Abstract
Polar bodies are as diverse as the organisms that produce them. Although in many animals these cells often die following meiotic maturation of the oocyte, in other organisms they are an essential and diverse part of embryonic development. Here we highlight some of this diversity and summarize the evolutionary basis for their utility.
Keywords: polar body, scale insect, parasitic wasps, endosperm, polyembryonic development
A polar body is the byproduct of an oocyte meiotic division. It is the small cell that normally apoptoses, and in textbook figures, it usually just disappears. This portrayal though does not do the cell justice.
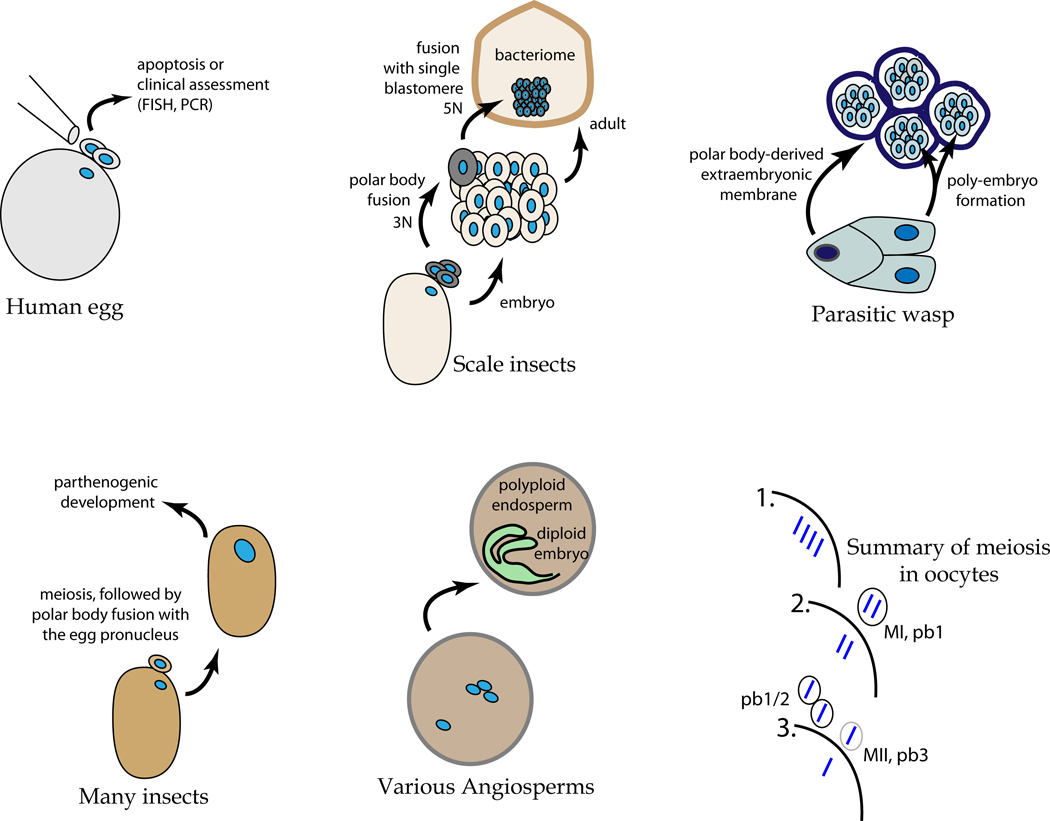
Polar bodies typically form by asymmetric cytokinesis: cytosol and organelles are shunted into the secondary oocyte during meiosis I, and then into the egg in meiosis II [Fig. 1]. This leaves the ovum’s sister and aunt (or cousins, if the first polar body also undergoes meiosis II) with relatively little cytoplasm and so in most organisms, these polar bodies simply degenerate. The polar body of human oocytes, for example, apoptoses in 17–24 hours following formation and the resulting fragments remain entrapped within the zona pellucida (Longo, 1997). Polar bodies were first reported in 1824 by Carus in gastropods, but their role was not clarified until the work of Butschli in 1875, Giard in 1876, and finally Hertwig in 1877 (see Korschelt and Heider, 1902). These structures were often confused with egg fragments or expelled yolk masses, but were eventually referred to as directional bodies (or Richtungskorper), a term implying the place where the maturation divisions start. The common names "polocytes" and "polar bodies" derive from their polar position in the eggs.
