Summary
Endogenous mechanisms in the resolution of acute inflammation are of interest since excessive inflammation underlies many pathologies. We report a new aspirin-triggered DHA metabolome that biosynthesizes a potent product in inflammatory exudates and human leukocytes, namely aspirin-triggered Neuroprotectin D1/Protectin D1 [AT-(NPD1/PD1)]. The complete stereochemistry of AT-(NPD1/PD1) proved to be 10R,17R-dihydroxydocosa- 4Z,7Z,11E,13E,15Z,19Z-hexaenoic acid. The chirality of hydroxyl groups and geometry of the conjugated triene system essential for bioactivity were established by matching biological materials with stereochemically pure isomers prepared by organic synthesis. AT-(NPD1/PD1) reduced neutrophil (PMN) recruitment in murine peritonitis in a dose-dependent fashion where neither a Δ15-trans-isomer nor DHA was effective. With human cells, AT-(NPD1/PD1) decreased transendothelial PMN migration as well as enhanced efferocytosis of apoptotic human PMN by macrophages. These results indicate that AT-(NPD1/PD1) is a potent antiinflammatory-pro-resolving molecule.
Introduction
Neutrophils (PMN) are first to arrive in the acute inflammatory response and play an important protective role in innate immunity and host defense. However, excessive accumulation of PMN within tissues can lead to tissue damage, amplification of the inflammatory response, injury from within and prolongation of the signs of inflammation (Majno et al., 1982). The control of neutrophil infiltration is of wide interest, as new anti-inflammatory agents are needed to control excess neutrophil responses that can give rise to chronic inflammatory diseases (Dinarello, 2010). Along these lines, evidence was sought for the endogenous mechanism(s) controlling PMN infiltration and natural tissue resolution, since protective PMN (i.e. acute inflammatory responses) are programmed to be self-limited and tightly controlled (Serhan et al., 2002; Serhan and Savill, 2005). Lipid mediators such as prostaglandins and leukotrienes play pivotal roles in the initiation of acute inflammation (Samuelsson, 1983), whereas resolvins and protectins promote and stimulate active resolution (Bazan et al., 2010; Serhan et al., 2002; Serhan and Savill, 2005). In excess, prostaglandins and leukotrienes are pro-inflammatory (Samuelsson, 1983).
Unlike many of the current anti-inflammatories, which delay complete resolution and are considered toxic to this vital process (i.e., resolution toxic) (Gilroy et al., 1999; Schwab et al., 2007), aspirin is unique in that it jump-starts resolution by novel previously unrecognized mechanisms that involve the biosynthesis of aspirin-triggered (AT) lipid mediators (Gilroy and Perretti, 2005; Serhan, 2007). For example, aspirin-triggered lipoxins were the first aspirintriggered lipid mediators uncovered (Clària and Serhan, 1995). Aspirin is well appreciated for its ability to inhibit COX-1 and inactivate this enzyme, blocking both prostaglandin and thromboxane production in cells that possess these biosynthetic pathways (Samuelsson, 1982; Vane, 1982). The mechanism of aspirin’s action involves acetylation of COX within the enzyme’s catalytic region. This prevents alignment of the substrate arachidonic acid for oxygenation within the catalytic center that produces the prostaglandin endoperoxide intermediate (PGG2) required for the biosynthesis of both thromboxanes and prostaglandins via COX-1.
Aspirin’s action within cells that possess COX-2 is different. The catalytic region of COX-2 is larger than that of COX-1 and when acetylated by aspirin the biosynthesis of endoperoxide is blocked. Yet, unlike COX-1, acetylated COX-2 remains active producing lipoxygenase-like products such as 15-HETE from arachidonic acid but with the oxygen insertion in the R configuration rather than S as is the case with lipoxygenases (Clària and Serhan, 1995; Rowlinson et al., 2000). The COX-2-produced 15R-HETE is converted to the potent bioactive aspirin-triggered lipoxins that retain the carbon 15 position alcohol in the R configuration as in AT-15R-(epi)-lipoxin A4, which is produced in vivo in humans (Chiang et al., 2004). AT-LXA4 is longer acting in vivo than LXA4 because the 15R alcohol is less efficiently enzymatically converted to the inactive 15-oxo-LXA4 metabolite (Serhan et al., 1993). This aspirin-triggered pathway was recently demonstrated in humans, where low-dose aspirin is anti-inflammatory and triggers the endogenous biosynthesis of AT-LXA4, which in turn stops PMN infiltration in skin blisters (Morris et al., 2009 and references within).
Docosahexaenoic acid (DHA), an omega-3 fatty acid, is enriched in neural tissues (Bazan, 2006; Lands, 2005; Salem et al., 2001) and is also converted to potent mediators including resolvins and protectins in resolving inflammatory exudates (Serhan et al., 2002). One of the protectins, 10,17-docosatriene, is produced in murine ischemic stroke and is a potent regulator of PMN infiltration, reducing stroke-mediated tissue damage (Marcheselli et al., 2003; Serhan et al., 2002). Given its potent protective actions in the retina and brain, we initially termed this DHA-derived mediator neuroprotectin D1 (NPD1) (Bazan et al., 2010; Mukherjee et al., 2004; Stark and Bazan, 2011). Since this potent chemical mediator has a broader range of activities in the immune, cardiovascular and renal systems, for non-neuronal local biosynthesis and actions we used the name protectin D1 (PD1). The complete stereochemistry and antiinflammatory actions of NPD1/PD1 (10R,17S-dihydroxy-docosa-4Z,7Z,11E,13E,15Z,19Zhexanenoic acid) were unambiguously established (Serhan et al., 2006) and its immunoregulatory roles were demonstrated (Ariel et al., 2006; Ariel et al., 2005; Serhan and Savill, 2005). Of interest, recent results indicate that NPD1/PD1 is also renoprotective (Hassan and Gronert, 2009), induces corneal nerve regeneration (Cortina et al., 2010) and stimulates cardiac and neural stem cell differentiation at nanomolar potencies (Yanes et al., 2010).
Aspirin-triggered resolvins and related ω-3-derived mediators were first identified in selflimited resolving murine exudates as well as brain, and their production documented with isolated human cells (i.e., leukocytes, microglia and vascular cells) (Serhan et al., 2002) and in stroke (Marcheselli et al., 2003). The complete stereochemistry and bioactions of aspirintriggered 10,17-docosatriene remained to be established. Here, we report the complete stereochemistry of the aspirin-triggered protectin pathway from murine exudates and human PMN and report that AT-(NPD1/PD1) displays potent protective bioactions comparable to NPD1/PD1 in vitro and in vivo, reducing both PMN infiltration and enhancing the removal of apoptotic PMN by macrophages.
Results
Stereochemical characterization of synthetic AT-(NPD1/PD1) isomers
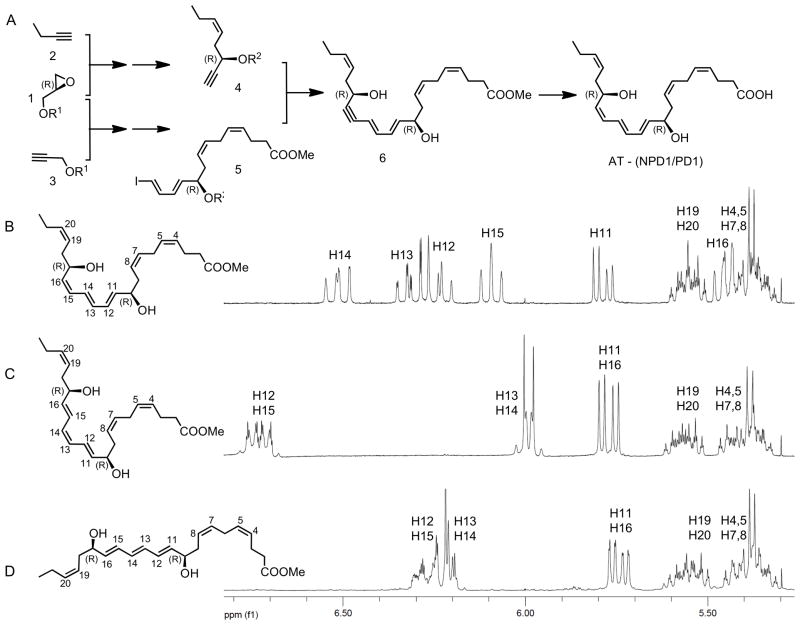
The structure and stereochemistry of each of the synthetic AT-(NPD1/PD1) stereoisomers (Figure 1) was fully confirmed by NMR analysis (Figure 2). Since the hydroxyl group chirality was directly incorporated from an enantiomerically pure starting material, the final R/S assignments at the C-10 and C-17 positions was made unambiguously (e.g. Figure 2A). The isomeric purity and double bond geometry for each stereoisomer was confirmed by NMR spectroscopy. The chemical shifts and coupling constants for each stereoisomer showed a distinct and characteristic profile in the NMR olefinic region in the NMR (Figure 2B,C,D).
