Abstract
Endogenous ligands such as high-mobility group box 1 (HMGB1) and nucleic acids are released by dying cells and bind Toll-like receptors (TLRs). Because TLR9 sits at the interface of microbial and sterile inflammation by detecting both bacterial and endogenous DNA, we investigated its role in a model of segmental liver ischemia-reperfusion (I/R) injury. Mice were subjected to 1 h of ischemia and 12 h of reperfusion prior to assessment of liver injury, cytokines and reactive oxygen species (ROS). Wild-type (WT) mice treated with an inhibitory CpG (iCpG) sequence and TLR9−/− mice had markedly reduced serum alanine aminotransferase (ALT) and inflammatory cytokines following liver I/R. Liver damage was mediated by bone marrow derived cells as WT mice transplanted with TLR9−/− bone marrow were protected from hepatic I/R injury. Injury in WT mice partly depended on TLR9 signaling in neutrophils, which enhanced production of ROS, IL-6, and TNF. In vitro, DNA released from necrotic hepatocytes increased liver non-parenchymal cell (NPC) and neutrophil cytokine secretion via a TLR9-dependent mechanism. Inhibition of both TLR9 and HMGB1 caused maximal inflammatory cytokine suppression in neutrophil cultures and conferred even greater protection from I/R injury in vivo.
Conclusions
TLR9 serves as an endogenous sensor of tissue necrosis that exacerbates the innate immune response during liver I/R. Combined blockade of TLR9 and HMGB1 represents a clinically relevant, novel approach to limiting I/R injury.
Keywords: Sterile inflammation, danger signals, neutrophils, high-mobility group box 1
Liver I/R injury is an unavoidable consequence of partial hepatectomy, liver transplantation, and hypovolemic shock and remains a significant clinical problem. In addition to hepatocyte necrosis and apoptosis, severe liver I/R injury can induce a dysregulated systemic inflammatory response that culminates in multiple organ failure.1, 2 The current treatment of liver I/R injury is merely supportive care and thus new therapeutic strategies are needed.
Hepatic I/R generates a complex array of signals that are initially confined to the liver milieu. The ensuing sequence of events is characterized by ischemia-induced cytolysis of hepatocytes and the generation of ROS. Subsequently, secondary activation of the innate immune system occurs with upregulation of inflammatory cytokines and chemokines that promote additional hepatocyte death. Inflammatory agents known to potentiate hepatic I/R injury have been well described and include TNF, IL-1β and IL-12.3–5 In particular, TNF induces adhesion molecule and chemokine expression leading to rapid infiltration of neutrophils, which are among the principal effectors of liver I/R injury.6
TLRs are pattern-recognition receptors (PRRs) that recognize conserved pathogen-associated molecular patterns. Activation of innate immunity through TLR ligation occurs in microbial infection. However, it is now apparent that TLRs can also recognize endogenous ligands. Liver I/R injury is exacerbated via activation of TLR4 by HMGB1, a damage-associated molecular pattern (DAMP) protein released from dying cells.7–9 Meanwhile, TLR2 does not appear to play a role in liver I/R injury as TLR2−/− mice have similar serum ALT as WT mice.10 The role of other individual TLRs in liver I/R is unknown. TLR9 is an endosomal protein that recognizes bacterial CpG as well as self-DNA.11, 12 Since liver I/R results in hepatocyte death and potential DNA release, we hypothesized that TLR9 contributes to the associated immune response. Furthermore, because the TLR9-mediated responses of dendritic cells and macrophages to bacterial DNA in vitro have been shown to be augmented by HMGB1,13, 14 we sought to determine the relationship between TLR9 and HMGB1 in liver I/R.
MATERIALS AND METHODS
Animals and procedures
8–16-week-old WT CD45.1 and CD45.2 C57BL/6 (B6) mice were purchased from the Jackson Laboratory. TLR9−/− (CD45.2) mice on a B6 background (obtained from S. Akira, Osaka University, Japan) were bred in our facility. Neutrophil depletion was accomplished with an i.p. injection of 500 μg anti-Ly6G antibody (1A8) or isotype control (RatIgG2a; BioXCell) 24 and 2 h before I/R. Flow cytometry revealed that this regimen resulted in 100% depletion of CD11bhiLy6G+ neutrophils within the liver, spleen, and bone marrow 24 h after the second dose. TLR9 blockade was accomplished with a s.c. injection of 100 μg iCpG or control DNA sequence (InvivoGen).15 HMGB1 blockade was achieved by i.p. injection of 50 μg anti-HMGB1 monoclonal antibody (gift from K.J. Tracey, Manhasset, NY) or mouse IgG2bκ isotype control (Sigma-Aldrich) 1 h prior to I/R. Bone marrow chimeric mice were generated using WT (CD45.1) and TLR9−/− (CD45.2) mice. 5×106 T cell-depleted bone marrow cells were injected i.v. within 2 hours of lethal irradiation (1,300 rads) using a 137Cs source. Greater than 90% of the hematopoietic cells in the spleen were of donor origin 8 weeks later. Serum was obtained by direct cardiac puncture. Animals were maintained in a pathogen-free animal housing facility at Memorial Sloan-Kettering Cancer Center. All procedures were approved by the Institutional Animal Care and Use Committee.
Model of liver ischemia-reperfusion
A model of segmental (70%) warm hepatic ischemia was used as previously described with minor modifications.7 Briefly, under ketamine (1 mg/ml) and xylazine (1 mg/ml) anesthesia, an upper midline abdominal incision was made and the liver hilum exposed. The vasculature supplying the left and median lobes (ischemic lobes) of the liver was occluded with a microvascular clamp (Roboz Surgical Instruments) for 60 min. Evidence of ischemia during the clamping period was confirmed by tissue blanching. After removal of the clamp, evidence of reperfusion was confirmed by immediate color change of the ischemic lobes. Sham mice underwent the same procedure without clamping. Mice were euthanized by carbon dioxide inhalation.
Liver damage assessment
Serum ALT was measured using the Olympus AU400 Chemistry Analyzer. Formalin-fixed liver samples were embedded in paraffin. 5 μm sections were stained with hematoxylin-eosin (H&E) and examined with an Axioplan 2 widefield microscope (Zeiss).
