second round.
The questionnaires captured information on socio–demographic characteristics, self-reported medical history and symptoms, measured height and weight, and medication usage. No laboratory data were recorded as a part of this study. If, in the second census round, a client was found to have died since the first census round, a verbal-autopsy form was administered, by the CHBC provider, to a member of the client's family (Anon., 1997). The autopsy forms captured information on symptoms experienced prior to death, medical history, and the documented causes of death. Each autopsy form was reviewed by two physicians, who independently coded the primary and contributory causes of death, using a condensed list from the tenth revision of the International Classification of Diseases (Anon., 1997).
Analysis
Data were entered into electronic databases using the Epi Info 2004 software package (Centers for Disease Control and Prevention, Atlanta, GA). They were validated by sampling 10% of the questionnaires, with less than one error/five pages being considered acceptable. Analysis was carried out using Epi Info 2004 and the JMP IN 2005 software package (SAS Institute, Cary, NC). Limited clinical staging was performed using the following criteria (WHO, 2005): weight loss and herpes zoster (stage 2); diarrhoea for >1 month, fever for >1 month, oral thrush, pulmonary tuberculosis within the previous year, and severe bacterial infections (stage 3); and oral or genital ulcers for >1 month, presumed to result from infection with Herpes simplex virus (stage 4). These clinical criteria were recorded by the CHBC providers on the basis of the clients' self-reported clinical histories (during both census rounds), with the corresponding staging assigned during the data analysis. The level of mortality recorded between the census rounds was expressed as deaths/100 person-years of observation. Survival curves were constructed using the Kaplan–Meier method and compared using the log-rank test. Bivariable analysis was performed to determine which of the first-round characteristics of the clients were significantly associated with mortality by the second census round. A P-value of <0.05 was considered indicative of a statistically significant difference.
Research Ethics
Ethical approval for the study was obtained from the Kilimanjaro Christian Medical Centre's Research Ethics Committee, an Institutional Review Board of Duke University Medical Center, and the Tanzania National Institute of Medical Research's National Medical Research Coordinating Committee. All clients included in the second census round gave their written informed consent for use of the data collected on them in both census rounds.
Results
Overall, 378 CHBC clients were surveyed during the initial census round, which was carried out as an internal quality-improvement activity. Although age eligibility (≥18 years at the time of the first round) and the availability of identifying information allowed follow-up to be attempted, in the second census round, on 257 clients, only 226 (88%) of these were successfully followed up. The 31 clients lost to follow-up had all reportedly moved out of the study area between the two census rounds. The median time elapsed between the interviews in the two census rounds was 16.6 months (range=12.1–24.6 months).
Demographic Characteristics
The socio–demographic characteristics recorded, in the first census round, for the 257 clients for whom follow-up was attempted are summarized in Table 1. All districts of the Kilimanjaro Region were represented. Most (75.2%) of the clients for whom follow-up was attempted were female, most (62.4%) were farmers, and most (92.1%) had completed no more than primary education. Only 34% were married. Most (62.3%) spent ≤U.S.$6.68/week on household expenses (Anon., 2007b). The median age of these clients was 38 years (range=20–66 years).
Table 1. The socio–demographic characteristics, in the first census round, of the 257 clients on whom follow-up was attempted in the second census round.
| Characteristic | Value |
|---|---|
| no. and (%) of clients: | |
| Female | 182 (75.2) |
| District of residence | |
| Hai | 50 (19.5) |
| Moshi Rural | 41 (16.0) |
| Moshi Urban | 46 (17.9) |
| Mwanga | 17 (6.6) |
| Rombo | 42 (16.3) |
| Same | 61 (23.7) |
| Occupation | |
| Farming | 146 (62.4) |
| Business | 55 (23.5) |
| Skilled work | 12 (5.1) |
| Other | 14 (9.0) |
| Education | |
| Primary or less | 220 (92.1) |
| Secondary | 19 (7.9) |
| Marital status | |
| Single | 73 (31.5) |
| Married | 80 (34.5) |
| Widowed | 49 (21.1) |
| Other | 30 (12.9) |
| Weekly household expenditure (Tanzanian shillings) | |
| 0–7000 (U.S.$0–6.68) | 154 (62.3) |
| >7000 (>U.S.$6.68) | 93 (37.7) |
| Median age and (range) (years) | 38 (20–66) |
Morbidity
Table 2 depicts the morbidity reported by clients during the first census round. Most (66.9%) of the clients were categorized as being in WHO stage 3, with a history of severe bacterial infections being the most common stage-3-defining condition (52.5% of all clients). The median body-mass index (BMI) was 20.3 kg/m2 (range=12.5–39.3 kg/m2), and having to stay in bed for part of the day over most of the previous month was the predominant functional capacity among the clients (50.4%).