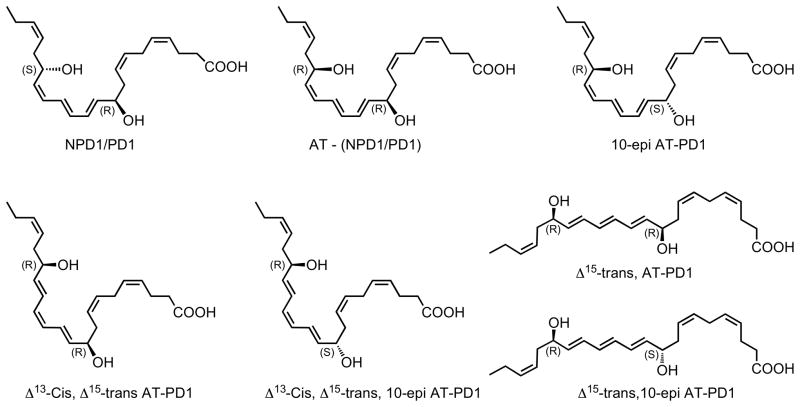
Figure 1. Synthetic protectins prepared for lipidomics and matching.
Neuroprotectin D1/protectin D1, aspirin-triggered neuroprotectin D1/protectin D1 and related isomers.
Figure 2. Strategy for the total organic synthesis of isomerically pure protectin isomers and their stereochemical assignment by NMR spectroscopy.
The total synthesis of AT- (NPD1/PD1) (Panel A) started with the epoxide opening of the R-glycidol derivative 1 by metallated derivatives of alkynes 2 and 3 leading after several steps to the synthesis of key intermediates 4 and 5, which were subjected cross-coupling to form the acetylenic precursor 6, what after selective hydrogenation and ester hydrolysis afforded AT-(NPD1/PD1). The NMR assignments of the olefinic region of three AT-(NPD1/PD1) stereoisomers, are shown in Panels B, C and D, show the anticipated chemical shifts and coupling constants for each isomer.
Stereochemical assignment of AT-(NPD1/PD1)
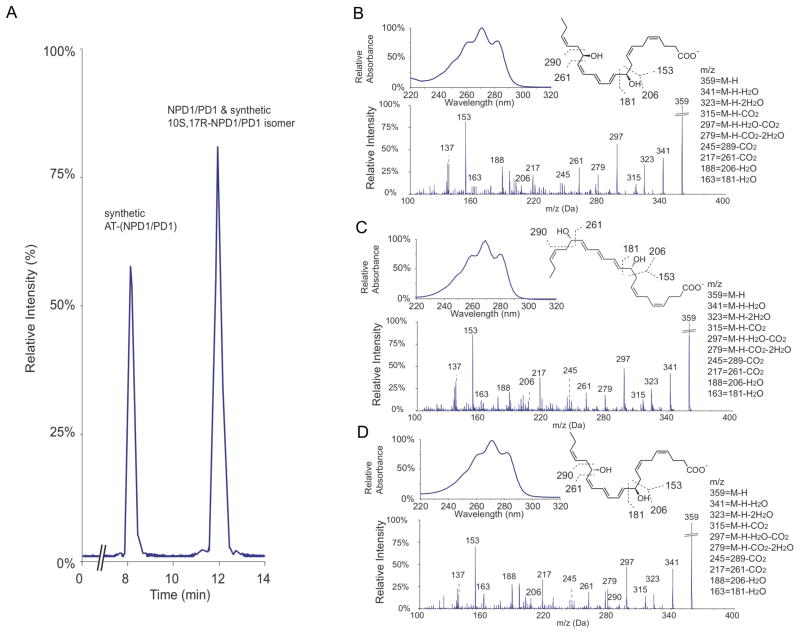
To determine the complete stereochemical assignment and bioactions of AT-(NPD1/PD1), we directly compared the physical and biological properties of DHA derived AT-(NPD1/PD1) and related 10,17 dihydroxy-docosatriene stereoisomers produced by leukocytes to those prepared in stereochemically pure form by total organic synthesis. Figure 3A is a representative MRM analysis of 10R,17R-dihydroxydocosa-4Z,7Z,11E,13E,15Z,19Z-hexaenoic acid (denoted as synthetic AT-(NPD1/PD1)), 10S,17R-dihydroxydocosa-4Z,7Z,11E,13E,15Z,19Z-hexaenoic acid (denoted as 10S,17R-PD1 isomer) and NPD1/PD1. Synthetic AT-(NPD1/PD1) and NPD1/PD1 were separated in this liquid chromatography conditions (for details, see Experimental Procedures), with retention times at 8.2 and 12.0 min respectively, while the 10S,17R-PD1 isomer co-eluted with NPD1/PD1. This 10S,17R-PD1 isomer was found to be biologically inactive (Serhan et al., 2006) and did not co-elute with the aspirin-triggered product; therefore, we next focused on determining the complete stereochemistry of AT-(NPD1/PD1). As expected, spectroscopic properties of synthetic AT-(NPD1/PD1), Δ15-trans-AT-(NPD1/PD1) and NPD1/PD1 are essentially identical (Figure 3B, 3C and 3D, respectively). The UV spectra of synthetic AT-(NPD1/PD1), Δ15-trans-AT-(NPD1/PD1) and NPD1/PD1 displayed essentially the same triplet bands of absorbance with λmax MeOH at ~271nm. Also, tandem mass spectra of these three compounds shared essentially the same fragmentation patterns. The major ions of three tandem mass spectra were assigned as following: m/z 341, 323, 315, 297, and 279 were neutral loss ions, which were consistent with two hydroxyl groups and one carboxyl group of each compound (see figure insets); m/z 153, 181, 206, and their corresponding neutral loss ions, m/z 163 and 188, originated from fragmentations around C-10; and the cleavages between C-16 and C-17 generated m/z 261 and its neutral loss m/z 217. These results are consistent with earlier findings (Hong et al., 2003; Serhan et al., 2006), and also further confirmed the chemical integrity of synthetic AT-(NPD1/PD1). Together, synthetic AT-(NPD1/PD1) and NPD1/PD1 were able to be discriminated using their reverse phase liquid chromatography retention times which were different (Figure 3A), while their UV and mass spectra were virtually indistinguishable, as was that of the biologically inactive AT-(NPD1/PD1) isomer using LC-UV-MS-MS retention times (vide infra).
Figure 3. LC-MS-MS lipidomics and properties of synthetic AT-(NPD1/PD1), NPD1/PD1,and their isomers.
MRM chromatogram (m/z 359 > 153) co-injection of synthetic AT- (NPD1/PD1), 10S,17R-AT-(NPD1/PD1) isomer and NPD1/PD1. AT-(NPD1/PD1) and NPD1/PD1 separate in this LC system with retention times of 8.2 and 12.0 min. Representative tandem mass and UV spectra of synthetic AT-(NPD1/PD1) (B), Δ15-trans-NPD1/PD1 (C) and NPD1/PD1 (D).
Matching of biologic AT-(NPD1/PD1) with synthetic AT-(NPD1/PD1) candidates
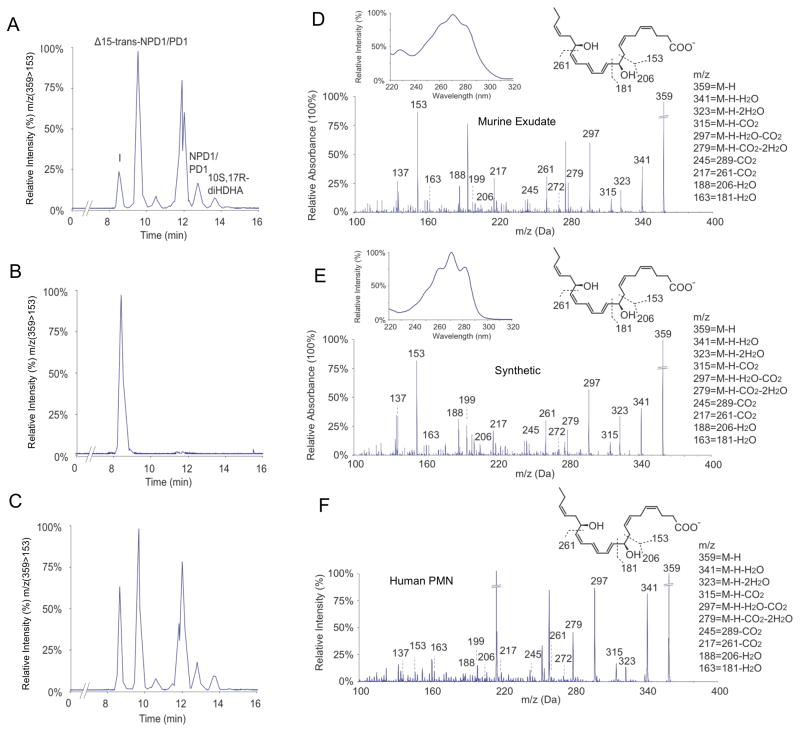
AT-(NPD1/PD1) was identified in resolving murine exudates treated with aspirin (Serhan et al., 2002). Hence biologic AT-(NPD1/PD1) was obtained from resolving murine exudates in vivo. Briefly, peritonitis was initiated via intra-peritoneal (i.p.) administration of zymosan A (1 mg/mouse), and exudates were harvested 24 hours post injection (i.e. within the resolution phase (Bannenberg et al., 2005)). Exudates were subject to solid phase extraction and analyzed using LC-UV-MS-MS-based mediator lipidomics. Representative MRM (m/z 359 > 153) is shown in Figure 4A. Among the candidates, synthetic AT-(NPD1/PD1) matched peak I with retention time at 8.2 min (Figure 4B), which was confirmed by co-injection (Figure 4C). Synthetic AT-(NPD1/PD1) gave a UV spectrum and tandem mass spectrum that was essentially identical to the material beneath Peak I (Figure 4D and 4E), which further confirmed the matching. AT-(NPD1/PD1) was also isolated and identified from activated human leukocytes, namely, aspirin treated human PMN. A representative tandem mass spectrum of the human PMN aspirin triggered product is shown in Figure 4F. Collectively, by matching biologic materials from both human and murine exudates AT-(NPD1/PD1) with synthetic candidates these results established the complete stereochemistry of endogenous AT-(NPD1/PD1) as 10R,17R-dihydroxydocosa-4Z,7Z,10E,12E,14Z,19Z-hexaenoic acid.