Cell isolation and adoptive transfer
Liver NPCs and bulk splenocytes were isolated as previously described.16 Bulk CD45+ hematopoietic cells were isolated from liver NPCs using immunomagnetic beads (Miltenyi Biotec) as per the manufacturer’s instructions. WT hepatocytes were separated from NPCs following in situ perfusion with collagenase (type IV, 1 mg/ml; Sigma-Aldrich) and gentle mechanical disruption of liver tissue. This was followed by five cycles of centrifugation (50 × g for 2 min) in which the hepatocytes were separated from the supernatant. Hepatocyte purity exceeded 90% as assessed by light microscopy. For the isolation of neutrophils, splenocytes were incubated with an anti-Ly6G (1A8) biotinylated antibody and then anti-biotin microbeads according to the manufacturer’s protocol (Miltenyi Biotec). Purity was routinely greater than 96% and cell viability was greater than 97% by trypan blue exclusion. 2–4 × 106 TLR9−/− (CD45.2) or WT (CD45.1 or CD45.2) freshly isolated neutrophils were injected into the spleens of anesthetized TLR9−/− or WT mice just prior to I/R.
Flow cytometry
Flow cytometry was performed on a FACSAria (BD Biosciences). Fc receptors were blocked with 1μg anti-FcγRIII/II antibody (2.4G2; Monoclonal Core Facility, Sloan-Kettering Institute) per 106 cells. Neutrophils were defined as CD11bhiLy6G+. Cells were stained with fluorescent-conjugated CD11b (M1/70) and Ly6G (1A8) antibodies (BD Biosciences). Data was analyzed using FlowJo software (Tree Star).
In vitro co-culture assays
WT hepatocytes were rendered necrotic by incubation at 60°C for 60 min. Flow cytometry confirmed that greater than 98% of hepatocytes (side scatter high) were necrotic (PI+). Necrotic hepatocytes were plated at 106 or 5 ×106 cells/well in 96 well plates containing serum free media (RPMI 1640 containing 10 mM Hepes, 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM L-Glutamine; Media Preparation Core Facility, Sloan-Kettering Institute). Supernatant was harvested after a 12 h incubation period at 37°C and was used as conditioned media in subsequent co-culture assays. The concentrations of single and double stranded DNA in conditioned media were 504 ± 18 μg/ml and 84 ± 8 μg/ml, respectively. Bulk CD45+ liver NPCs or purified neutrophils from unmanipulated WT or TLR9−/− mice were cultured overnight at 106 or 5 × 106 cells/ml in media. Rabbit polyclonal anti-HMGB1 (10 μg/ml; Abcam) was added to certain wells containing conditioned media from necrotic hepatocytes. In additional experiments, conditioned media was pre-treated for 2 h with DNAse I (100 μg/ml; Sigma-Aldrich) at 25 °C.
Assays for oxidative burst and cytokines
Measurement of oxidative burst as gauged by the conversion of dihydrorhodamine (DHR) 123 to its oxidized form, rhodamine (R) 123, was determined by flow cytometry using the Phagoburst Kit (Orpegen) according to the manufacturer’s protocol. Ischemic liver CD45+ NPCs following sham procedure or I/R were cultured at a concentration of 106 cells/ml in media containing 10% FCS for 24 h. Purified neutrophils were cultured at 5 × 106 cells/ml in media with 10% FCS or conditioned media for 12 h. Supernatant and serum cytokine levels were determined using a cytometric bead array (Mouse Inflammation Kit, BD Biosciences). None of the tested cytokines were detected in control wells containing conditioned media only. Addition of polymixin B (10μg/ml, Sigma), which blocks endotoxin, to the in vitro conditioned media cultures did not alter the cytokine production by liver NPCs or neutrophils (unpublished data).
Statistical analysis
Results are expressed as mean ± SEM. Group comparisons were performed using Student’s t test using statistical software (Prism 5.0; GraphPad Software, Inc.). P<0.05 was deemed significant.
RESULTS
TLR9 signaling increases liver I/R injury
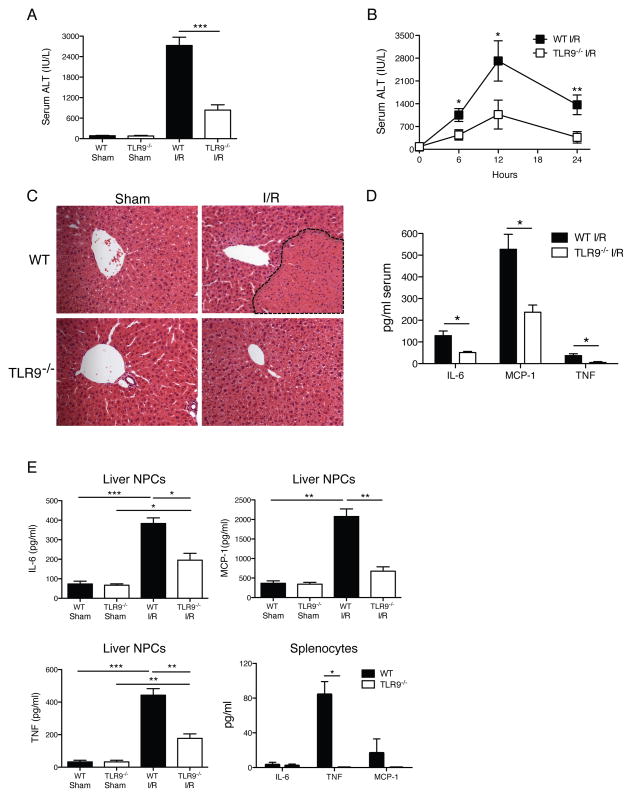
To determine the relative importance of TLR9 signaling in liver inflammation, we measured serum ALT in WT and TLR9−/− mice after 12 h of I/R. ALT levels in TLR9−/− mice were significantly reduced when compared with WT animals (Fig. 1A). Because both WT and TLR9−/− mice experienced maximal liver injury 12 h after I/R (Fig. 1B), subsequent experiments were performed at this time point. Liver histology was consistent with the ALT findings. Severe hepatocellular necrosis was evident in WT mice whereas TLR9−/− mice exhibited minimal damage (Fig. 1C). Accordingly, TLR9−/− mice had significantly less inflammatory cytokines in the serum, ischemic liver NPC cultures, and splenocyte cultures after I/R (Figs. 1D, E). Because TLR9 activation can result in type I interferon production, we measured circulating and local production of IFN-α. We did not observe any significant differences in IFN-α levels between WT and TLR9−/− mice within the serum or supernatant of ischemic liver NPC cultures after sham or 12 h of I/R (unpublished data).