Table 2. Common symptoms and conditions experienced during the first census round among the 257 clients on whom follow-up was attempted in the second census round.
| Symptom/condition | No. and (%) of clients |
|---|---|
| world health organization disease stage | |
| 1 | 26 (10.1) |
| 2 | 31 (12.1) |
| 3 | 172 (66.9) |
| 4 | 28 (10.9) |
| activity level in previous month | |
| No symptoms | 71 (28.9) |
| Had symptoms, but normal activities possible | 51 (20.7) |
| Bedridden for less than half of each day | 22 (8.9) |
| Bedridden for most of each day | 102 (41.5) |
| body-mass index (kg/m2)* | |
| <18.5 (underweight) | 60 (30.2) |
| 18.5–24.9 (healthy weight) | 121 (60.1) |
| 25.0–29.9 (overweight) | 12 (6.0) |
| ≥30.0 (obese) | 6 (3.0) |
| weight change over previous 3 months | |
| Increased | 15 (6.3) |
| Decreased | 196 (81.7) |
| No change | 29 (12.1) |
| Weakness for >1 month | 193 (78.5) |
| Chronic headache | 129 (52.7) |
| Previous bacterial infection | 125 (52.5) |
| Fever for >1 month | 125 (51.4) |
| Skin rash | 96 (39.3) |
| Oral or genital ulcers | 81 (34.6) |
| Herpes zoster in previous 5 years | 70 (29.4) |
| Diarrhoea for >1 month | 69 (27.6) |
| Pulmonary tuberculosis in past year | 66 (27.4) |
| Vaginal candidiasis >1 month | 46 (25.3 of women) |
| Swollen lymph nodes | 57 (23.4) |
| Painful swallowing | 51 (20.9) |
| Oral thrush | 46 (18.6) |
| Urethral discharge in men | 10 (16.7 of men) |
Categorization of underweight, healthy weight, overweight and obese according to Anon. (2006).
The predominant symptoms reported included any weight loss in the previous 3 months (81.7%), weakness for >1 month (78.5%), chronic headache (52.7%), and fever for >1 month (51.4%).
Mortality
Of the 226 clients who were successfully followed up in the second census round, 102 (45%) had died since the first round [Fig. (a)]. There were 51 deaths/100 person-years of observation. The median time to death, since interview in the first census round, was 5.6 months (range=0.2– 25.1 months).
FIG.
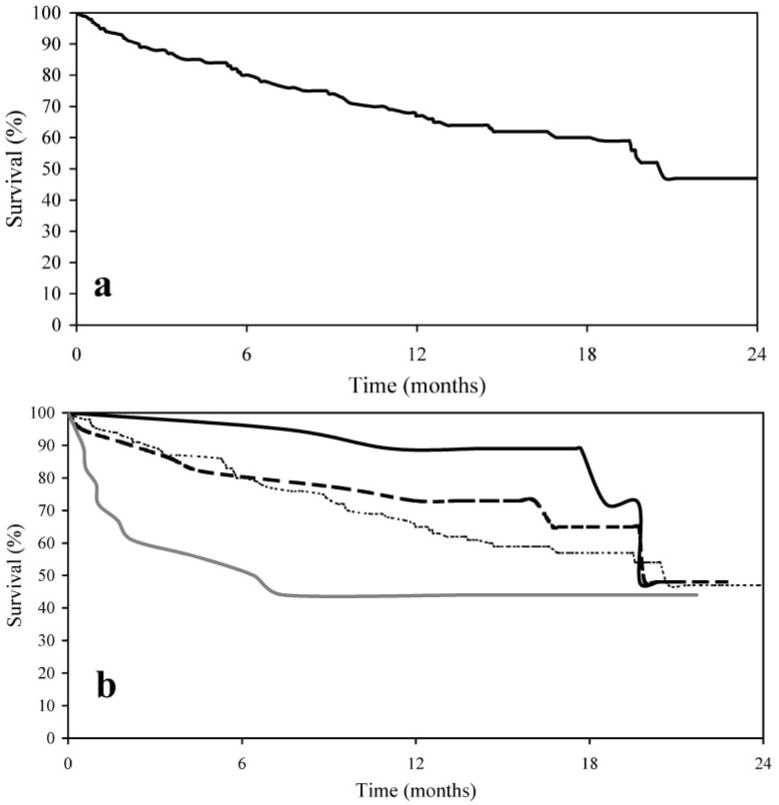
Kaplan–Meier survival curves for 189 of the 226 clients surveyed in both the first and second census rounds for whom follow-up or death dates were available. The curves show the overall results (a) and the results stratified according to whether, in the first census round, the clients were in (World Health Organization) stages 1 (
 ), 2 (
), 2 (
 ), 3 (
), 3 (
 ) or 4 (
) or 4 (
 ) of their HIV disease (b). Of the 189 living clients considered from the first census round (time ‘0’), 149, 125, 115 and 110 remained alive 6, 12, 18 and 24 months later, respectively.
