Abstract
The oculomotor and spatial attention systems are inter-connected. Whereas a link between motor commands and spatial shifts in visual attention is demonstrated, it is still unknown whether the recently discovered proprioceptive signal in somatosensory cortex impacts on visual attention, too. This study investigated whether visual targets near the perceived direction of gaze are detected more accurately than targets further away, despite the equal eccentricity of their retinal projections. We dissociated real and perceived eye position using left somatosensory repetitive transcranial magnetic stimulation (rTMS), which decreases cortical processing of eye muscle proprioceptive inflow and produces an underestimation of the rotation of the right eye. Participants detected near-threshold visual targets presented in the left or right visual hemifield at equal distance from fixation. We have previously shown that when the right eye is rotated to the left of the para-sagittal plane, TMS produces an underestimation of this rotation, shifting perceived eye position to the right. Here we found that, in this condition, TMS also decreased target detection in the left visual hemifield and increased it in the right. This effect depended on the direction of rotation of the right eye. When the right eye was rotated rightward and TMS, we assume, shifted perceived gaze direction in opposite direction, leftward, visual accuracy decreased now in the right hemifield. We suggest that the proprioceptive eye position signal modulates the spatial distribution of visual processing resources, producing “pseudo-neglect” for objects located far relative to near the perceived direction of gaze.
INTRODUCTION
The motor command issued to the extraocular eye muscles can bias the allocation of attention in space. For instance, just before the eyes move, visual detection increases in the direction of the planned movement (Deubel & Schneider, 1996; Hoffman & Subramaniam, 1995; Kowler, Anderson, Dosher, & Blaser, 1995). This shift in attention occurs involuntarily, even when optimal task performance would require that the focus of attention remains unchanged (Deubel & Schneider, 1996), suggesting that the motor command can affect the spatial distribution of visual processing resources.
The recent discovery of a proprioceptive eye position signal in somatosensory cortex (Balslev & Miall, 2008; Wang, Zhang, Cohen, & Goldberg, 2007) prompts the question whether this sensory signal of eye position, in analogy with the motor command, impacts on the spatial distribution of attention.
To test whether the perceived direction of gaze modulates the distribution of attention in visual space, it is necessary to present visual targets at equal distance from the fovea, but at different distances from the perceived direction of gaze. We have recently shown that 1 Hz repetitive transcranial magnetic stimulation (rTMS) over left anterior parietal cortex (APC) dissociates the real eye position from the perceived eye position by targeting the proprioceptive representation of the right eye in left somatosensory cortex (Balslev & Miall, 2008). In this previous study, participants viewed an LED array placed 50 cm in front of their right eye in a dark room. They moved a lit LED until they perceived it as being right in front of their nose. After 15 min of 1 Hz TMS over left APC, the location of the LED perceived straight ahead was shifted to the left, corresponding to a shift in perceived position of the right eye by 3° to 4° to the right. This TMS-induced shift in perceived eye position was modulated by passive eye movement, suggesting that TMS over somatosensory cortex reduces the ability to perceive and correct for ocular proprioceptive perturbations.
In monkey somatosensory cortex, each cell that codes eye proprioception is tuned for one radial direction and the firing rate of each cell increases monotonically with ocular eccentricity in its preferred direction. For example, a rightward coding cell’s response increases for increasing angles if eye is rotated from sagittal to the right, but does not change its response despite increasing angles if the eye is rotated from sagittal to the left (Wang et al., 2007). Because the cells with various directional sensitivities are located next to one another, the function across the population of cells is likely to be V-shaped, with higher population firing for larger orbital eccentricities, regardless of their direction. Assuming that human somatosensory cortex has a similar organization, inhibitory TMS will cause a reduction of neuronal firing and an underestimation of the rotation angle of the contralateral eye around the parasagittal direction. This assumption fits with our previous finding. When fixating on a target located in front of the nose and near the eye, binocular convergence dictates that the right eye is rotated leftward. In this condition, TMS shifts the perceived position of the eye toward the right (Balslev & Miall, 2008).
Here we used this procedure to dissociate real and perceived eye positions in order to investigate whether perceived eye position modulates the distribution of attention in the visual space. Subjects detected visual targets presented at equal retinal eccentricity in the left or right visual field while fixating their gaze on a target placed in the mid-sagittal plane at 57 cm from them, corresponding to a rotation of the right eye of approximately 3° to 4° to the left (Experiment 1). We predicted that the TMS would increase visual detection in the right versus left visual field, corresponding to the right-shift in the perceived direction of gaze that we have previously demonstrated (Balslev & Miall, 2008) (Figure 1, left).
Figure 1.
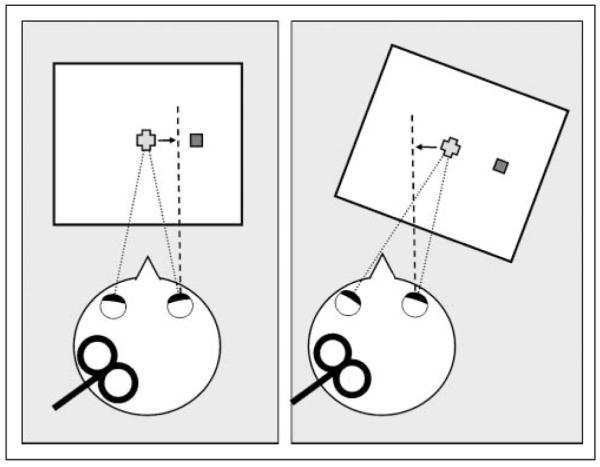
Experimental set-up. Left APC-rTMS produces an underestimation of the angle of rotation of the right eye relative to the parasagittal plane (Experiment 1, left). This displaces the perceived eye position toward the right (dashed line), when the fixation point is central, and toward the left, when the fixation point is 14° rightward (Experiment 2, right). The figure-of-eight coil was positioned over left MC or left APC.