Figure 1. TLR9−/− mice have less injury after liver I/R.
WT and TLR9−/− mice were subjected to liver I/R or sham procedure and serum ALT was measured (A) 12 h later or (B) at serial time points. (C) Liver sections are shown from the ischemic lobes of WT and TLR9−/− mice at 12 h (magnification x200). Non-viable tissue is shown by dashed lines. Liver sections are representative of 4 independent experiments (5 mice/group). (D) Serum cytokines were determined in WT and TLR9−/− mice after 12 h of I/R. (E) Ischemic liver CD45+ NPCs or bulk splenocytes from WT and TLR9−/− mice after 12 h of I/R or sham procedure were cultured in media without restimulation. Supernatant cytokine levels were measured 24 h later. Cytokines were undetectable in splenocyte cultures from mice undergoing sham procedure (unpublished data). Data in A, B, D and E represent means ± SEM and are representative of at least 3 independent experiments (5 mice/group). *p<0.05; **p<0.01; ***p<0.001.
Inhibitory CpG (iCpG) protects WT mice from liver I/R injury
Since TLR9−/− mice demonstrated less liver I/R injury, we postulated that TLR9 blockade might protect WT mice. A single dose of an iCpG sequence17 that disrupts co-localization of CpG with TLR9 in endosomal vesicles was used. Injection of iCpG immediately prior to I/R reduced serum ALT in WT mice to levels comparable to TLR9−/− mice (Fig. 2A). In fact, WT mice were protected by TLR9 blockade as late as 6 h after the initiation of I/R, suggesting that this approach may be useful clinically where there is often a delay in diagnosis and therapy. TLR9 blockade in WT mice resulted in lower serum and cultured NPC cytokine levels (Figs. 2B, C) and less liver injury by histology (Fig. 2D).
Figure 2. TLR9 inhibition protects WT mice from liver I/R injury.
WT mice received 100 μg iCpG s.c. immediately before, or 6 or 8 h following the onset of I/R or 100 μg control oligodeoxynucleotide (Ctrl ODN) sequence just prior to I/R. (A) Serum ALT and (B) serum cytokines were measured at 12 h. (C) Ischemic liver CD45+ NPCs from WT mice treated with Ctrl ODN or iCpG just prior to initiation of ischemia as in (B), were cultured in media after 12 h of I/R. Supernatant levels of cytokines were determined 24 h later. (D) Representative liver H&E staining at 12 h is shown (magnification x200). Non-viable patches of ischemic lobes are demarcated by dashed lines. Data in (A–C) represent means ± SEM and in (A–D) are representative of at least 3 independent experiments (5 mice/group). *p<0.05.
TLR9-mediated injury during I/R depends on liver NPCs
We generated bone marrow chimeric mice to identify whether hepatic I/R injury requires TLR9 signaling in liver parenchymal cells or NPCs. Irradiated WT mice reconstituted with TLR9−/− bone marrow cells and TLR9−/− mice transplanted with TLR9−/− bone marrow were protected from I/R injury to a similar degree as non-irradiated TLR9−/− mice (Fig. 3A). In contrast, TLR9−/− mice transplanted with WT bone marrow cells had increased serum ALT, serum cytokines (Fig. 3B), and liver injury by histology (Fig. 3C) after I/R.
Figure 3. TLR9 in bone marrow derived cells mediates liver injury after I/R.
Bone marrow chimeras were generated using WT (CD45.1 or CD45.2) and TLR9−/− (CD45.2) mice. Non-irradiated WT and TLR9−/− mice served as controls (non-chimeras). 12 h after I/R, (A) serum ALT and (B) serum cytokines were measured. (C) H&E-stained sections of ischemic liver lobes from TLR9−/− chimeric mice 12 h after I/R (magnification x100). Non-viable patches are demarcated by dashed lines. Data in (A) and (B) represent means ± SEM and in (A–C) are representative of 3 independent experiments (5 mice/group). *p< 0.05; **p<0.01.
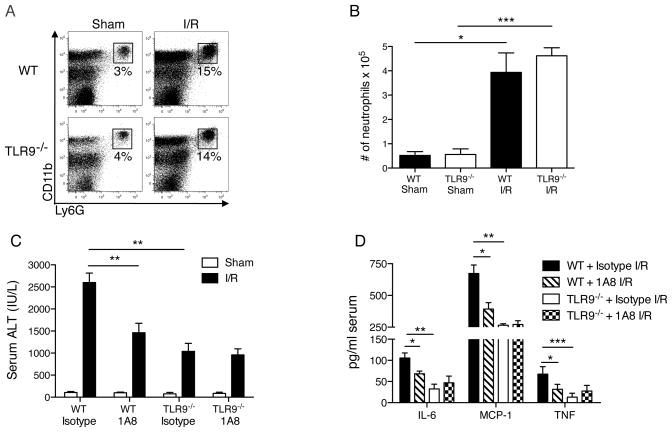
Neutrophil depletion in TLR9−/− mice does not alter liver injury
Since neutrophils are known to express TLR918 and are considered key mediators of liver I/R injury,19, 20 we sought to determine whether they had reduced function in TLR9−/− mice. Despite the known role of TLR9 in promoting neutrophil trafficking and accumulation at primary sites of bacterial infection,21, 22 we found that the percentage of neutrophils comprising NPCs and the absolute number of neutrophils recruited to the ischemic liver after I/R were surprisingly similar in WT and TLR9−/− mice (Figs. 4A, B). CpG has been shown to promote TNF production by neutrophils in vitro without affecting IL-10.23 To compare the functional capacity of neutrophils in WT and TLR9−/− mice, we depleted neutrophils with a monoclonal antibody (1A8)24 prior to I/R. WT mice had reduced serum ALT (Fig. 4C) and serum cytokines (Fig. 4D). In contrast, neutrophil depletion in TLR9−/− mice did not affect liver injury. Collectively, these data suggest that neutrophils exacerbate the local and systemic inflammatory response to liver I/R in WT but not TLR9−/− mice.