Figure 4. LC-MS-MS matching: comparisons for biologic and synthetic AT-(NPD1/PD1).
(A) Representative MRM analysis (m/z 359 > 153) of incubations of cells from zymosan A (1mg/mouse) induced peritonitis with exudate taken at 24 h. Cells (50 × 106/ml) were treated with aspirin (2mM, 20 min, 37ºC), and then incubated with DHA (5 μg/mL) and A23187 (5 μM) for 20 min at 37ºC. Panels B (methyl formate fraction obtained from mouse peritonitis exudates) and C are representative MRM analyses (m/z 375 > 153) of synthetic AT-(NPD1/PD1) and coinjection of synthetic AT-(NPD1/PD1). Panels D and E are representative online UV (insets) and tandem mass spectra of exudate AT-(NPD1/PD1) from mouse peritonitis exudates (Panel D) and synthetic AT-(NPD1/PD1) (Panel E). Panel F, representative tandem mass spectrum of AT- (NPD1/PD1) obtained from incubations of isolated human PMN. Human PMN (50 × 106/ml) were incubated with TNFα (100ng/mL, 4h, 37ºC) followed by aspirin (2 mM), DHA (5 μg/ml), and A23187 (5 μM) for 20 min at 37ºC. (n=3)
AT-NPD1/PD1 limits neutrophil infiltration in vivo
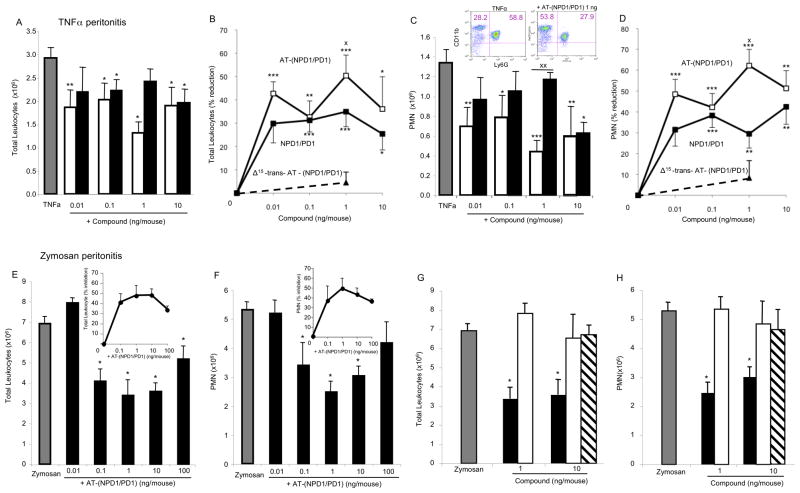
The complete stereochemical assignment for AT-(NPD1/PD1) also relied on determining in parallel its biological actions. Earlier results indicated that NPD1/PD1 exerted potent antiinflammatory actions regulating leukocyte trafficking in murine systems (Hong et al., 2003; Serhan et al., 2006; Serhan et al., 2002). Hence we sought to compare directly the bioactions of NPD1/PD1 to that of AT-(NPD1/PD1) carried out in parallel with the physical matching experiments (Figures 3 and 4). Synthetic AT-(NPD1/PD1) limited PMN infiltration into the peritoneum in TNFα-stimulated peritonitis (Figure 5). Both NPD1/PD1 (0.1 ng-10 ng) and AT-(NPD1/PD1) (0.01ng-10.0 ng) proved to be significant regulators of TNFα-stimulated leukocyte infiltration into the peritoneum. AT-(NPD1/PD1) reduced total leukocyte population of the exudate including PMN, monocyte and lymphocyte infiltrates (Figure 5A and B) reaching a maximal reduction at 1 ng/mouse by as much as 50.4% ±8.8%. The PMN population was also reduced (Figure 5C and D) with AT-(NPD1/PD1) reaching a maximal PMN reduction at 1 ng/mouse (62.2% ±7.8%). Representative flow cytometry dot plots obtained with murine exudates indicated a reduction in the Ly6G+CD11b+ population as compared to TNFαalone (Figure 5C, inset). Of note, theΔ 15-trans-AT-(NPD1/PD1) isomer did not reduce either the total exudate leukocyte population or PMN infiltration (Figure 5B and D, upper panels).
Figure 5. AT-(NPD1/PD1) reduces leukocyte infiltration in peritonitis: direct comparison to NPD1/PD1.
Panels A-D: Peritonitis was initiated by peritoneal injection of 500 ng TNFαalone, TNFαplus synthetic AT-(NPD1/PD1) (0.01 – 10.0 ng) or NPD1/PD1 (0.01 – 10 ng). Peritoneal lavages were obtained at 4 h and leukocytes were enumerated (see Experimental Procedures). (A) Total leukocyte numbers, (B) dose-response: percent reduction of total leukocytes, (C) total PMN numbers and (D) dose-response: percent reduction of PMN infiltration. Representative flow cytometry dot plots (C, inset). TNFα, gray bars; AT- (NPD1/PD1), black bars; NPD1/PD1, white bars. Values are mean + SEM of n=7. Panels E-H: AT-(NPD1/PD1) dose response and direct comparison to DHA. Zymosan A alone (100 μg/mouse), zymosan A plus AT-(NPD1/PD1) (0.01 – 100 ng/mouse) or zymosan A plus DHA (1 and 10 ng/mouse) were injected (i.p.) and lavages were obtained at 4 h. (E and G) Total exudate leukocyte (PMN, monocyte, lymphocyte) numbers, (F and H) total PMN. Panels E and F insets: percent reduction in total leukocytes and PMN. Zymosan A, gray bars; AT-(NPD1/PD1), black bars; DHA, white bars; Δ15-trans-AT-(NPD1/PD1), striped bars. All values mean + SEM of n=3. *p<0.05, **p<0.01, ***p<0.001 versus vehicle; Xp< 0.05, XXp<0.01 AT-(NPD1/PD1) versus NPD1/PD1. Using one-way and two-way ANOVA indicated that AT-NPD1 and NPD1/PD1 were not significantly different from each other, P > 0.05.
Recently, we found that NPD1/PD1 limited PMN infiltration in zymosan A-initiated peritonitis (Serhan et al., 2006). Therefore, we determined whether AT-(NPD1/PD1) also reduced PMN infiltration in zymosan A-stimulated peritonitis. Indeed, AT-(NPD1/PD1) (0.1ng- 100.0 ng) significantly reduced total leukocyte (Figure 5E), as well as PMN infiltration (Figure 5F) reaching a maximal reduction of 47.8 ±10.0% and 49.1 ±11.9%, respectively. In comparison to AT-(NPD1/PD1) (1.0 and 10.0 ng), equal doses of either the precursor DHA or Δ15-trans-AT-NPD1/PD1 did not reduce either total leukocyte infiltration or PMN infiltration (Figure 5G and H). Together, these results indicate that AT-(NPD1/PD1) regulates inflammatory responses induced by the pro-inflammatory cytokine TNFαand the TLR ligand, zymosan A. Moreover, these results indicate that the actions of AT-(NPD1/PD1) and NPD1/PD1 are stereospecific in limiting PMN accumulation in vivo in both murine types of inflammation.
