Abstract
Experimental autoimmune encephalomyelitis (EAE) is an animal model for studying multiple sclerosis (MS). Calpain has been implicated in many inflammatory and neurodegenerative events that lead to disability in EAE and MS. Thus, treating EAE animals with calpain inhibitors may block these events and ameliorate disability. To test this hypothesis, acute EAE Lewis rats were treated dose-dependently with the calpain inhibitor calpeptin (50 – 250 µg/kg). Calpain activity, gliosis, loss of myelin, and axonal damage were attenuated by calpeptin therapy, leading to improved clinical scores. Neuronal and oligodendrocyte death were also decreased with down regulation of pro-apoptotic proteins, suggesting that decreases in cell death were due to decreases in the expression or activity of pro-apoptotic proteins. These results indicate that calpain inhibition may offer a novel therapeutic avenue for treating EAE and MS.
Keywords: apoptosis, axonal damage, calpain, calpeptin, EAE
INTRODUCTION
Multiple sclerosis (MS) is a chronic and debilitating disease of the central nervous system (CNS) that affects nearly 400,000 adults between the ages of 20 to 40 in the United States (Noonan et al., 2002). While the exact etiology of MS has not been fully identified, studies in MS patients and animals with experimental autoimmune encephalomyelitis (EAE), an animal model of MS, have implicated an attack on myelin proteins by infiltrating auto-reactive T cells and other immune cells (Keegan and Noseworthy, 2002). These pro-inflammatory attacks contribute to the accumulation of neurological damage that results in disability in EAE and MS (Trapp and Nave, 2008).
Current therapies for MS, which are mainly immunomodulatory in nature, have shown some effect in reducing clinical symptoms; however, further research is warranted to identify novel therapeutic targets. Proteases as potential targets have been suggested for MS therapy (Govindarajan et al. 1974; Marks et al. 1974), including ubiquitous isoforms of the calcium (Ca2+)-activated neutral protease calpain, μ-calpain and m-calpain (Schaecher et al., 2001b; Guyton et al., 2006; Hassen et al. 2006), which are activated by µM and mM concentrations of Ca2+, respectively (Ray and Banik 2003). Calpain expression and activity were upregulated in immune cells, glial cells, and neurons in spinal cord and optic nerve of animals with EAE (Banik and Shields, 1999; Guyton et al., 2005). Calpain activity was increased in post-mortem brain tissue from MS patients (Shields et al., 1999b) and co-localized with damaged axons (Diaz-Sanchez et al., 2006). Calpain activity was also increased in polymorphonuclear cells (PBMCs) from MS patients during relapse, compared with MS patients in remission (Imam et al., 2007).
Calpain is involved in normal function but also associated with deleterious events when unregulated due to aberrant increases in intracellular free Ca2+ levels. Immunologic events include activation and migration of T cells and other immune cells (Soede et al., 1999; Butler et al., 2009), increased production of pro-inflammatory cytokines (Schaecher et al., 2001a; Imam et al., 2007), and cleavage of myelin into antigenic peptides (Deshpande et al., 1995) that can potentially lead to epitope spreading of T cells (Banik et al., 1994). Thus, calpain is very likely a key player in the inflammatory phase of MS. Calpain is also linked to neurodegenerative events such as demyelination (Shields and Banik, 1999), axonal damage, loss of neurons, and oligodendrocytes (Guyton et al., 2005; Guyton et al. 2006), and modulation of proteins involved in apoptotic pathways (Das et al., 2008).
