Abstract
Several novel multicomponent assembly processes have been developed for the rapid and efficient assembly of various heterocyclic scaffolds bearing a tetrahydroisoquinoline core, each of which allows for facile derivatization to access a diverse array of compounds. This work led to the serendipitous discovery of a new method for the synthesis of a fused quinazolone ring system, which was applied to a one-step total synthesis of the quinazolinocarboline alkaloid rutaecarpine.
The identification of potent and selective modulators of biological pathways is a key step in studying fundamental biology and discovering drug leads. Efforts to facilitate access to such compounds have fueled the development of several approaches for the generation of small molecule libraries.1 Collections of small molecules containing privileged scaffolds, which are skeletal frameworks that bind to multiple receptors and display a wide range of biological activities,2,3 often exhibit relatively high hit rates in screening assays. Initial hits can then be modified by introducing potency and selectivity enhancing substituents to give derivatives that exhibit favorable physiochemical properties.
We recently reported the design and development of a novel strategy for diversity-oriented synthesis (DOS) that featured a Mannich-type multicomponent assembly process (MCAP), followed by various cyclization reactions that were enabled by selective functional group pairing to construct substituted heterocyclic ring systems.4–6 This approach has been extended to the synthesis and diversification of compounds based on the benzodiazepine, tetrahydropyridine, and aryl piperidine scaffolds.7–9 We now report a further expansion of this approach for DOS and the development of several MCAPs that provide ready access to various privileged structures fused to a tetrahydroisoquinoline core, which itself is found in molecules that exhibit a wide array of biological activities, including antihypertensive,10 anti-tumor, 11 and anti-malarial properties.12
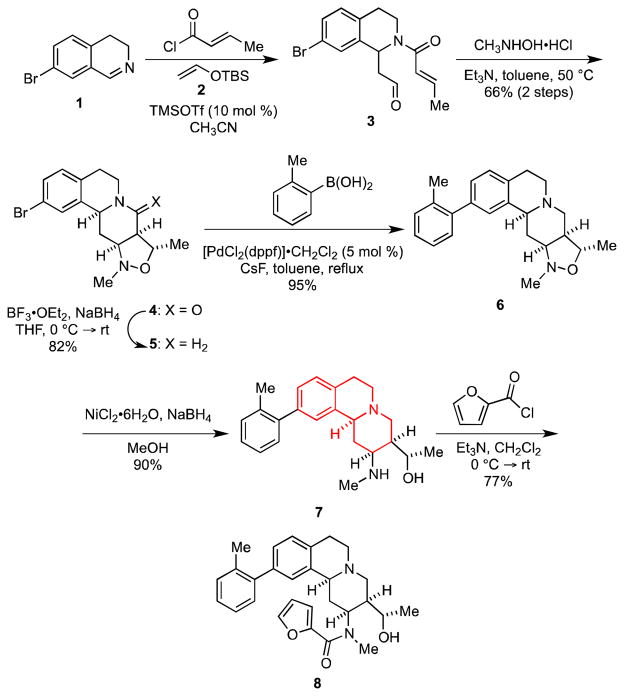
We chose the readily available 7-bromodihydro-isoquinoline (1) 13 as the common input for these MCAPs, because it allows for preparation of various derivatives via cross-coupling reactions with the aryl bromide moiety (Scheme 1). Inasmuch as compounds incorporating the highlighted tricyclic ring system in 7 are known to be potent α2-adrenoceptor antagonists,14 we sought to develop an efficient entry to this ring system.
Scheme 1.
Synthesis and Diversification of 7
Accordingly, treatment of 1 with trans-crotonyl chloride and silyl enol ether 2 in the presence of a catalytic amount of TMSOTf provided the aldehyde 3, which upon condensation with N-methylhydroxylamine gave an intermediate nitrone that underwent facile 1,3-dipolar cycloaddition to provide isoxazolidine 4 in 66% yield from 1; no other stereoisomers were isolated. The relative stereochemistry in 4 was unambiguously determined by single crystal x-ray analysis. Notably, 4 is easily prepared on a multi-gram scale without column chromatography. Subsequent reduction of the lactam moiety in 4, followed by a Suzuki cross-coupling provided the biaryl isoxazolidine 6. Upon treatment with nickel boride, 6 underwent N,O-bond cleavage to give the secondary amine 7 in 90% yield.15 The amino group in 7 is an obvious point for further diversification as illustrated by its reaction with 2-furoyl chloride in the presence of triethylamine to furnish amide 8 in 77% yield.
