Abstract
Recently our group identified in murine bone marrow (BM) and human cord blood (CB), a rare population of Very Small Embryonic-Like (VSEL) stem cells. We hypothesize that these cells are deposited during embryonic development in BM as a mobile pool of circulating pluripotent stem cells (PSC) that play a pivotal role in postnatal tissue turnover both of non-hematopoietic and hematopoietic tissues. During in vitro co-cultures with murine myoblastic C2C12 cells, VSELs form spheres that contain primitive stem cells. Cells isolated from these spheres may give rise to cells from all three germ layers when plated in tissue specific media. The number of murine VSELs and their ability to form spheres decreases with the age and is reduced in short-living murine strains. Thus, developmental deposition of VSELs in adult tissues may potentially play an underappreciated role in regulating the rejuvenation of senescent organs. We envision that the regenerative potential of these cells could be harnessed to decelerate aging processes.
Keywords: Oct-4, Nanog, SSEA, CXCR4, VSELs, embryonic stem cells
Introduction
Aging results from decrease in rejuvenation/regeneration potential of several vital organs and tissues. Since tissue regeneration depends on a proper function of stem cells, multipotent stem/primitive cells become a major focus of interest in regenerative medicine (Asahara et al., 1999; Brazelton et al., 2000; Corti et al., 2004; Dawn et al., 2005; Ratajczak et al., 2007b). Aging processes could be explained by i) age related stem cell dysfunction, ii) accumulation of mutations in stem cell pool over a life-span and iii) shortening of the telomeres as result of decrease in telomerase activity. Recently, based on our data showing a presence of Very Small Embryonic-Like (VSEL) stem cells in murine BM (Kucia et al., 2006a; Zuba-Surma et al., 2007a) and human cord blood (CB) (Kucia et al., 2007a) as well as in several solid organs (Kucia et al., 2007d), we proposed a hypothesis that these cells are deposited during embryonic development in adult tissues as a mobile pool of circulating pluripotent stem cells (PSC) and that the number of these cells decreases with the age (Ratajczak et al., 2007a). Thus, aging could be at least partially explained by aging-dependent exhaustion of pool of circulating PSC in adult tissues.
We hypothesize that VSELs are a dormant quiescent population of small CXCR4+ SSEA+ Oct-4+ Nanog+ PSC that i) are descendants of epiblast-derived stem cells (EPSC) including some primordial germ cells (PGC) ii) are deposited during development and reside in BM and other adult tissues, iii) after being mobilized from BM into peripheral blood, these cells may participate in turnover of other tissue specific (monopotent) stem cells that are located in peripheral niches, iv) when mobilized may also play a role in tissue/organ regeneration, as seen during stress situations or organ injuries, and v) number of these cells in the adult tissues decreases with the age (Kucia et al., 2006a; Ratajczak et al., 2007a). On the other hand, we hypothesize that since there is “a thin red line” between regeneration and tumor formation, it is likely that this population of very primitive stem cells may give rise to some types of cancer (e.g., pediatric sarcomas, teratomas, germinal tumors) when exposed to mutagenic environment (Buzzeo et al., 2007; Kucia et al., 2007d; Li et al., 2006; Liu et al., 2006; Oakley et al., 2007).
In this review we will describe the most important features of VSELs and discuss a potential role of these cells in rejuvenation/regeneration of tissues in steady state conditions as well as during emergencies related to tissue damage. We will also present an evidence that the number of VSELs and their ability to form spheres, composed of primitive stem cells that possess ability to differentiate into cells from all three germ layers, decreases in mice with the age and is reduced in short-living murine strains.
Bone marrow as a home of versatile stem cells
Recent evidence indicates that BM contains a heterogeneous population of non-hematopoietic stem cells in addition to well described hematopoietic stem cells (HSC) (Kucia et al., 2005; Orkin et al., 2002). The cells of non-hematopoietic compartment of BM display several features of pluripotent stem cells (PSC) and have been variously described in the literature as i) mesenchymal stem cells (MSC) (Anjos-Afonso et al., 2007; Friedenstein et al., 1966; Peister et al., 2004; Prockop, 1997), ii) multipotent adult progenitor cells (MAPC) (Jiang et al., 2002), iii) marrow-isolated adult multilineage inducible (MIAMI) cells (D'Ippolito et al., 2004), very small embryonic-like (VSEL) stem cells (Kucia et al., 2007a; Kucia et al., 2006a) or iv) multipotent adult stem cells (MASC) (Beltrami et al., 2007). Unexpectedly, BM was recently also identified as a potential source of precursors of germ cells (oocytes and spermatogonial cells) (Johnson et al., 2005; Nayernia et al., 2006). We envision the possibility that in some cases similar or overlapping populations of primitive stem cells in the BM were detected using different experimental strategies and hence were assigned different names.
All the BM-derived non-hematopoietic stem cells listed above (MAPC, MIAMI, VSEL and MASC) have been demonstrated to be able in vitro cultures to differentiate into cells from all three germ layers. However, despite several attempts made so far, there is no convincing data proving that any type of these cells may contribute to the development of multiple organs and tissues when injected into the developing blastocyst. Thus, this important criterion for in vivo pluripotentiality is still missed for stem cells isolated from adult tissues. The potential explanation for this “deficiency” will be discussed latter on in this paper.
Generally, it has been hypothesized that the potential presence of PSC in the BM was a result of the “developmental migration” of stem cells during ontogenesis and that these cells found a permissive microenvironment in bone marrow tissue (Kucia et al., 2007d; Ratajczak et al., 2007a). To support this hypothesis, we established that VSELs express CXCR4 (α-chemokine Gαi protein-coupled seven transmembrane span receptor) and c-met (tyrosine kinase receptor) which are responsible for the robust response of VSELs to the cognate ligands of these receptors - stromal derived factor-1 (SDF-1) and hepatocyte growth factor/scatter factor (HGF/SF), respectively (Ratajczak et al., 2006). Since SDF-1 and HGF/SF are secreted by BM microenvironment, both SDF-1-CXCR4 and HGF/SF-c-met axes may play a pivotal role in accumulation of VSELs in BM tissue (Ratajczak et al., 2006). On the other hand, VSELs residing in the BM could be released/mobilized into circulation if needed, such as during pharmacological G-CSF induced mobilization or during stress related processes following tissue/organ injury (Kucia et al., 2004; Kucia et al., 2006b).
Isolation of Very Small Embryonic LIKE (VSEL) Stem Cells
Very small embryonic-like stem cells (VSELs) have been recently isolated and characterized by our team. These cells were detected in adult bone marrow and other organs as the first adult tissue-derived primitive population with embryonic-like features that have been purified at the single cell level (Kucia et al., 2006a; Kucia et al., 2007d; Zuba-Surma et al., 2007a).
VSELs were isolated by multiparameter FACS sorting as a population of rare (~0.01% of BM MNC) Sca-1+ lin− CD45− cells (Kucia et al., 2006a). They express (as determined by RQ-PCR and immunhistochemistry) markers of pluripotent stem cells such as SSEA-1, Oct-4, Nanog, Rex-1 and Rif-1 telomerase protein. Direct electron microscopical analysis revealed that these cells display several features typical for embryonic stem cells such as i) a small size (~3.5 µm in diameter), ii) a large nucleus surrounded by a narrow rim of cytoplasm, and iii) open-type chromatin (euchromatin) (Kucia et al., 2006a). Analysis employing ImageStream system (Figure 1) confirmed a very small size of VSELs as well as other features related to their primitivity such as high nuclear to cytoplasmic ratio (Zuba-Surma et al., 2007a). We found that VSELs may be released from BM and circulate in blood during tissue/organ injury (e.g., heart infarct, stroke, skeletal muscle and liver damage). This suggests that they may play an underappreciated role in tissue/organ regeneration in pathological emergency situations (Kucia et al., 2004; Kucia et al., 2006b).