Neurons in APC carry not only eye proprioception but also visual, attention-related, and oculomotor signals. To exclude these potential confounds and to tighten the link between ocular proprioception and the effect of anterior parietal TMS on visual attention, we conducted a second experiment that manipulated perceived gaze position in the opposite direction (Experiment2). Based on the organization of the proprioceptive eye representation in the monkey and in analogy with our previous results, we assumed that when the right eye is rotated to the right, an underestimation of this rotation would shift the perceived eye position to the left (Figure 1, right). Thus, we predicted that if perceived gaze direction is responsible for the effect of anterior parietal TMS on visual attention, then directing the gaze to the right will reverse the effect producing an increase in visual detection in the left relative to the right visual hemifield. However, if the TMS effect is the result of the alteration of the visual, oculomotor, and attention-related signals in left parietal cortex, then altering the direction of gaze should not bias the laterality of the change in visual detection after TMS.
Because the effect of APC rTMS on the actual position of the eyes or on the direction of incidental saccades while fixating a visual target is unknown, we also measured eye position during periods of fixation. A systematic lateral shift in eye position during fixation may change the relative eccentricity of the targets producing changes in visual accuracy that do not reflect a change in visual attention. Similarly, because saccade planning is known to bias visual attention in the direction of a saccade (Deubel & Schneider, 1996; Hoffman & Subramaniam, 1995; Kowler et al., 1995), an eventual effect of rTMS on visual attention may be mediated by a change in the direction of the incidental saccades. To investigate the effect of TMS on the position of the eyes at fixation, as well as on the direction of the incidental saccades and microsaccades, we recorded eye position before and after TMS.
To rule out a potential effect of rTMS on the motor representation of the extraocular muscles, we used primary motor cortex (MC) as a control site. The location of this control site, anterior to APC, and thus, closer to the eye, also rules out any direct effect of TMS on the eye muscles. We predicted that MC TMS would have no effect on visual detection.
METHODS
Subjects
All participants were healthy, right-handed adults with normal vision who gave written informed consent to participate in the study. The study was approved by the School of Psychology Ethics Committee at the University of Birmingham. We recruited 6 participants (median age = 27 years, range = 19–53 years, 2 women, 5 right eye dominant) for Experiment 1 and 10 participants (median age = 22.5 years, range = 19–53 years, 6 women, 8 right eye dominant) for Experiment 2. One participant took part in both experiments.
Design
We conducted two experiments that tested visual detection. The experiments differed by the position of the right eye in the head at fixation. In Experiment 1, the right eye was rotated to the left by ~3° to 4° similar with our previous experiment (Balslev & Miall, 2008). In Experiment 2, the right eye was rotated to the right by ~10° (Figure 1).
Participants performed a cued attention task, repeated identically before and after 15 min of off-line rTMS. Thus, the design of each experiment was 2 × 2 × 2 × 2 factorial with factors: (i) target side (left vs. right), (ii) cue validity (valid vs. invalid), (iii) session (pre-TMS or post-TMS), and (iv) area where rTMS was applied (APC or MC). We used an exogenous cue to increase the sensitivity of the task for lateral changes in visual detection. Thus, we hypothesized that in Experiment 1, for instance, not only the rightward targets would be more salient than the leftward targets, but also the rightward cues would be more salient than the leftward cues. Because of the difference in the salience of the cues, the rightward validly cued targets would be better detected than the leftward valid targets and the rightward invalidly cued targets would be better detected than the leftward invalid targets. The right–left difference in the salience of the cues would thus produce a bias in visual detection in the same direction as the right–left difference in the salience of the targets, synergistically enhancing the magnitude of this bias.
Task
Participants sat in front of an LCD screen (1024 × 768 pixels, 29 × 36 cm, 32° × 40° visual angle) positioned horizontally on the table with its center at 57 cm from their eyes. Hence, the binocular convergence angle was about 6° to 8°. The head was in a chin rest. The short axis of the screen was either parallel with the participant’s mid-sagittal plane (Experiment 1) or angled at 14° to the right of it (Experiment 2, Figure 1). Fourteen degrees was the largest possible angle of gaze where the participants were still able to see the whole display given our set-up. The participants placed their head in a chin rest andtheir right hand on the table near their body’s midline. They used their left hand to respond. A fixation cross (0.6° × 0.6°) was presented at the center of the screen for a pseudorandom duration between 501 and 1000 msec. A cue (red empty square, 1° × 1°) was then shown at 14° visual angle to the left or right of the fixation cross for 40 msec, then extinguished. After a 100-msec delay, a gray-scale target (0.5° × 0.5°) was presented for 80 msec either at the same location as the cue (valid trials) or on the opposite side of the screen, symmetrical to the cue (invalid trials). Immediately after the target was presented, two masks (red empty squares, 1° × 1°) were shown for 100 msec at both locations where the target could have appeared. The participants responded by pressing one of two keys with their index and middle fingers, the left-side key for left targets and the right-side key for right targets. They were required to refrain from pressing any key if they were not sure they saw the target. To check their compliance with this instruction, there were catch trials where no target was presented. Participants had 1150 msec to respond before moving to the next trial. The hit rate and the false positive rate for the responses were calculated. To measure lateral asymmetries in visual detection, we computed a laterality index as HR − HL/HR + HL, where HR and HL were hit rates for right and left targets, respectively. This laterality index has values between +1 and −1; the more positive the value, the larger the rightward bias and the more negative, the larger the leftward bias.