AT-NPD1/PD1 reduced human PMN transendothelial migration and enhanced efferocytosis
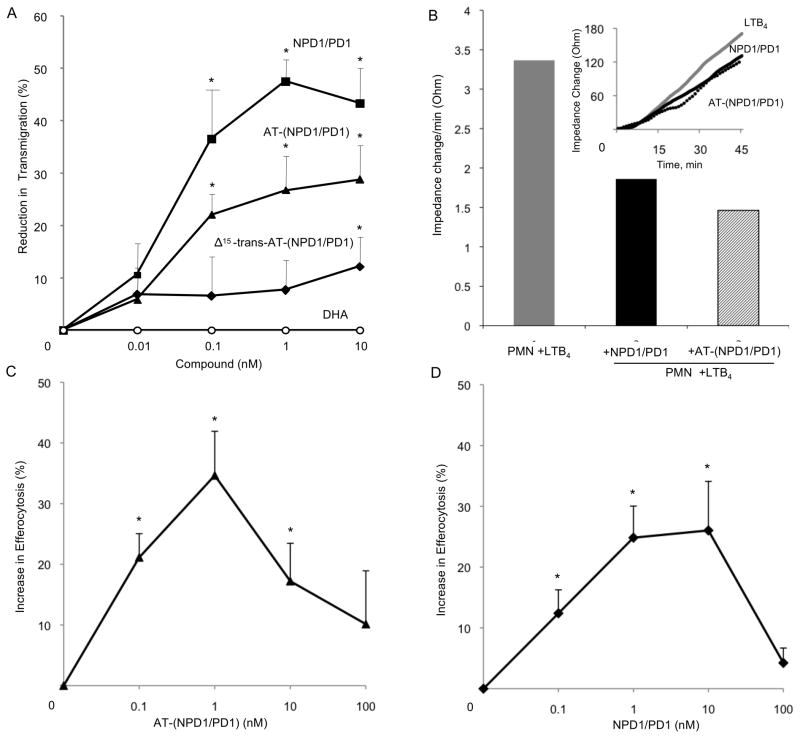
Since this aspirin-triggered compound reduces PMN infiltration in murine systems, we next questioned whether it impacts PMN transendothelial migration using isolated human cells because this is the first committed step of leukocytes in acute inflammation. AT-(NPD1/PD1) and NPD1/PD1 (0.1–10.0 nM) significantly reduced (~30% and ~50%, respectively) PMN transendothelial migration induced by LTB4 (Figure 6A). In comparison equal concentrations of the Δ15-trans isomer of AT-(NPD1/PD1) where the conjugated triene portion of the molecule was in the trans rather than cis configuration did not significantly reduce PMN transendothelial migration. Again in this system, the precursor DHA did not reduce LTB4-stimulated PMNtransendothelial migration (Figure 6A). To corroborate these findings, we next used an electric cell-substrate impedance sensing system (ECIS) that sensitively quantitates cellular responses in two cell systems by real-time monitoring of barrier impedance (Tsikitis et al., 2004). Both AT- (NPD1/PD1) and NPD1/PD1 (1nM) decreased LTB4-stimulated PMN-transendothelial migration by ~40 and 30%, respectively (Figure 6B). AT-(NPD1/PD1) also enhanced the uptake of apoptotic human PMN by human macrophages at concentrations as low as 0.1 nM, as did NPD1/PD1 when compared directly (Figure 6C and D). The response was bell-shaped and consistent with the dose response relationship observed for efferocytosis and proresolving lipid mediators such as RvE1 (Hong et al., 2008).
Figure 6. AT-(NPD1/PD1) limits human PMN transmigration across human endothelial cells and enhances human macrophage efferocytosis of apoptotic human PMNs.
Panel A: Neutrophils (106 cells per monolayer) were exposed to vehicle containing buffer or indicated concentrations of NPD1/PD1 (square), AT-(NPD1/PD1) (triangle), Δ15-trans-AT-NPD1/AT-PD1 (diamond) or DHA (circle) (15 min, pH 7.45, PBS-/-, 37ºC). Transmigration was initiated with addition of 10-8 M LTB4 (90 min, 37ºC). Results are mean ±SEM obtained from 3-5 separate PMN donors, each point in triplicate. Panel B: HUVECs were plated (0.2×106/well) in an ECIS chamber for 24 h. After 24 h, PMN were isolated from human donors and incubated with AT- (NPD1/PD1) or NPD1/PD1 (1 nM) for 15 min, 37ºC. LTB4 (10nM) and PMN (106 cells) were then added to the HUVECS. Impedance changes were monitored using Applied Biophysics ECIS software. (B) Representative real time tracing of impedance changes. Results are mean ± S.E.M. of n=3 separate donors and HUVEC preparations, *p<0.05. Using a two-way ANOVA, NPD1/PD1 was not significantly different from AT-NPD1/PD1 in reducing PMN transendothelial migration, P > 0.05.
Panels C and D: Human macrophages (105 per well) were exposed to vehicle containing buffer or indicated concentrations of AT-(NPD1/PD1) (black triangles) or NPD1/PD1 (black squares) (15 min, pH 7.45, PBS -/-, 37ºC). Uptake was initiated with addition of CFDA-labeled human apoptotic PMNs (3×105/well, 60 min, 37ºC). Increase in efferocytosis was determined by monitoring total fluorescence from PMNs; mean ± SEM, *p < 0.05 vs. vehicle, n = 3 healthy subjects.
Discussion
In this report, we present evidence for the formation, the complete stereochemistry, antiinflammatory and pro-resolving actions of a novel aspirin-triggered DHA metabolome that produces AT-(NPD1/PD1). On the basis of physical and biological properties in matching results with material from human cells, murine exudates and total organic syntheses, the complete stereochemistry of AT-(NPD1/PD1) proved to be 10R,17R-dihydroxydocosa- 4Z,7Z,11E,13E,15Z,19Z-hexaenoic acid (Figures 1–4). The new aspirin-triggered product demonstrated potent regulatory actions with leukocytes in vivo (Figure 5) and reduced human PMN transendothelial migration and enhances efferocytosis (Figure 6). This matching approach was deemed necessary because typically less than nanogram amounts of a given lipid mediator are produced in vivo and >100 μg of isolated product is required for direct NMR determinations of conjugated double bonds that might give assignments for stereochemistry (Serhan et al., 2006; Spite et al., 2009; Sun et al., 2007).
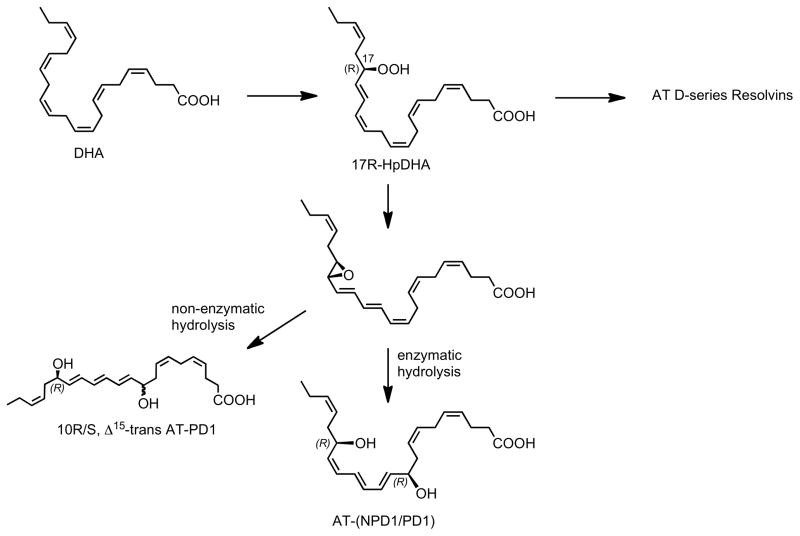
Similar to the inversion of stereochemistry observed in the aspirin-triggered lipoxin and resolvin pathways, we anticipated that AT-(NPD1/PD1) was likely to possess the carbon 17R stereochemistry (Serhan et al., 2002), rather than the 17S chirality present in NPD1/PD1 produced via a lipoxygenase reaction (Bazan et al., 2010). Aspirin acetylation of COX-2 gives rise to a catalytic activity that converts DHA to 17R-hydroxy-containing products (Serhan et al., 2002). It is noteworthy to point out that the chirality at the C-10 position and the double bond geometry of the triene system could not be reliably predicted based on LC-MS-MS lipidomics alone. As proposed for other potent polyunsaturated fatty acid-derived local mediators, it is likely that the aspirin-triggered protectin pathway involves the conversion of the hydroperoxide 17R-HpDHA to an epoxide intermediate (Figure 7). Given the significant conformational differences anticipated for these DHA-derived products, the stereochemical mode of the enzymatic epoxide opening and the resulting triene geometry needed to be verified by matching with isomers of known stereochemistry. In order to achieve the complete stereochemical assignments for the bioactive product(s), therefore, we prepared several geometric isomers of the 10,17R-dihydroxydocosa-4Z,7Z,11,13,15,19Z-hexaenoic acid with specific changes in R/S and Z/E configurations, and confirmed their stereochemistry using NMR spectroscopy (Figures 1 and 2). These synthetic materials were also used for the direct matching and comparison with biologically produced AT-(NPD1/PD1).