Figure 1.
Diversity of polar body function and fate in various organisms. [A] Polar bodies in most animals normally apoptose shortly following formation. In humans, the genetic material of polar bodies, both RNA and DNA, is useful to determine oocyte quality in a minimally invasive assay. Specific genomic DNA amplification and sequencing may reveal genetic abnormalities inherent within the oocyte. RNA detected by qPCR may be used to reflect genetic and epigenetic activity to reveal additional oocyte functionality. [B] In scale insects, the polar bodies survive and form the bacteriome, an essential nutrient structure of the adult. To construct the bacteriome, the polar bodies first fuse with each other and then with one diploid cell of the resulting embryo. This polyploidy cell (5n) then replicates many times to encompass a population of bacteria maternally derived and which is needed by the adult to convert the sugary sap diet into amino acids and other essential nutrients. [C] The polar cell, a meiotically-derived cell replicates its nuclei without cytokinesis, and the resulting syncytium eventually migrates as a tissue to encompass the many embryos that form from a single fertilized egg. [D] Many insects develop by parthenogenic activation. Amongst the great diversity of this developmental mechanism are cases of polar body fusion with the egg. This creates a diploid cell and activation of development. [E] Angiosperm plants have diverse strategies in forming embryos and the supporting endosperm tissues. Shown is one example in which the polyploid endosperm forms by nuclear fusion of the meiotic products i.e. equivalent to a polar body, with each other and with one of the sperm at fertilization. [F] Diagrammatic representation of the meiotic process of the oocyte (large line) and the sequential formation of the polar bodies with reductive DNA divisions. Shown n blue are the four sister chromatids of one chromosome. In some organisms, the first polar body also divides (pb1/2) giving rise to three total polar bodies. pb = polar body; MI and MII refer to meiosis 1 and 2, respectively.
Some may not consider the polar body to meet the standards of a cell, but indeed it does. Human polar bodies, for example, have a nucleus, ribosomes, Golgi, mitochondria and cortical granules (Zamboni, 1970), although the extent of their functionality is largely unknown. Polar bodies may, however, give rise to human embryoids if fertilized and twins may even result when one spermatozoa fertilizes the ovum and a second sperm fertilizes its sister polar body (Bieber et al., 1981). No twin pairs resulting from fertilization of the second (haploid) polar body have been reported (Machin, 2009), but clearly polar bodies have cellular and developmental potentials beyond apoptosis, even in humans.
Human polar bodies have become increasingly important clinically for human disease assessment and as metrics of embryonic potential. For example, the polar body is currently being used as a DNA source representative of the oocyte for genetic testing (Verlinsky et al., 1997; 1999). Removal of one or both polar bodies resulting from meiotic divisions I and II enables testing of various genes and transcripts by qPCR or by fluorescence in situ hybridization (FISH). Such preimplantation genetic diagnoses (PGD) enable predictive characters for Mendelian disorders and are preferable in many cases to an embryo biopsy, in which a blastomere is removed from the embryo for similar analysis. Polar body mRNA also is being considered as a metric for the oocyte in tests for embryonic viability (Klatsky et al., 2010).
But in many other organisms, polar bodies are not dispensable. They are not simply waste bins for three-quarters of the genetic material produced during oogenesis, and they do not disappear following meiosis.
Though we are most familiar with animals in which sperm-egg fusion initiates development, diversity is the way of life. Many animals begin development instead through naturally occurring parthenogenetic activation, that is, without incorporation of a sperm genome [Fig. 1D]. Indeed, animals use multiple mechanisms of parthenogenesis, most of which are dependent upon polar bodies. In this form of developmental activation, a haploid pronucleus forms in the egg along with one or two haploid polar body nuclei. Following completion of meiosis, two nuclei fuse to restore the somatic number of chromosomes, either the pronucleus and a polar body, or even two polar bodies alone. The fusion of a polar body with the oocyte nucleus to restore diploidy was first observed in 1889 by Hertwig in parthenogenic starfish eggs (see Korschelt and Heider, 1902). In animals, polar bodies participate in four basic mechanisms to restore the 2n chromosome content in parthenogenic organisms. These are: 1. The second polar body (haploid) fuses with the haploid oocyte pronucleus; 2. The second polar body (haploid) fuses with a haploid daughter nucleus from division of the first polar body (diploid); 3. A daughter nucleus from division of the first polar body fuses with the pronucleus; or 4. Two haploid nuclei fuse from cleavage. All parthenogenic animals employ one of these mechanisms for egg activation and restoration of chromosome number (Strand and Grbic, 1997).