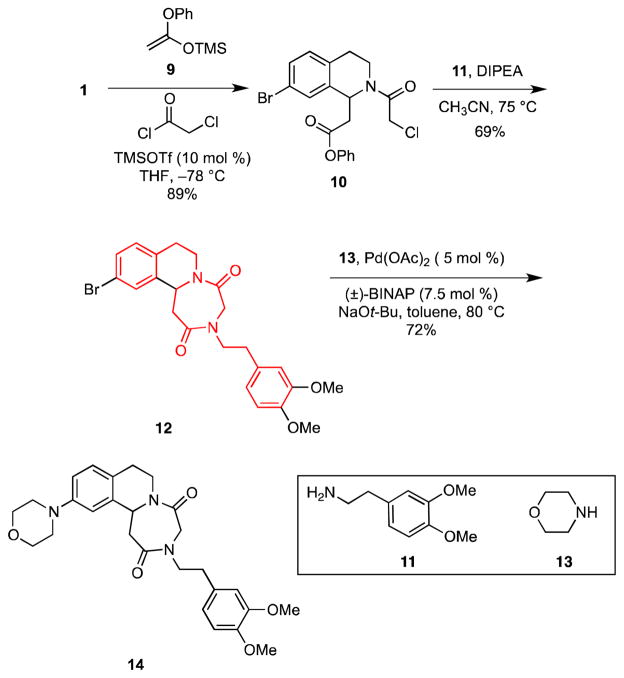
We also wanted to explore methods for elaborating heterocyclic rings directly onto the parent dihydroisoquinoline 1, and it occurred to us that appending a 1,4-diazepine-2,5-dione ring might be of considerable use. For example, N,N′-diphenethyl-1,4-diazepine-2,5-diones (highlighted portion of 12) are of interest as inhibitors of the UCB13-UEV enzyme complex and hence may be useful as anti-tumor agents.16 Since 12 represents a novel, constrained analog of these systems, we set to the task of preparing such compounds via our MCAP/cyclization strategy. In the event, reaction of 1 with silyl ketene acetal 9 and chloroacetyl chloride in the presence of a catalytic amount of TMSOTf at −78 °C provided amide 10 in 89% yield (Scheme 2). When 10 was heated with primary alkyl amines in the presence of Hünig’s base in CH3CN the corresponding N-alkyl 1,4-diazepine-2,5-diones were obtained. For example, reaction of 10 with 11 provided 12 in 69% yield. Buchwald-Hartwig cross-coupling of 12 with morpholine (13) gave amine 14 in 72% yield.
Scheme 2.
Synthesis of 1,4-Diazepine-2,5-diones
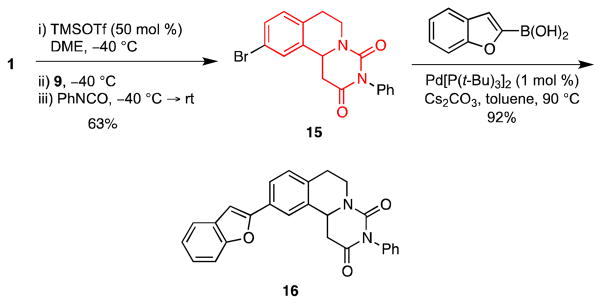
The molecular framework embodied in 15 and 16 (6,7-dihydropyrimido[6,1-a]isoquinoline-2,4(3H,11bH)-dione) is present in compounds that exhibit hypotensive and diuretic effects.17 We were thus were intrigued by the possibility that we might exploit an MCAP/cyclization sequence to access this ring system by a route that would be more expedient than those previously reported.17a,18 Indeed, sequential treatment of 1 with TMSOTf, the silyl ketene acetal 9 and phenyl isocyanate provided 15 in 63% yield (Scheme 3). Because an isocyanate is implemented as the electrophilic component, this series of reactions represents a significant expansion of our MCAP chemistry to give products containing urea functionality. Preliminary studies using isothiocyanates as electrophilic inputs have not been as successful. Compound 15 was further elaborated via a Suzuki cross-coupling to give the aryl substituted tricyclic scaffold 16 in 92% yield.
