Abstract
Family relationships help shape species-typical social and emotional development, but our understanding of how this shaping occurs is still relatively limited. Prairie voles are a socially monogamous and biparental species that is well situated to complement traditional animal models, such as rats and mice, in investigating the effects of family experience. In this series of studies, we aimed to test hypotheses relating to how prairie vole families function under undisturbed, standard laboratory conditions. In the first study, we compared the parental behavior of primiparous biparental (BP) and single-mother (SM) prairie vole family units for 12 postnatal days and then tested for sex differences, behavioral coordination, and family structure effects. Under BP conditions, nest attendance was coordinated and shared equally by both sexes, while pup-directed and partner-directed licking and grooming (LG) were coordinated in a sex and social-context-dependent manner. Contrary to our expectations, SMs showed no evidence of strong parental compensation in response to the lack of the father, indicating a minimal effect of family structure on maternal behavior but a large effect on pup care. In the second study, we examined the effects of these BP and SM rearing conditions on family dynamics in the next generation and found that SM-reared adult parents exhibited lower rates of pup-directed LG in comparison to BP-reared counterparts. Situated in the context of human family dynamics and psychology, these results suggest that the study in prairie voles may help improve our understanding of family systems and how perturbations to these systems can affect adults and offspring.
Keywords: family, family dynamics, family structure, prairie vole, biparental care, monogamy, maternal behavior, paternal behavior, coordination
Introduction
Humans often exhibit selective social bonds between adult partners and biparental care of offspring (Fortunato & Archetti, 2010; Low, 2007; Murdoch, 1981; Sbarra & Hazan, 2008; Schor, 2003). Human studies have revealed an important link between these family relationships and corollaries such as adult pair-bond stability, maternal and paternal investment, parental coordination, and family structure and long-term behavioral, and mental health outcomes, including cognitive performance, emotional regulation, behavioral control, and sociality, in adults and children (Amato, 2000; Boyum & Parke, 1995; Feinberg, 2002; Maccoby, 2000; Matheson et al., 2005; McEwen & Flouri, 2009; McLanahan & Sandefur, 1994; Meaney, 2001; Schor, 2003; Shear & Shair, 2005; Wen, 2008). While extraordinarily informative for our understanding of the importance of family life to shape individuals, ethical constraints prohibit experiments that can test direct causation. It is therefore unclear how and to what extent family life directly influences long-term outcomes.
One approach to filling this gap is the use of animal models. Laboratory strains of rats and mice are well characterized both behaviorally and neurobiologically, and the use of these species has been essential to our understanding of how family factors such as early environment and maternal care affect the behavioral development (Branchi et al., 2006; Fleming et al., 2002; Meaney, 2001). Laboratory rats and mice, however, do not form selective social bonds between mates nor do they provide biparental (BP) care. This limits their utility as animal models for the study of more complex family dynamics.
BP and pair-bonding species, such as prairie voles (Microtus ochrogaster), California mice (Peromyscus californicus), and Mongolian hamsters (Meriones unguiculatus) are well positioned to complement the work of these traditional animal models (Bredy, Lee, Meaney, & Brown, 2004; Cantoni & Brown, 1997; Carter, DeVries, & Getz, 1995; Frazier, Trainor, Cravens, Whitney, & Marler, 2006; Gromov, 2009; Lonstein & De Vries, 2000; Wright & Brown, 2002; Young & Wang, 2004). Over the last several decades, prairie voles have become a well-established laboratory model species for the study of sociality (Carter et al., 1995; McGraw & Young, 2009; Young & Wang, 2004). Of particular focus has been their utility in revealing the neurobiological mechanisms involved in selective partner preferences. Partner preference behavior is well characterized in these animals, and experimental manipulation of this phenomenon has identified the importance of the reward circuitry (e.g., nucleus accumbens), oxytocin and vasopressin neuropeptide systems, and specific genetic elements (e.g., avpr1a microsatellite) in the initiation and modulation of this behavior (Donaldson & Young, 2008; McGraw & Young, 2009; Young & Wang, 2004). Importantly, the relevance of this research has not been limited to prairie voles. These findings have helped drive strikingly similar discoveries in humans and nonhuman primates (Bales, Mason, Catana, Cherry, & Mendoza, 2007; Donaldson & Young, 2008; Smith, Agmo, Birnie, & French, 2010; Walum et al., 2008).
Pair bonding is not the only type of family-relevant behavior displayed by prairie voles. Nest sharing, mate guarding, paternal and BP care, spontaneous alloparenting, and communal nesting are also exhibited, and like humans there is wide individual variation in the expression of these traits (Aragona et al., 2006; Carter et al., 1995; Getz & Carter, 1996; Getz, McGuire, Pizzuto, Hofmann, & Frase, 1993; Lonstein & De Vries, 2000). For example, in the field, prairie vole pups are variably reared by large communal families, lone breeder-pairs, and single-mothers (SMs); there are also variations in the levels of maternal, paternal, and alloparental investment (Carter et al., 1995; Getz & Carter, 1996; Lonstein & De Vries, 2000; Young & Wang, 2004). Collectively, these findings suggest that prairie voles could serve as a broadly relevant animal model of complex family dynamics—but only if there is a better understanding of how they function as family units under controlled laboratory conditions.
Despite the obvious human parallels, there has been little effort to test specific hypotheses about undisturbed prairie vole communal, BP, and SM family dynamics in standard laboratory environments, and what literature exists is marked by inconsistencies in testing conditions and contradictory findings (Hartung & Dewsbury, 1979; Lonstein & De Vries, 1999; McGuire, Parker, & Bemis, 2007; Solomon, 1993; Wang & Novak, 1992). Both issues make it difficult to predict the outcome of pharmacological and brain-region-specific experimental manipulations.
In this set of studies, we aimed to get a better handle on prairie vole family behavior in the laboratory and directly test several key hypotheses about primiparous prairie vole family dynamics under undisturbed laboratory conditions. In Experiment 1, we tested (1) whether both parents would contribute equally to pup care across multiple measures of direct parenting behavior, (2) whether parenting behavior would change over time as pups mature, (3) whether parents were capable of coordinating parenting activities, as is common in BP birds (Lack, 1968), and (4) whether maternal behavior would differ significantly under BP and SM conditions. In Experiment 1, we hypothesized that BP-fathers would invest less time in pup-directed care (e.g., lower nest attendance and lower rates of pup-directed licking and grooming [LG]); we predicted that behaviors related to pup-directed care would decrease significantly throughout the postnatal period; and we expected that BP parents would show evidence of parental coordination in the form of coordinated nest occupancy and social-context-dependent LG. Finally for Experiment 1, we predicted differences in maternal behavior across family structures. In particular, we expected that under laboratory conditions SMs would attend to the nest and LG pups at different rates than BP-mothers. One possibility was that SMs might compensate for the lack of the father and increase pup-directed care; alternatively, without help, SMs might decrease maternal investment.
In Experiment 2, we tested the hypothesis that ethologically relevant manipulations of family structure in one generation can affect primiparous family behavior in the next. In particular, we expected SM-reared animals to exhibit significantly lower levels of pup-directed nest attendance and LG.
Materials and Methods
Animals
All animals were 3- to 4-month-old, sexually, behaviorally, and pup-exposure-naive Illinois stock prairie voles reared and maintained in our Emory University colony (Atlanta, GA). All breeder pairs were housed in large polycarbonate, ventilated cages (34 cm wide × 30 cm long × 18 cm high) lined with bedding (bed-o-cob, Maumee, OH) and a single cotton nestlet. All weanlings were housed in same sex, same group, pairs, or trios in smaller polycarbonate, ventilated cages (15 cm wide × 30 cm long × 18 cm deep) with bedding, a cotton nestlet, and shredded paper for nesting. Animals had ad libitum access to rabbit chow (LabDiet/PMI International, Richmond, IN) in hanging baskets and to water. All cages were maintained at 22° C on a 14:10 light/dark schedule in a cubicle separated from the rest of the colony to allow undisturbed observations. Cages were changed weekly. Every effort was made to minimize the use of animals and all procedures were approved by Emory University's IACUC and are in accord with national and state guidelines.