Calpain activity of dependent on the ratio of calpain to its endogenous inhibitor calpastatin (Higuchi et al., 2005) and, once activated, calpain degrades calpastatin (Blomgren et al., 1999). Unfortunately, calpastatin is a large molecule and is not a good candidate for drug therapy. Synthetic small molecule calpain inhibitors ameliorate inflammation and neurodegeneration in many CNS disorders (Ray and Banik, 2003). Our studies have demonstrated that calpain inhibition reduced infiltration of immune cells into the spinal cord of EAE animals, reduced axonal degeneration, and reduced neuronal loss (Guyton et al., 2006). A recent study also demonstrated that a novel calpain inhibitor CYLA reduced clinical signs of EAE (Hassen et al., 2006). We have chosen calpeptin for calpain inhibition in the current study because it is a cell permeable peptide aldehyde inhibitor, which binds to the active site of calpain and reversibly inhibits protease activity (Tsujinaka et al., 1988). In addition, calpeptin inhibits neurodegeneration following spinal cord injury, suggesting that calpeptin enters the CNS (Ray et al., 1999). Our current results show that calpeptin reduced microgliosis, astrogliosis, axonal damage, and neuron and oligodendrocyte death in EAE spinal cord. These findings further identify calpain as a viable target for treating EAE and MS.
MATERIALS AND METHODS
EAE Induction
Adult male Lewis rats (180 – 200 g) were purchased from Charles River Breeding Laboratories (Wilminton, MA) to perform our experiments, which were approved by the Institutional Animal Care and Use Committee, in accordance with the Laboratory Welfare Act and the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (Bethesda, MD). To induce EAE, rats were immunized subcutaneously with 0.2 mL of complete Freund’s adjuvant (CFA) containing 10 mg/mL of Mycobacterium tuberculosis H37Ra (Difco, Detroit, MI) and phosphate-buffered saline (PBS) containing guinea pig spinal cord homogenate (200 mg/mL) and MBP (200 µg/mL) in a 1:1 emulsion. Control (CON) animals received PBS/CFA alone. Two hours later all rats received an intraperitoneal (ip) injection of Pertussis toxin (1.25 µg/rat).
Calpeptin Therapy and Tissue Collection
On days 1 to 9 post-EAE induction, rats received ip injections of either vehicle (1.0% DMSO in saline) or calpeptin (50 – 250 µg/kg) twice daily. Rats were monitored daily for weight loss and signs of clinical disability due to EAE based on the following grades: 0, no change; 1, limp tail; 2, hind-limb weakness with difficulty righting; 3, hind limb partial paralysis; 4, hind-limb complete paralysis with front-limb weakness; and 5, quadriplegic or moribund. Animals were sacrificed under anesthesia (95 mg/kg of ketamine, 5 mg/kg of xylazine) on day 9 post-EAE induction. Lumbar spinal cord regions were removed and cut into 2 sections. One segment was snap-frozen in tissue freezing media (Fisher Scientific, Fair lawn, NJ) for in situ immunofluorescent labelings and the other segment was snap-frozen for Western blotting. In subsequent studies, animals were treated twice daily with calpeptin (250 µg/kg on days 1 to 9 post-EAE induction (before disease onset) or days 7 to 9 post-EAE induction (at disease onset) and clinical scores monitored until animals recovered (day 15 post-EAE induction) or were sacrificed at day 10 post-EAE induction, and spinal cord tissues were collected for evaluation of immune cell infiltration via hematoxylin & eosin (H&E) staining.
H&E Staining
Paraffin-embedded spinal cord tissues were sliced into 5 µm sections. Immune cell infiltration into the spinal cord and perivascular cuffing were examined following H&E staining of the tissue sections, as we described previously (Shields et al., 1998).
Protein Extraction and Western Blot Analysis
The methods used to detect changes in protein levels were described previously (Das et al., 2008). All antibodies for Western blotting were purchased from Santa Cruz and diluted at a concentration of 1:200, unless otherwise stated. We used 10 to 15 µg of protein for loading per lane for resolving on 5–20% SDS-PAGE gels and then transferred to nitrocellulose blots. Blots were incubated for 24 hours with antibodies against m-calpain, calpastatin, capase-8, tBid, Bax, Bcl-2, caspase-3, or MBP (1:1000) diluted in Tris-buffered saline (TBS) with 0.1% Tween-20 plus 5% (w/v) fat-free milk then incubated with horseradish peroxidase (HRP)-conjugated anti-rabbit (1:2000) or anti-mouse antibody (1:2000) in 0.1% TBS with Tween-20 for 45 minutes. Calpain and caspase-3 activities were determined using antibody against α-spectrin, which detected the calpain-cleaved 145-kDa spectrin breakdown products (SBDP) and caspase-3-cleaved 120-kDa SBDP, respectively. Protein bands were detected by alkaline HRP-catalyzed oxidation of luminol in the presence of H2O2 using enhanced chemiluminescence (Amersham Life Sciences, Buckinghamshire, UK). Blots were exposed immediately to X-OMAT XAR-2 film, scanned, and imaged using Photoshop software (Adobe Systems, San Jose, CA). Bands were quantified using NIH Image software. All proteins were normalized to β-actin, and expressed as % change in protein level, compared with CON-0 set at 100% or as a ratio.