Scheme 3.
Synthesis of Dihydropyrimidine-2,4-diones
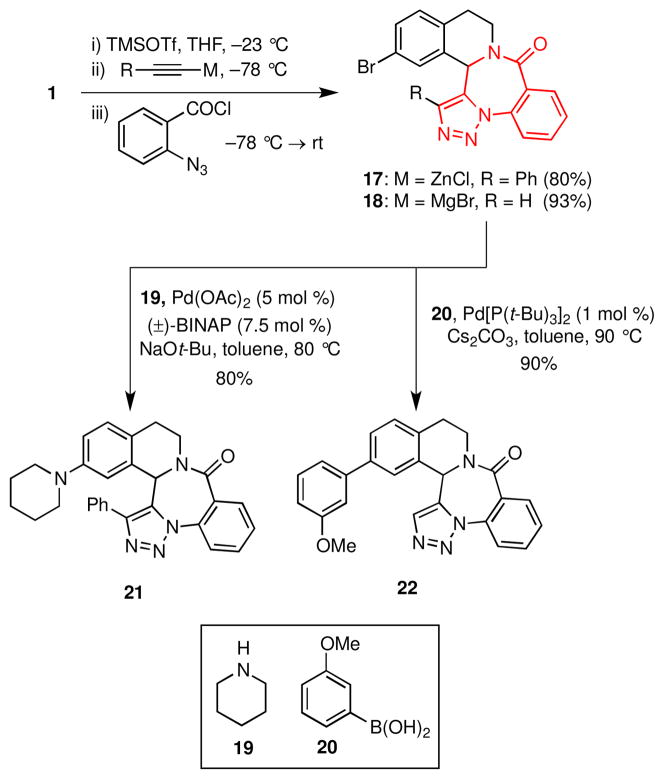
The 1,5-benzodiazepin-2-one framework is present in many compounds that bind to multiple targets including the interleukin-1β converting enzyme (ICE)19 and voltage-gated potassium channels.20 More specifically, the triazolo 1,5-benzodiazepin-2-one scaffold, highlighted in 17 and 18, is present in compounds reported to inhibit serine protease21 and to bind to the benzodiazepine receptor.22 Toward incorporating this ring system in fused isoquinolines, 1 was first allowed to react with either zinc phenylacetylide or ethynylmagnesium bromide in the presence of TMSOTf (Scheme 4). The intermediate adducts were then trapped with o-azidobenzoyl chloride, and upon warming to room temperature, the amide thus produced readily underwent a dipolar cycloaddition to furnish the novel triazolo 1,5-benzodiazepin-2-ones 17 and 18 in 80% and 93% yields, respectively. Subsequent Buchwald-Hartwig or Suzuki cross-coupling reactions generated derivatives 21 and 22.
Scheme 4.
Synthesis of Triazolo 1,5-Benzodiazepin-2-ones
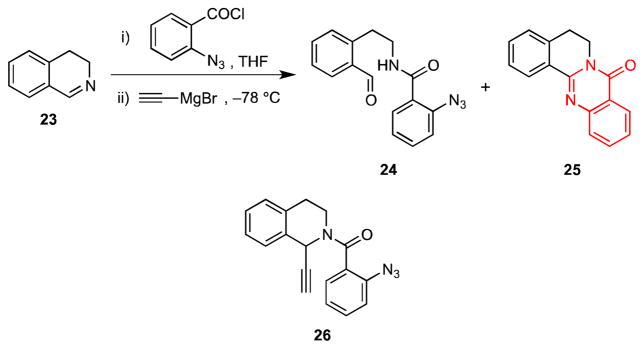
During initial experiments that were directed toward developing the sequence outlined in Scheme 4, we discovered that acylation of dihydroisoquinoline 23 with o-azidobenzoyl chloride at room temperature, followed by addition of ethynylmagnesium bromide did not give the expected amide 26; the benzaldehyde 24 and the quinazolone 25 were obtained instead (Scheme 5). Although 25 was isolated as a minor product, its formation suggested that we had serendipitously uncovered a new entry to quinazolones, a heterocyclic ring system present in a variety of pharmaceuticals and biologically active natural products.3a,23
Scheme 5.