Experiment 1: Biparental and Single-Mother Family Dynamics
Twenty-one sexually naive, adult female prairie voles were paired with an equal number of adult, sexually naive male prairie voles in breeder cages. On Day 18 postpairing, all males were handled and shaved dorsally to distinguish them from the females. Ten of the males were removed from the breeder cages and housed in a separate room, where they remained throughout the study. The other 11 were returned to their female partners. This created 10 SM and 11 BP family units. Eighteen dams gave birth between 23 and 30 days postpairing (SM = 9 [90%], BP = 9 [81%]). The number of days that had elapsed from pairing to birth was recorded, pups were checked and culled if necessary, and then pups were returned to their biological parents. Litters with more than four pups were culled to four so that all litters contained 2–4 pups. Two BP and two SM family units were excluded from the statistical analyses because pups died or the family unit was not observed for a full 12 days. Sample sizes of n = 7 for each condition were used in the statistical analysis. Pups were checked for sex at weaning to determine average sex ratios for each group. No litters with only one sex were included, as rodents have been shown to LG male and female pups at different rates (Moore & Morelli, 1979).
Following birth (day of birth designated PND1), each family unit was observed for 12 postnatal days (PNDs). Observation sessions occurred three times per day for 1 hr each: lights ON (06:00–07:00 hr), middle of the day (12:30–13:30 hr), and lights OFF (20:00–21:00 hr). Dark phase observations were made under red-light (15 W) conditions.
Data were collected by visual spot-checks as described previously (Ahern & Young, 2009; Hammock & Young, 2005), and the behaviors of the mother and father were logged according to three complementary categories: (1) nest occupancy, (2) activity, and (3) nursing. Nest occupancy recorded whether parents occupied the nest or not as well as whether the parents were huddling together on the nest or not. The activity category included pup-directed LG, partner-directed LG, autogrooming (self-directed LG), eating, drinking, wandering (walking or running), digging, gnawing, and climbing/jumping. The nursing category logged whether the pups were attached and able to nurse or unattached and unable to nurse. Because prairie vole pups have milk teeth, they are attached to the mother almost continuously when she is on the nest. This prevents direct observation of nursing behavior.
The visual spot-checking method resulted in 10 data points per observation session for each behavior for each parent. If the behavior occurred during the spot-check, it received a 1; if it did not, it received a 0. Behavioral frequencies for each parent were then calculated by averaging the 1s and 0s. Initially, averages were taken for each of the three separate observation sessions per PND. Preliminary analyses, however, revealed no main effect of time of day; therefore, time of day was not included as a factor in subsequent analyses. We further simplified the data by pooling PNDs into 3-day bins (PND1–3, 4–6, 7–9, 10–12, designated as PND bins 1–4, respectively). Aggregating the data in this manner simplified the data set overall, while still conveying changes over time. Occasionally, data were aggregated even further by averaging over all 12 PNDs. This allowed us to test how different types of behaviors interact (e.g., LG behavior in reference to nest occupancy).
Of particular interest in this study was a quantitative analysis of parental coordination within BP family units. To test for coordination in nest occupancy, we compared the “observed” exposure rate, which was calculated from the raw data, against a theoretical “expected” exposure rate, which was calculated by multiplying the probability of finding each parent off the nest: [mother-off frequency] × [father-off frequency] = [expected nest exposure frequency]. If parents come and go from the nest independently, and therefore do not coordinate nest occupancy, then the observed and expected exposure rates should be equal. If instead parents do coordinate, then the observed frequency should be significantly different than the expected frequency.
All data were tested for normality (Shapiro–Wilk's test) and homogeneity of variance (Levene's test) prior to statistical analysis. For most analyses, separate mixed repeated measures two-way ANOVAs were used to test within (e.g., PND bin) and between (e.g., Parent) subject effects. Planned Tukey post hoc tests were performed when appropriate. In situations where averages had been taken over the entire 12 PND observation period, independent or paired Student's t-tests were performed, depending on the nature of the dependent variable. p < .05 was accepted as significant and was Bonferroni corrected for multiple comparisons when noted. All statistical tests were performed in SPSS 17.
Experiment 2: The Effect of Family Structure on Parenting in the Next Generation
Thirty-one adult, sexually naive prairie voles of each sex were paired in large breeder cages. Eighteen days postpairing, males were handled. Seventeen males were removed to another room, while 14 were returned to their partners. This created 17 SM and 14 BP family units. Twelve of 14 (85%) BP pairs and 15 of 17 (88%) SM pairs gave birth within 23–30 days postpairing. The animals were left undisturbed and unobserved until pups were weaned at PND22. Weanlings were housed in the small, ventilated cages described above in same sex, same group pairs or trios until approximately 90 days of age. From these adult weanlings, 27, sexually naive, prairie voles of each sex were paired in large, ventilated breeder cages. Each breeder pair consisted of one BP-reared male plus one BP-reared female (BP/BP pairs = 12) or one SM-reared male plus one SM-reared female (SM/SM pairs = 15). Each original litter provided at most one male and one female to these pairs, and no pairs consisted of siblings.
Because previous work from our group has shown a long-term effect of family structure on partner preference formation (Ahern & Young, 2009), we conducted partner preference tests on 12 females from the BP/BP group and 12 females from the SM/SM group as a positive control for the effect of rearing condition on adult social behavior. Partner preference testing has been described in full detail elsewhere (Ahern, Modi, Burkett, & Young, 2009; Williams, Carter, & Insel, 1992). In brief, 24 hr after pairing, breeder cages were moved to a behavior room. Males were removed from the homecage and tethered at one end of a three-chambered apparatus. A colony male of similar sociosexual experience was tethered to the other end. The test female was then placed in the center chamber and allowed to roam freely for 3 hr. Sessions were video-recorded to mpeg video files and DVD for archiving. At the end of the test, each test female and each male partner were returned to the homecage. Homecages were returned to the colony cubicle, where they remained for the rest of the study. Video files were then processed for huddling time and locomotor activity (center chamber entries) using an automated behavioral analysis software system (SocialScan 2.0, CleverSys Inc., Reston, VA). This system has been optimized for high-reliability, high-accuracy, and high-throughput partner preference analysis (Ahern et al., 2009). Student's t-tests were used to compare time spent huddling with the partner versus the stranger for each group. Significance levels were Bonferroni adjusted for multiple comparisons.
Eighteen to 20 days after the initial BP/BP and SM/SM pairings, males were handled and shaved dorsally to distinguish them from females during observations. Twenty-six pairs produced pups (BP/BP = 11 of 12 [92%]; SM/SM = 15 of 15 [100%]). After birth, BP/BP and SM/SM family units were observed following the procedures outlined for Experiment 1, with the exception that the observations were conducted at the following times: lights ON (06:00–07:00 hr), noon (12:00–13:00 hr), and late afternoon (16:00–17:00hr). The postnatal observation period lasted for six PNDs. The observational log consisted of the same three categories.
Results
Experiment 1: Biparental and Single-Mother Family Dynamics
Gestation
Differences in family structure were generated by either handling and removing the sire (SM family units) or handling and returning sire (BP family units) 18 days after pairing. To ensure that mothers did not experience significantly different times from separation to birth, we logged how many days elapsed between male handling and/or removal and birth. As shown in Table 1, most BP and SM gave birth around 24 or 25 days postpairing. Only one litter from each group took more than 25 days from pairing to birth. We also tracked the number and sex of the pups for which the parents (or parent) cared. Each group consisted of approximately equal numbers of 2-, 3-, and 4-pup litters and there was no evidence of a difference in sex ratio (Tab. 2). No litter in either group consisted of only one sex.
Table 1. Experiment 1 Gestation Times.
| Group | n | Days from Pairing to Birtha | Days from Male Handling or Removal to Birtha | % Born 24 or 25 Days Postpairing | % Born >25 Days Postpairing |
|---|---|---|---|---|---|
| BP | 7 | 24.86±.55 | 6.86±.55 | 86 (6/7) | 14 (1/7); Day 28 |
| SM | 7 | 24.71±.42 | 6.71±.55 | 86 (6/7) | 14 (1/7); Day 29 |
Note. Each group consisted of seven family units. Pairs were cohabitated for 18 days and then the males were either handled and removed (SM group) or handled and returned (BP group). The number of days that elapsed between pairing and birth (most often 24 or 25 days) did not differ significantly between groups (p = .841). Values represent mean ± SEM or percentage (number/total).