Immunofluorescent Labeling of Tissue Sections
Spinal cord tissues were sliced into 10 µm cross-sections, fixed with 95% ethanol, and stained, as described previously (Guyton et al., 2005). Microgliosis and astrogliosis were determined using the antibodies specific for CD11b (OX-42, 1:100; eBiosource, Camarillo, CA) and glial fibrillary acidic protein (GFAP, 1:400, Chemicon, Billerica, MA), respectively. Briefly, sections were incubated for 1 hour in blocking buffer containing 2% horse serum in phosphate-buffered saline (PBS), followed by incubation with OX-42 or GFAP antibody for 3 to 4 hours. For detection of axonal degeneration, slides were first autoclaved for 5 minutes in 0.1 M citrate buffer, then blocked as described above for 1 hour. Next, tissue sections were incubated overnight at 4°C with SMI-311 antibody (1:1000; Sternberger Monoclonals, Lutherville, MD), which could detect de-phosphorylated neurofilament protein (de-NFP). The sections were incubated for 30 minutes in the dark with horse anti-mouse IgG secondary antibody conjugated to fuorescein isothiocyanate (FITC, 1:100; Vector Laboratories, Burlingame, CA) to detect each cell marker. The slides were mounted with Vectashield Mounting Media (Vector Laboratories) and immediately viewed under a fluorescent microscope at 200× magnification.
Combined TUNEL and Immunoflourescent Labelings of Tissue Sections
To detect cell death in specific cells in spinal cord tissue, we used the combined terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick-end labeling (TUNEL) and immunofluorescent labelings. As we previously described (Ray et al., 2000), spinal cord tissue was sliced and fixed as described above and then post-fixed in 4% methanol-free formaldehyde (in PBS) for 15 minutes. The slides were incubated with TUNEL reaction mixture containing 10xPCR DIG labeling mixture with digoxigenin (DIG)-11-dUTP (2.5 µl; Roche Diagnostics, Indianapolis, IN) and terminal TdT (25 units; Promega, Madison, WI) in TdT buffer. The slides were covered with Hybrislip hybridization cover slips (Research Products, Mount Prospect, IL) and incubated at 37°C for 1 hour. The slides were stained with mouse anti-NeuN antibody (1:100; Chemicon) to detect neurons or for 36 hours with O-4 (1:100; Chemicon) to detect mature oligodendrocytes as described above. After incubation for 1 hour in the dark with sheep anti-DIG IgG antibody conjugated to rhodamine (1:100; Roche Diagnostics) and horse anti-mouse IgG secondary antibody conjugated to FITC (1:100; Vector Laboratories), slides were viewed under a fluorescent microscope at 200× magnification.
Statistical Analysis
All statistical tests were chosen based on standard recommendations for analyzing data from EAE studies (Fleming et al., 2005) and performed using SAS® statistics software (SAS Institute, Cary NC). Clinical scores were analyzed with the non-parametric Friedman test for ordinal data. Tests for normality of the protein expression indicated that data were not normally distributed. Thus, overall significant differences in protein expression were analyzed using the non-parametric Kruskal-Wallis test followed by Mann-Whitney-U tests for pair-wise comparisons. Protein expression data from control animals treated with vehicle (CON-0) or 250 µg/kg calpeptin (CON-250) were similar and combined to analyze statistical differences, compared with EAE groups. The null hypothesis for each analysis was rejected at P ≤ 0.05.