Formation of Quinazolone 25 as a Minor Side Product
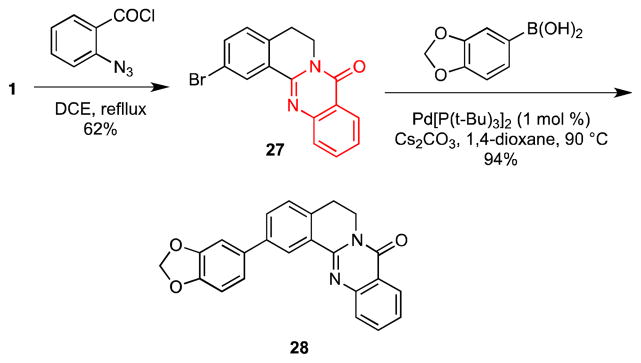
After some optimization, we found that treatment of 1 with o-azidobenzoyl chloride in 1,2-dichloroethane under reflux provided quinazolone 27 in 62% yield (Scheme 6); no aldehyde was observed in the 1H NMR spectrum of the crude reaction mixture. To the best of our knowledge, this represents the first example of an intramolecular cyclization of an organic azide onto a putative N-acyliminium ion to form a quinazolone.24 Subsequent Suzuki cross-coupling of 27 provided the biaryl quinazolone 28 in 94% yield.
Scheme 6.
Synthesis of Quinazolones 27 and 28
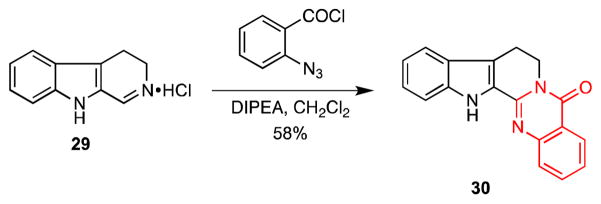
In order to showcase the utility of this novel route to quinazolones, we applied it to a one-step synthesis of the quinazolinocarboline alkaloid rutaecarpine (30). Rutaecarpine was isolated from the dried, unripe fruit of Evodia Rutaecarpa and displays various biological activities including being a potent and selective inhibitor of cytochrome P450 in human liver microsomes.25,26 Simply treating dihydro-β-carboline hydrochloride (29)27 with o-azidobenzoyl chloride in the presence of Hünig’s base in dichloromethane delivered rutaecarpine (30) in 58% yield (Scheme 7).24a,28
Scheme 7.
Total Synthesis of Rutaecarpine (30)
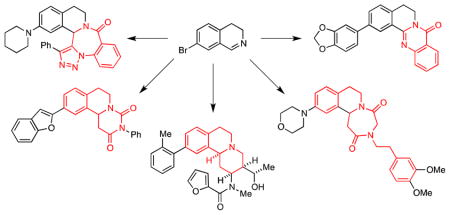
In summary, we have developed several new MCAPs for the rapid synthesis of complex heterocyclic scaffolds in which tetrahydroisoquinolines are fused to 1,4-diazepine-2,5-dione, dihydropyrimidine-2,4-dione, 1,5-benzodiazepin-2-one, and quinazolone rings. These heterocycles bear functionality that may be further exploited in a variety of cross-coupling reactions to generate small compound libraries for submission to the NIH Molecular Libraries Small Molecule Repository (MLSMR). An important feature of this approach to DOS is that the members of these libraries typically have clogP values in the approximate range of 3.0–4.5; these compounds thus have excellent promise as potential leads. We also discovered a novel method to form fused quinazolone rings and have applied this discovery to a one-step synthesis of the quinazolinocarboline alkaloid rutaecarpine. Significantly, these novel reaction sequences can be applied to other imines, including those that are generated in situ by four-component assembly processes we have previously reported.6–9 Further applications of this and related approaches to the syntheses of unique compound libraries are in progress, and the results of these investigations will be reported in due course.