In other animals and some plants, polar bodies give rise to vital tissues that protect and nourish the embryo. These apparent “oddities” reveal the diverse mechanisms used during reproductive development and may even allude to the origins of meiotic strategies. In the least, they challenge our understanding of sibling rivalry and cooperation by collapsing these interactions within a single chimeric entity – that is, an “organism” with nuclei, cells, or even tissues of non-identical genomes. After all, the essence of meiosis is genetic recombination, so that each meiotic division produces a distinct sibling. Embryos that retain their polar bodies during development thus have two or more different genomes, the degree of difference depending on the extent of cross-over between chromatids. Yet despite their genetic disparities, discrete cell lineages may coexist intimately. Diversity in the determination of developmental fates in meiotic products’ illustrates the complexity of these relationships.
Polar body formation of a nutritive tissue
The polar bodies of some scale insects (Diaspididae, which includes many agricultural pests; see Fig. 2A) actually live full and productive, if downright bizarre lives (Normark, 2004a; see Fig. 1B). Rather than degenerating after meiosis, they fuse together into a single triploid cell. Once the ovum has been fertilized and the embryo begins to develop, the polar-body derived triploid cell fuses again, but this time with a single diploid embryonic cell. This new cell is thus pentaploid, containing three maternal DNA chromosome sets derived from the polar bodies, one maternal DNA chromosome set from the developing embryo, and one chromosome set from the sperm DNA acquired at fertilization. The pentaploid cells proliferate to form a structure called the bacteriome, and the developing embryo surrounds and incorporates this structure into the larvae. The bacteriome then becomes an ‘organ’ housing endosymbiotic bacteria necessary for the insect’s nutrition.
Figure 2.

(A) An adult female armored scale insect (Quadraspidiotus juglansregiae) with its protective scale removed; (B) an adult female parasitoid wasp (Copidosoma floridanum) laying its egg into that of its host, the cabbage looper moth (Trichoplusia ni); (C) adult onions (Allium cepa). Photo credits: (A) Benjamin B. Normark, (B) Michael R. Strand, (C) Ranier Haessner.
The mature scale insect is thus chimeric: a pentaploid bacteriome within a normal haplodiploid body (males lose their paternal genome as they develop; Ross et al., 2010). No other adult animals live as obligate chimeras (Normark, 2004a, 2004b). What’s going on here?
One explanation interprets this chimerism as a strategy for gender crypsis, that is, preventing detection of the maternal or paternal genome (Normark, 2004a, 2004b). Scale insects rely on their bacterial endosymbionts to manufacture amino acids that are lacking in their own diet of sugary sap. These bacteria are only transmitted maternally, meaning bacteria in males are, evolutionarily, as good as dead. So some bacterial lineages have evolved to suicidally kill any male embryos they find themselves in, in order to free up resources for the dead males’ sisters and their endosymbionts (Hurst, 1991; Majerus, 2003; Normark, 2004c). Scale insects may use their pentaploid bacteriomes to thwart this kind of insurrection. Whether in a male or female, each bacteriome always contains two full copies of its mother’s diploid genome and one full copy of its father’s haploid genome, preventing the bacteria inside from determining the sex of their host.