Figure 7. Proposed biosynthesis scheme for aspirin-triggered protectins and AT- (NPD1/PD1).
The stereochemistry and anti-inflammatory-proresolving bioactions of the members of this pathway are determined (see text for details). The stereochemistry of the epoxide intermediate is shown in tentative configuration.
This aspirin-triggered pathway was first identified in resolution phase inflammatory exudates and brain tissues of mice treated with aspirin and its basic structure was proposed, e.g. 10,17R-dihydroxydocosa-4Z,7Z,11,13,15,19Z-hexaenoic acid (Marcheselli et al., 2003; Serhan et al., 2002). To confirm this structure elucidation as well as to establish its complete stereochemistry, it is essential to determine its physical and spectroscopic properties and compare them with those of candidates with known stereochemistries using LC-UV-MS-MS (Yang et al., 2011). Among these, 10S,17R-dihydroxydocosa-4Z,7Z,11E,13E,15Z,19Zhexaenoic acid (denoted as 10S,17R-NPD1/PD1 isomer in Figure 3) is the non-superimposable mirror image of NPD1/PD1 (10R,17S-dihydroxydocosa-4Z,7Z,11E,13E,15Z,19Z-hexaenoic acid) (Serhan et al., 2006). Therefore, the two are enantiomers, and as expected they display essentially identical chemical and physical properties in a symmetric environment (Carey and Sundberg, 2007). Indeed, 10S,17R-NPD1/PD1 isomer was indistinguishable from NPD1/PD1 in terms of chromatographic behavior (regular reverse phase liquid chromatography retention times), UV and tandem mass spectrum patterns (Figure 3), yet proved biologically inactive (Serhan et al., 2006) and did not coelute with the authentic human- or mouse-derived AT material with LC-MS-MS (Figure 3A).
The present matching results indicate that in this aspirin-triggered DHA metabolome, the stereochemical configuration of AT-(NPD1/PD1) differs from NPD1/PD1 only at carbon C-17, so the two are diastereomers. Results from earlier investigations have shown that the differences in the MS and UV spectra between lipid mediator diastereomers are minimum if any (Clària and Serhan, 1995; Spite et al., 2009; Sun et al., 2007), which is consistent with the present results. For example, tandem mass spectra of the AT-17R-containing product and NPD1/PD1 were essentially identical, and both compounds displayed essentially the same triplet-band of absorbance-shaped UV spectra with λmax MeOH at 271 nm (Figure 3B and 3C), characteristic of “ol-triene-ol” structures (Borgeat and Samuelsson, 1979; Serhan et al., 2006). Some diastereomers can possess different physical properties (Carey and Sundberg, 2007) by which they can be physically differentiated, Indeed, NPD1/PD1 and its aspirin-triggered isoform were well separated using reverse phase lipid chromatography conditions (Figure 3A). Diastereomers can give different biological properties, such as LTB4 and its natural isomer 12-epi-6-trans- LTB4, which is log orders of magnitude less active (Borgeat and Samuelsson, 1979; Lewis et al., 1981). Along these lines, the aspirin-triggered diastereomer of NPD1/PD1 separated in chromatographic retention time directly compared to NPD1/PD1 but shared its potent biological action (Figures 5 and 6).
We confirmed the actions of this aspirin-triggered response with synthetic AT- (NPD1/PD1), which was potent, giving activity at picogram amounts/mouse (Figure 5). TNFαis a well-known cytokine for its role in host defense, however aberrant or uncontrolled TNFαresponses are associated with several inflammatory disorders (Dinarello, 2010). Although anti- TNF therapies are widely available and in clinical use, it is becoming increasingly clear that a complete blockade of TNFαmay promote adverse side effects (Antoni and Braun, 2002), thus indicating limitations in this approach (Lin et al., 2008). Importantly, AT-(NPD1/PD1) is a potent regulator of PMN infiltration in vivo yet did not completely block PMN recruitment, and hence is not immunosuppressive. This is consistent with the unique counterregulatory actions of resolvins and specialized proresolving mediators that do not compromise host defense via immune suppression of effector cell function (Spite et al., 2009). Of interest, with human PMN transmigration, 1–10 nM amounts of AT-(NPD1/PD1) reduced LTB4-stimulated PMN transmigration, and the Δ15-trans isomer of AT-(NPD1/PD1) was dramatically less potent, underscoring the stereoselectivity of AT-(NPD1/PD1) and the counterregulatory yet not immunosuppressive actions of pro-resolving mediators. Along these lines, NPD1/PD1 promotes corneal epithelial wound healing (Gronert et al., 2005) and regulates adiponectin (González Périz et al., 2009). Given the present results, these processes may also be amenable to AT- (NPD1/PD1) regulation.
In the biosynthesis of protectins, two endogenous forms of the pathway exist, namely the aspirin-dependent as documented here, and the lipoxygenase-initiated pathway for NPD1/PD1 (Bazan, 2006; Bazan et al., 2010; Hong et al., 2003; Mukherjee et al., 2004; Serhan et al., 2002). Hence, NPD1/PD1 joins the other specialized anti-inflammatory–pro-resolving mediators that are biosynthesized in both aspirin-dependent COX-2 routes (i.e., 17R dominant) as well as lipoxygenase-initiated (17S-dominant chirality) routes (Serhan, 2007). COX-2 is induced in human PMN (Pouliot et al., 1998), and in the presence of aspirin the acetylated enzyme converts DHA to a 17R-hydroperoxy-containing DHA-derived intermediate 17R-HpDHA identified as predominantly 17R-hydroxy-containing for its chirality at the carbon 17 position (Serhan et al., 2002). Next, this hydroperoxide intermediate of DHA is converted to both 17R-AT D-series resolvins and/or AT-(NPD1/PD1), as outlined in Figure 7. The biosynthesis of active mediators carrying potent selective actions involves conversion of the 17-hydroperoxy intermediate to a 16R,17R-epoxide that stereoselectively opens with an apparent enzymatic production of AT- (NPD1/PD1) with the E,Z,Z triene configuration (Figure 7). This double bond configuration proved critical for potent bioactivity, since the Δ15-trans-AT-(NPD1/PD1) isomer was essentially inactive.
Taken together the results presented here confirm that the structure of AT-(NPD1/PD1) is 10R,17R-dihydroxy-docosa-4Z,7Z,11E,13E,15Z,19Z-hexaenoic acid. Notably, this assignment implies that the postulated enzyme-catalyzed opening of the 16R,17R epoxide precursor of AT- (NPD1/PD1) would proceed, in theory, via the introduction of the C-10 hydroxyl from the same side as the epoxide oxygen atom to give the 10R,17R configuration, likely from water. This addition would be opposite of the observed opening of the 16S,17S epoxide-containing intermediate precursor of NPD1/PD1 that leads to the 10R,17S configuration. This difference suggests that the C-10 hydroxyl group is introduced via an enzyme-catalyzed process, and that the enzyme involved has a strong preference for the formation of the 10R isomer, rather than the stereochemical inversion of the epoxide precursor. These observations are consistent with the formation of a rigid cationic-type intermediate at the enzyme active site, which undergoes stereospecific attack by a water molecule from the same direction to afford the 10R product. Also, the well known antarafacial hydrogen abstraction in LTA4 biosynthesis in this case to produce the 16,17-epoxide-containing intermediate from DHA might be similar to the biosynthetic mechanism of LTA4 from 5-HpETE as carried out by the 5-LOX (Shimizu et al., 1984; Wetterholm et al., 1995). It is possible in the present scheme (Figure 7) that the 17- hydroperoxide intermediate is converted to an epoxide at C16-C17 position that could give to cis or trans epoxides that require enzymatic processing in situ to give the potent active stereochemical structure of AT-(NPD1/PD1). It is presently not known whether 15-LOX, 12- LOX or other lipoxygenases use the same reaction mechanism as 5-LOX to produce cis-epoxidecontaining LTA4-like intermediates or whether unanticipated new enzymatic mechanisms are involved.
In humans, low-dose aspirin triggers 15-epi-lipoxin production, which reduces PMN infiltration following challenge (Morris et al., 2009) and is formed in healthy subjects (Chiang et al., 2004) as well as demonstrates reduced mortality from colorectal cancer in long-term studies with daily aspirin (Rothwell et al., 2010). Omega-3 EFA (EPA and DHA) at ~1g/day reduce cardiovascular mortality (Skulas-Ray et al., 2011). Thus, the combination of aspirin with omega-3 EFA and the aspirin-triggered metabolome (i.e., AT-LX, AT-Rv) can have a beneficial therapeutic impact in many diseases associated with ongoing inflammation and host-mediated local tissue injury. Indeed, in a recent clinical study, the combination of both aspirin (81 mg/day) and omega-3 EPA and DHA significantly reduced periodontal disease in patients with chronic periodontitis (El-Sharkawy et al., 2010), presumably by jump-starting resolution of periodontal inflammation via production of aspirin-triggered mediators and inhibition of prostanoids (Van Dyke and Serhan, 2006). Hence, it is likely that the aspirin inhibition of prostanoids and concomitant biosynthesis of aspirin-triggered lipid mediators such as AT-lipoxins, AT-resolvins and AT-protectins, including the new AT-(NPD1/PD1) structure established herein, may each contribute to some of these beneficial outcomes.