Either session (pre- or post-TMS) comprised 134 trials for Experiment 1 and 72 trials for Experiment 2. Of those, six trials (Experiment 1) and four trials (Experiment 2) had no target presentation (catch trials). The rest of the trials were divided equally between conditions (validly cued left-side target, invalidly cued left-side target, validly cued right-side target, and invalidly cued right-side target) and presented in randomly interleaved order. Fewer trials were included in Experiment 2 due to the additional eye tracking task (see below for details). Each session took less than 8 min to complete.
Before the experiment, participants practiced the task for 10 min. During this practice interval, we ran the Quest algorithm (Watson & Pelli, 1983) with each participant to adjust the contrast of the gray-scale target to correspond to a hit rate of 30%. The rationale for this procedure was to avoid floor or ceiling effects.
Eye Tracking
During Experiment 2, the participants wore a head-mounted eye tracker (IRIS, Skalar Medical, Delft, The Netherlands) connected to the computer through an analog input board. The input was filtered through a 100-Hz low-pass filter, digitized to 12-bit resolution, and then sampled at intervals of 5msec (200 Hz).
In order to assess the effect of TMS on eye position, horizontal eye-in-head position was recorded on-line during each trial of Experiment 2 for the first 500 msec of the 501–1000 msec fixation period before the presentation of the cue. To assess eye position during mid-sagittal fixation, we conducted a control experiment with an identical set-up as Experiment 1, where participants were instructed to fixate a cross while eye position was recorded for 60 sec. The recording was performed within the same session as Experiment 2, following both the pre- and post-TMS visual attention task described above.
Participants were seated with the head in a chin rest to align the head midline to their trunk mid-sagittal plane. The position of the head was verified at the beginning of each session using a laser pointer attached to the eye tracker. The head was then fixed in position using a bite bar. Before each session (pre- or post-TMS) and for each position of the screen (mid-sagittal or rightward), the eye tracker was calibrated by asking the participants to move their eyes to follow a dot (0.5° × 0.5°) that jumped between a set of seven predefined screen locations in random order, each location being presented twice. The locations were at the screen center and at 2°, 4°, and 6° from center to the left and to the right on the screen horizontal midline. A second-order polynomial was fit to the eye tracker data and its co-efficients were used for calibration.
We did not use an eye tracker in our main experiment (Experiment 1) in order to maximize the number of task trials presented in the effective post-TMS time window. A conservative rule of the thumb is to assume that the effect of rTMS would last for about 50% of the duration of stimulation (Robertson, Theoret, & Pascual-Leone, 2003). Thus, after 15 min of TMS, there is an effective time window of 7 to 8 min for the post-TMS session. After TMS repositioning and calibrating the eye tracker took a maximum of 2 min. To accommodate the delay introduced by setting up the eye tracker, calibrations, and the 1-min recording of eye position, the number of trials in Experiment 2 was reduced to approximately half of the number of trials used in Experiment 1. The task during Experiment 2 took about 4 min to complete.
Eye Movement Analysis
For each session, data were averaged over the two eyes after removing linear drift from each monocular dataset. In order to investigate the effect of TMS on the position of the eyes at fixation, as well as on the direction of the incidental saccades and microsaccades, we parsed eye position time series into fixations, saccades, glissades, and blinks using a velocity criterion (Nystrom & Holmqvist, 2010). We computed the mean eye position at fixation and the proportion of saccades directed to the left or right from fixation. A laterality index for the direction of the saccades was computed as NR − NL/NR + NL, where NR and NL were the number of saccades directed to the right and left, respectively.
Transcranial Magnetic Stimulation
Each rTMS session consisted of 900 biphasic stimuli produced by a Magstim Rapid Stimulator (Dyfed, UK) and delivered with a frequency of 1 Hz over 15 min. One of two identical, standard 70-mm-diameter figure-of-eight coils was centered over the stimulation site, and maintained in this position by a coil holder; the participant’s head was restrained by a chin rest and forehead rest-pad. The stimulation site was mapped in each subject in relation to the “motor hotspot” of the left hemisphere, which is the scalp projection of primary MC. The motor hotspot was defined as the point of maximum evoked motor response in the relaxed first dorsal interosseus (FDI) muscle of the right hand. The anterior parietal site of stimulation (APC) was located 3 cm posterior to the motor hotspot, measured on a line oriented at 45° from the sagittal plane and perpendicular to the central sulcus (Balslev & Miall, 2008). The placement of the coil is compatible with our recent fMRI data that show a proprioceptive representation for the right eye in left sensorimotor cortex at approximately 3 cm posterior and inferior to the motor hotspot for the right hand (Balslev, Albert, and Miall, in press). The control site was at the motor hotspot. Stimulation intensity was set at 110% of resting motor threshold of the right FDI muscle. To identify the threshold, the subjects were asked to rest the right hand on the table with the fingers slightly spread. The resting motor threshold was then defined as the lowest intensity that elicited a visible twitch in the FDI muscle in three out of five trials when the stimulation was given over the motor hotspot. Careful visual inspection has previously been shown to provide similar threshold estimates as EMG recordings do (Balslev, Braet, McAllister, & Miall, 2007; Pridmore, Fernandes Filho, Nahas, Liberatos, & George, 1998). During rTMS, the coil was positioned tangential to the scalp with the long axis of the figure-of-eight coil oriented 45° to the sagittal plane. The current flow of the initial rising phase of the biphasic pulse in the TMS coil induced a current flowing from posterior to anterior in the brain. During each session, the active coil was exchanged for the spare coil after 4 and 11 min of rTMS to avoid overheating. All subjects were tested within a period of 8 min after the cessation of the rTMS train. For Experiment 1, the median resting motor threshold was 55% stimulator output (range 47–65%) for APC stimulation and 51% (48–65%) for MC stimulation. For Experiment 2, the resting motor threshold was 59.5% (48–68%) for APC stimulation and 56.5% (45–67%) for MC stimulation. Each participant received rTMS at both the active site and the control site on two separate days. The order was counterbalanced across participants.