) of their HIV disease (b). Of the 189 living clients considered from the first census round (time ‘0’), 149, 125, 115 and 110 remained alive 6, 12, 18 and 24 months later, respectively.
Causes of Death
Coding of the verbal-autopsy forms (Table 3) revealed that the primary cause of death was infectious for 94 (92.2%) of the deceased clients and non-infectious or unknown for the other eight (7.8%). Among the infectious causes, respiratory and/or gastro-intestinal infections accounted for the majority of the deaths (85.3% of all deaths). Of the deaths attributed to respiratory and/or gastro-intestinal infections, 25 (24.5% of all deaths) were attributed only to acute or chronic diarrhoea, 17 (16.7%) to pulmonary tuberculosis, 11 (10.8%) only to chronic cough, and 31 (30.4%) to a combination of chronic cough and chronic diarrhoea. Among the eight deaths with non-infectious or unknown causes, two (2.0% of all deaths) were attributed to complications of pregnancy/childbirth, and two (2.0%) to suicide. The most common contributory causes of death included malnutrition (81.4%), anaemia (42.2%), infectious skin diseases (38.2%), and liver diseases (26.5%).
Table 3. The primary and contributory causes of death for the 102 clients who died between the first and second census rounds.
| No. and (%) of dead clients | ||
|---|---|---|
| Cause of death* | Primary cause | Contributory cause |
| infectious | 94 (92.2) | – |
| Acute diarrhoea | 10 (9.8) | 9 (8.8) |
| Chronic diarrhoea | 15 (14.7) | 2 (2.0) |
| Chronic cough | 11 (10.8) | 15 (14.7) |
| Chronic diarrhoea and chronic cough | 31 (30.4) | 1 (1.0) |
| Pulmonary tuberculosis, probable or confirmed | 17 (16.7) | – |
| Acute respiratory-tract infection | 3 (2.9) | 4 (3.9) |
| Respiratory disease, unknown cause | – | 1 (1.0) |
| Febrile illness, acute or chronic | 4 (3.9) | 1 (1.0) |
| Infectious skin disease | 2 (2.0) | 39 (38.2) |
| Herpes zoster | – | 1 (1.0) |
| Meningitis | 1 (1.0) | 13 (12.7) |
| non-infectious/unknown | 8 (7.8) | – |
| Maternal death | 2 (2.0) | 2 (2.0) |
| Intentional self-harm/suicide | 2 (2.0) | – |
| Alcohol-related | – | 13 (12.7) |
| Anaemia | – | 43 (42.2) |
| Malnutrition | – | 83 (81.4) |
| Renal failure | – | 6 (5.9) |
| Diabetes mellitus | – | 4 (3.9) |
| Hypertensive diseases | – | 6 (5.9) |
| Blood loss | 1 (1.0) | 5 (4.9) |
| Gastric/duodenal ulcer | – | 2 (2.0) |
| Excessive vaginal bleeding | – | 7 (6.9) |
| Liver disease | 1 (1.0) | 27 (26.5) |
| Skin disease | – | 2 (2.0) |
| Skin cancer | – | 2 (2.0) |
| Lymphoma/leukaemia | – | 2 (2.0) |
| Cancer, unspecified | – | 1 (1.0) |
| Nervous system problem | 1 (1.0) | 8 (7.8) |
| Epilepsy/status epilepticus | – | 2 (2.0) |
| Paraplegia/quadriplegia | – | 2 (2.0) |
| Headache | – | 2 (2.0) |
| Blindness | – | 1 (1.0) |
| Ear disease | – | 1 (1.0) |
| Other family member died of HIV | – | 1 (1.0) |
| Unknown | 1 (1.0) | – |
Results were obtained by coding verbal autopsy forms using a condensed version of the tenth revision of the International Classification of Diseases (Anon., 1997).