RESULTS
Calpeptin Therapy Reduced Clinical Signs of EAE
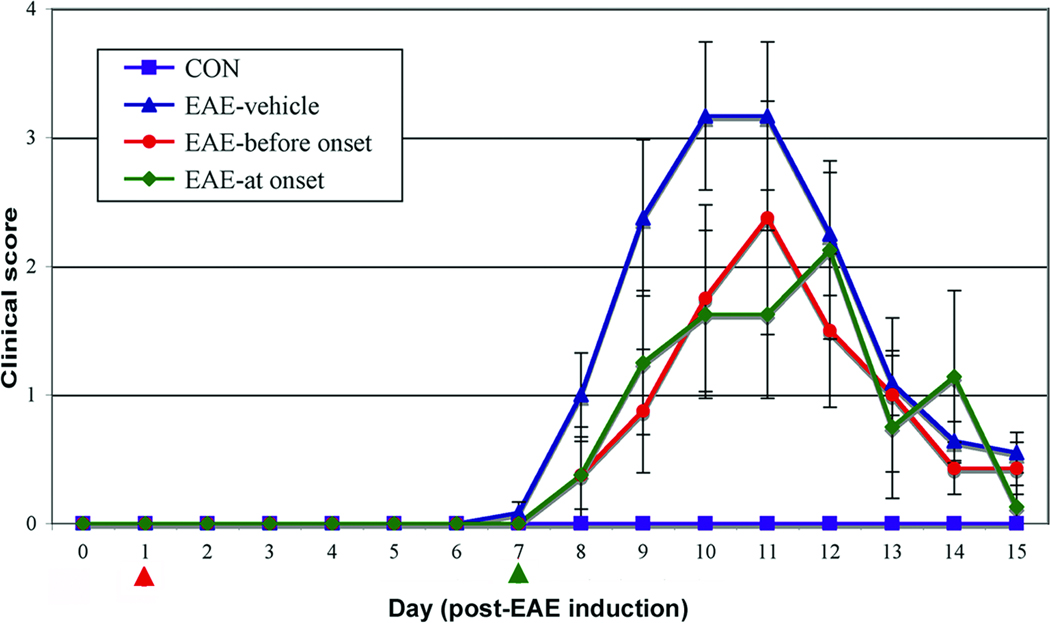
The effects of calpain inhibition on clinical signs of disease were assessed in an acute EAE model by treating control (CON) and EAE animals with intraperitoneal (ip) injections of either vehicle (1.0% DMSO in saline) or calpeptin (50 – 250 µg/kg) twice daily during days 1 to 9 post-EAE induction (Fig. 1). The doses of calpeptin were chosen based on efficacious effects achieved at this dose range in other neurological disorders (Ray et al., 1999). As expected, CON animals that received either vehicle or calpeptin (250 µg/kg) therapy did not show clinical signs of EAE. But EAE rats that did not receive calpeptin (EAE-0) showed classic signs of EAE, including tail limpness and hind-limb paralysis, with a cumulative clinical score of 45 (Table 1). A decrease in cumulative clinical score (P = 0.0652) was noted in EAE animals that received 100 µg/kg of calpeptin (EAE-100); however, the decrease did not reach a self-imposed significance level set at P ≤ 0.05. In contrast, the cumulative clinical score was significantly decreased (P = 0.0113) in animals that received the 250µg/kg of calpeptin (EAE-250), when compared with the EAE-0 group. The EAE rats that received 50µg/kg of calpeptin (EAE-50) twice daily did not differ from EAE-0 animals. The onset of disease was not significantly different for any EAE groups. On the peak day of disease (day 10), all animals were sacrificed for collection of spinal cord tissues for examination of neurodegenerative changes.
Fig. 1.
Calpeptin reduced clinical symptoms of disease in acute EAE animals. Lewis rats with acute EAE were ip injected twice-daily with calpeptin (250 µg/kg) or vehicle on days 1 to 9 (before onset, red arrow) or on days 7 to 9 post-EAE induction (at onset, green arrow) and monitored for clinical signs of disease until day 15 (n = 8 to 12 rats per group). Clinical scores for EAE-vehicle group significantly increased (P ≤ 0.05) over EAE-calpeptin-before onset and calpeptin-at onset. There was no significant difference between calpeptin treated groups.