Figure 4. TLR9 is necessary for neutrophil-mediated I/R injury.
Neutrophil (CD11bhiLy6G+) recruitment to the ischemic liver after 12 h of I/R or sham surgery is represented as (A) percentage of hepatic CD45+ leukocytes or (B) absolute number of neutrophils. WT or TLR9−/− mice were depleted of neutrophils and (C) serum ALT and (D) serum cytokines were determined 12 h after I/R. Data in (B–D) represent means ± SEM and in (A–D) are representative of at least 2 independent experiments (4–6 mice/group). *p<0.05; **p<0.01; ***p<0.001.
TLR9 signaling in neutrophils increases hepatic injury
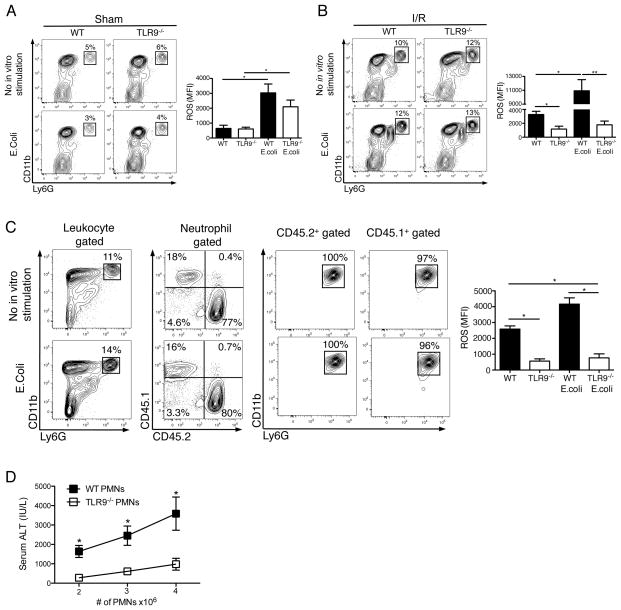
Because neutrophil ROS production mediates liver I/R injury,25 we asked whether it depended on TLR9 signaling. Although neutrophils from WT and TLR9−/− mice had similar oxidative burst after sham procedure both at baseline and after culture with E.coli (Fig. 5A), neutrophils from WT mice had much greater ROS generation after I/R (Fig. 5B). In particular, TLR9 activation during I/R appeared to prime the neutrophil response to subsequent stimulation with E.coli in vitro (Fig. 5B).
Figure 5. Liver I/R increases neutrophil oxidative burst via TLR9.
CD45+ NPCs from the ischemic livers of WT and TLR9−/− mice 12 h after (A) sham laparotomy or (B) I/R were isolated. Contour plots depict neutrophils as a percentage of hepatic leukocytes in the presence or absence of E.coli stimulation in vitro. Bar graphs depict mean ROS production ± SEM by WT and TLR9−/− neutrophils pooled from 3 separate experiments, each with similar results. (C) 4 × 106 WT (CD45.1+) neutrophils were injected into TLR9−/−(CD45.2+) recipients just prior to I/R. 12 h later, ischemic liver CD45+ NPCs were isolated and then assessed for the presence of donor and recipient neutrophils. The bar graph depicts mean ROS production ± SEM by donor and recipient neutrophils pooled from 2 separate experiments, each with similar results. (D) I/R was performed in TLR9−/− mice following intrasplenic injection of varying numbers of WT or TLR9−/− neutrophils (PMNs). Serum ALT was measured 12 h later. Data in (D) are representative of 3 independent experiments. (A-D) were performed with (4–6 mice/group). *p<0.05; **p<0.01.
To determine if the magnitude of liver I/R injury depended on TLR9 signaling in neutrophils specifically, we performed adoptive transfer experiments. Congenic WT (CD45.1+) neutrophils were injected into TLR9−/− (CD45.2+) recipients just prior to induction of hepatic ischemia. Analysis of donor and native neutrophils within the ischemic lobes after I/R revealed that WT neutrophils exhibited greater ROS production than their TLR9−/− counterparts (Fig. 5C). Adoptive transfer of WT neutrophils increased serum ALT after I/R in a dose-dependent manner while injury was considerably less after injection of TLR9−/− neutrophils (Fig. 5D). Interestingly, neutrophil expression of TLR9 in WT mice did not change after 12 h of I/R (unpublished data). Taken together, these findings demonstrate that neutrophil-TLR9 signaling regulates the inflammatory response during liver I/R.
DNA from necrotic hepatocytes increases liver NPC and neutrophil cytokine production in a TLR9-dependent manner
RNA released by dying host cells was recently shown to regulate the inflammatory response to polymicrobial sepsis through TLR3.26 We addressed the possibility that the limited inflammatory response in TLR9−/− mice during liver I/R was caused by their inability to respond to endogenous DNA released by necrotic hepatocytes. Therefore, we performed a series of in vitro experiments in which WT and TLR9−/− hepatic NPCs were cultured with supernatant from necrotic WT hepatocytes (conditioned media) for 24 h. In cultures of WT NPCs, the addition of conditioned media significantly increased the levels of IL-6, TNF and MCP-1 above baseline (media alone) in a dose dependent manner (Fig. 6A). Pre-treatment of conditioned media with DNAse prior to culture with WT NPCs reduced cytokine production. In contrast, conditioned media failed to induce TLR9−/− NPC cytokine production to levels attained by WT NPCs. Moreover, the presence of DNAse in conditioned media did not alter cytokine production by TLR9−/− NPCs (Fig. 6A). To validate the TLR9 dependency of the response to conditioned media, we found that the cytokine response of WT NPCs to conditioned media was abrogated by iCpG to a similar extent as DNAse (unpublished data).
Figure 6. Liver NPCs are activated by endogenous hepatocyte DNA through TLR9.
WT and TLR9−/− (A) CD45+ NPCs or (B) neutrophils were cultured in media alone or with conditioned (Con) media from 106 or 5 × 106 necrotic hepatocytes. Some wells containing conditioned media (from 5 × 106 necrotic hepatocytes) were pre-treated with DNAse I for 2 h prior to co-culture with NPCs or neutrophils. Supernatant cytokines were measured 24 (NPCs) or 12 h (neutrophils) later. Levels of MCP-1 were undetectable for neutrophil cultures in (B) (unpublished data). Data represent means ± SEM and are representative of 3 independent experiments. *p<0.05; **p<0.01.