Predictors of Mortality
Advanced WHO stage at the first census round was found to be significantly positively associated with between-census mortality [log-rank test χ2=8.115; P=0.044; Fig. (b)]. Certain first-round symptoms were also strongly associated with mortality, the clients who died between censuses being more than twice as likely to have reported weakness for >1 month [odds ratio (OR)= 2.64; P=0.008], oral thrush (OR=2.32; P=0.015), and painful swallowing (OR= 2.02; P=0.036) during the first round than the clients who survived to the second round. Other first-round conditions found to be significantly associated with mortality by the second round included having to stay in bed for part of the day over most of the previous month (OR=1.94; P=0.017), fever for >1 month (OR=1.95; P=0.016), and a history of severe bacterial infections (OR=1.80; P=0.036).
Medication Use
At the start of the present study, ART in Tanzania was only available to those who could afford to pay for it themselves, at a cost of approximately U.S.$35/month. The Tanzanian government began providing free ART to patients with disease of WHO stages 3 or 4 in September 2004 (i.e. shortly before the second census round), starting at referral hospitals and expanding to regional and district hospitals.
At the time of the first census round, ART use — reported by 27 (12.9%) of the clients investigated at that time — was uncommon. It remained just as uncommon by the time of the second census round, when only 15 (12.7%) of the clients were recorded as using ART (P=0.970). The use of SXT prophylaxis was much more wide-spread and increased, among the clients investigated, during the present study. Overall, 108 (44.6%) of the clients investigated in the first census round and 77 (63.6%) of the clients surviving at the time of the second round were recorded as receiving such prophylaxis (at the time of the first and second rounds, respectively; P<0.001). Neither the use of ART at the time of the first round nor the use of SXT at that time was, however, significantly associated with survival to the second round.
Discussion
In the present study, baseline morbidity and mortality in a cohort of HIV-infected adults receiving CHBC in northern Tanzania were found to be high. The majority of the clients had advanced, symptomatic HIV disease for which treatment with ART was indicated. The level of morbidity and causes of mortality indicate that CHBC programmes need to improve access to basic medications and resources, in addition to expanding ART, in order for effective care to be delivered to HIV-infected patients.
The socio–demographic characteristics of the present CHBC cohort were generally representative of the wider Tanzanian population (Anon., 2002), with the majority of clients being farmers and having only a primary or lower level of education. The proportion of women in the cohort was, however, high, which may reflect KIWAKKUKI's original status as a women's organization (although it now serves both men and women). There is some gender bias in HIV infection in Tanzania, with females accounting for 58.5% of the HIV burden in individuals aged ≥15 years (Anon., 2008).
A high level of morbidity was seen at the time of the first census round, with the majority of clients having WHO stage-3 disease and almost half having had to stay in bed for part of the day over most of the previous month. The symptoms reported are similar to those found in a cohort in Malawi, in which 95% of patients were clinically diagnosed with AIDS-related disease and were receiving home-based care (HBC) services from teams consisting of community volunteers and healthcare workers (Bowie et al., 2006). The majority of the patients in the Malawian cohort had disease of WHO stages 3 or 4 (95%), and experienced symptoms including weight loss (94%), headache (74%), and fever (70%). The level of debility in both the Malawian and the present, Tanzanian cohort can be attributed to the progression of HIV disease in the absence of wide-spread ART. In addition, the prevalence and range of symptoms indicate that basic medications and resources, such as antimicrobials, antipyretics and adequate nutrition, were also scarce in the Tanzanian cohort, with many clients probably lacking the financial resources and physical strength to visit centralized medical centres.