Table 1.
Clinical parameters of disease
| Group | CS 3–5a | Onsetb | Cumulative CSc |
|---|---|---|---|
| CON | 0/10 | na | 0 |
| EAE-0 | 8/14 (57%) | 8.13 ± 0.64 | 45 |
| EAE-50 | 6/12 (50%) | 7.67 ± 0.82 | 47 |
| EAE-100 | 6/14 (43%) | 8.56 ± 0.53 | 32 |
| EAE-250 | 4/14 (29%) | 7.57 ± 0.53 | 33d |
Incidence of animals with a clinical score (CS) of 3 to 5/total number of animals per group.
Day of onset presented as mean ± standard deviation (SD).
Cumulative CS for all animals within each group from days 1 to 9.
P ≤ 0.05, EAE-0 versus EAE-250.
Calpain activity is upregulated in splenocytes as early as day 4 post-EAE induction (Shields et al., 1999a) and by day 9 in spinal cord, which correlates with onset of clinical signs of disease (Schaecher et al., 2002). These previously reported experiments were designed to show that calpain activity was inhibited throughout the development of EAE. However, treating animals at the onset of disease is more clinically relevant for developing therapies for MS patients. Thus, we designed current set of experiments in which EAE animals were treated with calpeptin (250 µg/kg) on days 1 to 10 post-EAE induction (before onset) or on days 7 to 10 (at onset) that corresponded to the period when EAE rats showed the first clinical signs of paralysis. In order to determine if calpeptin therapy reduced EAE over the course of disease or delayed peak disease until after day 10 (when the first set of rats were sacrificed), all animals were monitored for clinical signs of disease until day 15 (Fig. 1). While calpeptin therapy did not significantly alter the onset of disease symptoms, the treatment before onset and the more clinically relevant treatment at onset significantly reduced clinical scores of disease and therapy at onset was as effective as therapy before onset.
Calpeptin Therapy attenuated Immune Cell Infiltration and Perivascular Cuffing in EAE
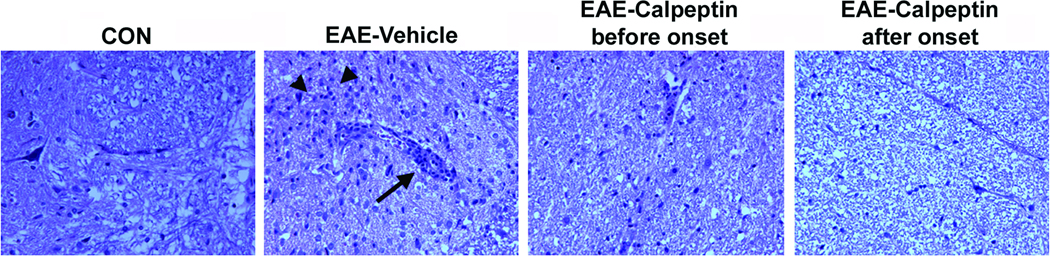
Perivascular cuffing of immune cells in the spinal cord of EAE animals is a common inflammatory event that occurs at the onset of EAE (Shields et al., 1998). Treating EAE rats with calpeptin before the onset of EAE is thought to block T cell activation and migration, but whether or not treating at the onset (day 7) of disease would reduce immune cell infiltration and perivascular cuffing is unknown. In order to address his question, a second set of EAE animals were treated twice daily with calpeptin (250 µg/kg) before the onset of disease (days 1 – 9) and at onset of disease (days 7 – 9), as described above; however, animals were sacrificed at the peak of disease (day 10) for collection of spinal cord tissues and examination of immune cell infiltration via H&E staining (Fig. 2). Tissues from EAE animals treated with vehicle alone demonstrated marked increase in perivascular cuffing of immune cells and increase in infiltration of these cells into the surrounding tissue. Animals treated with calpeptin before onset and at onset, in contrast, demonstrated fewer immune cells in the spinal cord tissue and less perivascular cuffing. These data suggest that treating EAE animals as late as the day of onset can reduce clinical signs of disease, in part by reducing infiltration of immune cells.