Polar body formation of a protective tissue
Polar bodies play a similarly vital role in the life cycles of Copidosoma floridanum [Fig. 2B] and some other parasitoid wasps (Strand and Grbic, 1997; see Fig. 1C). Development in these insects begins when the adult deposits its eggs into an egg of the cabbage looper moth, Trichoplusia ni. It is within this other egg and its subsequent caterpillar that the parasitic wasp embryos and larvae will develop, eventually killing their host. The wasp egg has a distinct polarity and is enclosed within a thin chorion. During early development, the polar body nucleus separates from the egg’s pronucleus, and migrates to the future anterior of the embryo. These polar body remain viable and form a polar body cell. Within this cell, the polar body nucleus divides without cytokinesis to form a syncytial compartment at the anterior of the egg that eventually migrates as an extraembryonic tissue surrounding the dividing blastomeres. The embryo then ruptures out of its chorion and continues development now enveloped by the polar body-derived extraembryonic structure. This polar-body derived tissue mediates nutrient flow from the host caterpillar’s body to the yolkless wasp embryos, and may fend off the host’s immune system. Crucially, it also organizes embryo proliferation in these wasps, subdividing the original embryo into multiple embryos, each of which develops autonomously from the others within its own polar body-derived sheath. The polar body tissue invades divisions between the embryos and separates them from each other, leading even to differentiation between larval castes (Strand and Grbic, 1997). And this extraembryonic tissue may also play a role in kin recognition, allowing the sterile, precocious soldier larvae, which kill embryos from other wasp eggs, to spare their siblings in the later-forming reproductive caste. C. floridanum’s lineage of polar body-derived cells persists for about ten days, and during that time it seems to dominate most aspects of embryonic development.
Polar bodies and the plant’s endosperm
Parasitoid polyembryonic wasp larvae, the genomic contortions of scale insects’ bacteriomes, and those parthenogenic animals may seem alien, even highly atypical of early development. Fair, we agree. However we are all intimately dependent on a similarly strange entity – the endosperm. The seeds of flowering plants – largely endosperm – nourish both the growing angiosperm embryo and humanity. Cereal grains, the endosperm tissues of domestic grasses, provide about half the world’s calories (Paterson et al., 2006). The endosperm tissues of flowering plants originate during double fertilization: one spermatocyte of the plant pollen fertilizes the ovum while his twin fuses with two, four, or more syncitial nuclei of the central cell (part of the female gametophyte). In most taxa – over 70% of those studied embryologically and likely in the common ancestor of all angiosperms - these are cleavage nuclei sister to the ovum (Rudall, 2006; Williams and Friedman, 2004). But in onions (Allium cepa; Musial et al., 2005; Fig. 2C), black pepper (Piper nigrum; Kanta, 1962; Madrid and Friedman, 2009), and many other angiosperms, these nuclei may descend from two or four distinct meiotic products (Maheshwari, 1948; Maheshwari, 1950; Yadegari and Drews, 2004). One central cell nucleus derives from the same meiotic product as the ovum, but the others descend from one (or all three) of the polar body nuclei, which are not segregated by plasma membranes after meiosis [Fig. 1E]. In these plants, the polar bodies’ genomes live on in the polyploid endosperm – at least until the endosperm is absorbed by the growing embryo.
Why would some plants call on their polar bodies to contribute to the endosperm? The evolution of the endosperm is often explained by kin selection (Queller, 1983). When both nuclei of the central cell are sister to the ovum, and as the sperm that fertilizes them is the brother of the sperm that fertilizes the ovum, then the endosperm is genetically very similar to the embryo (though with an extra copy of the maternal genome). Because this endosperm is more related to the embryo than the female gametophyte is, it may be more likely to sacrifice itself to provision the embryo, and in angiosperms it has indeed displaced the female gametophyte from the nutritive role. But when all four meiotic products of oogenesis contribute nuclei to the central cell, as in Piper, the endosperm (like the scale insect’s bacteriome) shares only 40% of its genome with the embryo. Kin selection hardly seems to apply in this case.
So what’s going on?
The fraction of the endosperm genome (or extra-embryonic tissue from the parasitic wasp, or the bacteriome of scale insects) shares with the embryo, when coupled with a complete abdication of fitness, would confound an explanation based on kin selection. But the polar bodies that these structures developed from have zero fitness to begin with. Instead, polar-body-derived nutritive and protective tissues might be reconceptualized simply as a bizarre form of maternal provisioning. Selection has favored females that co-opt their polar bodies this way because their offspring benefit from these tissues. The onion’s endosperm, the parasitoid wasp’s extra-embryonic membrane, and the scale insect’s bacteriome are not altruistically sacrificing their fitness. Instead, they are manipulated to benefit the diploid embryo. Nowak et al. (2010) point out in their recent and controversial paper on the origins of insect eusociality (the highest level of social organization) “descent is from queen to queen, with the worker force generated as an extension of the queen … in each generation. Selection acts on the traits of the queen and the extrasomatic projection of her personal genome”. Since plant, wasp, and scale insect polar bodies, even more fundamentally than Copidosoma’s soldier larvae, will not pass on their genes, they may similarly be viewed as soma-like, fitness-boosting accessories to the germ line. Like “normal” polar bodies, they are sacrificed to give the embryo a better start in life.