A two-tailed t-test, BP versus SM, p < .841.
Table 2. Experiment 1 Litter Size and Sex Ratio by Group.
| Groupa | n | 2-Pup Littersa | 3-Pup Littersa | 4-Pup Littersa | Average Pups/Litter | Average Percent Males/Litter |
|---|---|---|---|---|---|---|
| BP | 7 | 1 | 3 | 3 | 3.28 ±.28 | 52 |
| SM | 7 | 2 | 2 | 3 | 3.14 ±.34 | 48 |
Note. Groups show similar numbers of 2-, 3-, and 4-pup litters as well as pup sex ratios based on sex checks at weaning. Values represent total count, mean ± SEM, or average percentage.
A two-tailed 3 × 2 Fisher's exact test revealed no statistically significant difference between BP and SM family units in the number of 2-, 3-, and 4-pup litters (p = .825).
Nest Occupancy
We predicted that there would be differences between BP-mothers, BP-fathers, and SMs in how they attended to the nest and the pups. Specifically, we expected that, in comparison to BP-mothers, BP-fathers would occupy the nest at a lower frequency, whereas SMs would occupy the nest at a higher frequency. Contrary to this expectation, BP-fathers and SMs occupied the nest at roughly the same rate as BP-mothers, with all three showing a decrease in nest occupancy throughout the postnatal period (Fig. 1A; two-factor ANOVA; PND: F(1,3) = 10.423, p < .001; parent [BP-mother, BP-father, SM]: F(1,18) = 2.478, p = .112; PND × parent interaction: F(1,6) = .573, p = .750; Tukey post hoc tests: BP-mother vs. BP-father: p = .812; BP-mother vs. SM: p = .295; BP-father vs. SM: p = .106). BP-mothers and BP-fathers huddled on the nest together far more often than they occupied the nest alone and they occupied the nest alone at essentially equal frequencies (Fig. 1A; two-factor ANOVA; PND: F(1,3) = 2.471, p = .072; parent-alone: F(1,18) = 273.638, p < .001; PND × parent-alone interaction: F(1,6) = 4.474, p = .001; Tukey post hoc tests: BP-mother vs. BP-father: p = .781; BP-mother vs. SM: p < .001; BP-father vs. SM: p < .001).
FIGURE 1.
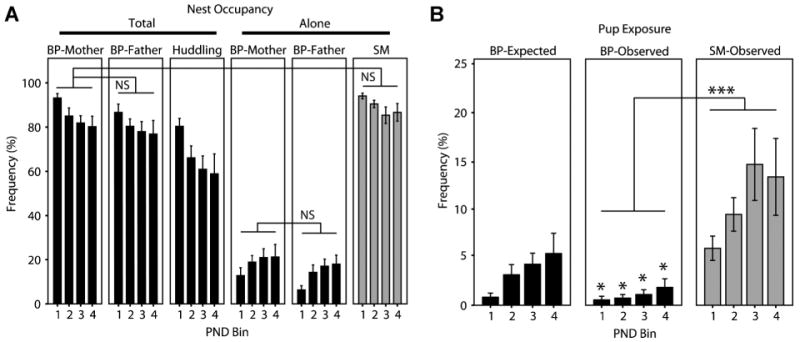
Nest occupancy and coordination in Experiment 1. For each data set, the first 12 PNDs were grouped into 3-day bins for analysis and for graphing: PND1 –3 = PND Bin 1, PND4–6 = PND Bin 2, etc. (A) Parents attended to the nest at approximately equal rates regardless of sex or group, and all decreased nest attendance over time. In the BP-group, BP-mothers and BP-fathers huddled on the nest at a high rate and were alone with their pups at approximately equal rates. (B) BP-parents left the nest exposed less than expected, suggesting behavioral coordination, for all five PND bins (*p < .05, paired t-tests), and BP pups were left exposed significantly less than SM pups. Bars represent mean ± SEM. ***p < .001, NS, not significant. Huddling indicates the time when both male and female were huddling together on the nest.
Pup Exposure
With two parents in the BP condition to attend to the nest and pups and only one parent in the SM condition, we predicted BP-reared pups would be exposed less frequently than SM-reared pups, even within the confines of standard laboratory caging. Our analysis of pup exposure revealed that BP primiparous prairie vole parents left the nest and pups exposed at a very low rate—approximately 2–4% of observations (Fig. 1B, “BP-observed”)—which was significantly lower than the SM nest exposure rate (Fig. 1B; two-factor ANOVA; PND: F(1,3) = 2.706, p = .060; group [BP vs. SM]: F(1,12) = 25.992, p < .001; PND × group interaction: F(1,3) = 2.095, p = .118). BP and SM pup-exposure rates tended to increase over time.
Nest Coordination
While the pup exposure data matched our expectations, we were less sure whether there would be any quantifiable evidence of parental coordination in the BP condition. Nest exposure leaves the pups vulnerable. In theory, two parents could coordinate their behavior to decrease the vulnerability of the nest even more than if the two parents simply came and went from the nest independently.
To test for coordination, we compared the “observed” exposure rate against a theoretical “expected” exposure rate (see Experiment 1 in the Materials and Methods Section). We predicted that the parents would indeed coordinate to decrease nest and pup vulnerability and thus the observed rate would be significantly lower than expected. This is precisely what we observed (Fig. 1B; two-factor ANOVA: PND: F(1,3) = 3.668, p = .032; observed/expected: F(1,1) = 17.555, p = .006; PND × observed/expected: F(1,3) = 5.182, p = .009). In fact, post hoc paired t-tests revealed that the observed frequencies were significantly lower than expected for each of the PND bins (p < .05).
Licking and Grooming
Prairie voles LG their pups, each other, and themselves. We predicted that BP-mothers would LG pups more than BP-fathers. We also expected that SMs would compensate for the lack of the father and LG pups more than BP-mothers. As shown in Figure 2A–C, the frequency of LG differed dramatically based on the object of attention (note the difference in scale between Fig. 2A and 2B & C), and pup-directed LG decreased over time regardless of group (three-way ANOVA: PND: F(1,3) = 38.587, p < .001; parent: F(1,18) = .100, p = .905; LG-object: F(1,2) = 335.408, p < .001; PND × parent interaction: F(1,6) = 1.410, p = .214; PND × LG interaction: F(1,6) = 34.161, p < .001; PND × par-parent × LG interaction: F(1,12) = 2.551, p = .004). Follow-up post hoc Tukey analyses revealed several other interesting findings: (1) pups were the object of the highest frequency of LG bouts (Fig. 2A–C; pups > partner, p < .001; pups > self, p < .001); (2) as predicted, BP-fathers LG pups at a significantly lower frequency than BP-mothers (Fig. 2A; p = .016); (3) contrary to our expectations, SMs LG pups at a statistically indistinguishable rate compared to BP-mothers throughout the postnatal period (Fig. 2A; p = .985); (4) partner-directed LG is significantly more pronounced in BP-fathers than in BP-mothers (Fig. 2B; p = .001); and (5) BP-fathers participated in significantly more autogrooming (or self-directed LG) than did BP-mothers (Fig. 2C; p < .001), while there was no statistical difference in autogrooming between BP-mothers and SMs (p = .473).
FIGURE 2.