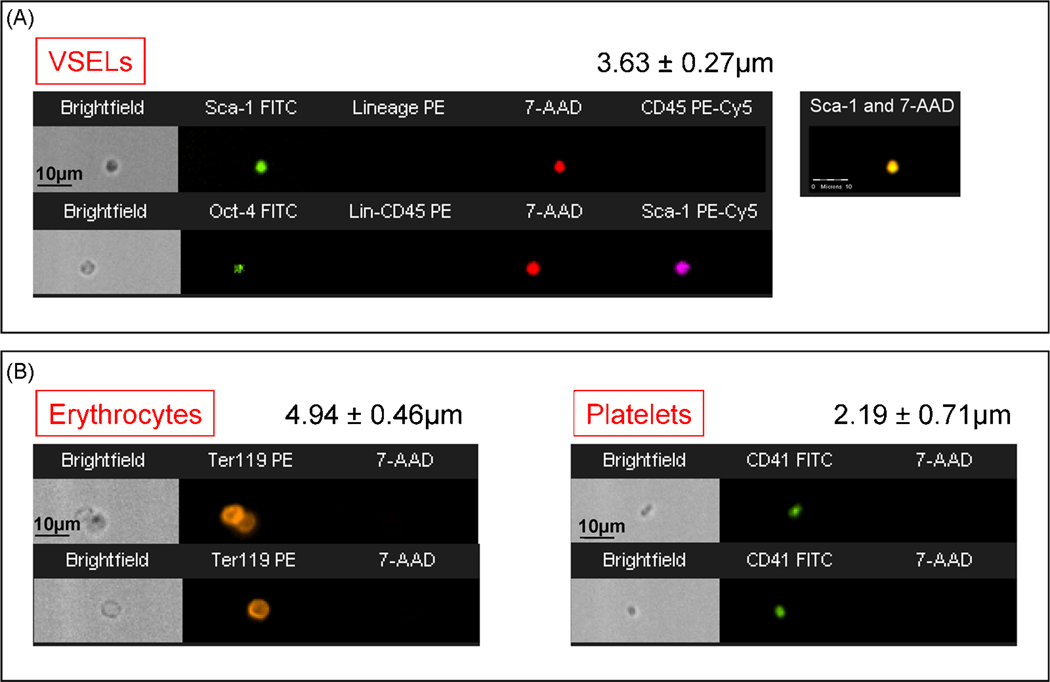
Figure 1. Morphological comparison between murine VSELs, erythrocytes and platelets by ImageStream system.
Murine VSELs shown on Panel A were stained for Sca-1 (FITC, green), Lineage markers (PE, orange) and CD45 (PE-Cy5, magenta) as shown on the upper image or for Oct-4 (FITC, green), Lineage markers, CD45 (PE, orange) and Sca-1 (PE-Cy5, magenta) as presented on the lower image. Murine erythrocytes were stained for Ter119 (PE, orange), while murine platelets for CD41 (FITC, green) (Panel B). Following fixation, all samples were stained with 7-aminoactinomycin D (7-AAD, red) to visualize nuclei. Erythrocytes and platelets do not possess nuclei, while VSELs exhibit cellular structure containing nuclei. Average size of each population is shown in each panel (Mean ± SEM).
VSELs are smaller than erythrocytes and larger than platelets (Kucia et al., 2007d). They can be distinguished from large platelets not only based on different surface markers, but also because of the content of nuclei (Figure 1). Interestingly, despite their small size, VSELs posses diploid number of chromosomes and contain numerous mitochondria. They do not express MHC molecules of class I and II as well as CD90, CD105 and CD29 antigens. Thus, for the first time a sorting procedure allowing purification of distinct population of adult BM- derived very primitive embryonic-like stem cells have been developed and morphology as well as surface markers of these rare cells were described at the single cell level (Kucia et al., 2006a; Zuba-Surma et al., 2007a).
We found that ~5–10% of purified VSELs co-cultured over a C2C12 murine sarcoma cell feeder layer are able to form spheres that resemble embryoid bodies. Cells from these VSEL-derived spheres (VSEL-DS) are composed of immature cells with large nuclei containing euchromatin, and similarly to purified VSELs, exhibit CXCR4+SSEA-1+Oct-4+ phenotype. Furthermore, VSEL-DS, after re-plating over C2C12 cells, may again (up to 5–7 passages) grow new spheres or, if plated into cultures promoting tissue differentiation, give rise into cells from all three germ-cell layers (Kucia et al., 2006a). The observation that VSELs isolated from GFP+ mice grew GFP+ VSEL-DS showing a diploid content of DNA, confirms the VSELs’ origin of VSEL-DS as well as excludes their development from the supportive C2C12 cell line and excludes the possibility of cell fusion (Kucia et al., 2006a). Similar spheres were also formed by VSELs isolated from murine fetal liver, spleen and thymus.
Since VSELs express several markers of primordial germ cells including fetal-type alkaline phosphatase, Oct-4, SSEA-1, CXCR4, Mvh, Stella, Fragilis, Nobox, and Hdac6, they could be closely related to the population of epiblast-derived PGC (Kucia et al., 2006a; Ratajczak et al., 2007a). VSELs, as mentioned above, are also highly mobile and respond robustly to SDF-1 gradient, adhere to fibronectin and fibrinogen, and may interact with BM-derived stromal fibroblasts. Confocal microscopy and time laps studies revealed that these cells attach rapidly to, migrate beneath and undergo emperipolesis in marrow-derived fibroblasts (Kucia et al., 2006a; Kucia et al., 2007c). Since fibroblasts secrete SDF-1 and other chemoatractants they may create a homing environment for small CXCR4+ VSELs. These robust interactions of VSELs with BM-derived fibroblasts do have an important implication, namely that isolated BM stromal cells may be contaminated by these tiny cells from the beginning when isolated and cultured in vitro. Importantly, this observation may explain the unexpected “plasticity” of marrow-derived fibroblastic cells (e.g., MSC or MAPC).
There is also another potential explanation why VSELs as described above may hide in fibroblastic cells. Since VSELs do not express MHC-I molecules and could become a potential target for elimination by NK cells, they may evoke attack from NK cells while hiding inside of fibroblasts. Similar mechanism called entosis was recently described for cancer cells that in certain circumstances may lead to promoting survival of cancer cells that hide inside other cells (Overholtzer et al., 2007).
Finally, recently a very similar population of cells that show similar morphology and markers to murine BM-derived VSELs was purified from human cord blood (CB) (Kucia et al., 2007a). The major antigenic phenotype of these human CB- derived cells has been established as CD133+CXCR4+Lin−CD45− (Kucia et al., 2007a). In conclusion it is anticipated that VSELs isolated from BM as well as CB could become an important source of pluripotent stem cells for regeneration. At this point, however, it is not clear whether VSEL contribute to the blastocyst development.
Reprogramming of somatic cells – inducible plurippotent stem cells (iPSC) versus VSELs
Recent evidence shows that an appropriate “cocktail” of expression vectors encoding four transcription factors such as Oct-4, Sox2, Myc and Klf4 can reprogram murine fibroblasts to an embryonic status. Such manipulation reawakens silenced segments in the genome of already differentiated cell and reestablishes pluripotency of its nucleus (Meissner et al., 2007; Takahashi et al., 2007). This interesting approach was already confirmed by three independent laboratories. Cells from the clones of cells developmentally reverted by this strategy contribute to blastocyst development not only in standard assay but also give rise to the development of a whole embryo during tetraploid complementation assay. Cells generated by this strategy, however have the potential to develop teratomas if injected into syngeneic mice. Thus, the potential clinical application of this strategy to obtain iPSC by “debugging cellular reprogramming” by multiple transduced genes requires further validation.