But the mechanisms preventing these tissues from competing with the diploid embryo beyond the asymmetrical cytokinesis in meiosis I are not well known. Presumably this fate is determined by more than the absence of apoptotic mechanisms found in human polar bodies, and instead is a partitioning of factors that leads to different fates. An alternative explanation is that the genome of the oocyte is in some way different from that of the polar body, leading to differences in developmental potential. In this regard it is perhaps important to note that in each case of polar body functionality, except for parthenogenesis, that they become either polyploid or syncitial. These characters by themselves may limit the developmental potential of the polar body-derived tissues to single fates and to low fitness. Since sibling rivalry is all about fitness, competition between the modified, polar body derivatives and the embryonic tissues is eliminated. It is, however, not yet clear mechanistically how this lack of rivalry is communicated. Sperm undergo meiosis too, but their fates are strictly as an individual, not as a part of sibling cells in a developing organism. Improved understanding of the determinants of polar bodies’ developmental fates may offer insight into the evolutionary forces driving competition and cooperation between cell lineages in general. Indeed, some diseases of pregnancy are apparently due to conflicts between the genetically distinct tissues of mother and fetus (Haig, 2002).
Conclusion
Organisms with polar body-derived tissues illustrate that the differentiation between ovum and polar body is not necessarily as direct as it seems. Although more limited in fate than the oocyte and embryo, the polar bodies of these bizarre examples are capable of developing into complex, highly organized structures, or even enabling parthenogenesis. The relationship between meiotic products and their descendent cell lineages pertains to some of evolution’s most vexing questions and polar body-derived tissues demonstrate the potential variability of this relationship. We therefore encourage a broader and more refined look at the diversity of polar body development.
Acknowledgments
Funding: no funding was used in this study.
Abbreviations
- DNA
deoxyribonucleic acid
- FISH
fluorescence in situ hybridization
- PGD
preimplantation genetic diagnoses
- C. floridanum
Copidosoma floridanum
References
- 1.Bieber FR, Nance WE, Morton CC, Brown JA, Redwine FO, Jordan RL, Mohanakumar T. Genetic Studies of an Acardiac Monster: Evidence of Polar Body Twinning in Man. Science. 1981;213:775–777. doi: 10.1126/science.7196086. [DOI] [PubMed] [Google Scholar]
- 2.Haig D. Genomic imprinting and kinship. New Brunswick, N.J: Rutgers University Press; 2002. [Google Scholar]
- 3.Hurst LD. The incidences and evolution of cytoplasmic male killers. Proc R Soc B. 1991;244:91–99. [Google Scholar]
- 4.Kanta K. Morphology and embryology of Piper nigrum L. Phytomorphology. 1962;12:207–221. [Google Scholar]
- 5.Klatsky PC, Wessel GM, Carson SA. Detection and Quantification of mRNA in Single Human Polar Bodies: a Minimally Invasive Test of Gene Expression During Oogenesis. [17 September 2010];Mol Hum Reprod. 2010 doi: 10.1093/molehr/gaq077. epub. [DOI] [PubMed] [Google Scholar]
- 6.Korschelt E, Heider K. Allgemeiner Theil. Jena: Fischer Verlag; 1902. Lehrbuch der vergleichenden Entwidklungsgeschichte der Wirbellosen Thiere; p. 750. [Google Scholar]
- 7.Longo FJ. Fertilization. New York: Chapman & Hall; 1997. [Google Scholar]
- 8.Machin G. Non-identical monozygotic twins, intermediate twin types, zygosity testing, and the non-random nature of monozygotic twinning: A review. Am J Med Genet. 2009;151C:110–127. doi: 10.1002/ajmg.c.30212. [DOI] [PubMed] [Google Scholar]