To identify whether neutrophils have a similar response as NPCs to endogenous DNA, we performed analogous experiments by culturing WT or TLR9−/− neutrophils in conditioned media. The production of IL-6 and TNF by neutrophils also exhibited TLR9 dependence (Fig. 6B). Compared with WT neutrophils, TLR9−/− neutrophils produced less cytokines in culture with conditioned media and were not affected by DNAse treatment of conditioned media. Other endogenous ligands derived from necrotic cells such as HMGB1 and RNA have also been shown to activate immune cells.8, 27 In order to confirm that the difference in cytokine production between WT and TLR9−/− immune cells in our in vitro system did not result from differential sensitivity to other potentially activating ligands, we cultured NPCs and purified neutrophils with recombinant HMGB1, LPS or poly(I:C). Both NPCs and purified neutrophils responded similarly to each stimulus irrespective of TLR9 expression (unpublished data). Collectively, these data demonstrate that DNA released from necrotic hepatocytes contributes to the inflammatory response evoked in NPCs and neutrophils via TLR9.
Blockade of HMGB1 and TLR9 confers the greatest protection from I/R injury
In addition to its role as a promoter of inflammation and organ damage in hepatic I/R.7 HMGB1 was recently identified as a CpG-ODN-binding protein that potentiates the cytokine response of DCs and macrophages via TLR9.13, 14 Therefore, we tested the relationship between HMGB1 and TLR9 in hepatic I/R injury. Using our conditioned media culture system, we found that antibody-mediated blockade of HMGB1 lowered inflammatory cytokine production by both WT and TLR9−/− NPCs (Fig. 7A). DNAse in combination with anti-HMGB1 suppressed WT NPC cytokine production further, and as expected, did not affect TLR9−/− NPCs. A similar relationship between HMGB1 and TLR9 was observed using purified neutrophils. Maximal suppression of WT neutrophil cytokine production was achieved in the presence of DNAse-treated conditioned media combined with anti-HMGB1 (Fig. 7B).
Figure 7. Absence of TLR9 combined with HMGB1 blockade increases hepatic resistance to I/R injury.
WT and TLR9−/− (A) CD45+ NPCs or (B) neutrophils were cultured in conditioned (Con) media from necrotic hepatocytes and αHMGB1. Certain wells containing αHMGB1 were pre-treated with DNAse I for 2 h. After 12 h or 24 h, supernatant cytokines were measured. (C) WT and TLR9−/− mice were injected with αHMGB1 or isotype control 1 h prior to I/R. Serum ALT was measured 12 h later. (D) The percentage of neutrophils in the ischemic livers of mice subjected to 12 h of I/R following treatment with αHMGB1 or isotype is shown. (E) WT mice were pre-treated with iCpG, αHMGB1 or both just prior to I/R. Serum ALT was measured 12 h later. Data represent means ± SEM and are representative of 3 (A, B) or 2 (C, D, E) independent experiments (4–6 mice/group). *p<0.05; **p<0.01.
To test the relevance of our observations in vivo, we performed I/R in WT and TLR9−/− mice that were pre-treated with a neutralizing monoclonal antibody to HMGB1. As expected, blockade of HMGB1 in WT mice resulted in reduced levels of serum ALT after I/R (Fig. 7C). TLR9−/− mice that received anti-HMGB1 experienced the greatest protection from liver I/R injury with lower serum ALT, TNF, IL-6 and MCP-1 (unpublished data). To gain additional insight into how TLR9−/− mice were protected in the presence of HMGB1 blockade, we examined cell recruitment. Consistent with its role as an immune chemoattractant,8 we found that in vivo neutralization of HMGB1 lowered recruitment of neutrophils to the ischemic lobes of I/R-treated WT and TLR9−/− mice (Fig. 7D). Pre-treatment of WT mice with iCpG and anti-HMGB1 conferred maximal protection from I/R injury (Fig. 7E). Together, these data demonstrate that simultaneous blockade of two DAMP signaling pathways during liver I/R ameliorates the ensuing hyperinflammatory response.
DISCUSSION
During homeostatic conditions, degradation of endogenous mammalian DNA is a tightly controlled process. Apoptotic cells are engulfed by macrophages and autologous DNA is degraded in lysosomes. Regulated compartmentalization of self-DNA and RNA in this manner prevents a potentially maladaptive inflammatory response to host nucleic acids.28 Recently, the TLR system has garnered considerable attention as nucleic acid receptors have been implicated in host injury by responding to endogenous signals.29 The purpose of this study was to test the notion that TLR9 regulates the inflammatory response during sterile liver inflammation. Our results highlight neutrophil TLR9 activation as a critical determinant of the inflammatory response that follows liver I/R.
We discovered that the absence of TLR9 during liver I/R was associated with lower serum ALT, limited liver necrosis, as well as reduced systemic and local inflammatory cytokines. Importantly, these features in TLR9−/− mice were replicated in WT mice via the administration of a single dose of an inhibitory CpG sequence. I/R induces a biphasic pattern of injury. While the acute phase (0–6 h) is characterized by ischemia-induced hepatocyte death and generation of inflammatory cytokines and chemokines, the subacute phase (>6 h) is dominated by the activation and influx of neutrophils.19 Protection conferred by iCpG as late as 6 h into I/R demonstrates that continued TLR9 activation is necessary for maximal liver injury and suggests that neutrophils recruited to the liver during the subacute phase may be involved.