If we compare reprogrammed somatic cells (e.g., fibroblasts) and VSELs, important analogies and differences occur. On one hand, fibroblasts to be transduced with the cocktail of transcription factors, in contrast to VSELs possess a proper somatic imprint on maternal and paternal chromosomes. On the other hand, however, VSELs, which our preliminary data already suggests erased somatic imprint, have constitutive expression of all these genes that have to be first over-expressed in fibroblasts (e.g., Oct-4, Sox-2, Myc and Klf4). Therefore, one of the potential strategies to obtain stem cells that will display all the features of PSC (including potential for blastocyst complementation) would be “debugging cellular reprogramming” by transducing cells by multiple transcription factors (Oct-4, Sox-2, Myc and Klf4) (1st scenario). The other one would rely on the re-establishment of the proper somatic imprint in VSELs (2nd scenario). Our team is currently focused on this second possibility – re-establishment of a proper somatic imprint in VSELs.
Very small cells in adult tissues
Data obtained in our laboratory clearly demonstrate the presence of small primitive nucleated cells in adult BM and other tissues that are slightly larger than blood platelets yet smaller than erythrocytes (Figure 1). Employing ImageStream system we could visualize the VSELs and compare them directly with erythrocytes and platelets. ImageStream is a novel system which similarly to flow cytometry analyzes cells in suspension but also takes the images of acquired objects (Zuba-Surma et al., 2007b; Zuba-Surma et al., 2007c; Zuba-Surma et al., 2007d).
Generally, the presence of cells possessing similar small size (< 5 µm) in adult murine tissues has been postulated recently by Vacanti and colleagues (Vacanti et al., 2001). These cells were introduced as “spore-like stem cells”. Unfortunately the isolation strategy of spore-like stem cells was not clearly described by the authors in the original paper and thus it was not specified how these cells were isolated from adult tissues (Vacanti et al., 2001). In contrast, by employing FACS-based phenotypic analysis of single cell suspensions prepared from murine brain, blood, and intestinal epithelium, Howell et al. revealed the presence of very small CD45−Sca-1+c-kit− cells in varying degrees that may represent universal pluripotent stem cells residing at different levels in multiple murine tissues (Howell et al., 2003). Very small stem cells with neuroblast activity were also recently found in the subventricular zone of the murine brain (Scheffler et al., 2005). Finally, Hung and colleagues described a population of very small cells residing in human BM during isolation of mesenchymal stem cells (MSCs) on double layer culture plates containing 3-µm pores employed to sieve out the relatively large mesenchymal stem cells (Hung et al., 2002).
VSEL number and function in aging
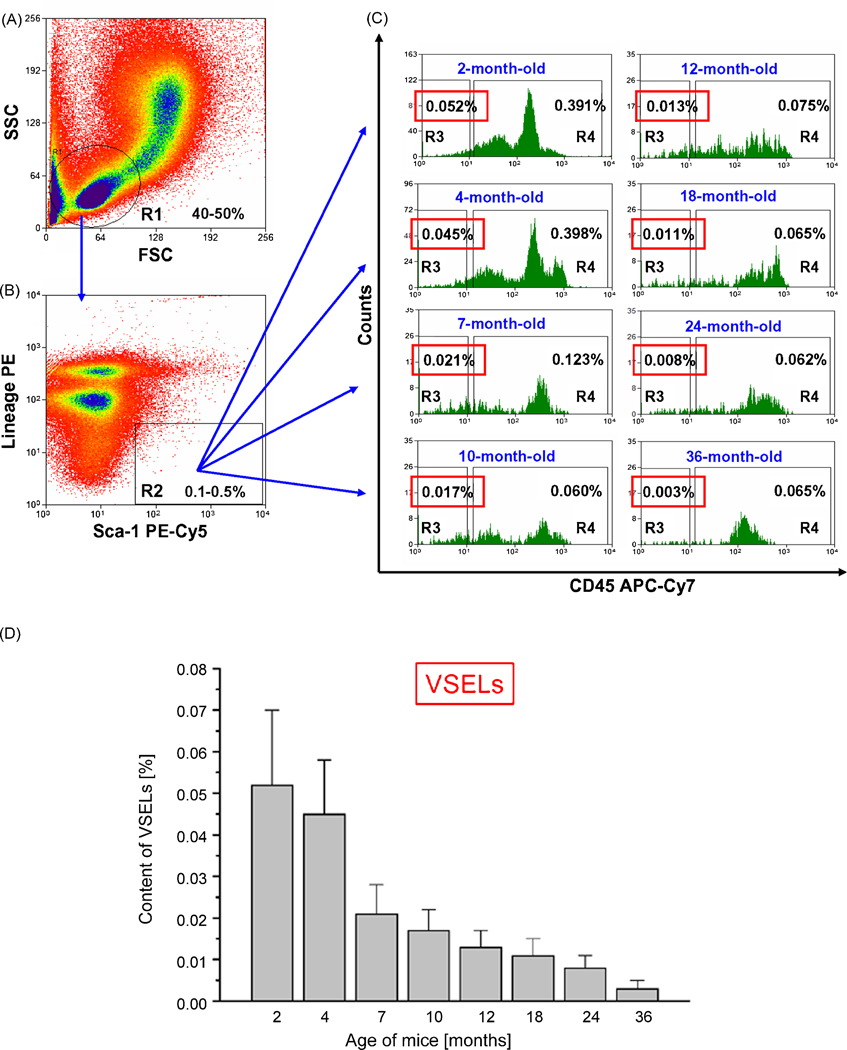
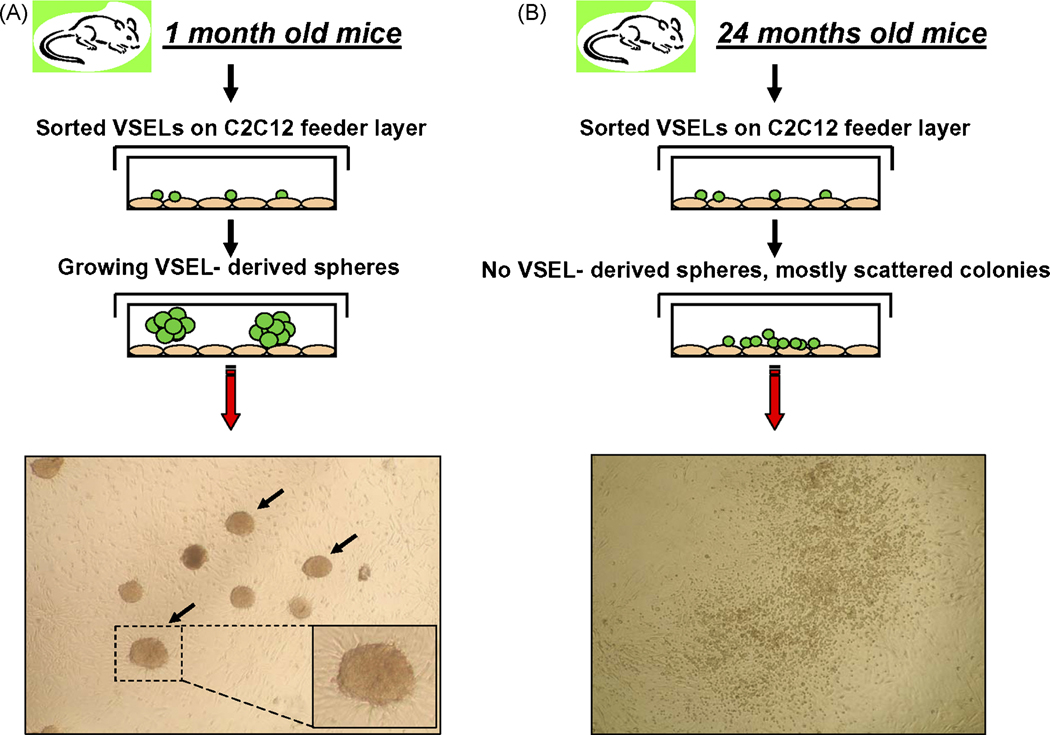
Employing flow cytometry, we evaluated the content of VSELs among small BM- derived Sca-1+Lin− nucleated cells isolated from mice at different ages (2 months – 3 years) (Figure 2). Cells that display VSEL phenotype were also identified in several other adult organs (Figure 3). However, we noticed that the number of these cells (Sca-1+Lin−CD45−) in BM from C57BL/6 mice gradually decreases over time from 0.052±0.018% to 0.003±0.002% between age of 2-months and 3-years, respectively (Figure 2 panel D). More importantly, not only a number of these cells in adult organs decreases with the age but also their ability to form spheres containing primitive stem cells (VSEL-DS) declaims with time (Figure 4). This age-dependent content of VSELs in BM may explain why the regeneration processes are more efficient in younger individuals, when compared to older organisms.