Significance
The results of the present report establish the stereochemistry of AT-(NPD1/PD1) and several of its natural AT-isomers (Figure 7) generated by human leukocytes and murine tissues during inflammation. Moreover, they demonstrate the potent stereoselective anti-inflammatory and pro-resolving actions of AT-(NPD1/PD1) in regulating PMN tissue accumulation and provide new avenues to mark the impact of DHA and its endogenous anti-inflammatory–proresolving mediators (Serhan, 2007) that can be evoked with aspirin treatment. Reducing PMN excess accumulation in tissues by these new strategies can both shorten the resolution interval of acute inflammation (Schwab et al., 2007; Serhan and Savill, 2005) and impact the transition from innate to adaptive immunity without compromising host defense.
Experimental Procedures
Materials
DHA was from Cayman Chemicals (Ann Arbor, MI). Calcium ionophore, A23187, and aspirin (ASA) were purchased from Sigma (St. Louis, MO). Zymosan A and 5- carboxyfluorescein diacetate, acetoxymethyl ester (CFDA) were from Invitrogen (Carlsbad, CA). Recombinant murine TNFαwas from Peprotech (Rocky Hill, NJ). PerCp-Cy5.5 anti-mouse CD11b (clone M1/70) and FITC-anti-mouse-Ly6G (clone RB6-8C5) were purchased from eBiosciences (San Diego, CA). Costar 5.0 μm transwells were from Corning (Corning, NY). Human umbilical vein endothelial cells (HUVECs) were purchased from Lonza Walkersville Inc. (Walkersville, MD).
LC-MS-MS-based Mediator Lipidomics
LC-UV-MS-MS-based mediator lipidomics was performed using linear ion trap quadrupole mass spectrometer (3200 QTRAP, Applied Biosystems, Foster City, CA) equipped with two HPLC pumps (LC20AD, Shimazu, Columbia, MD) coupled to a reverse phase column (Lunar C18, 150mm × 2mm × 3μm, Phenomenex, Torrance, CA). The mobile phase consisted of methanol:water:acetic acid 70:30:0.01 (v/v/v) with flow rate at 0.2 ml/min; after 25 min, the mobile phase was ramped to 100:0:0.01 over next 5 min, and kept at 100:0:0.01 for 10 min to wash column. All intact cell incubations and in vivo exudates were stopped with 2 volumes of cold methanol and kept at -20ºC for at least 30 min. Samples were extracted using C18 solid phase extraction (SPE) and further analyzed using LC-MS-MS. Detailed procedures and LC-MSMS instrument settings as well as isolation, quantitation, and structural determination of AT- (NPD1/PD1) and related lipid-derived mediators used in the present experiments for elucidation of novel products were as reported in (Hong et al., 2003; Serhan et al., 2006; Serhan et al., 2002).
Preparation of Biologic AT-(NPD1/PD1)
Biologic AT-(NPD1/PD1) was isolated using murine exudates from zymosan-initiated peritonitis as well as human PMN as in (Serhan et al., 2006). Briefly, peritonitis was initiated by intraperitoneal (i.p.) administration of 1mg zymosan A in 1 ml of sterile saline using 6- to 8- week-old FVB male mice (Charles River Laboratories, Wilmington, MA) that were fed Lab Diet 5001. After 24 h, mice were sacrificed, and peritoneal exudates were harvested. All animal experiments were carried out in accordance with the Harvard Medical School Standing Committee on Animals guidelines for animal care (Protocol 02570). Exudate cells (50 × 106 cells/ml) were suspended in 1 ml DPBS+/+ and treated with aspirin (2mM, 20 min, 37ºC) followed by incubation with HPLC-purified DHA (5μg, 5min, 37ºC), and stimulated with A23187 (5μM, 20 min, 37ºC).
Human neutrophils (PMN) were isolated by dextran–Histopaque (1077) double gradient from whole blood from healthy volunteers (de-identified) who denied taking medications 2 weeks before donation (Partners Human Research Committee Protocol no. 88–02642) and anticoagulated with heparin (1U/mL) (Krishnamoorthy et al., 2010). Informed consent was obtained from all donors. To induce COX-2, PMN (50×106 cells/incubation) were stimulated with recombinant human TNFα (10ng/mL) for 4 h, 37ºC as in (Pouliot et al., 1998). Afterward, aspirin (2 mM) was added (20 min, 37ºC) followed by A23187 (5 μM) and DHA (5 μg/mL) and incubated for 20 min, 37ºC. Incubations were stopped with 2 volumes of cold methanol and kept at -20ºC for at least 30 min as above.
Total synthesis and stereochemical characterization of AT-(NPD1/PD1)
Several isomerically pure stereoisomers of NPD1/PD1 having either the 10R,17R or the 10S,17R stereochemistry were prepared by total synthesis, by utilizing enantiomerically pure starting materials and by employing highly stereocontrolled processes (see results in Figures 1 and 2). The hydroxyl group stereochemistry at both the C-10 and C-17 positions was secured from the corresponding protected glycidol derivative. The following isomers were synthesized: 10R,17R-dihydroxy-docosa-4Z,7Z,11E,13E,15Z,19Z-hexanenoic acid, 10R,17R-dihydroxydocosa- 4Z,7Z,11E,13Z,15E,19Z-hexanenoic acid, 10R,17R-dihydroxy-docosa- 4Z,7Z,11E,13E,15E,19Z-hexanenoic acid, 10S,17R-dihydroxy-docosa-4Z,7Z,11E,13E,15Z,19Z-hexanenoic acid, 10S,17R-dihydroxy-docosa-4Z,7Z,11E,13Z,15E,19Z-hexanenoic acid, and (VI) 10S,17R-dihydroxy-docosa-4Z,7Z,11E,13E,15E,19Z-hexanenoic acid. The methyl ester of each isomer was fully characterized by high field NMR spectroscopy that unambiguously confirmed its double bond geometry.
Acute inflammatory exudates: murine peritonitis
Peritonitis was carried out as above. Briefly, the methyl ester of each new synthetic stereoisomer or DHA were each administered via intraperitoneal injection (i.p.) with either zymosan A (100 μg/mL) or TNFα (500 ng/ml) in 1 mL of saline. After 4 h, the mice were sacrificed in accordance with the Harvard Medical Area Standing Committee on Animals (Protocol no. 02570). Peritoneal lavages were harvested immediately, cells were enumerated, cell viability was determined using Trypan blue exclusion, and differential cell counts were monitored by Wright-Giemsa staining and flow cytometry (BD FACS Canto). PMN were identified based on LY6G+CD11b+ staining and analyzed via FlowJo Software.
Human Neutrophil Transmigration
HUVEC confluent monolayers were grown on polycarbonate membranes (5.0-μm pore size, 0.1% gelatin) for 24 hours as in (Gimbrone et al., 1974; Serhan et al., 2006).
PMN were isolated as above and fluorescently labeled with CFDA for 20 min, room temperature. Excess CFDA was removed (1000 rpm, 10 min, RT) and PMN were incubated with either vehicle-containing buffer or compound for 15 min at 37ºC. A chemotactic gradient was established by placing the transwell inserts in media containing 10nM LTB4. PMN (106 cells) were added in the upper chambers, and transmigration was carried out at 37ºC for 90 min. Transmigrated PMN were quantified via fluorescence using a Victor3 plate reader.
Electric cell-substrate impedance sensing (ECIS)
Human PMN and human endothelial interactions were assessed using changes in electric cell-substrate impedance sensing (ECIS) 1600R system (Applied Biophysics). ECIS measures real-time cellular responses by monitoring impedance changes of cells placed atop specific electrodes (Tsikitis et al., 2004; Wang et al., 2010). HUVECs (~0.2 × 106 cells/well) were cultured to confluence (24 h) on an 8W10E+ electrode array. Next, fresh human PMN were isolated (106 cells/well) following venipuncture and exposed to either vehicle containing buffer (EMB serum free media), AT-(NPD1/PD1) (1 nM), or NPD1/PD1 (1 nM) for 15 min, 37ºC. PMN were added to each well and impedance baseline was set at 4 kHz. Cells were then exposed to LTB4 (10 nM) and changes in impedance reflecting transendothelial PMN migration were monitored in real time via ECIS software (v1.2.35.0) for 45 min, 37ºC, 0.5% CO2.