Fig. 2.
Calpeptin attenuated perivascular cuffing and immune cell infiltration in acute EAE. Lewis rats with acute EAE were treated twice-daily ip with calpeptin (sacrificed on day 10 for evaluation of histopathology via H&E staining). Arrows indicate perivascular cuffing and arrowheads indicate immune cell infiltration into the spinal cord (n = 3 for CON group and n = 8 per EAE group).
Calpeptin Therapy Attenuated Calpain:Calpastatin Ratio and Calpain Activity in EAE
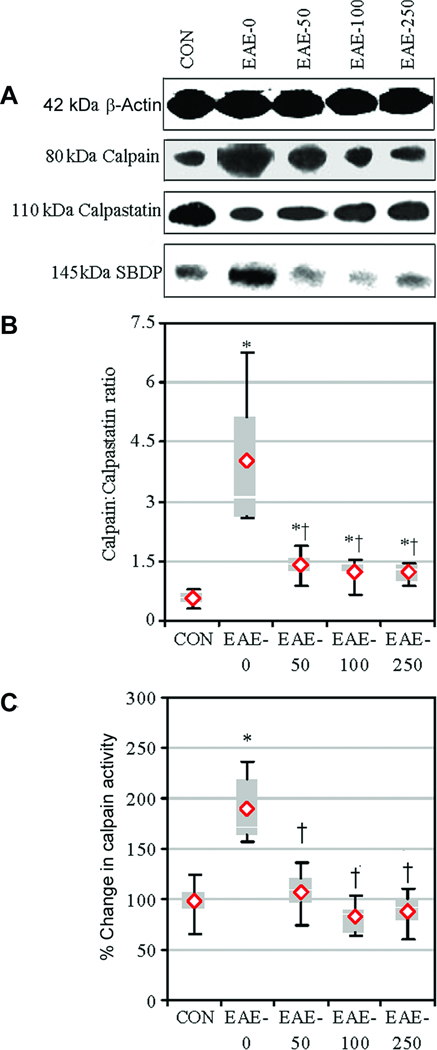
The effects of calpeptin therapy on calpain:calpastatin ratio and calpain activity were determined via Western blot analyses of spinal cord tissue from CON and EAE animals that were treated with vehicle or calpeptin (50 – 250 µg/kg) and sacrificed on day 9 post-EAE induction (Fig. 3). The calpain:calpastatin ratio was significantly increased in EAE-0 animals, compared with CON animals (Fig. 3A, B). In contrast, calpain:calpastatin ratios were significantly reduced in all treatment groups, when compared with EAE-0 group. In line with these findings, calpain activity, as measured in formation of calpain-specific 145-kDa SBDP, was significantly increased in spinal cord from vehicle treated EAE-0 group, compared with CON group (Fig. 3A, C). Calpeptin therapy significantly decreased calpain activity to levels similar to those seen in CON animals in three calpeptin groups.
Fig. 3.
Calpeptin attenuated calpain expression and activity in acute EAE. Expression of calpain and calpastatin and activity of calpain were examined in tissues via Western blotting. (A) Representative blots showed calpain and calpastatin expression. Calpain activity was assessed using antibody against spectrin, which recognized the calpain-cleaved 145-kDa SBDP. (B) The mean calpain:calpastatin ratio compared with CON and (C) calpain activity as % change compared with CON-0 set at 100%. Results were presented as box-plot of inter-quartile data with the white line denoting the median expression. Diamonds represented mean protein expression (n = 4–5 per group, * P ≤ 0.05 compared with CON, and † P ≤ 0.05 for EAE-0 versus EAE-calpeptin).