Figure 2. Multicolor flow cytometric analysis of VSEL content in fraction of nucleated cells isolated from bone marrow of 2-, 4-, 7-, 10-, 12-, 18-, 24- and 36-months old mice.
Percent content of VSELs was analyzed in total bone marrow cell population by MoFlo cell sorter (DAKO) following the lysis of red blood cells and immunostaining for murine Sca-1, CD45 and hematopoietic lineages markers. Specific antibodies conjugated with following fluorchoromes: PE-Cy5, APC-Cy7 and PE, respectively (BD Pharmingen), were employed for the staining. Based on the comparison with beads particles (Flow Cytometry Size Calibration Kit, Invitrogen) only small cells (app. 2–10 µm in size) were included into region R1 (Panel A). Cells from region R1 are visualized based on their expression of Sca-1 and lineage markers on the next dot-plot (Panel B). Panel C shows histograms of Sca-1+ lin− cells derived from region R2 when the bone marrow cells of mice in different age were analyzed. VSELs are indicated as a fraction of cells which is negative for CD45 and lineage markers and do express Sca-1 antigen (region R3, Panel C). Sca-1+ lin− CD45+ hematopoietic stem cells (HSCs) are shown in region R4 on each histogram. Average content of VSELs and HSCs is shown for each age group of mice. Panel D. Age dependent decrease in the content of VSELs in murine bone marrow. VSEL content among BM cells was analyzed as described on Figure 2. Total nucleated bone marrow cells were stained for Sca-1, CD45 and lineage markers and subsequently analyzed by MoFlo. The graph shows the average content of VSELs according to the age of animals (Mean ± SEM).
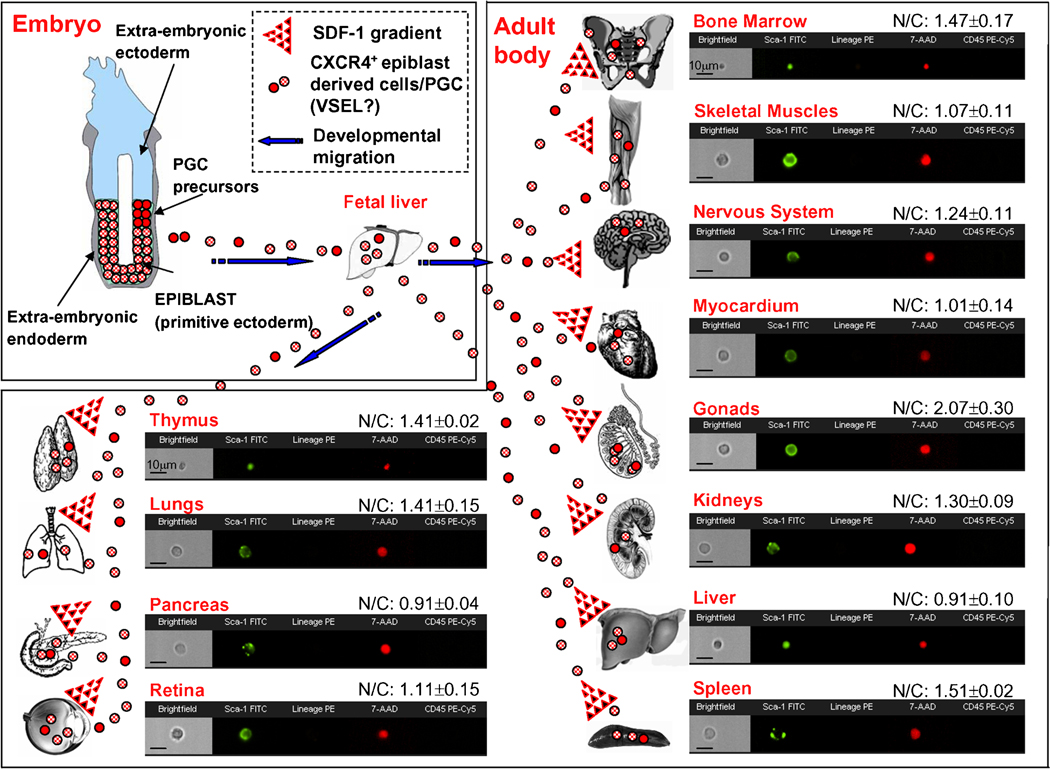
Figure 3. Hypothesis of developmental deposition of Oct-4+ epiblast-derived embryonic stem cells (VSELs?) in adult tissues.
During early gastrulation epiblast is a source of pluripotent stem cells for all three germ layers (meso-, ecto- and endoderm) and primordial germ cells (PGC) as well. We hypothesize that at this stage some epiblast derived stem cells could be deposited as Oct-4+ PSC in peripheral tissues/organs (red circles). Similarly, some migrating PGC could go astray from their major migratory route to the genital ridges and become deposited as well. Generally, the presence of Oct-4+ stem cells in the fetal liver, BM and other tissues could be explained by the developmental deposition of CXCR4+ epiblast derived stem cells (e.g., VSELs) following an SDF-1 gradient in different organs (mainly BM). The potential relationship of these cells to early epiblast/primordial germ cells (PGC) requires further investigation (Ratajczak et al., 2007a). The presence of Sca-1+ lin− CD45− cells was analyzed in various organs by ImageStream system and the images of these cells are shown as examples. Cells were stained for Sca-1 (FITC, green), Lineage markers (PE, orange) and CD45 (PE-Cy5, magenta). Nuclei were stained with 7-aminoactinomycin D (7-AAD, red) following fixation. The average values of nuclear to cytoplasmic (N/C) ratios of Sca-1+ lin− CD45− populations from each organ are shown with the images (Mean ± SD). The high values of N/C ratios of Sca-1+ lin− CD45− cells detected in several organs, comparable to BM- derived VSELs, indicate primitive character of these populations.
Figure 4. Sphere formation by VSELs isolated from young but not old mice.
Isolated by FACS Sca-1+lin−CD45− VSELs from 1 month old murine BM form spheres if plated over exponentially growing murine C2C12 myoblastic cells line in Dulbecco's MEM (DMEM) supplemented with 2% heat inactivated fetal bovine serum (FBS). In these co-culture conditions ~ 5–10% of plated VSELs form by day 7–10 spheres (VSEL-DS) that consist of primitive stem cells which express Oct-4, Nanog and SSEA-1 antigen. Cells from VSEL-DS if plated in secondary cultures supplemented with appropriate combinations of differentiation promoting factors may differentiate into cells from all three germ layers. Panel A – Typical formation of sphere by murine VSELs from 1 month old mice. Panel B – VSELs isolated from 2 years old mice do not grow spheres but instead scattered colonies of adherent cells. Representative images are shown.