RESULTS
Experiment 1
A summary of the pre- and post-TMS hit rates is given in Table 1. Before TMS, the laterality index was −0.02 ± 0.09 (mean and standard deviation; Figure 2), not significantly different from zero (one-sample t test, p > .5). APC-rTMS significantly increased the laterality index to 0.21 ± 0.31 (Wilcoxon signed rank test, p = .02), thus increasing visual detection in the right relative to the left visual hemifield. This change was specific to APC-rTMS. Thus, after rTMS over the control area in MC, the laterality index was −0.01 ± 0.15, showing no difference from its pre-TMS value (Wilcoxon signed rank test, p > .6) (Figure 2). Pairwise comparisons pre- versus post-TMS confirmed the increase in hit rate in the right visual field (Table 1, Wilcoxon signed rank test, p = .04), as well as the decrease in hit rate in the left visual field (Wilcoxon signed rank test, p =.02).
Table 1.
Hit Rate (%) before and after TMS
| Anterior Parietal Cortex |
Motor Cortex |
|||
|---|---|---|---|---|
| Left Hemifield |
Right Hemifield |
Left Hemifield |
Right Hemifield |
|
| Experiment 1 | ||||
| Pre-TMS | 37.7 (13.7) | 34.1 (14.1) | 40.8 (13.6) | 42.1 (16.2) |
| Post-TMS | 29.9 (16.3)* | 45.1 (15.6)* | 36.4 (9.6) | 37.21 (15.16) |
| Experiment 2 | ||||
| Pre-TMS | 37.1 (19.1) | 49.1 (21.7) | 32.9 (23.1) | 42.6 (27.5) |
| Post-TMS | 35.5 (21.4) | 34.1 (29.3)* | 30.2 (27.6) | 47.3 (33.8) |
The table shows the mean and the standard deviation values.
Post- vs. pre-TMS pairwise comparisons significant at p <.05 (Wilcoxon signed rank test).
Figure 2.
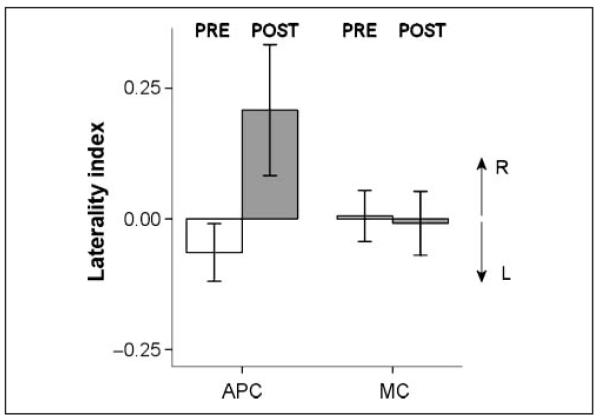
Experiment 1. The change in laterality index [(HR – HL)/(HR + HL), where HR and HL were hit rates for right and left targets, respectively] from pre- to post-TMS sessions for the APC and MC stimulation. The right eye was rotated to the left of the sagittal plane. White bars = pre-TMS session. Gray bars = post-TMS session. Positive values for laterality index correspond to larger hit rates in the right versus left visual field. Error bars show one standard error.
Experiment 2
A summary of the pre- and post-TMS hit rates is given in Table 1. The laterality index in the pre-TMS data was 0.11 ± 0.36 (mean and standard deviation; Figure 3), not significantly different from zero (one-sample t test p > .3), and not significantly different from the laterality index of the pre-TMS data of Experiment 1 (independent-samples t test, p >.3).
Figure 3.
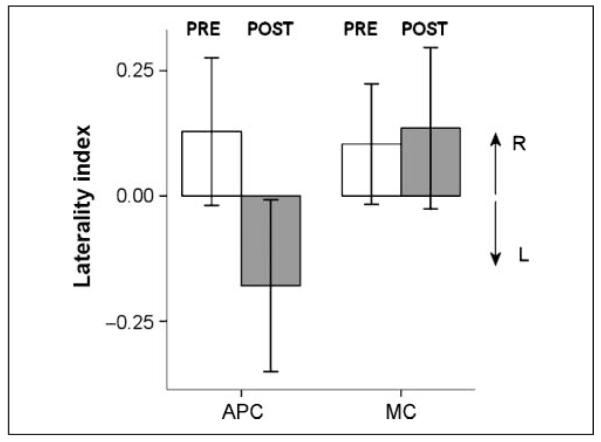
Experiment 2. The change in laterality index after TMS of APC versus MC. The right eye was rotated to the right of the sagittal plane. Same notation conventions as in Figure 2.