Efferocytosis: Uptake of apoptotic PMN by human macrophages
Monocytes were isolated from human whole blood and cultured in RPMI with 10 ng/mL human GM–CSF (R&D Systems) at 37 ºC for 7 days (Geissler et al., 1989; Krishnamoorthy et al., 2010). For efferocytosis of apoptotic PMNs, isolated human PMNs were allowed to undergo apoptosis in RPMI (2 × 106 cells/mL) for 16–18 h then labeled with carboxyfluorescein diacetate (10 μM, 30 min at 37 ºC; Molecular Probes). Macrophages (1.0 × 105 cells/well) were incubated with either AT-(NPD1/PD1), NDP1/PD1 or vehicle (15 min at 37 ºC). Apoptotic PMNs were added at 1:3 (Macrophage:PMN), and incubations carried out at 37 ºC for 60 min. Supernatants were aspirated and trypan blue (0.03% in DPBS+/+ for ~60 s) was added to quench extracellular fluorescence. Fluorescence was measured using a M3 SpectraMax plate reader (Molecular Devices).
Statistical analysis
The significance of differences between groups was evaluated using the two-tailed Student’s t-test. In addition, two one-way and two-way ANOVA were carried out for evaluating significance of difference among more than two groups and for dose-response curves. P values of less than 0.05 were considered statistically significant.
Highlights.
Aspirin-triggered Neuroprotectin D1/Protectin D1 proved to be 10R,17Rdihydroxydocosa-4Z,7Z,11E,13E,15Z,19Z-hexaenoic acid.
The chirality of carbon-10 and -17 alcohols and geometry of conjugated triene essential for bioactivity of the aspirin-triggered product was established by matching with materials prepared by total organic synthesis.
These results establish a new aspirin-triggered metabolome that can contribute to and enhance aspirin’s anti-inflammatory and pro-resolving actions.
Acknowledgments
We thank Mary H. Small for expert assistance in manuscript preparation. This work was supported in part by National Institutes of Health (NIH, Bethesda, MD) grant no. RC2AT005909 (C.N.S., N.A.P., N.G.B.) The content is solely the responsibility of the authors and does not necessarily represent the official views of NCCAM or the NIH.
Abbreviations used in this paper
- AT
aspirin-triggered
- COX
cyclooxygenase
- LOX
lipoxygenase
- PMN
polymorphonuclear leukocyte
- SPM
specialized pro-resolving mediators
- DHA
C22:6, docosahexaenoic acid
- EPA
C20:5, eicosapentaenoic acid
- NSAID
non-steroidal anti-inflammatory drug
- NPD1/PD1
neuroprotectin D1/protectin D1 (10R,17S-dihydroxy-docosa-4Z,7Z,11E,13E,15Z,19Z-hexaenoic acid)
- AT-(NPD1/PD1)
aspirin-triggered neuroprotectin D1/protectin D1 (10R,17R-dihydroxy-docosa-4Z,7Z,11E,13E,15Z,19Z-hexaenoic acid, determined herein)
- LC-MS-MS
liquid chromatography tandem mass spectrometry
- NMR
nuclear magnetic resonance
Footnotes
Disclosure
C.N.S. is inventor on patents assigned to Brigham and Women's Hospital on the resolvins, protectins, and related compounds, their analogs and uses that are licensed for clinical development. C.N.S retains founder stock in Resolvyx Pharmaceuticals. N.A.P. is inventor on patents assigned to the University of Southern California on the resolvins, protectins, and related compounds, their analogs and uses that are licensed for clinical development. N.A.P. retains stock in Resolvyx Pharmaceuticals. The other authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antoni C, Braun J. Side effects of anti-TNF therapy: current knowledge. Clin Exp Rheumatol. 2002;20:S152–157. [PubMed] [Google Scholar]
- Ariel A, Fredman G, Sun YP, Kantarci A, Van Dyke TE, Luster AD, Serhan CN. Apoptotic neutrophils and T cells sequester chemokines during immune response resolution via modulation of CCR5 expression. Nat Immunol. 2006;7:1209–1216. doi: 10.1038/ni1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariel A, Li PL, Wang W, Tang WX, Fredman G, Hong S, Gotlinger KH, Serhan CN. The docosatriene protectin D1 is produced by TH2 skewing and promotes human T cell apoptosis via lipid raft clustering. J Biol Chem. 2005;280:43079–43086. doi: 10.1074/jbc.M509796200. [DOI] [PubMed] [Google Scholar]
- Bannenberg GL, Chiang N, Ariel A, Arita M, Tjonahen E, Gotlinger KH, Hong S, Serhan CN. Molecular circuits of resolution: formation and actions of resolvins and protectins. J Immunol. 2005;174:4345–4355. doi: 10.4049/jimmunol.174.7.4345. [DOI] [PubMed] [Google Scholar]
- Bazan NG. Cell survival matters: docosahexaenoic acid signaling, neuroprotection and photoreceptors. Trends Neurosci. 2006;29:263–271. doi: 10.1016/j.tins.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Bazan NG, Calandria JM, Serhan CN. Rescue and repair during photoreceptor cell renewal mediated by docosahexaenoic acid-derived neuroprotectin D1. J Lipid Res. 2010;51:2018–2031. doi: 10.1194/jlr.R001131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgeat P, Samuelsson B. Arachidonic acid metabolism in polymorphonuclear leukocytes: Effects of ionophore A23187. Proc Natl Acad Sci USA. 1979;76:2148–2152. doi: 10.1073/pnas.76.5.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey FA, Sundberg RJ. Advanced Organic Chemistry, Part A: Structure and Mechanisms. New York: Springer Science; 2007. [Google Scholar]
- Chiang N, Bermudez EA, Ridker PM, Hurwitz S, Serhan CN. Aspirin triggers anti-inflammatory 15-epi-lipoxin A4 and inhibits thromboxane in a randomized human trial. Proc Natl Acad Sci USA. 2004;101:15178–15183. doi: 10.1073/pnas.0405445101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clària J, Serhan CN. Aspirin triggers previously undescribed bioactive eicosanoids by human endothelial cell-leukocyte interactions. Proc Natl Acad Sci USA. 1995;92:9475–9479. doi: 10.1073/pnas.92.21.9475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortina MS, He J, Li N, Bazan NG, Bazan HE. Neuroprotectin D1 synthesis and corneal nerve regeneration after experimental surgery and treatment with PEDF plus DHA. Invest Ophthalmol Vis Sci. 2010;51:804–810. doi: 10.1167/iovs.09-3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA. Anti-inflammatory agents: present and future. Cell. 2010;140:935–950. doi: 10.1016/j.cell.2010.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sharkawy H, Aboelsaad N, Eliwa M, Darweesh M, Alshahat M, Kantarci A, Hasturk H, Van Dyke TE. Adjunctive treatment of chronic periodontitis with daily dietary supplementation with omega-3 fatty acids and low-dose aspirin. J Periodontol. 2010;81:1635–1643. doi: 10.1902/jop.2010.090628. [DOI] [PubMed] [Google Scholar]
- Geissler K, Harrington M, Srivastava C, Leemhuis T, Tricot G, Broxmeyer HE. Effects of recombinant human colony stimulating factors (CSF) (granulocytemacrophage CSF, granulocyte CSF, and CSF-1) on human monocyte/macrophage differentiation. J Immunol. 1989;143:140–146. [PubMed] [Google Scholar]
- Gilroy DW, Colville-Nash PR, Willis D, Chivers J, Paul-Clark MJ, Willoughby DA. Inducible cycloxygenase may have anti-inflammatory properties. Nat Med. 1999;5:698–701. doi: 10.1038/9550. [DOI] [PubMed] [Google Scholar]
- Gilroy DW, Perretti M. Aspirin and steroids: new mechanistic findings and avenues for drug discovery. Curr Op Pharmacol. 2005;5:405–411. doi: 10.1016/j.coph.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Gimbrone MA, Jr, Cotran RS, Folkman J. Human vascular endothelial cells in culture: growth and DNA synthesis. J Cell Biol. 1974;60:673–684. doi: 10.1083/jcb.60.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Périz A, Horrillo R, Ferré N, Gronert K, Dong B, Morán-Salvador E, Titos E, Martínez-Clemente M, López-Parra M, Arroyo V, et al. Obesity-induced insulin resistance and hepatic steatosis are alleviated by omega-3 fatty acids: a role for resolvins and protectins. FASEB J. 2009;23:1946–1957. doi: 10.1096/fj.08-125674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronert K, Maheshwari N, Khan N, Hassan IR, Dunn M, Schwartzman ML. A role for the mouse 12/15-lipoxygenase pathway in promoting epithelial wound healing and host defense. J Biol Chem. 2005;280:15267–15278. doi: 10.1074/jbc.M410638200. [DOI] [PubMed] [Google Scholar]
- Hassan IR, Gronert K. Acute changes in dietary omega-3 and omega-6 polyunsaturated fatty acids have a pronounced impact on survival following ischemic renal injury and formation of renoprotective docosahexaenoic acid-derived protectin D1. J Immunol. 2009;182:3223–3232. doi: 10.4049/jimmunol.0802064. [DOI] [PubMed] [Google Scholar]