Moreover, we have also established the differences in the content of VSELs among BM- derived nucleated cells isolated from long- and short-lived murine strains. We reported that the number of these cells is much higher in BM of long-lived (e.g., C57BL/6) as compared to short-lived (DBA/2J) mice (Kucia et al., 2006a). In our opinion, it would be interesting to identify genes that are responsible for tissue distribution/expansion of these cells as they could be involved in controlling the life span of mammals.
However, there are more scientific challenges coming out from recent VSELs’ studies. Based on our animal data it would be interesting to study whether the number of VSELs depends also on human age. It would be also important to evaluate if there are some differences in VSELs number in BM and other tissues as function of senescence between members of long- and short-living families.
Developmental origin of VSELs
An important question to be answered is the developmental origin of VSEL stem cells. We hypothesize that these cells are specified early during development at the stage of so called “cylinder embryo” in the primitive ectoderm (epiblast). We also envision that VSELs are direct descendants from the germ lineage. From developmental point of view, this is the most important cell lineage in the body (Drenos et al., 2005; Kucia et al., 2007b; Ratajczak et al., 2007a; Weismann, 1885; Zwaka et al., 2005). The significance of this statement will be justified below.
It has been widely accepted that from the developmental and evolutionary point of view, the main goal of the multicellular organism is to pass genes to the next generations. This process is orchestrated by the highly developed interactions between germ and somatic cell lines (Weismann, 1885). The germ line carries the genome (nuclear and mitochondrial DNA) from one generation to the next generation being the only cell lineage which preserve true developmental totipotency (Donovan, 1998; Zwaka et al., 2005). In this context, we can envision that all somatic cells are descendants from the germ line and help germ cells to accomplish this mission effectively.
To support this concept, we can look at the zygote, derived directly during fertilization from the fusion of two germ cells (female oocyte and male sperm), as the most primitive totipotent germ stem cell capable to form both embryo and extra-embryonic tissues (placenta). During fertilization the haploid number of chromosomes derived from an oocyte is combined with haploid number of chromosomes of a male sperm, and thus the zygote could be envisioned as a mother cell to the germ lineage which “down the road” will give rise to i) more differentiated cells from the germ lineage - gametes (responsible for the transfer of genome to the next generation) and ii) other somatic lineages that provide the soma to fulfill this mission. These somatic cells develop from the germ lineage during embryogenesis. In this context, the body/soma becomes a “vehicle” protecting the germ lineage and allowing to pass the genomic information to the progeny (Ratajczak et al., 2007a).
The germ potential of the zygote is retained in the i) first blastomers, ii) cells of developing morula and iii) cells from the inner cell mass (ICM) of a blastocyst. However, at this time of development, some level of specification already occurs and the trophoectoderm “buds out” from the germ line. Trophoectoderm is responsible for formation of the placenta and the remaining part of the blastocyst, while ICM will give rise to the embryo including epiblast. Epiblast stem cells (EPSC) have been also demonstrated to preserve pluripotency as well as retain germ lineage potential (Matsui et al., 1992; Shamblott et al., 1998; Yamazaki et al., 2003). EPSC express following antigens related to their pluripotent stage: SSEA-1 (mice), SSEA-3/4 (human), Oct-4 and Nanog. They give rise to all three germ layers, ecto-, meso- and endoderm including primordial germ cells (PGC). Thus, the epiblast, through the process of gastrulation, is the source of all stem cells for all the germ layers. The stem cells in these layers give rise to all of the tissues and organs in the embryo. Thus, EPSC could be envisioned as a founder population of PSC for multipotent stem cells of ecto-, endo- or mesoderm that subsequently give rise to unipotent stem cells which develop several somatic cell lineages in the next stages of embryonic development (Ratajczak et al., 2007a; Rossant, 2007; Yamanaka et al., 2006). These Oct-4+ EPSC may survive in the tissues and organs into adulthood as a founder population of stem cells (Figure 3). Furthermore, there is also evidence that also some of the epiblast-derived PGC may go astray during their migration through the embryo to the genital ridges and seed to peripheral tissues (Upadhyay et al., 1982).
Status of somatic imprint – functional implications for potential PSC in adult organs/tissues?
The most valuable assay to demonstrate that a given stem cell is truly pluripotent is an assay based on the developmental complementation of the blastocyst. In this assay, candidate PSC is injected into developing blastocyst and subsequently the embryo or the neonatal mouse is examined if the potential candidate cell contributed to development of organs and tissues belonging to all three germ layers (Hochedlinger et al., 2003; Hochedlinger et al., 2006; Prelle et al., 2002). In fact this had been not shown yet in a reproducible manner for any type of stem cell isolated from the adult tissues.
We hypothesize that the problem with providing such evidence could be explained by the possibility that all PSC that reside in adult tissues erase “somatic imprint” that means different methylation of some maternally and paternally inherited genes (e.g., H19, Igf-2, Igf-2R, Snrpn) (Lee et al., 2002; Mann, 2001). For example, Igf-2 is expressed from paternal and H19 from maternal chromosome. The erasure of somatic imprint on these genes by demethylation process protects developing organism from parthenogenesis and teratoma formation. However, on the other hand, prevents the contribution of surviving into adulthood PSC to complete blastocyst development (Lee et al., 2002; Mann, 2001).
The phenomenon of erasure of the somatic imprint was studied extensively in a case of epiblast derived PGC (Lee et al., 2002; Mann, 2001). Accordingly, shortly before the epiblast is about to give rise to all three germ layers (ectoderm, mesoderm and endoderm), the first morphologically identifiable precursors of primordial germ cells (PGC) in mice become specified ~ 6.0–6.5 days post conception (dpc) in the proximal part of the epiblast as the first population of stem cells in the embryo at the beginning of gastrulation (McLaren, 1992; McLaren, 2003). PGC in mice subsequently move for a short period of time first to the basis of alantois, which is located in the extraembryonic mesoderm and then migrate into the embryo proper towards the genital ridges - where finally they will undergo developmental differentiation to oocytes or sperm, respectively (Molyneaux et al., 2004).
Interestingly, the migrating PGC, in contrast to EPSC, loose their pluripotency. Namely, migrating PGC, freshly isolated from embryo, proliferate in vitro only for a few days, and then disappear either because they differentiate or die (De Felici et al., 1983). Furthermore, while the nuclei of even terminally differentiated somatic cells (e.g., fibroblasts, lymphocytes) can be successfully used as donors for nuclear transfer and may give rise to the clonote, nuclei from PGC at 11.5 dpc and later are incompetent to support full-term development of clonote (Yamazaki et al., 2003). This is somehow intriguing, taking into consideration that PGC are the population of stem cells that carries “developmental totipotency” for oocytes and sperm.
However, on the other hand, when PGC are cultured over murine embryonic fibroblasts and exposed ex vivo to following growth factors: kit ligand (KL), leukemia inhibitory factor (LIF) and basic fibroblast growth factor (FGF-2), they continue proliferation and form large colonies of embryonic germ cells (EG) which similarly to embryonic stem cells (ESC) can be expanded indefinitely (Matsui et al., 1992; Shamblott et al., 1998; Turnpenny et al., 2003). Such EG have been derived from pre- and post-migratory, as well as from migratory PGC in both mice and humans and were described as cells preserving pluripotency (Matsui et al., 1992; Shamblott et al., 1998). Namely, EG in contrast to PGC, fully contribute to all three germ layers in blastocyst complementation assay giving rise to all somatic lineages and germ cells of the developing embryo.