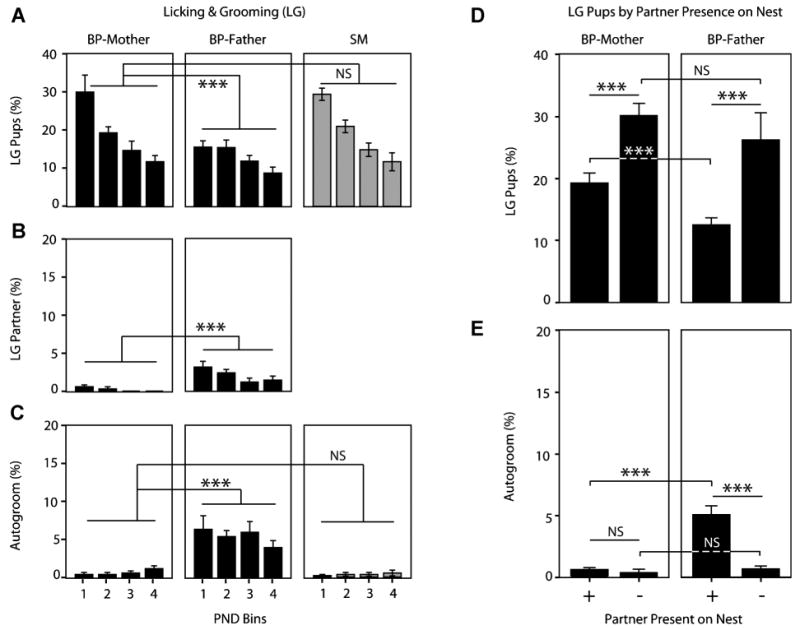
Licking and grooming (LG). For each data set, the first 12 PNDs were grouped into 3-day bins for analysis and for graphing: PND1–3 = PND Bin 1, PND4–6 = PND Bin 2, etc. (A) BP-mothers LG pups at a higher rate than BP-fathers, but there was no difference between BP-mothers and SMs in pup-directed LG. (B) BP-fathers exhibit partner-directed LG at a higher rate than BP-mothers. (C) BP-fathers also show a greater rate of allogrooming than BP-mothers; BP-mothers and SMs show no difference in allogrooming. (D) BP-mothers LG pups at a higher rate than BP-fathers when both parents are attending to the nest; when attending to the pups alone, however, BP-mothers and BP-fathers LG pups at essentially the same rate. Both parents increase pup-directed LG in response being alone with the pups. (E) BP-mothers show little allogrooming, regardless of whether they are alone on the nest or accompanied; BP-fathers show significantly more allogrooming than BP-mothers when attending to the nest together, but little autogrooming when attending to the pups alone. Bars represent mean ± SEM. *** p < .001, NS, not significant.
Licking and Grooming Coordination
The analyses above indicate that, as predicted, BP parents exhibit a sex difference in pup-directed LG behavior. We wondered whether this might be due to behavioral coordination. We predicted that perhaps BP-fathers might be shifting their LG focus from the pups to the mother when the parents were attending to the nest simultaneously.
To examine this, LG frequencies were averaged over the entire 12-day postnatal period, normalized to nest occupancy, and then analyzed in reference to whether the BP parents were attending to the pups on the nest together or alone (two-way mixed ANOVA; partner-location: F(1,1) = 25.398, p < .001; parent: F(1,12) = 2.774, p = .122; partner-location × parent interaction: F(1,1) = .342, p = .570). Following this analysis, four planned independent t-tests were conducted and the p-value was Bonferroni adjusted to < .0125. When attending to the nest together, BP-fathers LG pups at a lower frequency than BP-mothers (Fig. 2D; p = .005). When alone with the pups, however, BP-fathers and BP-mothers LG pups at statistically indistinguishable rates (p = .477). Attending to the nest alone significantly increased pup-directed LG behavior (paired t-tests; p = .016 and .009 for BP-father and BP-mother, respectively), with the increase being more pronounced for BP-fathers.
Autogrooming was also altered by the presence or absence of the partner on the nest, at least in males (two-way ANOVA; partner-location: F(1,1) = 25.518, p < .001; parent: F(1,12) = 24.740, p < .001; partner-location × parent interaction: F(1,1) = 20.320, p = .001). Again adjusted t-test comparisons were made (Bonferroni adjusted level p < .0125). When accompanied on the nest by the BP-mother, BP-fathers exhibited significantly more autogrooming than when alone with the pups (planned paired t-test; p < .002). BP-mothers show little change in response to partner presence on the nest (planned paired t-test; p = .515). Moreover, the rate of autogrooming by BP-fathers only differed from BP-mothers when both were on the nest together (independent t-test; p = .002), whereas there was no sex difference when alone with the pups (BP-mother vs. BP-father; independent t-test; p = .468).
Nursing
Nursing occurs almost exclusively when the mother is on the nest. Therefore, in addition to our prediction that SMs would increase nest occupancy, we also predicted that SMs might be available to nurse more often. Not surprisingly, since BP-mothers and SMs showed no statistically significant difference in overall nest occupancy, nursing rates did not differ significantly between BP- and SM-family units, although there was a near significant trend (Fig. 3A; two-way ANOVA; PND: F(1,3) = 5.615, p = .003; group: F(1,12) = 4.474, p = .054; PND × group interaction: F(1,3) = 1.251, p= .305).
FIGURE 3.
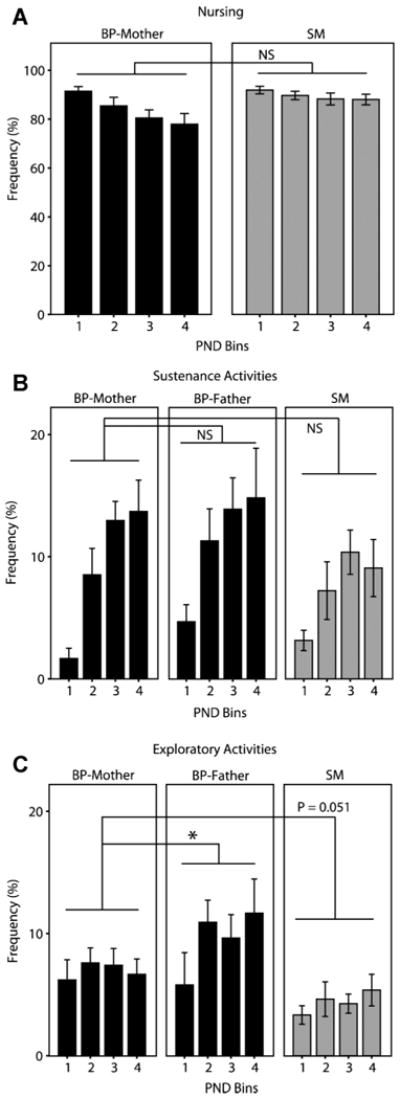
Nursing, sustenance, and exploratory activities. For each data set, the first 12 PNDs were grouped into 3-day bins for analysis and for graphing: PND1–3 = PND Bin 1, PND4–6 = PND Bin 2, etc. (A) BP-mothers and SMs nurse pups at essentially equal rates. (B) There are also no differences in sustenance-related eating and drinking activities. (C) BP-fathers engage in a slightly higher frequency of exploratory activities (wandering, gnawing, digging, climbing, jumping) than BP-mothers; BP-mothers have a trend toward a higher rate than SMs (p = .051). Bars represent mean ± SEM. *p < .05, NS, not significant.
Sustenance-Related Activities
Eating and drinking, or sustenance-related activities, were compared across parents. We suspected that SMs might either decrease these activities to focus more time on pup-care or increase these activities to sustain higher levels of care toward the pups. Statistical analysis revealed no differences in the rate of sustenance-related activities between BP-mothers and BP-fathers or BP-mothers and SMs (Fig. 3B; two-way ANOVA; PND: PND: F(1,3) = 23.480, p < .001; parent: F(1,18) = 1.069, p = .364; PND × par-parent interaction: F(1,6) = .770, p = .597; p > .163 for both Tukey comparisons). We cannot conclude from our data whether SM dams consume a different amount of food than BP mothers in a single food bout.
Exploratory Activities
There were minor differences in the frequency of time animals spent in “exploratory” activities, which equaled the sum total of all the gnawing, wandering, digging, jumping, and climbing frequencies (two-way ANOVA; PND: F(1,2) = 3.609, p = .036; parent: F(1,18) = 3.865, p = .037; PND × parent interaction: F(1,4) = .605, p = .661). During the postnatal period, BP-fathers engaged in a significantly higher frequency of exploratory activities compared to BP-mothers (Fig. 3C; Tukey, p = .035), and BP-mothers exhibited a trend toward engaging in a higher rate of exploratory activities than SMs (Tukey, p = .051).
Experiment 2: The Effect of Family Structure on Parenting in the Next Generation
We predicted that changing family dynamics by altering family structure would influence parenting in the next generation. We tested this by examining the adult primiparous parenting behavior of pairs that had been BP-reared (BP/BP animals) and pairs that had been SM-reared (SM/SM animals).