In the present study, as described in several other investigations, mortality was found to be associated with advanced WHO clinical stage (Jerene and Lindtjørn, 2005). Weakness, oral thrush, painful swallowing, fever, severe bacterial infections, and being bedridden were all independently associated with mortality. These are same or similar to the predictors of mortality detected in other studies. Lindan et al. (1992), for example, described low BMI, chronic diarrhoea, a history of herpes zoster disease and oral thrush as being associated with mortality in a cohort of HIV-infected women in Kigali, Rwanda.
The mortality rate in the Tanzanian cohort was high but similar, as far as can be seen from the limited data available, to that of other HIV-infected cohorts receiving HBC but without widespread access to ART. Among a cohort of HIV-infected Ugandan children receiving HBC services from a state hospital, for example, O'Hare et al. (2005) observed mortality of 26% over a period of 10 months. Similarly, of the HIV-infected patients receiving HBC in Malawi investigated by Bowie et al. (2006), 56% died within the first 18 months. In contrast, only 5% of a cohort of HIV-infected Ugandan adults receiving SXT and ART as part of their HBC died over a period of 16 weeks (Mermin et al., 2008).
The use of ART by the clients investigated in the present study remained low, even though most of the clients had advanced HIV disease. The Tanzanian government began distributing free ART to government healthcare centres in September 2004, shortly before the second census round. It is therefore likely that, during the period of the present study, patients in rural communities would not have access to such medications. The use of SXT increased over the study period, a trend that may indicate that CHBC services are becoming more informed and effective at providing clients with some essential medications and services. The lack of a statistically significant association between ART or SXT use and survival, although disappointing, is probably the result of factors such as the targeting of medications towards the sicker clients and the sporadic access to such medications.
The primary and contributory causes of death, as well as the conditions associated with mortality, indicate that improved access to ART, as well as the provision of other medications, nutrition and care, is essential. A basic prevention and care package, tailored to the region and resources available, has previously been advocated to reduce morbidity and mortality in HIV-infected patients living in Africa (Mermin et al., 2005). In the present cohort, of HIV-infected patients in northern Tanzania, much of the mortality was attributable to chronic illness and infections of the respiratory and gastro-intestinal systems, with malnutrition and anaemia among the most common contributory causes of death. Besides ART, SXT and isoniazid prophylaxis, micronutrient supplementation and the provision of safe drinking water have been shown to prevent respiratory and gastro-intestinal disease in people living with HIV in Africa, and many such interventions have been shown to be cost-effective (Rose, 1998; Anglaret et al., 1999; Fawzi et al., 2004; Lule et al., 2005). The present results indicate that a treatment package targeted at respiratory and gastro-intestinal disease may be of benefit to HIV-infected patients receiving CHBC.
Following the dissemination of the data collected in the present study to KIWAKKUKI, a CHBC liaison officer was placed fulltime at the Kilimanjaro Christian Medical Centre (KCMC) — the government referral hospital serving northern Tanzania. This liaison officer now helps link CHBC clients with the government services offering free ART. Given the need for a two-pronged approach, with provision of both ART and basic resources, the liaison officer also provides KCMC patients with information on KIWAKKUKI's CHBC programme, through which patients could receive home visits and basic treatment needs. It is recommended that four basic and cost-effective components be incorporated into all CHBC programmes in order to improve care for HIV-infected patients: SXT and isoniazid prophylaxis; micronutrient provision; oral rehydration therapy; and supplies to generate clean water. The trends in morbidity and mortality following the incorporation of such improvements (which would be useful topics for future research) need to be carefully monitored, so that CHBC programmes can be optimised.
Some limitations in the present study must be noted. As mentioned above, most of the clients investigated were female. The main findings are not, however, gender-specific. Approximately 12% of the initial cohort was lost to follow-up, apparently because they had moved out of the region. It is unknown whether the clients lost to follow-up had travelled home to die, or whether they were travelling because they were well. The WHO staging was based on an abbreviated list of criteria. More detailed staging would, however, only be expected to show increased severity of a client's HIV-associated disease.
Along with efforts to scale-up access to ART and prevent the further spread of the HIV epidemic in sub-Saharan Africa, there is great need for basic care and treatment to be delivered to the large numbers of patients living with HIV. CHBC and other alternative methods of delivering healthcare are becoming increasingly important in managing some of this healthcare burden. The present results indicate that countries need to address both basic interventions and ART access when scaling-up CHBC programmes for HIV-infected patients living in resource-limited settings.