As predicted, and in contrast with Experiment 1, APC-rTMS now biased visual detection toward the left, decreasing the laterality index to −0.17 ± 0.54 (Wilcoxon signed rank test, p = .03). This decrease was specific for APC-rTMS. Thus, MC rTMS did not significantly change the laterality index (0.13 ± 0.5, p >.7) (Figure 3). Pre- versus post-TMS pairwise comparisons confirmed the decrease in hit rate in the right visual field (Table 1; Wilcoxon signed rank test, p = .04). The hit rate in the left visual field, however, was unchanged ( Wilcoxon signed rank test, p >.5).
Cue Validity Effects
The hit rate for validly cued targets was higher than for invalid targets for both experiments [2 × 2 × 2 × 2 ANOVA, main effect of cue validity: F(1, 5) = 12.62, p =.01 for Experiment 1, and F(1, 9) = 23.75, p = .001 for Experiment 2]. The reason for using a cued-attention paradigm here was to increase the sensitivity of the task to detecting lateral asymmetries in visual detection. Thus, we did not expect TMS to change the effect of the cue on visual detection. Indeed, although the hit rates in both experiments showed a significant main effect of cue validity, there was no statistically significant three-way interaction between Cue validity × TMS session × Brain area, or a four-way interaction between Cue validity × Target side × TMS session × Brain area ( p > .6, both experiments). In line with our prediction that APC-rTMS would induce a lateral difference in hit rates regardless of cue validity, both these analyses of hit rates yielded a significant three-way interaction between Target side × TMS session × Brain area [Experiment 1: F(1, 5) = 8.75, p = .03; Experiment 2: F(1, 9) = 6.35, p = .03].
False Alarm Rate
The mean accuracy of catch trials was 95.83% for Experiment 1 and 75% for Experiment 2. A false-positive response was defined as a keypress indicating that the target was presented in one visual field even though no target had been presented there and could occur during either a catch trial or a trial where the target was presented in the opposite visual field. For Experiment 1, the mean false-positive rate was under 1% for either false-left or false-right responses across all sessions and all subjects. For these false responses, we found no significant three-way interaction between brain area, TMS session, and visual hemifield ( p > .5). For Experiment 2, participants made, on average, 1.62% false-left responses and 6.15% false-right responses across all sessions and all subjects. There were significantly more false-right than false-left responses [main effect of target side: F(1, 9) = 8.2, p = .01]. This right bias in response in Experiment 2 may be related to the participants’ gaze being directed toward the right. Notably, APC-rTMS had no effect on the left–right distribution of false alarms [2 × 2 × 2 × 2 ANOVA, interaction between brain area, TMS session and visual hemifield: F(9, 1) = 1.13, p >.3].
Reaction Times
The instruction to the participants emphasized accuracy rather than speed of response, therefore, we have analyzed our data in terms of hit rate. For a more complete picture of the data, a summary of the unspeeded reaction times is given in Table 2. We did not find any statistically significant difference in these reaction times between pre- and post-TMS conditions (Wilcoxon signed rank test, p >.1).
Table 2.
Unspeeded Reaction Time (msec) before and after TMS
| Anterior Parietal Cortex |
Motor Cortex |
|||
|---|---|---|---|---|
| Left Hemifield |
Right Hemifield |
Left Hemifield |
Right Hemifield |
|
| Experiment 1 | ||||
| Pre-TMS | 814 (186) | 796 (186) | 804 (169) | 757 (123) |
| Post-TMS | 770 (180) | 740 (180) | 726 (220) | 723 (180) |
| Experiment 2 | ||||
| Pre-TMS | 821 (137) | 867 (115) | 882 (96) | 843 (110) |
| Post-TMS | 820 (107) | 791 (73) | 834 (127) | 793 (123) |
The table shows the mean and the standard deviation. None of the pre vs. post pairwise comparisons were statistically significant (paired-samples t tests, p > .05).
Eye Position and Saccade Direction
A summary of the position of the eyes at fixation is given in Table 3.
Table 3.
Eye Position at Fixation (Degrees Visual Angle) before and after TMS
| APC | MC | |
|---|---|---|
| Mid-sagittal Fixation | ||
| Pre-TMS | 0.3 (1.5) | −0.6 (1.8) |
| Post-TMS | 0.4 (2.3) | −1.4 (2.6) |
| Rightward Fixation | ||
| Pre-TMS | 0.1 (3.4) | 0.1 (2.9) |
| Post-TMS | −0.7 (3.1) | −1.6 (2.2) |
The table shows the mean and the standard deviation. Negative values are to the left of the fixation cross. None of the pre vs. post pairwise comparisons were statistically significant (paired-samples t tests, p >.05). APC = anterior parietal cortex; MC = motor cortex.
None of the pre- versus post-pairwise comparisons were significant (paired-samples t tests, p > .1). The full 2 × 2 × 2 ANOVA on eye position data yielded a non-significant three-way interaction between direction of gaze at fixation (Experiment 1 vs. 2), TMS session, and brain area ( p >.5).
Before TMS, the frequency of incidental saccades and microsaccades was 36.1 ± 20.8 saccades/minute for mid-sagittal fixation and 81.4 ± 37.1 saccades/minute for rightward fixation. These values are in line with the previous reports on the mean frequency of microsaccades around 18–72/minute (Abadi & Gowen, 2004; Engbert & Kliegl, 2003).
A summary of the laterality index for the incidental saccades during the period of fixation is given in Table 4. None of the pre- versus post-TMS pairwise comparisons were significant (paired-samples t tests, p > .1). The full 2 × 2 × 2 ANOVA on the laterality index for saccade direction yielded a nonsignificant three-way interaction between Direction of gaze × TMS session × Brain area ( p >.3).