Supplementary Material
Acknowledgments
We thank the National Institutes of Health (GM 24539 and 86192) and the Robert A. Welch Foundation (F-0652) for their generous support of this work. We also thank Dr. Vincent Lynch (The University of Texas at Austin) for performing X-ray crystallography.
Footnotes
Supporting Information Available. Experimental procedures, spectral data and copies of 1H and 13C NMR spectra for all new compounds, and the CIF file for 4. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Brenner S, Lerner RA. Proc Natl Acad Sci USA. 1992;89:5381–5383. doi: 10.1073/pnas.89.12.5381. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Njardarson JT, Gaul C, Shan D, Huang X-Y, Danishefsky SJ. J Am Chem Soc. 2004;126:1038–1040. doi: 10.1021/ja039714a. [DOI] [PubMed] [Google Scholar]; (c) Kaiser M, Wetzel S, Kumar K, Waldmann H. Cell Mol Life Sci. 2008;65:1186–1201. doi: 10.1007/s00018-007-7492-1. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Schreiber SL. Science. 2000;287:1964–1969. doi: 10.1126/science.287.5460.1964. [DOI] [PubMed] [Google Scholar]
- 2.Evans BE, Rittle KE, Bock MG, DiPardo RM, Freidinger RM, Whitter WL, Lundell GF, Veber DF, Anderson PS, Chang RSL, Lotti VJ, Cerino DJ, Chen TB, Kling PJ, Kunkel KA, Springer JP, Hirshfield J. J Med Chem. 1988;31:2235–2246. doi: 10.1021/jm00120a002. [DOI] [PubMed] [Google Scholar]
- 3.For leading references to privileged substructures, see: Welsch ME, Snyder SA, Stockwell BR. Curr Opin Chem Biol. 2010;14:347–361. doi: 10.1016/j.cbpa.2010.02.018.DeSimone RW, Currie KS, Mitchell SA, Darrow JW, Pippin DA. Comb Chem High Throughput Screening. 2004;7:473–493. doi: 10.2174/1386207043328544.Horton DA, Bourne GT, Smythe ML. Chem Rev. 2003;103:893–930. doi: 10.1021/cr020033s.Constantino L, Barlocco D. Curr Med Chem. 2006;13:65–85.
- 4.For the first example of this process, see: Martin SF, Benage B, Hunter JE. J Am Chem Soc. 1988;110:5925–5927.
- 5.For a review of such strategies, see: Sunderhaus JD, Martin SF. Chem-Eur J. 2009;15:1300–1308. doi: 10.1002/chem.200802140.
- 6.(a) Sunderhaus JD, Dockendorff C, Martin SF. Org Lett. 2007;9:4223–4226. doi: 10.1021/ol7018357. [DOI] [PubMed] [Google Scholar]; (b) Sunderhaus JD, Dockendorff C, Martin SF. Tetrahedron. 2009;65:6454–6469. doi: 10.1016/j.tet.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donald JR, Martin SF. Org Lett. 2011;13:852–855. doi: 10.1021/ol1028404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sahn JJ, Su JY, Martin SF. Org Lett. 2011;13:2590–2593. doi: 10.1021/ol200709h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hardy S, Martin SF. Org Lett. 2011;13:3102–3105. doi: 10.1021/ol201010s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gavras I, Vlahakos D, Melby JC, Gavras H. J Clin Pharm. 1984;24:343–350. doi: 10.1002/j.1552-4604.1984.tb02786.x. [DOI] [PubMed] [Google Scholar]
- 11.Asaoka T, Yazawa K, Mikami Y, Takahashi K. J Antibiot. 1982;35:1708–1710. doi: 10.7164/antibiotics.35.1708. [DOI] [PubMed] [Google Scholar]
- 12.Francois G, Bringmann G, Phillipson JD, Boyd MR, Assi LA, Schneider C, Timperman G. 5,639,761. U S Patent No. 1994
- 13.(a) Worrall DE. Org Synth. 1929;9:66. [Google Scholar]; (b) Schumacher RW, Davidson BS. Tetrahedron. 1999;55:935–942. [Google Scholar]; (c) Mjalli AMM, Gohimmukkula DR, Tyagi S. 7,208,601. U S Patent No. 2005
- 14.VanDyke JW, Jr, Havera HJ, Johnson RD. J Med Chem. 1971;15:91–93. [Google Scholar]
- 15.Jones AD, Knight DW, Thornton SR. J Chem Soc, Perkin Trans 1. 1999:3337–3344. [Google Scholar]
- 16.Okatsu TT, Sigmund JS, Peypoch AM, Ortiz AR, Pérez De Rozas GS, Masip IM, Fernández AM, Ruiz DG, León AM. 2008009758. PCT Int Appl WO. 2008:A1.