- 9.Madrid EN, Friedman WE. The developmental basis of an evolutionary diversification of female gametophyte structure in Piper and Piperaceae. [6 Feb 2009];Ann Bot. 2009 doi: 10.1093/aob/mcp011. epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maheshwari P. The angiosperm embryo sac. Bot Rev. 1948;14:1–56. [Google Scholar]
- 11.Maheshwari P. An introduction to the embryology of angiosperms. New York: McGraw Hill; 1950. [Google Scholar]
- 12.Majerus MEN. Sex wars: genes, bacteria, and biased sex ratios. Princeton, New Jersey: Princeton University Press; 2003. [Google Scholar]
- 13.Musial K, Bohanec B, Jakse M, Przywara L. The development of onion (Allium cepa L.) embryo sacs in vitro and gynogenesis induction in relation to flower size. In Vitro Cell Dev Bio –Plant. 2005;41:446–452. [Google Scholar]
- 14.Normark BB. The strange case of the armored scale insect and its bacteriome. PLoS Biology. 2004a;2:3. doi: 10.1371/journal.pbio.0020043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Normark BB. The sex lives of scales. Natural History. 2004b;113:38–44. [Google Scholar]
- 16.Normark BB. Haplodiploidy as an outcome of coevolution between male-killing cytoplasmic elements and their hosts. Evolution. 2004c;58:790–798. doi: 10.1111/j.0014-3820.2004.tb00412.x. [DOI] [PubMed] [Google Scholar]
- 17.Nowak MA, Tarnita CE, Wilson EO. The evolution of eusociality. Nature. 2010;466:1057–1062. doi: 10.1038/nature09205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paterson AH, Freeling M, Sasaki T. Grains of knowledge: genomics of model cereals. Genomes. 2006;46:97–116. doi: 10.1101/gr.3725905. [DOI] [PubMed] [Google Scholar]
- 19.Queller DC. Kin selection and conflict in seed maturation. Journal of Theoretical Biology. 1983;100:153–172. [Google Scholar]
- 20.Ross L, Pen I, Shuker DM. Genomic conflict in scale insects: the causes and consequences of bizarre genetic systems. [10 March 2010];Biol Rev. 2010 doi: 10.1111/j.1469-185X.2010.00127.x. epub. [DOI] [PubMed] [Google Scholar]
- 21.Rudall PJ. How many nuclei make an embryo sac in flowering plants? BioEssays. 2006;28:1067–1071. doi: 10.1002/bies.20488. [DOI] [PubMed] [Google Scholar]
- 22.Strand MR, Grbic M. The development and evolution of polyembryonic insects. Current Top Dev Biol. 1997;35:121–159. doi: 10.1016/s0070-2153(08)60258-6. [DOI] [PubMed] [Google Scholar]
- 23.Verlinsky Y, Rechitsky S, Cieslak J, Ivakhnenko V, Wolf G, Lifchez A, Kaplan B, Moise J, Walle J, White M, Ginsberg N, Strom C, Kuliev A. Preimplantation diagnosis of single gene disorders by two-step oocyte genetic analysis using first and second polar body. Biochem Mol Med. 1997;62:182–187. doi: 10.1006/bmme.1997.2635. [DOI] [PubMed] [Google Scholar]
- 24.Verlinsky Y, Rechitsky S, Verlinsky O, Ivachnenko V, Lifchez A, Kaplan B, Moise J, Valle J, Borkowski A, Nefedova J, Goltsman E, Strom C, Kuliev A. Prepregnancy testing for single-gene disorders by polar body analysis. Genet Test. 1999;3:185–190. doi: 10.1089/gte.1999.3.185. [DOI] [PubMed] [Google Scholar]
- 25.Williams JH, Friedman WE. The four-celled female gametophyte of Illicium (Illiciaceae; Austrobaileyales): Implications for understanding the origin and early evolution of monocots, eumagnoliads, and eudicots. Am J Bot. 2004;91:332–351. doi: 10.3732/ajb.91.3.332. [DOI] [PubMed] [Google Scholar]
- 26.Yadegari R, Drews GN. Female gametophyte development. The Plant Cell. 2004;16:S133–S141. doi: 10.1105/tpc.018192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zamboni L. Ultrastructure of mammalian oocytes and ova. Biol. Reprod. Suppl. 1970;2:44–63. [PubMed] [Google Scholar]