Table 4.
Laterality Index for the Direction of the Incidental Saccades during Fixation before and after TMS
| APC | MC | |
|---|---|---|
| Mid-sagittal Fixation | ||
| Pre-TMS | 0.2 (0.4) | −0.1 (0.4) |
| Post-TMS | 0.2 (0.3) | 0.2 (0.3) |
| Rightward Fixation | ||
| Pre-TMS | 0.1 (0.2) | −0.1 (0.2) |
| Post-TMS | 0.1 (0.1) | −0.1 (0.2) |
The table shows the mean and the standard deviation of the laterality index for saccade direction calculated as NR − NL/NR + NL, where NR and NL were the number of saccades directed to the right and left, respectively. None of the pre vs. post pairwise comparisons were statistically significant (paired-samples t tests, p > .05). APC = anterior parietal cortex; MC = motor cortex.
DISCUSSION
We have previously shown that left anterior parietal rTMS shifts the perceived eye position to the right of its real position when fixating a target located straight ahead (Balslev & Miall, 2008). Here we found that an identical procedure biases visual detection toward the right, increasing visual accuracy in the right visual field and decreasing it in the left visual field (Experiment 1). We suggest that the perceived gaze position biases the distribution of attention in the visual space, favoring visual detection near compared with far from the perceived direction of gaze.
Besides eye position, parietal cortex also carries visual, oculomotor and attention-related signals whose alteration may produce lateralized changes in visual accuracy. However, despite an identical TMS, when the right eye was rotated to the right, TMS now reduced visual perception in the right visual hemifield (Experiment 2). The dependence of the effect on visual perception on the direction of gaze makes it unlikely that the changes in visual accuracy in this study resulted from a direct effect of TMS on the visual, oculomotor, or attention signals at the site of stimulation. The leftward bias in visual attention in Experiment 2 is predicted by the assumption that left anterior parietal rTMS produces an underestimation of the rightward rotation of the right eye in the orbit. We have not tested this assumption, but have two arguments to justify it. First, the eye proprioceptive projection in the monkey shows increasing firing rate for increasing rotation of the eye, and all directions are represented in each hemisphere (Wang et al., 2007). Thus, a reduction of neural activity with TMS is expected to reduce the perceived rotation of the eye in the head, regardless of its direction. Second, our previous TMS study in humans showed that in central fixation, when the binocular convergence ensures that the right eye is rotated leftward, TMS produces a shift in perceived eye position to the right (Balslev & Miall, 2008), supporting the assumption that TMS produces an underestimation of the rotation of the right eye in the head.
In Experiment 2, the pairwise comparisons showed a decrease in hit rate pre- versus post-TMS in the right visual field, but no significant change in the left visual field (Table 1). The decrease in hit rate in the right visual field is in accord with our hypothesis. However, we failed to find a corresponding increase in visual detection in the left visual field. One explanation for the absence of an effect in the left visual field is the possibility that APC-rTMS may have caused both a relative increase in visual detection in the left versus right hemifield, as we hypothesized, and a global decrease in hit rate, common for both visual hemifields. A stroke (Bartolomeo & Chokron, 2002) or a TMS-induced virtual lesion in posterior parietal cortex (Hilgetag, Theoret, & Pascual-Leone, 2001) decreases visual detection in the egocentric space contralateral to the lesion. In Experiment 2, both targets were presented to the right of the body midline, and therefore, left parietal TMS may have produced a global decrease in visual accuracy common to both hemifields. A global decrease in hit rate in both hemifields would cancel out a relative increase in hit rate in the left versus right visual field and amplify a local decrease in hit rate in the right visual field, yielding post-TMS hit rates similar to ours. The current data do not allow us to conclude whether the measured hit rates in Experiment 2 reflect an isolated decrease in attention in the right visual hemifield or both a global decreased attention to targets located to the right of the body midline and an increase in attention in the left hemifield. The decrease in the laterality index after APC-rTMS suggests that TMS biases the distribution of attention in the visual space so that targets in the right visual hemifield are detected less than targets in the left visual hemifield.
Furthermore, a direct effect of the anterior parietal TMS on the eye muscles or the frontal eye fields is ruled out by the absence of an effect after TMS over MC. MC is located anterior to APC, hence, is closer anatomically to the eye muscles or the frontal eye fields.
Finally, a direct effect of APC-rTMS on the position of gaze at fixation or on the direction of the saccades is unlikely, given the negative results of the analyses of eye position. Thus, the change in hit rate in this experiment does not reflect an effect of rTMS on the actual as opposed to the perceived eye position or on the oculomotor command.
Hence, we found that a TMS procedure that produces a shift in perceived eye position by affecting the processing of proprioceptive input from the right eye (Balslev & Miall, 2008) also induced a change in visual detection during binocular viewing. We have not tested visual detection in monocular viewing. It is known that the eye position estimate for either eye integrates proprioceptive information across both eyes (Gauthier, Nommay, & Vercher, 1990). Therefore, we do not expect that the modality of viewing (monocular or binocular) or the viewing eye (left or right) would change the effect of TMS on visual detection.