Partner Preference Formation
As a positive control for family-structure-dependent changes in adult social behavior adult females from the BP/BP and SM/SM groups were assayed in the partner preference test (Ahern & Young, 2009). As predicted, BP-reared females showed a significant group partner preference, whereas SM-reared females did not (Fig. 4A; p < .001 and p = .107, respectively, Bonferroni adjusted p-value set to <.025), replicating our previous findings that, on average, SM-reared females require longer periods of time to develop stable partner preferences. Also as predicted, there was no group difference in locomotor activity (mean center chamber entries ± SEM: BP-reared female: 133 ± 19, SM-reared female = 143 ± 28; t-test: p = .773).
FIGURE 4.
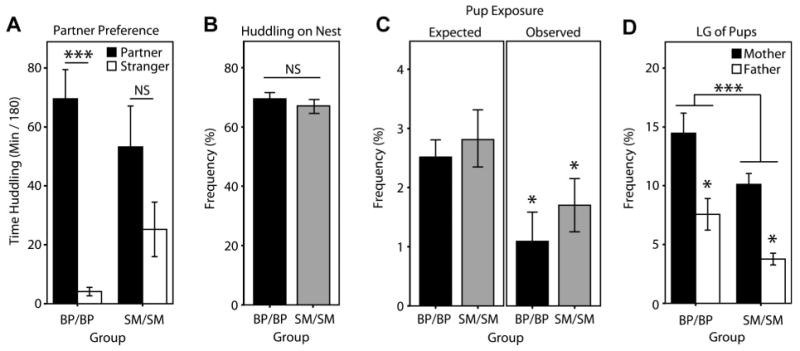
Rearing condition influences parenting of the next generation. (A) As a positive control for differences in adult social behavior between BP- and SM-reared animals, females from the BP/BP and SM/SM pairings were tested for partner preference. Similar to Ahern and Young (2009), as a group, BP-reared females exhibited a significant partner preference after 24 hr of cohabitation (p < .001), whereas SM-females did not (p = .107). (B) During the first six PNDs, BP/BP and SM/SM parents huddled at equal rates. (C) There were no differences in pup exposure and both groups showed nest coordination (observed exposure < expected, p < .05 for both). (D) SM-reared parents exhibited lower frequencies of pup-directed LG than BP-reared parents (p = .001), despite both groups showing a sex difference (BP: p = .006; SM: p < .001; Bonferroni adjusted p = .025). Bars represent mean ± SEM. ***p < .001, *p < .05, NS, not significant.
Gestation
Despite differences in social behavior, BP/BP and SM/SM pairs showed no differences in the time that elapsed from pairing until the birth of offspring, nor were there differences in the percentage of pairs that gave birth within a week of the first litter (Tab. 3). Analysis of litter size also revealed no group difference (Tab. 3).
Table 3. Experiment 2 Gestation Time, Birth Rate, and Litter Size by Group.
| Group | n | Time from Pairing to Birth of 1st Littera | Litters Brought to Termb | Litter Size at Birthc |
|---|---|---|---|---|
| BP/BP | 12 | 24.45±.37 | 92% (11/12) | 3.91±.31 |
| SM/SM | 15 | 24.87±.49 | 100% (15/15) | 3.86±.38 |
Note. BP/BP and SM/SM family units exhibited statistically indistinguishable gestation and fertility rates under laboratory conditions. Values represent mean ± SEM or percentage (number/total).
t-Test revealed no group difference in gestation (p = .532).
Two-tailed Fisher's exact test revealed no group difference in birth rate (p = .444).
Mann–Whitney nonparametric test revealed no group difference in litter size (p = .850).
Family Interactions
We hypothesized that, after birth, SM/SM pairs would exhibit lower huddling rates, lower rates of LG, and an altered parental nest coordination profile in comparison to BP/BP counterparts. Family unit observations revealed no differences in adult huddling frequency (Fig. 4B; two-way ANOVA; PND: F(1,1) = 2.693, p = .113; group: F(1,25) = .35, p = .853; PND × group: F(1,1) = .798, p=.380), and there were no differences in the frequency of nest exposure—either observed or how the observed differed from expected, suggesting that both groups coordinated nest occupancy equally well (Fig. 4C; two-way ANOVA; observed/expected: F(1,1) = 33.650, p < .001; group: F(1,25) = .633, p = .434; observed/expected × group interaction: F(1,1) = .125, p = .726).
Our analysis of LG behavior, however, did reveal an overall group effect (Fig. 4D; three-way ANOVA; object-of-LG: F(1,2) = 149.729, p < .001; group: F(1,50) = 6.963, p = .011; sex: F(1,1)= 11.127, p = .002; object-of-LG × group interaction: F(1,2) = 10.988, p < .001; object-of-LG × sex interaction: F(1,2) = 40.624, p < .001; group × sex interaction: F(1,1) = .020, p = .888; object-of-LG × group × sex interaction: F(1,1) = .124, p = .726). Post hoc analysis demonstrated that, while there were no differences in partner-directed LG or autogrooming measures (data not shown), BP/BP parents exhibited a higher frequency of pup-directed LG than SM/SM parents (p = .001). Both groups displayed a sex difference (post hoc t-tests; BP: p = .006; SM: p < .001; Bonferroni adjusted p = .025), with mothers engaging in a higher frequency of pup-directed LG than fathers.
Discussion
In this set of studies, we aimed to test several hypotheses pertaining to prairie vole family dynamics and structure. Three findings are of particular interest: (1) even as primiparous parents, BP prairie voles divide and coordinate multiple direct-parenting behaviors (Figs. 1 and 2); (2) SM prairie voles exhibit no statistically significant evidence of parental compensation in response to the removal of the father, even over extended observations, although there were some trends (Figs. 1–3); and (3) rearing condition significantly influences the primiparous parenting behavior of the next generation (Fig. 4).
Parenting and Parental Coordination Over Time
Under BP conditions, prairie vole parents engage in adult–adult interactions, such as huddling and partner-directed LG, and the pups receive attention from both parents (Fig. 1A). This creates a highly dynamic social environment in which each animal is receiving social stimulation from multiple sources, similar to human family systems. For prairie vole pups, this may be especially important, since stimulation is a well-known moderator of rodent development (Francis, Diorio, Liu, & Meaney, 1999; Kuhn & Schanberg, 1998; Meaney, 2001). It also creates a parental resource allocation scheme in which direct parental care, such as nest occupancy and LG, can be divided and coordinated.
Based on previous work, we had expected that mothers would be the primary nest attendant over the long-term (McGuire et al., 2007; Solomon, 1993). Contrary to this prediction, but in line with other studies (Hartung & Dewsbury, 1979; Lonstein & De Vries, 1999; Wang & Novak, 1992), we found that, overall, fathers and mothers occupied the nest at essentially equal rates throughout the postnatal period (Fig. 1A). They also attended to the nest alone at approximately equal frequencies. In short, the fathers were present and available to socially influence the pups at essentially the same rate as the mothers. The data also clearly show that pups regularly experience three distinct nest states, each of which might be important for development.
For animals with altricial young, managing nest occupancy is essential. In addition to discouraging predation of young nestlings (e.g., Getz, Larson, & Lindstrom, 1992), parental contact helps maintain warmth and the concomitant active interactions between adults and pups, such as pup-directed LG, help convey important social and environmental information (Meaney, 2001). Under BP conditions, the care of offspring could be coordinated. For example, in many avian species both parents can warm the chicks and provide sustenance; accordingly, monogamous birds often coordinate nest occupancy (Lack, 1968). In mammals, however, because only the mother can provide the necessary sustenance, there may be less incentive to develop the cognitive ability to coordinate nest occupancy and pup care.
To address this question in prairie voles, we analyzed pup exposure frequencies. Under BP conditions, pups were exposed on the nest very infrequently during the first 2 weeks of life. This suggested that the parents might be coordinating nest attendance rather than coming and going independently. Quantitative comparisons confirmed this prediction, with the observed exposure frequency being lower than expected (Fig. 1B).