We demonstrated that TLR9 in immune cells is necessary for severe hepatic damage during I/R. While we chose to focus on the role of TLR9 in neutrophils, we acknowledge that TLR9 may be exerting effects on other cells within the liver in a manner that may impact overall I/R injury. Our findings build upon those of Imaeda et. al who found that liver sinusoidal endothelial cells (LSEC) augment injury via TLR9 in a drug-induced model of hepatic inflammation.30 Notably, our in vitro experiments with NPCs were devoid of LSEC by virtue of CD45+ cell selection. Previously, we showed that immunomagnetic isolation of CD45+ NPCs is an effective method of separating LSEC from bone marrow-derived immune cells.31 Nonetheless, our in vivo experiments do not rule out the possibility that TLR9 signaling in LSECs may alter I/R injury via other mechanisms, such as activation of the inflammasome or changes in neutrophil activation in the ischemic liver. Interestingly, TLR9 deficiency reduced only ROS generation by neutrophils and not inflammatory monocytes or Kupffer cells (unpublished data). Collectively, these data highlight the complexity of signals occurring within a single organ during sterile inflammation, since the functional outcome of TLR9 signaling is not only cell specific but also dependent on nature of the initial insult (i.e. drug-induced vs. I/R injury).
TLR9-mediated activation of innate immune cells is known to promote cell survival.32 In addition, neutrophil survival has been reported to increase after transmigration across endothelia in response to TNF and IL-6.33 While this seems appropriate during infection, TLR9 activation in sterile inflammation can promote cell survival and potentiate a maladaptive innate immune response. However, we did not observe a difference in the number of apoptotic or necrotic neutrophils between WT and TLR9−/− mice after 12 h of I/R (unpublished data), making this potential mechanism of TLR9-mediated injury unlikely in our model. Although apoptosis is traditionally recognized as an anti-inflammatory mode of cell death, apoptotic mammalian DNA can activate TLR9 and promote inflammation.30, 34 We showed that DNA from necrotic hepatocytes promotes inflammation through its interaction with TLR9 in liver NPCs.
Neutrophils migrate rapidly to sites of inflammation to eliminate invading organisms and remove dead or dying cells. We found that neutrophil ROS and cytokine production, but not recruitment, depended on TLR9. Consistent with our data, it has been shown that pre-treatment of WT mice with CpG enhanced neutrophil ROS production and improved survival in polymicrobial infection.35 Our ROS data from WT mice reveal the cumulative indirect (i.e., via other cells) and direct effects of TLR9 activation on neutrophils. In order to identify the consequences of TLR9 activation exclusively in neutrophils, we performed adoptive transfer experiments in TLR9−/− mice. Using this model, we found that activation of TLR9 in neutrophils worsened liver I/R injury. While this is the first demonstration of the in vivo effect of TLR9 activation on neutrophils exclusively, others have shown that CpG-mediated activation of neutrophils in vitro triggers cytokine release, superoxide generation, and L-selectin shedding.36
Another mechanism by which neutrophils can influence outcome during inflammation is by generating cytokines.37 Co-incubation of WT neutrophils or NPCs with media from necrotic hepatocytes promoted the release of pro-inflammatory cytokines through a TLR9-dependent mechanism. Tissue necrosis leads to the release of cellular components such as HMGB1, nucleic acids (DNA and RNA) and heat shock proteins, all of which have been shown to serve as endogenous ligands to various TLRs.29, 38, 39 While the absence of TLR3, the receptor for viral and host RNA, has been shown to render macrophages more sensitive to TLR9 stimulation,26 we did not find differential sensitivity of WT and TLR9−/− cells to other DAMPs such as HMGB1 or poly(I:C). In addition, pre-treatment of conditioned media with RNAse failed to elicit a significant reduction in cytokine production by either WT or TLR9−/− NPCs (unpublished data).
The dose response observed with conditioned media suggests that activation of TLR9 requires a threshold concentration of DNA. The endosomal location of TLR9 has been shown to be a critical determinant in regulating the discrimination between self and non-self DNA.28 However, under certain conditions intracellular TLR9 has also been shown to respond to DNA molecules traditionally considered inactive. At high concentrations, DNA strands that lack canonical CpG motifs, contain phosphodiester bonds, and structurally resemble endogenous mammalian DNA can become immunostimulatory and trigger TLR9-dependent cytokine production.12, 40 In addition, necrosis itself may induce modifications in endogenous DNA that could potentially increase its binding or activation of TLR9. It is therefore conceivable that in situations such as liver I/R in which there is extensive uncontrolled necrosis, the host’s ability to control cell death is overwhelmed, resulting in a dysregulated inflammatory response.
HMGB1 is well recognized for its role as a DNA-binding protein that is passively released following necrosis.8, 41 HMGB1 has also been shown to exert its pro-inflammatory effect through multiple PRRs including TLR9.13, 38 In liver I/R, the failure of anti-HMGB1 to confer additional protection to TLR4−/− mice suggests that during hepatic inflammation HMGB1 acts predominantly through TLR4.7 We found no difference in neutrophil TLR4 expression between WT and TLR9−/− mice before or after I/R (unpublished data). While the functional outcome and signaling cascades that result from single TLR blockade are well described, the intricacies of signaling when multiple pathways are blocked remain unclear. Our data reveal that maximal cytokine suppression and increased resistance to hepatic ischemia can be achieved when both TLR9 and HMGB1 signaling are absent. These results confirm that DNA and HMGB1 play non-redundant roles as DAMPs in liver I/R as they promote inflammation and neutrophil-mediated collateral damage.
Our data show that TLR9−/− mice regulate local and systemic inflammation after liver I/R via impaired neutrophil function. While the use of iCpG to block a crucial DAMP pathway might serve as a therapeutic option in the treatment of liver I/R, blockade of multiple pathways can limit injury further. Understanding the role and interplay between pattern recognition receptors on immune cells may create novel and more innovative approaches to dealing with a variety of conditions associated with I/R.
Nonstandard abbreviations used
- ALT
alanine aminotransferase
- DAMP
danger associated molecular pattern
- DHR 123
dihydrorhodamine 123
- HMGB1
high-mobility group box 1
- iCpG
inhibitory CpG sequence
- I/R
ischemia-reperfusion
- LSEC
liver sinusoidal endothelial cell
- NPC
non-parenchymal cell
- PRR
pattern recognition receptor
- PMN
neutrophil
- PI
propidium iodide
- R 123
rhodamine 123
Footnotes
This work was supported by NIH grants DK068346 and AI70658 (RPD) and the Marshall K. Bartlett Fellowship, Massachusetts General Hospital (ZMB).
Conflict of interest. The authors have declared that no conflict of interest exists.
Contributor Information
Zubin M. Bamboat, Email: bamboatz@mskcc.org.