- 17.(a) Kaneko H, Nagatsuka T. 45009467. Jpn Kokai Tokkyo Koho JP. 1971; (b) Lombardino JG, McLaMore WM, Laubach GD. 3,021,331. U S Patent No. 1962
- 18.Angelov PA, Ivanov II, Venkov AP. Molecules. 2004;9:694–704. doi: 10.3390/90800694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Batchelor MJ, Bebbington D, Bemis GW, Fridman WH, Gillespie RJ, Golec JMC, Gu Y, Lauffer DJ, Livingston DJ, Matharu SS, Mullican MD, Murcko MA, Murdoch R, Nyce PL, Robidoux ALC, Wannamaker MW, Wilson KY, Zelle RE. 6,008,217. U S Patent No. 1999
- 20.Clareman DA, Liverton N, Selnick GG, Smith GR. 5,726,171. U S Patent No. 1998
- 21.Mohapatra DK, Maity PK, Shabab M, Khan MI. Bioorg Med Chem Lett. 2009;19:5241–5245. doi: 10.1016/j.bmcl.2009.06.107. [DOI] [PubMed] [Google Scholar]
- 22.Bertelli L, Biagi G, Giorgi I, Livi O, Manera C, Scartoni V, Martini C, Giannaccini G, Trincavelli L, Barili PL. Farmaco. 1998;53:305–311. doi: 10.1016/s0014-827x(98)00025-1. [DOI] [PubMed] [Google Scholar]
- 23.(a) Hughes AN, Rafi I, Griffin MJ, Calvert AH, Newell DR, Calvete JA, Johnston A, Clendeninn N, Body AV. Clin Cancer Res. 1999;5:111–118. [PubMed] [Google Scholar]; (b) López-Gresa MP, González MC, Primo J, Moya P, Romero V, Estornell E. J Antibiot. 2005;58:416–419. doi: 10.1038/ja.2005.54. [DOI] [PubMed] [Google Scholar]
- 24.For other methods of preparation, see: Eguchi S, Takeuchi H, Matsushita Y. Heterocycles. 1992;33:153–156.Wagner EC. J Org Chem. 1940;5:133–141.Grout RJ, Partridge MW. J Chem Soc. 1960:3551–3557.
- 25.Asahina Y, Kashiwaki K. J Pharm Soc Jpn. 1915:1293. [Google Scholar]
- 26.Ueng Y-F, Jan W-C, Lin L-C, Chen T-L, Guengerich FP, Chen C-F. Drug Met Disp. 2002;30:349–353. doi: 10.1124/dmd.30.3.349. [DOI] [PubMed] [Google Scholar]
- 27.Späth E, Lederer E. Ber Dtsch Chem Ges. 1930;63:2102–2111. [Google Scholar]
- 28.For selected synthesis of rutaecarpine, see: Asahina Y, Manske RHF, Robinson R. J Chem Soc. 1927:1708–1710.Kametani T, Higa T, Von Loc C, Ihara M, Koizumi M, Fukumoto K. J Am Chem Soc. 1976;98:6186–6188.Bergman T, Bergman S. J Org Chem. 1985;50:1246–1255.Zhang C, Kanta De C, Mal R, Seidel D. J Am Chem Soc. 2008;130:416–417. doi: 10.1021/ja077473r.Kamal A, Reddy MK, Reddy TS, Santos LS, Shankaraiah N. Synlett. 2011;1:61–64.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.