The quantitative comparison, however, was only one form of evidence. We also had anecdotal observations to corroborate this interpretation. During most spot-checks, parents were either huddled together with the pups or one brooded the pups while the other was off the nest. Occasionally, however, we directly observed the transition of one parent to the other. In some instances, the wandering parent ran to the nest as soon as the other left; in others, the parents would meet at the threshold of the nest and wrestle briefly before the transition either occurred or not; and in two instances, the nest-attending parent left the nest to fetch the other parent by grasping the hind-leg and pulling back to the nest. To our knowledge, this last type of enforced coordination has been noted in only one other rodent species, Microtus socialis guentheri (Libhaber & Eilam, 2002) and has not been previously reported in prairie voles (McGuire et al., 2007). While anecdotal, these observations support the interpretation that BP pairie vole parents are coordinating nesting behavior.
Next, we analyzed whether LG behavior might also be coordinated, or at least dependent on social context. Our initial LG analysis revealed that fathers engaged in a lower frequency of pup-directed LG than mothers (Fig. 2A), which is in line with some previous studies (McGuire et al., 2007; Solomon, 1993) but not others (Hartung & Dewsbury, 1979; Lonstein & De Vries, 1999). But why? Is the sex-difference inherent in LG capacity or is it a function of coordination? We predicted the latter: namely, that when BP animals are together on the nest, fathers shift some of their LG focus from the pups to their partners in a coordinated fashion. If true, two other phenomena should be present in the data: first, fathers should show higher rates of partner-directed LG than mothers; second, fathers and mothers should only show a sex difference when together on the nest (which occurs the majority of the time), not when alone with the pups. The data bear this out. Fathers do show higher rates of partner-directed LG than mothers (Fig. 2B) and there is only a sex difference in pup-directed LG when together on the nest, not when alone (Fig. 2D), indicating LG coordination. However, this coordination does not completely account for the sex difference in pup-directed LG. Fathers also engaged in a higher rate of autogrooming than mothers, particularly when there was dual occupancy of the nest (Fig. 2C and E), creating an inherent sex difference in pup-directed LG activity.
Overall, our data demonstrate that the active parenting exhibited by primiparous parents is complex, quantifiable, and dependent on an interplay between parent sex, division of labor, and behavioral coordination. But are these significant, albeit nuanced, findings repeatable? To be biomedically relevant, there needs to be clear evidence that these nuanced findings are robust, particularly in light of the inconsistencies in the literature. All observations for Experiment 1 had been conducted by T.H. Ahern. So we reprocessed a BP data set previously obtained by a different observer (E.A. Hammock) in a different building and in different caging and found strikingly similar nest and LG coordination results to those presented above (unpublished data). This analysis confirmed the robustness of the findings. In turn, the robustness of the findings suggests several experimental extensions of the study presented here. For example, brain lesions or targeted pharmacological treatments in conjunction with periodic family unit observations could be used to tease apart which neurobiological systems, for example, oxytocin, vasopressin, or prolactin, drive the different components of family interactions in each sex. Simultaneously, the effects of these manipulations on long-term pup development could be assessed.
Single-Mother Parenting
From a behavioral perspective, one approach to teasing apart the importance of family environment on behavior is to alter family structure. As shown above, BP prairie voles engage in high levels of coordinated, socially dependent, parenting behavior, which leads to high levels of huddling interaction between parents, minimal nest exposure, and multiple sources of social stimulation for the pups. If pups, in some evolutionary sense, “expect” this high level of parental contact, it is plausible that SMs might increase their rates of pup-directed care to compensate for the lack of a second parent. To test this hypothesis, we compared SM and BP family units.
To our knowledge, only two previous reports examined this question in a way that would be relevant to long-term outcomes (McGuire et al., 2007; Wang & Novak, 1992), and neither found behavioral compensation on the part of SMs. Both studies, however, used experienced parents in large semi-natural enclosures (1300 cm2), and the more detailed study (McGuire et al., 2007) only observed parents for the first three PNDs. All three factors introduce important confounds. First, experienced parents may have settled into a set parental pattern, which might work against behavioral compensation in subsequent litters. Second, the large semi-natural environments increase foraging demands and thus decrease the opportunity to compensate. Third, making observations during only the first three PNDs may have missed long-term compensation due to a ceiling effect during the immediate postpartum period (e.g., see Figs. 1A and 2A). Under our experimental conditions, we predicted that primiparous SMs would either show higher parental investment throughout the observation period or would at least maintain the high levels of pup-care longer. That prediction is only one of two competing alternate hypotheses, however. It was also plausible that SMs might decrease their maternal investment in the absence of their mates.
Contrary to both alternative hypotheses, we found no statistically significant evidence of parental compensation at any point on any measure by SMs. SMs were on the nest only as often as BP-mothers (Fig. 2A), and they exhibited pup-directed LG at frequencies approximately equal to that of BP-mothers throughout the postnatal period. Under our experimental conditions, there were also no statistically significant differences in nursing, sustenance-related activities, or exploration (although exploration did show a nonsignificant trend; Fig. 3C). Together, our analyses suggest that, while mothers may adjust LG behavior in response to a brief departure by the father (Fig. 2D), over the long-term, mothers showed little evidence of behavioral compensation in response to the lack of the father.
We note, however, that our study consisted of relatively small samples, only seven family units per family structure. A qualitative analysis of the bar-charts (e.g., Figs. 1A and 3A and C) suggests that there might be modest levels of compensation on the part of SMs in the form of higher nest occupancy, higher nursing, and lower exploratory activity. In an experiment that included more family units, there might have been significant evidence of compensation.
What is clear, however, is that there were no large behavioral differences in maternal behavior that would be amenable to some form of “rescue” treatment. Based on our data, in most cases, offspring of BP and SM families are likely to experience approximately equal rates of maternal investment. From the perspective of the pups, however, this lack of dramatic compensation by SMs creates a situation in which BP- and SM-reared pups experience significant differences in the rates of social stimulation, including total huddle time and LG received, because of the father in the BP condition.
The importance of these family structure differences should not be overlooked. In this study, we examined parenting rates for a comparatively brief period of time each day and yet found highly significant differences in total LG received and nest exposure between rearing conditions. Compounded throughout the preweaning period, the differences in social stimulation are likely to be highly consequential. Indeed, there is converging evidence that differences in family structure within BP species do have long-term consequences. In BP species such as prairie voles and California mice, family structure can affect reproductive and survival rates, with SMs rearing fewer pups to maturity under ethological conditions (Cantoni & Brown, 1997; Getz et al., 1992; Wright & Brown, 2002). In the laboratory, experiments with California mice have revealed that family structure impacts the development of at least two learning and memory tasks, with SM-reared animals learning more slowly (Bredy et al., 2004). In Degus, another BP rodent, BP conditions produce offspring with more complex neuronal architecture in both the somatosensory and limbic systems in comparison to SM-reared counterparts (Helmeke et al., 2009; Ovtscharoff, Helmeke, & Braun, 2006; Pinkernelle, Abraham, Seidel, & Braun, 2009). Prairie voles are affected as well. SM-reared females exhibit lower levels of spontaneous alloparenting (Ahern & Young, 2009; Roberts, Cushing, & Carter, 1998) as well as delayed partner preference formation, both of which may result in part from differences in oxytocin gene expression (Ahern & Young, 2009). The study of family structure on alloparental care is especially interesting because different populations of prairie voles tend to respond differently to changes in family structure: for highly social Illinois prairie voles (like those in this study), the presence of the sire during development facilitates the expression of alloparental behavior in offspring, whereas the presence of the sire suppresses the development of alloparental behavior in the less social Kansas prairie vole (Roberts et al., 1998). Together, these data demonstrate how broadly important family behavior is and how changes in family structure can alter that behavior. In fact, in Experiment 2, we extend these findings to show the effects of family structure in one generation can extend into the next.
Family Structure Influences Parenting Behavior in the Next Generation
In Experiment 1 we established that BP- or SM-reared offspring experience significant differences in their early social environment. Previous studies have shown how these differences affect long-term behavioral outcomes such as bonding and alloparenting behavior. In Experiment 2, we asked whether differences in early family structure would alter parenting of the next generation.