To explain this phenomenon at the molecular level, we need to noticed that PGC display a proper somatic imprint (paternal and maternal pattern of methylation) of H19, Igf-2, Igf-2R, Snrpn genes until 9.5 dpc - that is crucial to maintain their pluripotency (Mann, 2001; Yamazaki et al., 2003). The somatic imprint, however, is erased in PGC by demethylation while these cells migrate towards the genital ridges ~ 10.5 dpc (Lee et al., 2002). The erasing of the methylation (imprint) of H19, Igf-2, Igf-2R, and Snrpn in early PGC could make these cells resistant to parthenogenesis or formation of teratomas, but on the other hand shuts down PGC developmental pluripotency (Macchiarini et al., 2004; Oosterhuis et al., 2005). A proper somatic imprint is subsequently again reestablished in developing gonads in haploid gametes (sperm and oocytes), so that a fertilized egg (zygote) expresses a developmentally proper somatic imprint of these crucial genes.
We hypothesize that an analogical mechanism of somatic imprint erasure takes place during development, not only in PGC but also in other EPSC-derived PSC that are deposited in the developing organs – e.g., VSELs (Figure 3). Due to the erasure of somatic imprint, deposited in adult tissues PSC loose their ability to complete blastocyst development. However, the fact that PGC- derived EG cells regain their pluripoteniality, demonstrates that the erasure of somatic imprint is reversible. It is also possible that similarly as PGC, also other PSC residing in adult tissues may regain a proper somatic imprint under certain circumstances (e.g., tissue/organ injury) (Ratajczak et al., 2007a). This may occur through epigenetic changes. It is no doubt that the identification of genes/proteins involved in this phenomenon will be crucial for manipulating pluripotentiality of somatic stem cells.
The potential role of VSELs in pathology
In parallel, evidence has accumulated that BM-derived stem cells may play an undesirable role in the development of some pathologies. We hypothesize, that if VSELs are mobilized at the wrong time and home to the wrong place (e.g., into areas of chronic inflammation) they may exert unwanted effects (Reya et al., 2001; Virchow, 1855). For example, BM-derived stem/progenitor cells have been implicated in the pathogenesis of lung fibrosis, ocular pterygia and diabetic neuropathy (Hasegawa et al., 2006; Hashimoto et al., 2004; Song et al., 2005).
It has been also found that BM-derived stem cells may also contribute to the development of some non-hematopoietic tumors. This possibility was recently demonstrated in a model of murine stomach cancer caused by a chronic Helicobacter pylori (HP) infection (Houghton et al., 2004). The potential role of VSELs in this phenomenon requires further studies. In addition, mounting evidence also suggests that BM may be a source of endothelial progenitor cells and stromal cells for developing cancer tissue (Dome et al., 2006; Tolar et al., 2007). Thus, BM-stem cells (e.g., VSELs?) may in addition in different ways contribute to both initiation and expansion of the growing tumor.
Conclusions
Data obtained in our laboratory indicate that VSELs could potentially provide a real therapeutic alternative to the controversial use of human ES cells and therapeutic cloning. Hence, while the ethical debate on the application of ES cells in therapy continues, the potential of VSELs is ripe for exploration. Further studies are also required on a role of VSELs in aging. Would it be possible to expand life span of aging mice by transplantation of VSELs isolated from young littermates? Alternatively, it is possible to isolate VSELs from the mouse at young age (by femur aspiration under general anestesia), store these autologous cells for one year, and than inject them into the bone of the same animal. Would this strategy extend life span of this mouse? Thus, researchers working with animal models must determine whether these cells could be efficiently employed in the clinic or whether they are merely developmental remnants found in the BM that cannot be harnessed effectively for regeneration. The coming years will bring important answers to these questions.
Acknowledgments
Supported by NIH grant R01 CA106281-01 to MZR.
References
- Anjos-Afonso FDB. Nonhematopoietic/endothelial SSEA-1+ cells define the most primitive progenitors in the adult murine bone marrow mesenchymal compartment. Blood. 2007;109:1298–1306. doi: 10.1182/blood-2006-06-030551. [DOI] [PubMed] [Google Scholar]
- Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- Beltrami AP, Cesselli D, Bergamin N, Marcon P, Rigo S, Puppato E, D'Aurizio F, Verardo R, Piazza S, Pignatelli A, Poz A, Baccarani U, Damiani D, Fanin R, Mariuzzi L, Finato N, Masolini P, Burelli S, Belluzzi O, Schneider C, Beltrami CA. Multipotent cells can be generated in vitro from several adult human organs (heart, liver and bone marrow) Blood. 2007;110:3438–3446. doi: 10.1182/blood-2006-11-055566. [DOI] [PubMed] [Google Scholar]
- Brazelton TR, Rossi FM, Keshet GI, Blau HM. From marrow to brain: expression of neuronal phenotypes in adult mice. Science. 2000;290:1775–1779. doi: 10.1126/science.290.5497.1775. [DOI] [PubMed] [Google Scholar]
- Buzzeo MP, Scott EW, Cogle CR. The hunt for cancer-initiating cells: a history stemming from leukemia. Leukemia. 2007;21:1619–1627. doi: 10.1038/sj.leu.2404768. [DOI] [PubMed] [Google Scholar]
- Corti S, Locatelli F, Papadimitriou D, Strazzer S, Comi GP. Somatic stem cell research for neural repair: current evidence and emerging perspectives. J Cell Mol Med. 2004;8:329–337. doi: 10.1111/j.1582-4934.2004.tb00322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ippolito G, Diabira S, Howard GA, Menei P, Roos BA, Schiller PC. Marrow-isolated adult multilineage inducible (MIAMI) cells, a unique population of postnatal young and old human cells with extensive expansion and differentiation potential. J Cell Sci. 2004;117:2971–2981. doi: 10.1242/jcs.01103. [DOI] [PubMed] [Google Scholar]
- Dawn B, Zuba-Surma EK, Abdel-Latif A, Tiwari S, Bolli R. Cardiac stem cell therapy for myocardial regeneration. A clinical perspective. Minerva Cardioangiol. 2005;53:549–564. [PubMed] [Google Scholar]
- De Felici M, McLaren A. In vitro culture of mouse primordial germ cells. Exp Cell Res. 1983;144:417–427. doi: 10.1016/0014-4827(83)90421-4. [DOI] [PubMed] [Google Scholar]
- Dome B, Timar J, Dobos J, Meszaros L, Raso E, Paku S, Kenessey I, Ostoros G, Magyar M, Ladanyi A, Bogos K, Tovari J. Identification and clinical significance of circulating endothelial progenitor cells in human non-small cell lung cancer. Cancer Res. 2006;66:7341–7347. doi: 10.1158/0008-5472.CAN-05-4654. [DOI] [PubMed] [Google Scholar]