Vinod P. Balachandran, Email: balachav@mskcc.org.
Lee M. Ocuin, Email: ocuinl@mskcc.org.
Hebroon Obaid, Email: obaidh@mskcc.org.
George Plitas, Email: plitag01@nyumc.org.
Ronald P. DeMatteo, Email: dematter@mskcc.org.
References
- 1.Lentsch AB, Kato A, Yoshidome H, McMasters KM, Edwards MJ. Inflammatory mechanisms and therapeutic strategies for warm hepatic ischemia/reperfusion injury. Hepatology. 2000;32:169–73. doi: 10.1053/jhep.2000.9323. [DOI] [PubMed] [Google Scholar]
- 2.Jaeschke H, Lemasters JJ. Apoptosis versus oncotic necrosis in hepatic ischemia/reperfusion injury. Gastroenterology. 2003;125:1246–57. doi: 10.1016/s0016-5085(03)01209-5. [DOI] [PubMed] [Google Scholar]
- 3.Colletti LM, Remick DG, Burtch GD, Kunkel SL, Strieter RM, Campbell DA., Jr Role of tumor necrosis factor-alpha in the pathophysiologic alterations after hepatic ischemia/reperfusion injury in the rat. J Clin Invest. 1990;85:1936–43. doi: 10.1172/JCI114656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kato A, Gabay C, Okaya T, Lentsch AB. Specific role of interleukin-1 in hepatic neutrophil recruitment after ischemia/reperfusion. Am J Pathol. 2002;161:1797–803. doi: 10.1016/S0002-9440(10)64456-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lentsch AB, Yoshidome H, Kato A, Warner RL, Cheadle WG, Ward PA, Edwards MJ. Requirement for interleukin-12 in the pathogenesis of warm hepatic ischemia/reperfusion injury in mice. Hepatology. 1999;30:1448–53. doi: 10.1002/hep.510300615. [DOI] [PubMed] [Google Scholar]
- 6.Jaeschke H. Mechanisms of Liver Injury. II. Mechanisms of neutrophil-induced liver cell injury during hepatic ischemia-reperfusion and other acute inflammatory conditions. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1083–8. doi: 10.1152/ajpgi.00568.2005. [DOI] [PubMed] [Google Scholar]
- 7.Tsung A, Sahai R, Tanaka H, Nakao A, Fink MP, Lotze MT, Yang H, Li J, Tracey KJ, Geller DA, Billiar TR. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med. 2005;201:1135–43. doi: 10.1084/jem.20042614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–5. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 9.Tsung A, Hoffman RA, Izuishi K, Critchlow ND, Nakao A, Chan MH, Lotze MT, Geller DA, Billiar TR. Hepatic ischemia/reperfusion injury involves functional TLR4 signaling in nonparenchymal cells. J Immunol. 2005;175:7661–8. doi: 10.4049/jimmunol.175.11.7661. [DOI] [PubMed] [Google Scholar]
- 10.Zhai Y, Shen XD, O’Connell R, Gao F, Lassman C, Busuttil RW, Cheng G, Kupiec-Weglinski JW. Cutting edge: TLR4 activation mediates liver ischemia/reperfusion inflammatory response via IFN regulatory factor 3-dependent MyD88-independent pathway. J Immunol. 2004;173:7115–9. doi: 10.4049/jimmunol.173.12.7115. [DOI] [PubMed] [Google Scholar]
- 11.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–5. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 12.Yasuda K, Yu P, Kirschning CJ, Schlatter B, Schmitz F, Heit A, Bauer S, Hochrein H, Wagner H. Endosomal translocation of vertebrate DNA activates dendritic cells via TLR9-dependent and -independent pathways. J Immunol. 2005;174:6129–36. doi: 10.4049/jimmunol.174.10.6129. [DOI] [PubMed] [Google Scholar]
- 13.Tian J, Avalos AM, Mao SY, Chen B, Senthil K, Wu H, Parroche P, Drabic S, Golenbock D, Sirois C, Hua J, An LL, Audoly L, La Rosa G, Bierhaus A, Naworth P, Marshak-Rothstein A, Crow MK, Fitzgerald KA, Latz E, Kiener PA, Coyle AJ. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol. 2007;8:487–96. doi: 10.1038/ni1457. [DOI] [PubMed] [Google Scholar]
- 14.Ivanov S, Dragoi AM, Wang X, Dallacosta C, Louten J, Musco G, Sitia G, Yap GS, Wan Y, Biron CA, Bianchi ME, Wang H, Chu WM. A novel role for HMGB1 in TLR9-mediated inflammatory responses to CpG-DNA. Blood. 2007;110:1970–81. doi: 10.1182/blood-2006-09-044776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cerullo V, Seiler MP, Mane V, Brunetti-Pierri N, Clarke C, Bertin TK, Rodgers JR, Lee B. Toll-like receptor 9 triggers an innate immune response to helper-dependent adenoviral vectors. Mol Ther. 2007;15:378–85. doi: 10.1038/sj.mt.6300031. [DOI] [PubMed] [Google Scholar]
- 16.Pillarisetty VG, Shah AB, Miller G, Bleier JI, DeMatteo RP. Liver dendritic cells are less immunogenic than spleen dendritic cells because of differences in subtype composition. J Immunol. 2004;172:1009–17. doi: 10.4049/jimmunol.172.2.1009. [DOI] [PubMed] [Google Scholar]
- 17.Krieg AM, Wu T, Weeratna R, Efler SM, Love-Homan L, Yang L, Yi AK, Short D, Davis HL. Sequence motifs in adenoviral DNA block immune activation by stimulatory CpG motifs. Proc Natl Acad Sci U S A. 1998;95:12631–6. doi: 10.1073/pnas.95.21.12631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsuda Y, Takahashi H, Kobayashi M, Hanafusa T, Herndon DN, Suzuki F. Three different neutrophil subsets exhibited in mice with different susceptibilities to infection by methicillin-resistant Staphylococcus aureus. Immunity. 2004;21:215–26. doi: 10.1016/j.immuni.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 19.Jaeschke H, Farhood A, Smith CW. Neutrophils contribute to ischemia/reperfusion injury in rat liver in vivo. Faseb J. 1990;4:3355–9. [PubMed] [Google Scholar]