As predicted, SM- and BP-reared animals again showed differences in partner preference formation, with BP-reared females seeming to form stable partner preferences more quickly than SM-reared females (Fig. 4A). On family measures, there were differences in LG behavior: both sexes in the SM/SM group showed decreased pup-directed LG in comparison to BP/BP counterparts (Fig. 4D). Rearing condition, however, did not significantly affect family social behavior more broadly, as there were not decreases in adult huddling rates or measures of parental coordination (Fig. 4B and C). Still, to our knowledge, this is the first evidence of altered primiparous parenting of the next generation in prairie voles. Interestingly, a similar phenomenon was recently observed after several generations of BP or SM rearing in Mongolian hamsters, Meriones unguiculatus (Gromov, 2009).
In prairie voles, the cause of this intergenerational effect on LG behavior is currently unclear, but it may depend on early life LG and oxytocin receptor (OTR) densities in the bed nucleus of the stria terminalis (BNST). Female rats that receive high levels LG during development have higher BNST OTR densities and they LG their own pups more than female rats that received low levels of LG during development (Francis, Champagne, & Meaney, 2000; Francis, Young, Meaney, & Insel, 2002). In prairie voles, SM-reared pups receive less LG than BP-reared animals; they also have lower densities of OTR in the BNST (Ahern & Young, 2009). Therefore, manipulations of family structure in one generation may indirectly affect care in the next generation by directly altering LG stimulation and subsequently OTR circuits. Future studies will be required to directly test this hypothesis.
Primiparous Prairie Voles as a Model of Family Dynamics
Another important aspect of the present study is that it highlights the use prairie voles and other BP species as a fast, easy, and ethologically relevant way of studying human-relevant family dynamics on a genetically diverse background. At present, one of the most popular and elegant methods for studying the effect of natural variations in early experience on long-term pup development is to screen laboratory strain (e.g., Sprague– Dawley) rat mothers for relative extremes of LG behavior and compare offspring (Francis et al., 1999; Meaney, 2001). As powerful as this technique has been, screening animals is time consuming and it generates, in a sense, artificial comparison groups in a genetically constricted line of uniparental rats, thus requiring complicated cross-fostering experiments. With BP prairie voles, there is a richer set of family-relevant social interactions and pairs can be assigned to one family group or another without the need for screening, thus reducing the risk of selection bias and the need for cross-fostering. In fact, the model becomes more powerful when considering other permutations, such as rearing pups under different conditions for different parts of the developmental period by simply adding or removing adults at will.
Lastly, there is the consideration of our use of primiparous parents. In this study, we specifically used primiparous parents to help address the questions of SM compensation and whether new parents were capable of coordination from the outset. While somewhat unconventional, the use of primiparous parents has other advantages as well. First, it maintains the greatest level of behavioral diversity. There are no a priori exclusions based on the failure to raise a first litter. In the future, identifying manipulations that decrease the likelihood of losing the first litter could be an important measure. Second, and looking forward, primiparous parents also make brain-based studies of undisturbed parenting behavior more accessible. Conducting surgeries on animals that require 1–2 months to gain parenting experience can create significant experimental complications. With this study demonstrating robust and complex family interactions even among primiparous parents, researchers can now easily add any adult prairie vole to a study of family dynamics. In short, the data presented above suggest that primiparous prairie voles are an excellent experimental animal model with which researchers can more easily test hypotheses relating to family life.
Conclusions
Selective social bonding between mates and BP care are rare among mammals, but common in humans and Illinois stock prairie voles. In this set of studies, we demonstrate the richness of prairie vole family interactions and show that manipulations of family structure have important long-lasting consequences: in particular, family dynamics can influence parenting behavior directed toward the next generation. Based on the findings presented here, future, targeted manipulations of the socially relevant neural systems (e.g., oxytocin, vasopressin, prolactin) are likely to reveal how the brain manages these complex sets of now well-characterized family interactions and how it responds to changes experience-dependent social development.
Acknowledgments
This work was supported by MH64692 and MH077776 (L.J.Y.), NIH RR00165, and a NIMH training grant MH0732505 (T.H.A.). We thank Lorra Mathews for her assistance with general animal care and Erika Ahern for her critical reading of the manuscript.
References
- Ahern TH, Modi ME, Burkett JP, Young LJ. Evaluation of two automated metrics for analyzing partner preference tests. Journal of Neuroscience Methods. 2009;182(2):180. doi: 10.1016/j.jneumeth.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahern TH, Young LJ. The impact of early life family structure on adult social attachment, alloparental behavior, and the neuropeptide systems regulating affiliative behaviors in the monogamous prairie vole (Microtus ochrogaster) Frontiers in Behavioral Neuroscience. 2009;3:17. doi: 10.3389/neuro.08.017.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amato PR. The consequences of divorce for adults and children. Journal of Marriage and Family. 2000;62(4):1269–1287. [Google Scholar]
- Aragona BJ, Liu Y, Yu YJ, Curtis JT, Detwiler JM, Insel TR. Nucleus accumbens dopamine differentially mediates the formation and maintenance of monogamous pair bonds. Nature Neuroscience. 2006;9(1):133. doi: 10.1038/nn1613. [DOI] [PubMed] [Google Scholar]
- Bales KL, Mason WA, Catana C, Cherry SR, Mendoza SP. Neural correlates of pair-bonding in a monogamous primate. Brain Research. 2007;1184:245. doi: 10.1016/j.brainres.2007.09.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyum LA, Parke RD. The role of family emotional expressiveness in the development of children's social competence. Journal of Marriage and the Family. 1995;57(3):593. [Google Scholar]
- Branchi I, D'Andrea I, Fiore M, Di Fausto V, Aloe L, Alleva E. Early social enrichment shapes social behavior and nerve growth factor and brain-derived neurotrophic factor levels in the adult mouse brain. Biological Psychiatry. 2006;60(7):690. doi: 10.1016/j.biopsych.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Bredy TW, Lee AW, Meaney MJ, Brown RE. Effect of neonatal handling and paternal care on offspring cognitive development in the monogamous California mouse (Peromyscus californicus) Hormones and Behavior. 2004;46(1):30. doi: 10.1016/j.yhbeh.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Cantoni D, Brown RE. Paternal investment and reproductive success in the California mouse, Peromyscus californicus. Animal Behaviour. 1997;54:377–386. doi: 10.1006/anbe.1996.0583. [DOI] [PubMed] [Google Scholar]
- Carter CS, DeVries AC, Getz LL. Physiological substrates of mammalian monogamy: The prairie vole model. Neuroscience and Biobehavioral Reviews. 1995;19(2):303. doi: 10.1016/0149-7634(94)00070-h. [DOI] [PubMed] [Google Scholar]
- Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322(5903):900. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- Feinberg ME. Coparenting and the transition to parenthood: A framework for prevention. Clinical Child and Family Psychology Review. 2002;5(3):173. doi: 10.1023/a:1019695015110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming AS, Kraemer GW, Gonzalez A, Lovic V, Rees S, Melo A. Mothering begets mothering: The transmission of behavior and its neurobiology across generations. Pharmacology, Biochemistry, and Behavior. 2002;73(1):61. doi: 10.1016/s0091-3057(02)00793-1. [DOI] [PubMed] [Google Scholar]
- Fortunato L, Archetti M. Evolution of monogamous marriage by maximization of inclusive fitness. Journal of Evolutionary Biology. 2010;23(1):149–156. doi: 10.1111/j.1420-9101.2009.01884.x. [DOI] [PubMed] [Google Scholar]