- Hong S, Gronert K, Devchand P, Moussignac RL, Serhan CN. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood and glial cells: autacoids in anti-inflammation. J Biol Chem. 2003;278:14677–14687. doi: 10.1074/jbc.M300218200. [DOI] [PubMed] [Google Scholar]
- Hong S, Porter TF, Lu Y, Oh SF, Pillai PS, Serhan CN. Resolvin E1 metabolome in local inactivation during inflammation-resolution. J Immunol. 2008;180:3512–3519. doi: 10.4049/jimmunol.180.5.3512. [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy S, Recchiuti A, Chiang N, Yacoubian S, Lee CH, Yang R, Petasis NA, Serhan CN. Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proc Natl Acad Sci USA. 2010;107:1660–1665. doi: 10.1073/pnas.0907342107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lands WEM. Fish, Omega-3 and Human Health. 2. Champaign, IL: AOCS Press; 2005. [Google Scholar]
- Lewis RA, Goetzl EJ, Drazen JM, Soter NA, Austen KF, Corey EJ. Functional characterization of synthetic leukotriene B and its stereochemical isomers. J Exp Med. 1981;154:1243–1248. doi: 10.1084/jem.154.4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Ziring D, Desai S, Kim S, Wong M, Korin Y, Braun J, Reed E, Gjertson D, Singh RR. TNFalpha blockade in human diseases: an overview of efficacy and safety. Clin Immunol. 2008;126:13–30. doi: 10.1016/j.clim.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majno G, Cotran RS, Kaufman N, editors. Current Topics in Inflammation and Infection. Baltimore: Williams & Wilkins; 1982. [Google Scholar]
- Marcheselli VL, Hong S, Lukiw WJ, Hua Tian X, Gronert K, Musto A, Hardy M, Gimenez JM, Chiang N, Serhan CN, Bazan NG. Novel docosanoids inhibit brain ischemia-reperfusion-mediated leukocyte infiltration and pro-inflammatory gene expression. J Biol Chem. 2003;278:43807–43817. doi: 10.1074/jbc.M305841200. [DOI] [PubMed] [Google Scholar]
- Morris T, Stables M, Hobbs A, de Souza P, Colville-Nash P, Warner T, Newson J, Bellingan G, Gilroy DW. Effects of low-dose aspirin on acute inflammatory responses in humans. J Immunol. 2009;183:2089–2096. doi: 10.4049/jimmunol.0900477. [DOI] [PubMed] [Google Scholar]
- Mukherjee PK, Marcheselli VL, Serhan CN, Bazan NG. Neuroprotectin D1: a docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc Natl Acad Sci USA. 2004;101:8491–8496. doi: 10.1073/pnas.0402531101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouliot M, Gilbert C, Borgeat P, Poubelle PE, Bourgoin S, Créminon C, Maclouf J, McColl SR, Naccache PH. Expression and activity of prostaglandin endoperoxide synthase-2 in agonist-activated human neutrophils. FASEB J. 1998;12:1109–1123. doi: 10.1096/fasebj.12.12.1109. [DOI] [PubMed] [Google Scholar]
- Rothwell PM, Wilson M, Elwin CE, Norrving B, Algra A, Warlow CP, Meade TW. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet. 2010;376:1713–1714. doi: 10.1016/S0140-6736(10)61543-7. [DOI] [PubMed] [Google Scholar]
- Rowlinson SW, Crews BC, Goodwin DC, Schneider C, Gierse JK, Marnett LJ. Spatial requirements for 15-(R)-hydroxy-5Z,8Z,11Z,13E-eicosatetraenoic acid synthesis within the cyclooxygenase active site of murine COX-2. J Biol Chem. 2000;275:6586–6591. doi: 10.1074/jbc.275.9.6586. [DOI] [PubMed] [Google Scholar]
- Salem N, Jr, Litman B, Kim HY, Gawrisch K. Mechanisms of action of docosahexaenoic acid in the nervous system. Lipids. 2001;36:945–959. doi: 10.1007/s11745-001-0805-6. [DOI] [PubMed] [Google Scholar]
- Samuelsson B. Les Prix Nobel: Nobel Prizes, Presentations, Biographies and Lectures. Stockholm: Almqvist & Wiksell; 1982. From studies of biochemical mechanisms to novel biological mediators: prostaglandin endoperoxides, thromboxanes and leukotrienes; pp. 153–174. [Google Scholar]
- Samuelsson B. Leukotrienes: mediators of immediate hypersensitivity reactions and inflammation. Science. 1983;220:568–575. doi: 10.1126/science.6301011. [DOI] [PubMed] [Google Scholar]
- Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447:869–874. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN. Resolution phases of inflammation: novel endogenous anti-inflammatory and pro-resolving lipid mediators and pathways. Annu Rev Immunol. 2007;25:101–137. doi: 10.1146/annurev.immunol.25.022106.141647. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Fiore S, Brezinski DA, Lynch S. Lipoxin A4 metabolism by differentiated HL-60 cells and human monocytes: conversion to novel 15-oxo and dihydro products. Biochemistry. 1993;32:6313–6319. doi: 10.1021/bi00076a002. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Gotlinger K, Hong S, Lu Y, Siegelman J, Baer T, Yang R, Colgan SP, Petasis NA. Anti-inflammatory actions of neuroprotectin D1/protectin D1 and its natural stereoisomers: assignments of dihydroxy-containing docosatrienes. J Immunol. 2006;176:1848–1859. doi: 10.4049/jimmunol.176.3.1848. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac RL. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter pro-inflammation signals. J Exp Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Rådmark O, Samuelsson B. Enzyme with dual lipoxygenase activities catalyzes leukotriene A4 synthesis from arachidonic acid. Proc Natl Acad Sci USA. 1984;81:689– 693. doi: 10.1073/pnas.81.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skulas-Ray AC, Kris-Etherton PM, Harris WS, Vanden Heuvel JP, Wagner PR, West SG. Dose-response effects of omega-3 fatty acids on triglycerides, inflammation, and endothelial function in healthy persons with moderate hypertriglyceridemia. Am J Clin Nutr. 2011;93:243–252. doi: 10.3945/ajcn.110.003871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spite M, Norling LV, Summers L, Yang R, Cooper D, Petasis NA, Flower RJ, Perretti M, Serhan CN. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature. 2009;461:1287–1291. doi: 10.1038/nature08541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark DT, Bazan NG. Neuroprotectin D1 induces neuronal survival and downregulation of amyloidogenic processing in Alzheimer’s disease cellular models. Mol Neurobiol. 2011;43:131–138. doi: 10.1007/s12035-011-8174-4. [DOI] [PubMed] [Google Scholar]
- Sun YP, Oh SF, Uddin J, Yang R, Gotlinger K, Campbell E, Colgan SP, Petasis NA, Serhan CN. Resolvin D1 and its aspirin-triggered 17R epimer: stereochemical assignments, anti-inflammatory properties and enzymatic inactivation. J Biol Chem. 2007;282:9323– 9334. doi: 10.1074/jbc.M609212200. [DOI] [PubMed] [Google Scholar]
- Tsikitis VL, Morin NA, Harrington EO, Albina JE, Reichner JS. The lectinlike domain of complement receptor 3 protects endothelial barrier function from activated neutrophils. J Immunol. 2004;173:1284–1291. doi: 10.4049/jimmunol.173.2.1284. [DOI] [PubMed] [Google Scholar]
- Van Dyke TE, Serhan CN. A novel approach to resolving inflammation. Sci Am Oral and Whole Body Health. 2006:42–45. [Google Scholar]
- Vane JR. Les Prix Nobel: Nobel Prizes, Presentations, Biographies and Lectures. Stockholm: Almqvist & Wiksell; 1982. Adventures and excursions in bioassay: the stepping stones to prostacyclin; pp. 181–206. [Google Scholar]
- Wang Z, Ginnan R, Abdullaev IF, Trebak M, Vincent PA, Singer HA. Calcium/calmodulin-dependent protein kinase II delta 6 (CaMKIIdelta6) and RhoA involvement in thrombin-induced endothelial barrier dysfunction. J Biol Chem. 2010;285:21303–21312. doi: 10.1074/jbc.M110.120790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetterholm A, Haeggström JZ, Samuelsson B, Yuan W, Munoz B, Wong CH. Potent and selective inhibitors of leukotriene A4 hydrolase: Effects on purified enzyme and human polymorphonuclear leukocytes. J Pharmacol Exp Ther. 1995;275:31–37. [PubMed] [Google Scholar]
- Yanes O, Clark J, Wong DM, Patti GG, Sánchez-Ruiz A, Benton HP, Trauger SA, Desponts C, Ding S, Siuzdak G. Metabolic oxidation regulates embryonic stem cell differentiation. Nat Chem Biol. 2010;6:411–417. doi: 10.1038/nchembio.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R, Chiang N, Oh SF, Serhan CN. Metabolomics-lipidomics of eicosanoids and docosanoids generated by phagocytes. Curr Protoc Immunol. 2011 doi: 10.1002/0471142735.im1426s95. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]