- 20.Jaeschke H, Farhood A, Bautista AP, Spolarics Z, Spitzer JJ, Smith CW. Functional inactivation of neutrophils with a Mac-1 (CD11b/CD18) monoclonal antibody protects against ischemia-reperfusion injury in rat liver. Hepatology. 1993;17:915–23. [PubMed] [Google Scholar]
- 21.El Kebir D, Jozsef L, Pan W, Wang L, Filep JG. Bacterial DNA activates endothelial cells and promotes neutrophil adherence through TLR9 signaling. J Immunol. 2009;182:4386–94. doi: 10.4049/jimmunol.0803044. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz DA, Quinn TJ, Thorne PS, Sayeed S, Yi AK, Krieg AM. CpG motifs in bacterial DNA cause inflammation in the lower respiratory tract. J Clin Invest. 1997;100:68–73. doi: 10.1172/JCI119523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bellocchio S, Moretti S, Perruccio K, Fallarino F, Bozza S, Montagnoli C, Mosci P, Lipford GB, Pitzurra L, Romani L. TLRs govern neutrophil activity in aspergillosis. J Immunol. 2004;173:7406–15. doi: 10.4049/jimmunol.173.12.7406. [DOI] [PubMed] [Google Scholar]
- 24.Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J Leukoc Biol. 2008;83:64–70. doi: 10.1189/jlb.0407247. [DOI] [PubMed] [Google Scholar]
- 25.Jaeschke H, Smith CV, Mitchell JR. Reactive oxygen species during ischemia-reflow injury in isolated perfused rat liver. J Clin Invest. 1988;81:1240–6. doi: 10.1172/JCI113441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cavassani KA, Ishii M, Wen H, Schaller MA, Lincoln PM, Lukacs NW, Hogaboam CM, Kunkel SL. TLR3 is an endogenous sensor of tissue necrosis during acute inflammatory events. J Exp Med. 2008;205:2609–21. doi: 10.1084/jem.20081370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kariko K, Ni H, Capodici J, Lamphier M, Weissman D. mRNA is an endogenous ligand for Toll-like receptor 3. J Biol Chem. 2004;279:12542–50. doi: 10.1074/jbc.M310175200. [DOI] [PubMed] [Google Scholar]
- 28.Barton GM, Kagan JC, Medzhitov R. Intracellular localization of Toll-like receptor 9 prevents recognition of self DNA but facilitates access to viral DNA. Nat Immunol. 2006;7:49–56. doi: 10.1038/ni1280. [DOI] [PubMed] [Google Scholar]
- 29.Marshak-Rothstein A. Toll-like receptors in systemic autoimmune disease. Nat Rev Immunol. 2006;6:823–35. doi: 10.1038/nri1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Imaeda AB, Watanabe A, Sohail MA, Mahmood S, Mohamadnejad M, Sutterwala FS, Flavell RA, Mehal WZ. Acetaminophen-induced hepatotoxicity in mice is dependent on Tlr9 and the Nalp3 inflammasome. J Clin Invest. 2009;119:305–14. doi: 10.1172/JCI35958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katz SC, Pillarisetty VG, Bleier JI, Shah AB, DeMatteo RP. Liver sinusoidal endothelial cells are insufficient to activate T cells. J Immunol. 2004;173:230–5. doi: 10.4049/jimmunol.173.1.230. [DOI] [PubMed] [Google Scholar]
- 32.Jozsef L, Khreiss T, Filep JG. CpG motifs in bacterial DNA delay apoptosis of neutrophil granulocytes. Faseb J. 2004;18:1776–8. doi: 10.1096/fj.04-2048fje. [DOI] [PubMed] [Google Scholar]
- 33.Simon HU. Neutrophil apoptosis pathways and their modifications in inflammation. Immunol Rev. 2003;193:101–10. doi: 10.1034/j.1600-065x.2003.00038.x. [DOI] [PubMed] [Google Scholar]
- 34.Rifkin IR, Leadbetter EA, Busconi L, Viglianti G, Marshak-Rothstein A. Toll-like receptors, endogenous ligands, and systemic autoimmune disease. Immunol Rev. 2005;204:27–42. doi: 10.1111/j.0105-2896.2005.00239.x. [DOI] [PubMed] [Google Scholar]
- 35.Weighardt H, Feterowski C, Veit M, Rump M, Wagner H, Holzmann B. Increased resistance against acute polymicrobial sepsis in mice challenged with immunostimulatory CpG oligodeoxynucleotides is related to an enhanced innate effector cell response. J Immunol. 2000;165:4537–43. doi: 10.4049/jimmunol.165.8.4537. [DOI] [PubMed] [Google Scholar]
- 36.Hayashi F, Means TK, Luster AD. Toll-like receptors stimulate human neutrophil function. Blood. 2003;102:2660–9. doi: 10.1182/blood-2003-04-1078. [DOI] [PubMed] [Google Scholar]
- 37.Cassatella MA. Neutrophil-derived proteins: selling cytokines by the pound. Adv Immunol. 1999;73:369–509. doi: 10.1016/s0065-2776(08)60791-9. [DOI] [PubMed] [Google Scholar]
- 38.Park JS, Svetkauskaite D, He Q, Kim JY, Strassheim D, Ishizaka A, Abraham E. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem. 2004;279:7370–7. doi: 10.1074/jbc.M306793200. [DOI] [PubMed] [Google Scholar]
- 39.Chen GY, Tang J, Zheng P, Liu Y. CD24 and Siglec-10 selectively repress tissue damage-induced immune responses. Science. 2009;323:1722–5. doi: 10.1126/science.1168988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yasuda K, Rutz M, Schlatter B, Metzger J, Luppa PB, Schmitz F, Haas T, Heit A, Bauer S, Wagner H. CpG motif-independent activation of TLR9 upon endosomal translocation of “natural” phosphodiester DNA. Eur J Immunol. 2006;36:431–6. doi: 10.1002/eji.200535210. [DOI] [PubMed] [Google Scholar]
- 41.Bustin M. Regulation of DNA-dependent activities by the functional motifs of the high-mobility-group chromosomal proteins. Mol Cell Biol. 1999;19:5237–46. doi: 10.1128/mcb.19.8.5237. [DOI] [PMC free article] [PubMed] [Google Scholar]