Acknowledgments
The authors are grateful to all the clients and their families who participated in the study, and also thank the staff and volunteers of KIWAKKUKI's CBHC programme, who were involved in the implementation of questionnaires. Doctors M. Harbison and S. Sharma are thanked for their efforts in coding the verbal autopsies. The authors are also grateful to NOVIB for funding the home-based care programme at KIWAKKUKI.
The investigators received financial support from the U.S. Department of State Fulbright Program (H.Y.C. and N.M.T.); the National Institutes of Health awards for Units for HIV Clinical Trials Networks (U01 AI069484; N.M.T., J.A.B. and J.A.C.); International Studies of AIDS-associated Co-infections (AI062563; R.A.K., N.M.T., J.F.S., J.A.B. and J.A.C.); the AIDS International Training and Research Program (D43 TW06732; R.A.K., N.M.T., J.F.S., J.A.B. and J.A.C.); the Center for HIV/AIDS Vaccine Immunology (U01 AI067854; J.A.B. and J.A.C.), and the Center for AIDS Research (AI64518; J.A.B.).
References
- Anglaret X, Chene G, Attia A, Toure S, Lafont S, Combe P, Manlan K, N'Dri-Yoman T, Salamon R The Cotrimo–CI Study Group. Early chemoprophylaxis with trimethoprim–sulphamethoxazole for HIV-1-infected adults in Abidjan, Cote d'Ivoire: a randomised trial. Lancet. 1999;353:1463–1468. doi: 10.1016/s0140-6736(98)07399-1. [DOI] [PubMed] [Google Scholar]
- Anonymous. Policy Implications of Adult Morbidity and Mortality: End of Phase One Report. Dar es Salaam: Ministry of Health; 1997. [Google Scholar]
- Anonymous. National Policy on HIV/AIDS. Dar es Salaam: United Republic of Tanzania Prime Minister's Office; 2001. [Google Scholar]
- Anonymous. Household Budget Survey 2000/01. Dar es Salaam: National Bureau of Statistics; 2002. [Google Scholar]
- Anonymous. 2002 Population and Housing Census. Dar es Salaam: National Bureau of Statistics; 2003. [Google Scholar]
- Anonymous. Overweight and Obesity: Defining Overweight and Obesity. Atlanta, GA: Department of Health and Human Services, Centers for Disease Control and Prevention; 2006. [Google Scholar]
- Anonymous. Report on the Global AIDS Epidemic. Geneva: Joint United Nations Programme on HIV/AIDS; 2008. [Google Scholar]
- Anonymous. Service Provision Assessment Survey 2006. Dar es Salaam: National Bureau of Statistics; 2007a. [Google Scholar]
- Anonymous. 2003 United Nations Operational Rates of Exchange. New York, NY: United Nations Treasury; 2007b. [Google Scholar]
- Anonymous. 2008 Report on the Global AIDS Epidemic. Geneva: Joint United Nations Programme on HIV/AIDS; 2008. [Google Scholar]
- Beckmann S, Rai P. HIV/AIDS, Work and Development in the United Republic of Tanzania. Geneva: International Labour Organization Programme on HIV/AIDS and the World of Work; 2004. [Google Scholar]
- Bowie C, Kalilane L, Cleary P, Bowie C. The pattern of symptoms in patients receiving home based care in Bangwe, Malawi: a descriptive study. BMC Palliative Care. 2006;5:1. doi: 10.1186/1472-684X-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu HY, Crump JA, Ostermann J, Oenga RB, Itemba DK, Mgonja A, Mtweve S, Bartlett JA, Shao JF, Thielman NM. Sociodemographic and clinical characteristics of clients presenting for HIV voluntary counselling and testing in Moshi, Tanzania. International Journal of STD and AIDS. 2005;16:691–696. doi: 10.1258/095646205774357307. [DOI] [PubMed] [Google Scholar]
- Fawzi WW, Msamanga GI, Spiegelman D, Wei R, Kapiga S, Villamor E, Mwakagile D, Mugusi F, Hertzmark E, Essex M, Hunter DJ. A randomized trial of multivitamin supplements and HIV disease progression and mortality. New England Journal of Medicine. 2004;351:23–32. doi: 10.1056/NEJMoa040541. [DOI] [PubMed] [Google Scholar]