A similar effect of the perceived direction of gaze on the spatial distribution of attention has been found in the auditory modality. Looking toward a sound source improves sound perception at that location in healthy people (Morais, Cary, Vanhaelen, & Bertelson, 1980), and this perceptual advantage can be used to alleviate the left auditory deficit in patients with auditory neglect (Pavani, Ladavas, & Driver, 2005). In these studies, however, it is unclear whether the benefit in auditory perception reflects an involuntary bias in spatial attention or is merely the result of the voluntary orienting of attention toward the region of space where gaze had to be maintained. The participants in our experiment saw the targets at equal distance from fixation, and were not aware of any manipulation in the perceived eye-in-head position. This supports the previously identified link between the focus of gaze and attention, and further demonstrates that visuospatial attention can be involuntarily biased by the static eye position signal, reducing the detection of targets presented distal relative to near the perceived direction of gaze.
Our study is not the first one to suggest a link between eye position and visuospatial attention. A previous study found that the benefit of a spatially congruent cue disappears when the target is presented at a location to which the eyes cannot move (Craighero, Nascimben, & Fadiga, 2004). This occurs, for instance, when the fixation cross is presented at 40° from the mid-sagittal plane, where the eye has reached its maximum possible lateral rotation. When the eye cannot move further laterally, a cue presented at the periphery of the retina can no longer produce a shift in visual attention. These findings support the idea that eye position impacts on the dynamic shifts in visual attention (Craighero et al., 2004). Further to this previous work, our study suggests that the static allocation of attention (as opposed to a dynamic shift) can also be biased by perceived eye position. According to our findings, the visual space located far from the perceived direction of gaze is “neglected” in comparison with the visual space located near the perceived direction of gaze.
The current study dissociated the perceived eye position from the real eye position using rTMS over the eye proprioceptive representation. Prism exposure can also dissociate the perceived and the real eye position. After wearing laterally displacing prism goggles, the perceived eye position shifts to the opposite direction of the prism displacement (Craske, 1967; Kalil & Freedman, 1966). In line with the present findings, the changes in visual attention induced by prism adaptation follow the shift in perceived eye position. Thus, left-displacing prism goggles produce a shift to the right in the perceived direction of gaze (Kalil & Freedman, 1966) and increase visual perception toward the right visual field in healthy participants (Loftus, Vijayakumar, & Nicholls, 2009). Similarly, right-displacing goggles produce a shift to the left in the perceived direction of gaze (Craske, 1967) and increase visual perception toward the left in patients with neglect (Berberovic, Pisella, Morris, & Mattingley, 2004). However, because prism adaptation induces sensorimotor changes beyond the shift in the perceived eye position or attention (Redding, Rossetti, & Wallace, 2005), these previous results could not demonstrate a causal link between perceived eye position and attention focus. Based on our current results, we speculated that the change in perceived eye position induced by prism exposure may be a possible mechanism for the effect of prism adaptation on the spatial distribution of attention.
Our findings contrast with the negative results of a previous study that used neck vibration or caloric vestibular stimulation to shift the perceived direction of gaze to the right and tested the perception of lateral targets presented at equal distance from fixation using a temporal order judgment task (Rorden, Karnath, & Driver, 2001). No statistically significant difference in the performance was found for left versus right targets after either manipulation. All testing took place in complete darkness, where only the target and the fixation LEDs were visible. In normal light conditions, the vibration-induced illusions of target movement disappear (Lennerstrand, Han, & Velay, 1996), suggesting that seeing the body can anchor targets to their real location in space despite an altered signal of gaze position. We speculate that this is the critical difference between this and our study that took place in normal light conditions, where the participants were able to see their hands and body during testing. These visual cues provide veridical information about the location of the targets relative to the body and allow for a spatial representation that places the two targets at different distances from the perceived direction of gaze.
To summarize, we found a link between perceived eye position and visual attention. We suggest that eye proprioception in somatosensory cortex influences the spatial distribution of visual processing resources in the visual space based on three arguments. Firstly, the procedure shown to influence eye proprioception (Balslev & Miall, 2008) also produced a change in visual attention. Secondly, this change in visual attention depended on the direction of rotation of the right eye in the orbit in a way that is in accordance with the organization of the eye proprioceptive representation in the monkey (Wang et al., 2007). Finally, there is a good agreement between the site of TMS and the cortical projection for eye proprioception in humans. In this study, TMS was applied 3 cm posterior and inferior to the motor hotspot for the right hand. In an fMRI study (Balslev, Albert, and Miall, unpublished), we mapped the human eye proprioceptive representation to lie 23 mm posterior and inferior to the motor hotspot for the right hand.
Acknowledgments
This work was supported by a postdoctoral fellowship from the Danish Medical Research Councils (D. B.) and by the Wellcome Trust (grant 069439).
We thank Jonathan Winter for technical assistance.