- Donovan PJ. The germ cell--the mother of all stem cells. Int J Dev Biol. 1998;42:1043–1050. [PubMed] [Google Scholar]
- Drenos F, Kirkwood TB. Modelling the disposable soma theory of ageing. Mech Ageing Dev. 2005;126:99–103. doi: 10.1016/j.mad.2004.09.026. [DOI] [PubMed] [Google Scholar]
- Friedenstein AJ, Piatetzky-Shapiro II, Petrakova KV. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol. 1966;16:381–390. [PubMed] [Google Scholar]
- Hasegawa T, Kosaki A, Shimizu K, Matsubara H, Mori Y, Masaki H, Toyoda N, Inoue-Shibata M, Nishikawa MTI. Amelioration of diabetic peripheral neuropathy by implantation of hematopoietic mononuclear cells in streptozotocin-induced diabetic rats. Exp Neurol. 2006;199:274–280. doi: 10.1016/j.expneurol.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Hashimoto N, Jin H, Liu T, Chensue SW, Phan SH. Bone marrow-derived progenitor cells in pulmonary fibrosis. J Clin Invest. 2004;113:243–252. doi: 10.1172/JCI18847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochedlinger K, Jaenisch R. Nuclear transplantation, embryonic stem cells, and the potential for cell therapy. N Engl J Med. 2003;349:275–286. doi: 10.1056/NEJMra035397. [DOI] [PubMed] [Google Scholar]
- Hochedlinger K, Jaenisch R. Nuclear reprogramming and pluripotency. Nature. 2006;441:1061–1067. doi: 10.1038/nature04955. [DOI] [PubMed] [Google Scholar]
- Houghton J, Stoicov C, Nomura S, Rogers AB, Carlson J, Li H, Cai X, Fox JG, Goldenring JR, Wang TC. Gastric cancer originating from bone marrow-derived cells. Science. 2004;306 doi: 10.1126/science.1099513. [DOI] [PubMed] [Google Scholar]
- Howell JC, Lee WH, Morrison P, Zhong J, Yoder MC, Srour EF. Pluripotent stem cells identified in multiple murine tissues. Ann N Y Acad Sci. 2003;996:158–173. doi: 10.1111/j.1749-6632.2003.tb03244.x. [DOI] [PubMed] [Google Scholar]
- Hung SC, Chen NJ, Hsieh SL, Li H, Ma HL, Lo WH. Isolation and characterization of size-sieved stem cells from human bone marrow. Stem Cells. 2002;20:249–258. doi: 10.1634/stemcells.20-3-249. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- Johnson J, Bagley J, Skaznik-Wikiel M, Lee HJ, Adams GB, Niikura Y, Tschudy KS, Tilly JC, Cortes ML, Forkert R, Spitzer T, Iacomini J, Scadden DT, Tilly JL. Oocyte generation in adult mammalian ovaries by putative germ cells in bone marrow and peripheral blood. Cell. 2005;122:303–315. doi: 10.1016/j.cell.2005.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucia M, Dawn B, Hunt G, Guo Y, Wysoczynski M, Majka M, Ratajczak J, Rezzoug F, Ildstad ST, Bolli R, Ratajczak MZ. Cells expressing early cardiac markers reside in the bone marrow and are mobilized into the peripheral blood after myocardial infarction. Circ Res. 2004;95:1191-119995–1199. doi: 10.1161/01.RES.0000150856.47324.5b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucia M, Halasa M, Wysoczynski M, Baskiewicz-Masiuk M, Moldenhawer S, Zuba-Surma E, Czajka R, Wojakowski W, Machalinski B, Ratajczak MZ. Morphological and molecular characterization of novel population of CXCR4(+) SSEA-4(+) Oct-4(+) very small embryonic-like cells purified from human cord blood - preliminary report. Leukemia. 2007a;21:297–303. doi: 10.1038/sj.leu.2404470. [DOI] [PubMed] [Google Scholar]
- Kucia M, Reca R, Campbell FR, Zuba-Surma E, Majka M, Ratajczak J, Ratajczak MZ. A population of very small embryonic-like (VSEL) CXCR4(+)SSEA-1(+)Oct-4+ stem cells identified in adult bone marrow. Leukemia. 2006a;20:857–869. doi: 10.1038/sj.leu.2404171. [DOI] [PubMed] [Google Scholar]
- Kucia M, Reca R, Jala VR, Dawn B, Ratajczak J, Ratajczak MZ. Bone marrow as a home of heterogenous populations of nonhematopoietic stem cells. Leukemia. 2005;19:1118–1127. doi: 10.1038/sj.leu.2403796. [DOI] [PubMed] [Google Scholar]
- Kucia M, Wu W, Ratajczak MZ. Bone marrow-derived very small embryonic-like stem cells: Their developmental origin and biological significance. Dev Dyn. 2007b;11 doi: 10.1002/dvdy.21180. [DOI] [PubMed] [Google Scholar]
- Kucia M, Wysoczynski MJ, Ratajczak J, Ratajczak MZ. Identification of Very Small Embryonic Like (VSEL) stem cells in bone marrow. Cell Tissue Res. 2007c;331:125–134. doi: 10.1007/s00441-007-0485-4. [DOI] [PubMed] [Google Scholar]
- Kucia M, Zhang YP, Reca R, Wysoczynski M, Machalinski B, Majka M, Ildstad ST, Ratajczak J, Shields CB, Ratajczak MZ. Cells enriched in markers of neural tissue-committed stem cells reside in the bone marrow and are mobilized into the peripheral blood following stroke. Leukemia. 2006b;20:18–28. doi: 10.1038/sj.leu.2404011. [DOI] [PubMed] [Google Scholar]
- Kucia M, Zuba-Surma EK, Wysoczynski M, Wu W, Ratajczak J, Machalinski B, Ratajczak MZ. Adult marrow-derived very small embryonic-like stem cells and tissue engineering. Expert Opin Biol Ther. 2007d;7:1499–1514. doi: 10.1517/14712598.7.10.1499. [DOI] [PubMed] [Google Scholar]
- Lee J, Inoue K, Ono R, Ogonuki N, Kohda T, Kaneko-Ishino T, Ogura A, Ishino F. Erasing genomic imprinting memory in mouse clone embryos produced from day 11.5 primordial germ cells. Development. 2002;129:1807–1817. doi: 10.1242/dev.129.8.1807. [DOI] [PubMed] [Google Scholar]
- Li HC, Stoicov C, Rogers AB, Houghton J. Stem cells and cancer: evidence for bone marrow stem cells in epithelial cancers. World J Gastroenterol. 2006;12:363–371. doi: 10.3748/wjg.v12.i3.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Chen Z, Chen Z, Zhang T, Lu Y. Multiple tumor types may originate from bone marrow-derived cells. Neoplasia. 2006;8:716–724. doi: 10.1593/neo.06253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macchiarini P, Ostertag H. Uncommon primary mediastinal tumours. Lancet Oncol. 2004;5:107–118. doi: 10.1016/S1470-2045(04)01385-3. [DOI] [PubMed] [Google Scholar]
- Mann JR. Imprinting in the germ line. Stem Cells. 2001;19:287–294. doi: 10.1634/stemcells.19-4-287. [DOI] [PubMed] [Google Scholar]
- Matsui Y, Zsebo K, Hogan BL. Derivation of pluripotential embryonic stem cells from murine primordial germ cells in culture. Cell. 1992;70:841–847. doi: 10.1016/0092-8674(92)90317-6. [DOI] [PubMed] [Google Scholar]
- McLaren A. Development of primordial germ cells in the mouse. Andrologia. 1992;24:243–247. doi: 10.1111/j.1439-0272.1992.tb02647.x. [DOI] [PubMed] [Google Scholar]
- McLaren A. Primordial germ cells in the mouse. Dev Biol. 2003;262:1–15. doi: 10.1016/s0012-1606(03)00214-8. [DOI] [PubMed] [Google Scholar]