- Jerene D, Lindtjørn B. Disease progression among untreated HIV-infected patients in South Ethiopia: implications for patient care. MedGenMed. 2005;7:66. [PMC free article] [PubMed] [Google Scholar]
- Kwesigabo G, Killewo JZJ, Sandstrom A, Winani S, Mhalu FS, Biberfeld G, Wall S. Prevalence of HIV infection among hospital patients in north west Tanzania. AIDS Care. 1999;11:87–93. doi: 10.1080/09540129948225. [DOI] [PubMed] [Google Scholar]
- Landman KZ, Kinabo GD, Schimana W, Dolmans WM, Swai ME, Shao JF, Crump JA. Capacity of health-care facilities to deliver HIV treatment and care services, northern Tanzania, 2004. International Journal of STD and AIDS. 2006;17:459–462. doi: 10.1258/095646206777689134. [DOI] [PubMed] [Google Scholar]
- Lindan CP, Allen S, Serufilira A, Lifson AR, van de Perre P, Chen-Rundle A, Batungwanayo J, Nsengumuremyi F, Bogaerts J, Hulley S. Predictors of mortality among HIV-infected women in Kigali, Rwanda. Annals of Internal Medicine. 1992;116:320–328. doi: 10.7326/0003-4819-116-4-320. [DOI] [PubMed] [Google Scholar]
- Lule JR, Mermin J, Ekwaru JP, Malamba S, Downing R, Ransom R, Nakanjako D, Wafula W, Hughes P, Bunnell R, Kaharuza F, Coutinho A, Kigozi A, Quick R. Effect of home-based water chlorination and safe storage on diarrhea among persons with human immunodeficiency virus in Uganda. American Journal of Tropical Medicine and Hygiene. 2005;73:926–933. [PubMed] [Google Scholar]
- Mermin J, Bunnell R, Lule J, Opio A, Gibbons A, Dybul M, Kaplan J. Developing an evidence-based, preventive care package for persons with HIV in Africa. Tropical Medicine and International Health. 2005;10:961–970. doi: 10.1111/j.1365-3156.2005.01488.x. [DOI] [PubMed] [Google Scholar]
- Mermin J, Were W, Ekwaru JP, Moore D, Downing R, Behumbiize P, Lule JR, Coutinho A, Tappero J, Bunnell R. Mortality in HIV-infected Ugandan adults receiving antiretroviral treatment and survival of their HIV-uninfected children: a prospective cohort study. Lancet. 2008;371:752–759. doi: 10.1016/S0140-6736(08)60345-1. [DOI] [PubMed] [Google Scholar]
- O'Hare BAM, Venables J, Nalubeg JF, Nakakeeto M, Kibirige M, Southall DP. Home-based care for orphaned children infected with HIV/AIDS in Uganda. AIDS Care. 2005;17:443–450. doi: 10.1080/09540120412331291779. [DOI] [PubMed] [Google Scholar]
- Ole-Nguyaine S, Crump JA, Kibiki GS, Kiang K, Taylor J, Schimana W, Bartlett JA, Shao JF, Hamilton JD, Thielman NM. HIV-associated morbidity, mortality, and diagnostic testing opportunities among inpatients at a referral hospital in northern Tanzania. Annals of Tropical Medicine and Parasitology. 2004;98:171–179. doi: 10.1179/000349804225003163. [DOI] [PubMed] [Google Scholar]
- Rose DN. Short-course prophylaxis against tuberculosis in HIV-infected persons: a decision and cost-effectiveness analysis. Annals of Internal Medicine. 1998;129:779–786. doi: 10.7326/0003-4819-129-10-199811150-00005. [DOI] [PubMed] [Google Scholar]
- Somi GR, Matee MI, Swai RO, Lyamuya EF, Killewo J, Kwesigabo G, Tulli T, Kabalimu TK, Ng'ang'a L, Isingo R, Ndayongeje J. Estimating and projecting HIV prevalence and AIDS deaths in Tanzania using antenatal surveillance data. BMC Public Health. 2006;6:120. doi: 10.1186/1471-2458-6-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Community Home-based Care in Resource-limited Settings: a Framework for Action. Geneva: WHO; 2002. [Google Scholar]
- World Health Organization. Interim WHO Clinical Staging of HIV/AIDS and HIV/AIDS Case Definitions for Surveillance. Geneva: WHO; 2005. [Google Scholar]