REFERENCES
- Abadi RV, Gowen E. Characteristics of saccadic intrusions. Vision Research. 2004;44:2675–2690. doi: 10.1016/j.visres.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Balslev D, Albert N, Miall RC. Eye muscle proprioception is represented bilaterally in sensorimotor cortex. Human Brain Mapping. doi: 10.1002/hbm.21050. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balslev D, Braet W, McAllister C, Miall RC. Inter-individual variability in optimal current direction for transcranial magnetic stimulation of the motor cortex. Journal of Neuroscience Methods. 2007;162:309–313. doi: 10.1016/j.jneumeth.2007.01.021. [DOI] [PubMed] [Google Scholar]
- Balslev D, Miall RC. Eye position representation in human anterior parietal cortex. Journal of Neuroscience. 2008;28:8968–8972. doi: 10.1523/JNEUROSCI.1513-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolomeo P, Chokron S. Orienting of attention in left unilateral neglect. Neuroscience and Biobehavioral Reviews. 2002;26:217–234. doi: 10.1016/s0149-7634(01)00065-3. [DOI] [PubMed] [Google Scholar]
- Berberovic N, Pisella L, Morris AP, Mattingley JB. Prismatic adaptation reduces biased temporal order judgements in spatial neglect. NeuroReport. 2004;15:1199–1204. doi: 10.1097/00001756-200405190-00024. [DOI] [PubMed] [Google Scholar]
- Craighero L, Nascimben M, Fadiga L. Eye position affects orienting of visuospatial attention. Current Biology. 2004;14:331–333. doi: 10.1016/j.cub.2004.01.054. [DOI] [PubMed] [Google Scholar]
- Craske B. Adaptation to prisms: Change in internally registered eye-position. British Journal of Psychology. 1967;58:329–335. doi: 10.1111/j.2044-8295.1967.tb01089.x. [DOI] [PubMed] [Google Scholar]
- Deubel H, Schneider WX. Saccade target selection and object recognition: Evidence for a common attentional mechanism. Vision Research. 1996;36:1827–1837. doi: 10.1016/0042-6989(95)00294-4. [DOI] [PubMed] [Google Scholar]
- Engbert R, Kliegl R. Microsaccades uncover the orientation of covert attention. Vision Research. 2003;43:1035–1045. doi: 10.1016/s0042-6989(03)00084-1. [DOI] [PubMed] [Google Scholar]
- Gauthier GM, Nommay D, Vercher JL. The role of ocular muscle proprioception in visual localization of targets. Science. 1990;249:58–61. doi: 10.1126/science.2367852. [DOI] [PubMed] [Google Scholar]
- Hilgetag CC, Theoret H, Pascual-Leone A. Enhanced visual spatial attention ipsilateral to rTMS-induced “virtual lesions” of human parietal cortex. Nature Neuroscience. 2001;4:953–957. doi: 10.1038/nn0901-953. [DOI] [PubMed] [Google Scholar]
- Hoffman JE, Subramaniam B. The role of visual-attention in saccadic eye-movements. Perception & Psychophysics. 1995;57:787–795. doi: 10.3758/bf03206794. [DOI] [PubMed] [Google Scholar]
- Kalil RE, Freedman J. Persistence of ocular rotation following compensation for displaced vision. Perceptual and Motor Skills. 1966;22:135–139. doi: 10.2466/pms.1966.22.1.135. [DOI] [PubMed] [Google Scholar]
- Kowler E, Anderson E, Dosher B, Blaser E. The role of attention in the programming of saccades. Vision Research. 1995;35:1897–1916. doi: 10.1016/0042-6989(94)00279-u. [DOI] [PubMed] [Google Scholar]
- Lennerstrand G, Han Y, Velay JL. Properties of eye movements induced by activation of neck muscle proprioceptors. Graefes Archive for Clinical and Experimental Ophthalmology. 1996;234:703–709. doi: 10.1007/BF00292357. [DOI] [PubMed] [Google Scholar]
- Loftus AM, Vijayakumar N, Nicholls ME. Prism adaptation overcomes pseudoneglect for the greyscales task. Cortex. 2009;45:537–543. doi: 10.1016/j.cortex.2007.12.011. [DOI] [PubMed] [Google Scholar]
- Morais J, Cary L, Vanhaelen H, Bertelson P. Postural determinants of frontal-position advantage in listening to speech. Perception & Psychophysics. 1980;27:141–148. doi: 10.3758/bf03204302. [DOI] [PubMed] [Google Scholar]
- Nystrom M, Holmqvist K. An adaptive algorithm for fixation, saccade, and glissade detection in eye-tracking data. Behavior Research Methods, Instruments, & Computers. 2010;42:188–204. doi: 10.3758/BRM.42.1.188. [DOI] [PubMed] [Google Scholar]
- Pavani F, Ladavas E, Driver J. Gaze direction modulates auditory spatial deficits in stroke patients with neglect. Cortex. 2005;41:181–188. doi: 10.1016/s0010-9452(08)70892-x. [DOI] [PubMed] [Google Scholar]
- Pridmore S, Fernandes Filho JA, Nahas Z, Liberatos C, George MS. Motor threshold in transcranial magnetic stimulation: A comparison of a neurophysiological method and a visualization of movement method. Journal of ECT. 1998;14:25–27. [PubMed] [Google Scholar]
- Redding GM, Rossetti Y, Wallace B. Applications of prism adaptation: A tutorial in theory and method. Neuroscience and Biobehavioral Reviews. 2005;29:431–444. doi: 10.1016/j.neubiorev.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Robertson EM, Theoret H, Pascual-Leone A. Studies in cognition: The problems solved and created by transcranial magnetic stimulation. Journal of Cognitive Neuroscience. 2003;15:948–960. doi: 10.1162/089892903770007344. [DOI] [PubMed] [Google Scholar]
- Rorden C, Karnath HO, Driver J. Do neck-proprioceptive and caloric-vestibular stimulation influence covert visual attention in normals, as they influence visual neglect? Neuropsychologia. 2001;39:364–375. doi: 10.1016/s0028-3932(00)00126-3. [DOI] [PubMed] [Google Scholar]
- Wang XL, Zhang MS, Cohen IS, Goldberg ME. The proprioceptive representation of eye position in monkey primary somatosensory cortex. Nature Neuroscience. 2007;10:640–646. doi: 10.1038/nn1878. [DOI] [PubMed] [Google Scholar]
- Watson AB, Pelli DG. Quest: A Bayesian adaptive psychometric method. Perception & Psychophysics. 1983;33:113–120. doi: 10.3758/bf03202828. [DOI] [PubMed] [Google Scholar]