Calpeptin Therapy Attenuated Gliosis in EAE
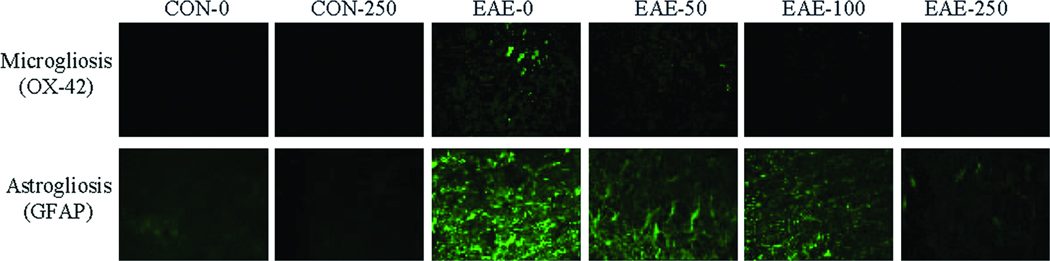
In order to examine the effects of calpeptin therapy on gliosis during acute EAE, spinal cord sections were stained with antibody against OX-42 (CD11b) and GFAP, which identified microgliosis and astrogliosis, respectively (Fig. 4). Treatment of EAE animals with vehicle alone exhibited notatble increases in microgliosis and astrogliosis, compared with CON animals treated with either vehicle or calpeptin (250 µg/kg). In contrast, stainings for OX-42 and GFAP in spinal cord tissues from Lewis rats with acute EAE were reduced in the EAE-100 and EAE-250 groups.
Fig. 4.
Calpeptin attenuated inflammatory events in acute EAE. Representative images of the spinal cord tissue collected from CON and acute EAE Lewis rats that were treated with vehicle or calpeptin (50 – 250 µg/kg) and stained for evaluation of microgliosis and astrogliosis using the antibodies against OX-42 and GFAP, respectively (n = 4 to 5), at magnification 200×.
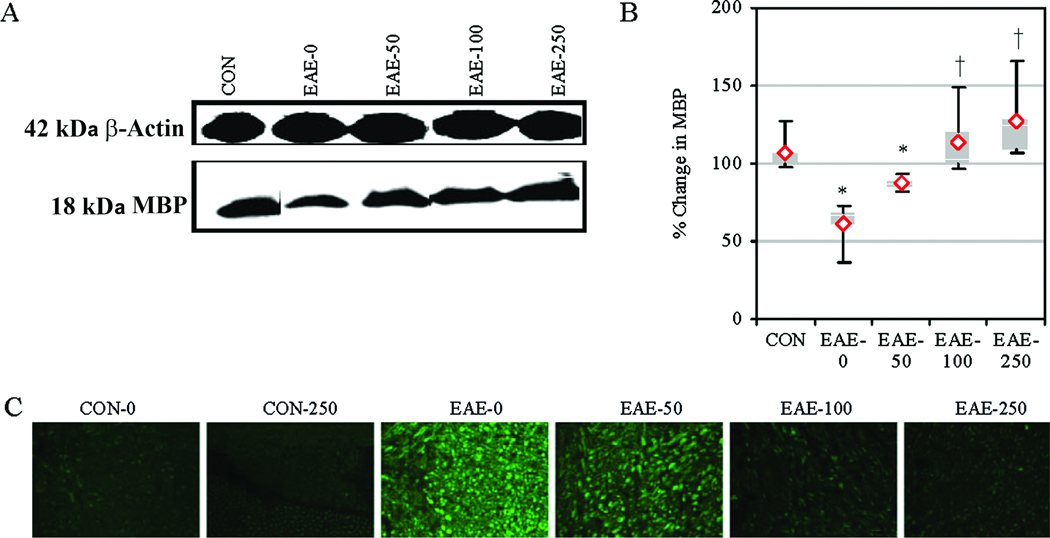
Calpeptin Therapy Attenuated MBP Degradation and Axonal Damage in EAE
In order to examine the effects of calpain inhibition on MBP degradation, we performed Western bloting using spinal cord homogenates from EAE animals after treatment with calpeptin (Fig. 5). Compared with CON-0 group, MBP expression was decreased in EAE-0 animals (Fig. 5A, B). While MBP degradation was not significantly altered in EAE-50 animals, the degradation was significantly decreased in the EAE-100 and EAE-250 groups, compared with the EAE-0 group. The effect of calpain inhibition on axonal damage was assessed in increase in de-NFP since NFP was reported to be dephosphorylated due to axonal damage (Trapp et al., 1998). The spinal cord tissue sections after staining with antibody that detected de-NFP (SMI-311) revealed an increase in de-NFP in EAE-0 group, compared with CON group (Fig. 5A, C). In contrast, stainings for de-NFP in the EAE-100 and EAE-250 groups were similar to those seen in CON group.