- Meissner A, Wernig M, Jaenisch R. Direct reprogramming of genetically unmodified fibroblasts into pluripotent stem cells. Nat Biotechnol. 2007;25:1177–1181. doi: 10.1038/nbt1335. [DOI] [PubMed] [Google Scholar]
- Molyneaux K, Wylie C. Primordial germ cell migration. Int J Dev Biol. 2004;48:537–544. doi: 10.1387/ijdb.041833km. [DOI] [PubMed] [Google Scholar]
- Nayernia K, Lee JH, Drusenheimer N, Nolte J, Wulf G, Dressel R, Gromoll J, Engel W. Derivation of male germ cells from bone marrow stem cells. Lab Invest. 2006;86:654–663. doi: 10.1038/labinvest.3700429. [DOI] [PubMed] [Google Scholar]
- Oakley EJ, Van Zant G. Unraveling the complex regulation of stem cells: implications for aging and cancer. Leukemia. 2007;21:612–621. doi: 10.1038/sj.leu.2404530. [DOI] [PubMed] [Google Scholar]
- Oosterhuis JW, Looijenga L. Testicular germ-cell tumors in a broader perspective. Nat Rev Cancer. 2005;5:210–222. doi: 10.1038/nrc1568. [DOI] [PubMed] [Google Scholar]
- Orkin SH, Zon LI. Hematopoiesis and stem cells: plasticity versus developmental heterogeneity. Nat Immunol. 2002;3:323–328. doi: 10.1038/ni0402-323. [DOI] [PubMed] [Google Scholar]
- Overholtzer M, Mailleux AA, Mouneimne G, Normand G, Schnitt SJ, King RW, Cibas ES, Brugge JS. A nonapoptotic cell death process, entosis, that occurs by cell-in-cell invasion. Cell. 2007;131:966–979. doi: 10.1016/j.cell.2007.10.040. [DOI] [PubMed] [Google Scholar]
- Peister A, Mellad JA, Larson BL, Hall BM, Gibson LF, Prockop DJ. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood. 2004;103:1662–1668. doi: 10.1182/blood-2003-09-3070. [DOI] [PubMed] [Google Scholar]
- Prelle K, Zink N, Wolf E. Pluripotent stem cells-model of embryonic development, tool for gene targeting, and basis of cell therapy. Anat Histol Embryol. 2002;31:169–186. doi: 10.1046/j.1439-0264.2002.00388.x. [DOI] [PubMed] [Google Scholar]
- Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- Ratajczak MZ, Machalinski B, Wojakowski W, Ratajczak J, Kucia M. A hypothesis for an embryonic origin of pluripotent Oct-4+ stem cells in adult bone marrow and other tissues. Leukemia. 2007a;21:860–867. doi: 10.1038/sj.leu.2404630. [DOI] [PubMed] [Google Scholar]
- Ratajczak MZ, Zuba-Surma E, Kucia M, Reca R, Wojakowski W, Ratajczak J. The pleiotropic effects of the SDF-1-CXCR4 axis in organogenesis, regeneration and tumorigenesis. Leukemia. 2006;20:1915–1924. doi: 10.1038/sj.leu.2404357. [DOI] [PubMed] [Google Scholar]
- Ratajczak MZ, Zuba-Surma EK, Machalinski B, Kucia M. Bone-marrow-derived stem cells-our key to longevity? J Appl Genet. 2007b;48:307–319. doi: 10.1007/BF03195227. [DOI] [PubMed] [Google Scholar]
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- Rossant J. Stem cells and lineage development in the mammalian blastocyst. Reprod Fertil Dev. 2007;19:111–118. doi: 10.1071/rd06125. [DOI] [PubMed] [Google Scholar]
- Scheffler B, Walton NM, Lin DD, Goetz AK, Enikolopov G, Roper SN, Steindler DA. Phenotypic and functional characterization of adult brain neuropoiesis. Proc Natl Acad Sci U S A. 2005;102:9353–9358. doi: 10.1073/pnas.0503965102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamblott MJ, Axelman J, Wang S, Bugg EM, Littlefield JW, Donovan PJ, Blumenthal PD, Huggins GR, Gearhart JD. Derivation of pluripotent stem cells from cultured human primordial germ cells. Proc Natl Acad Sci U S A. 1998;95:13726–13731. doi: 10.1073/pnas.95.23.13726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song YS, Ryu YH, Choi SR, Kim JC. The involvement of adult stem cells originated from bone marrow in the pathogenesis of pterygia. Yonsei Med J. 2005;46:687–692. doi: 10.3349/ymj.2005.46.5.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki MMN, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Tolar J, Nauta AJ, Osborn MJ, Panoskaltsis Mortari A, McElmurry RT, Bell S, Xia L, Zhou N, Riddle M, Schroeder TM, Westendorf JJ, McIvor RS, Hogendoorn PC, Szuhai K, Oseth L, Hirsch B, Yant SR, Kay MA, Peister A, Prockop DJ, Fibbe WE, Blazar BR. Sarcoma derived from cultured mesenchymal stem cells. Stem Cells. 2007;25:371–379. doi: 10.1634/stemcells.2005-0620. [DOI] [PubMed] [Google Scholar]
- Turnpenny L, Brickwood S, Spalluto CM, Piper K, Cameron IT, Wilson DI, Hanley NA. Derivation of human embryonic germ cells: an alternative source of pluripotent stem cells. Stem Cells. 2003;21:598–609. doi: 10.1634/stemcells.21-5-598. [DOI] [PubMed] [Google Scholar]
- Upadhyay S, Zamboni L. Ectopic germ cells: natural model for the study of germ cell sexual differentiation. Proc Natl Acad Sci U S A. 1982;79:6584–6588. doi: 10.1073/pnas.79.21.6584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacanti MP, Roy A, Cortiella J, Bonassar L, Vacanti CA. Identification and initial characterization of spore-like cells in adult mammals. J Cell Biochem. 2001;80:455–460. [PubMed] [Google Scholar]
- Virchow R. Editorial Archive fuer pathologische Anatomie und Physiologie fuer klinische. Medizin. 1855;8:23–54. [Google Scholar]
- Weismann A. The continuity of the germ-plasm as the foundationof a theory of heredity. Verlag von Gustav Fisher Jena. 1885 [Google Scholar]
- Yamanaka Y, Ralston A, Stephenson RO, Rossant J. Cell and molecular regulation of the mouse blastocyst. Dev Dyn. 2006;235:2301–2314. doi: 10.1002/dvdy.20844. [DOI] [PubMed] [Google Scholar]
- Yamazaki Y, Mann MR, Lee SS, Marh J, McCarrey JR, Yanagimachi R, Bartolomei MS. Reprogramming of primordial germ cells begins before migration into the genital ridge, making these cells inadequate donors for reproductive cloning. Proc Natl Acad Sci U S A. 2003;100:12207–12212. doi: 10.1073/pnas.2035119100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuba-Surma EK, Kucia M, Abdel-Latif A, Dawn B, Hall B, Singh R, Lillard JW, Ratajczak MZ. Morphological characterization of Very Small Embryonic-Like stem cells (VSELs) by ImageStream system analysis. J Cell Mol Med. 2007a Nov 20; doi: 10.1111/j.1582-4934.2007.00154.x. (PMID: 18031297) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuba-Surma EK, Kucia M, Abdel-Latif A, Lillard JW, Ratajczak MZ. The ImageStream system: a key step to a new era in imaging. Folia Histochem Cytobiol. 2007b;45:279–290. [PubMed] [Google Scholar]
- Zuba-Surma EK, Kucia M, Ratajczak MZ. “Decoding of Dot”: The ImageStream System (ISS) as a Supportive Tool for Flow Cytometric Analysis. CEJB. 2007c Dec 8; [Google Scholar]
- Zuba-Surma EK, Kucia M, Ratajczak MZ. Imagestream Technology – A Step Further than Flow Cytometry. Adv Cell Biol. 2007d;34:361–375. [Google Scholar]
- Zwaka TP, Thomson JA. A germ cell origin of embryonic stem cells? Development. 2005;132:227–233. doi: 10.1242/dev.01586. [DOI] [PubMed] [Google Scholar]