- Francis DD, Champagne FC, Meaney MJ. Variations in maternal behaviour are associated with differences in oxytocin receptor levels in the rat. Journal of Neuroendocrinology. 2000;12(12):1145. doi: 10.1046/j.1365-2826.2000.00599.x. [DOI] [PubMed] [Google Scholar]
- Francis D, Diorio J, Liu D, Meaney MJ. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science. 1999;286(5442):1155–1158. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- Francis DD, Young LJ, Meaney MJ, Insel TR. Naturally occurring differences in maternal care are associated with the expression of oxytocin and vasopressin (V1a) receptors: Gender differences. Journal of Neuroendocrinology. 2002;14(5):349. doi: 10.1046/j.0007-1331.2002.00776.x. [DOI] [PubMed] [Google Scholar]
- Frazier CRM, Trainor BC, Cravens CJ, Whitney TK, Marler CA. Paternal behavior influences development of aggression and vasopressin expression in male California mouse offspring. Hormones and Behavior. 2006;50(5):699. doi: 10.1016/j.yhbeh.2006.06.035. [DOI] [PubMed] [Google Scholar]
- Getz LL, Carter CS. Prairie-vole partnerships. American Scientist. 1996;84:56. [Google Scholar]
- Getz LL, Larson CM, Lindstrom KA. Blarina brevicauda as a predator on nestling voles. Journal of Mammalogy. 1992;73(3):591. [Google Scholar]
- Getz LL, McGuire B, Pizzuto T, Hofmann JE, Frase B. Social organization of the prairie vole (Microtus ochrogaster) Journal of Mammalogy. 1993;74(1):44. [Google Scholar]
- Gromov VS. Interactions of partners in family pairs, care of the offspring, and the role of tactile stimulation in formation of parental behavior of the Mongolian gerbil (Meriones unguiculatus) under laboratory conditions. Biology Bulletin. 2009;36(5):479. [PubMed] [Google Scholar]
- Hammock EAD, Young LJ. Microsatellite instability generates diversity in brain and sociobehavioral traits. Science. 2005;308(5728):1630. doi: 10.1126/science.1111427. [DOI] [PubMed] [Google Scholar]
- Hartung TG, Dewsbury DA. Parental behavior in 6 species of muroid rodents. Behavioral and Neural Biology. 1979;26(4):466. doi: 10.1016/s0163-1047(81)91034-7. [DOI] [PubMed] [Google Scholar]
- Helmeke C, Seidel K, Poeggel G, Bredy TW, Abraham A, Braun K. Paternal deprivation during infancy results in dendrite- and time-specific changes of dendritic development and spine formation in the orbitofrontal cortex of the biparental rodent Octodon degus. Neuroscience. 2009;163(3):790. doi: 10.1016/j.neuroscience.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Kuhn CM, Schanberg SM. Responses to maternal separation: Mechanisms and mediators. International Journal of Developmental Neuroscience. 1998;16(3–4):261. doi: 10.1016/s0736-5748(98)00034-3. [DOI] [PubMed] [Google Scholar]
- Lack D. Ecological adaptations for breeding in birds. London: Methuen and Company; 1968. [Google Scholar]
- Libhaber N, Eilam D. Social vole parents force their mates to baby-sit. Developmental Psychobiology. 2002;41(3):236–240. doi: 10.1002/dev.10075. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, De Vries GJ. Comparison of the parental behavior of pair-bonded female and male prairie voles (Microtus ochrogaster) Physiology & Behavior. 1999;66(1):33. doi: 10.1016/s0031-9384(98)00270-4. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, De Vries GJ. Sex differences in the parental behavior of rodents. Neuroscience and Biobehavioral Reviews. 2000;24(6):669. doi: 10.1016/s0149-7634(00)00036-1. [DOI] [PubMed] [Google Scholar]
- Low BS. Oxford Handbook of Evolutionary Psychology. New York, NY: Oxford University Press; 2007. Ecological and socio-cultural impacts on mating and marriage systems; pp. 449–462. [Google Scholar]
- Maccoby EE. Parenting and its effects on children: On reading and misreading behavior genetics. Annual Review of Psychology. 2000;51:1–27. doi: 10.1146/annurev.psych.51.1.1. [DOI] [PubMed] [Google Scholar]
- Matheson K, Kelly O, Cole B, Tannenbaum B, Dodd C, Anisman H. Parental bonding and depressive affect: The mediating role of coping resources. The British Journal of Social Psychology. 2005;44(Pt 3):371. doi: 10.1348/014466605X37477. [DOI] [PubMed] [Google Scholar]
- McEwen C, Flouri E. Fathers' parenting, adverse life events, and adolescents' emotional and eating disorder symptoms: The role of emotion regulation. European Child & Adolescent Psychiatry. 2009;18(4):206. doi: 10.1007/s00787-008-0719-3. [DOI] [PubMed] [Google Scholar]
- McGraw LA, Young LJ. The prairie vole: An emerging model organism for understanding the social brain. Trends in Neuroscience. 2009;33(2):103. doi: 10.1016/j.tins.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire B, Parker E, Bemis WE. Sex differences, effects of male presence and coordination of nest visits in prairie voles (Microtus ochrogaster) during the immediate postnatal period. American Midland Naturalist. 2007;157(1):187. [Google Scholar]
- McLanahan S, Sandefur GD. Growing up with a single parent: What hurts, what helps. Cambridge: Harvard University Press; 1994. [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annual Review of Neuroscience. 2001;24:1161. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Moore CL, Morelli GA. Mother rats interact differently with male and female offspring. Journal of Comparative and Physiological Psychology. 1979;93(4):677–684. doi: 10.1037/h0077599. [DOI] [PubMed] [Google Scholar]
- Murdoch GP. Atlas of World Cultures. Pittsburgh: Pittsburgh Press; 1981. [Google Scholar]
- Ovtscharoff W, Helmeke C, Braun K. Lack of paternal care affects synaptic development in the anterior cingulate cortex. Brain Research. 2006;1116(1):58. doi: 10.1016/j.brainres.2006.07.106. [DOI] [PubMed] [Google Scholar]
- Pinkernelle J, Abraham A, Seidel K, Braun K. Paternal deprivation induces dendritic and synaptic changes and hemispheric asymmetry of pyramidal neurons in the somatosensory cortex. Developmental Neurobiology. 2009;69(10):663. doi: 10.1002/dneu.20726. [DOI] [PubMed] [Google Scholar]
- Roberts RL, Cushing BS, Carter CS. Intraspecific variation in the induction of female sexual receptivity in prairie voles. Physiology & Behavior. 1998;64(2):209–212. doi: 10.1016/s0031-9384(98)00042-0. S0031-9384(98)00042-0[pii] [DOI] [PubMed] [Google Scholar]
- Sbarra DA, Hazan C. Coregulation, dysregulation, self-regulation: An integrative analysis and empirical agenda for understanding adult attachment, separation, loss, and recovery. Personality and Social Psychology Review. 2008;12(2):141–167. doi: 10.1177/1088868308315702. [DOI] [PubMed] [Google Scholar]
- Schor EL. Family pediatrics: Report of the task force on the family. Pediatrics. 2003;111(6 Pt 2):1541. [PubMed] [Google Scholar]
- Shear K, Shair H. Attachment, loss, and complicated grief. Developmental Psychobiology. 2005;47(3):253. doi: 10.1002/dev.20091. [DOI] [PubMed] [Google Scholar]
- Smith AS, Agmo A, Birnie AK, French JA. Manipulation of the oxytocin system alters social behavior and attraction in pair-bonding primates, Callithrix penicillata. Hormones and Behavior. 2010;57(2):255–262. doi: 10.1016/j.yhbeh.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon NG. Comparison of parental behavior in male and female prairie voles (Microtus ochrogaster) Canadian Journal of Zoology. 1993;71(2):434. [Google Scholar]
- Walum H, Westberg L, Henningsson S, Neiderhiser JM, Reiss D, Igl W. Genetic variation in the vasopressin receptor 1a gene (AVPR1A) associates with pair-bonding behavior in humans. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(37):14153–14156. doi: 10.1073/pnas.0803081105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZX, Novak MA. Influence of social-environment on parental behavior and pup development of meadow voles (Microtus pensylvanicus) and prairie voles (Microtus ochrogaster) Journal of Comparative Psychology. 1992;106(2):163. [Google Scholar]
- Wen M. Family structure and children's health and behavior: Data from the 1999 National Survey of America's Families. Journal of Family Issues. 2008;29(11):1492. [Google Scholar]
- Williams JR, Carter CS, Insel T. Partner preference development in female prairie voles is facilitated by mating or the central infusion of oxytocin. Annals of the New York Academy of Sciences. 1992;652:487. doi: 10.1111/j.1749-6632.1992.tb34393.x. [DOI] [PubMed] [Google Scholar]
- Wright SL, Brown RE. The importance of paternal care on pup survival and pup growth in Peromyscus californicus when required to work for food. Behavioural Processes. 2002;60(1):41–52. doi: 10.1016/s0376-6357(02)00101-8. S0376635702001018[pii] [DOI] [PubMed] [Google Scholar]
- Young LJ, Wang Z. The neurobiology of pair bonding. Nature Neuroscience. 2004;7(10):1048. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]