Fig. 5.
Calpeptin attenuated MBP degradation and axonal degeneration in acute EAE. (A) Representative blots and (B) average scanning densitometry presented as a % change in MBP compared with CON-0 set at 100%. Results were presented as box-plot of inter-quartile data with the white line denoting the median expression. Diamonds represented mean protein expression (n = 4 to 5 per group, * P ≤ 0.05 compared with CON, and † P ≤ 0.05 for EAE-0 versus EAE-calpeptin). (C) Representative images of axonal damage that was assessed by staining with SMI-311 antibody to detect de-NFP in the spinal cord tissues from CON and acute EAE Lewis rats after vehicle or calpeptin (50 – 250 µg/kg) therapy (n = 2 for CON groups, n = 4 to 5 for EAE groups, and magnification 200×).
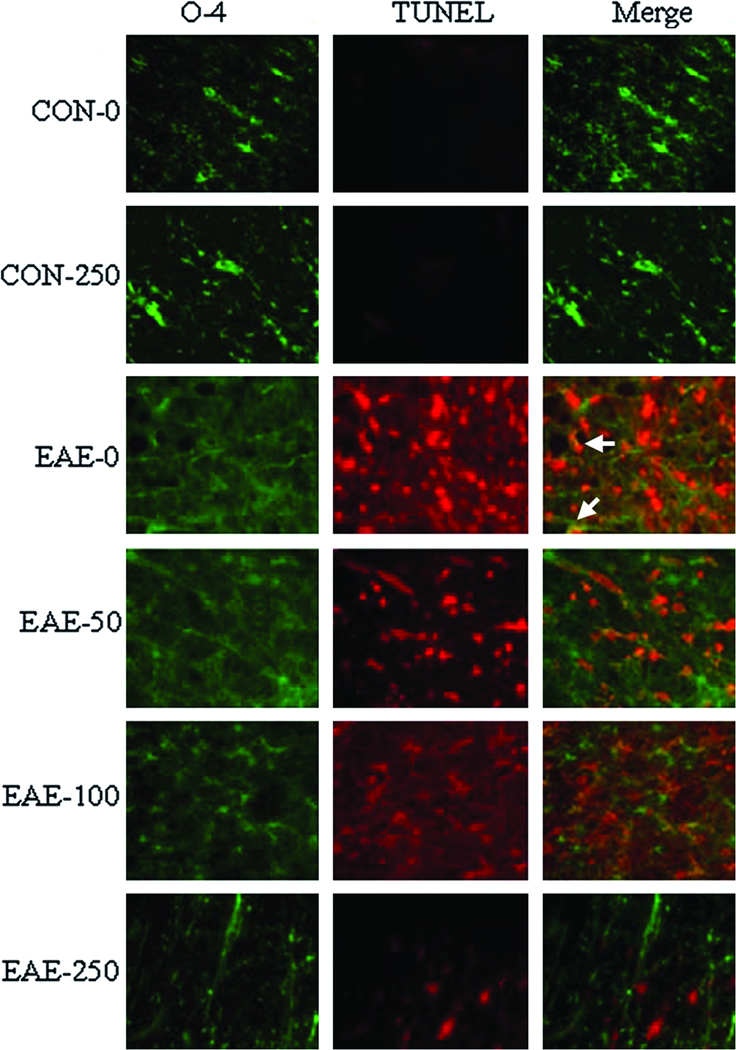
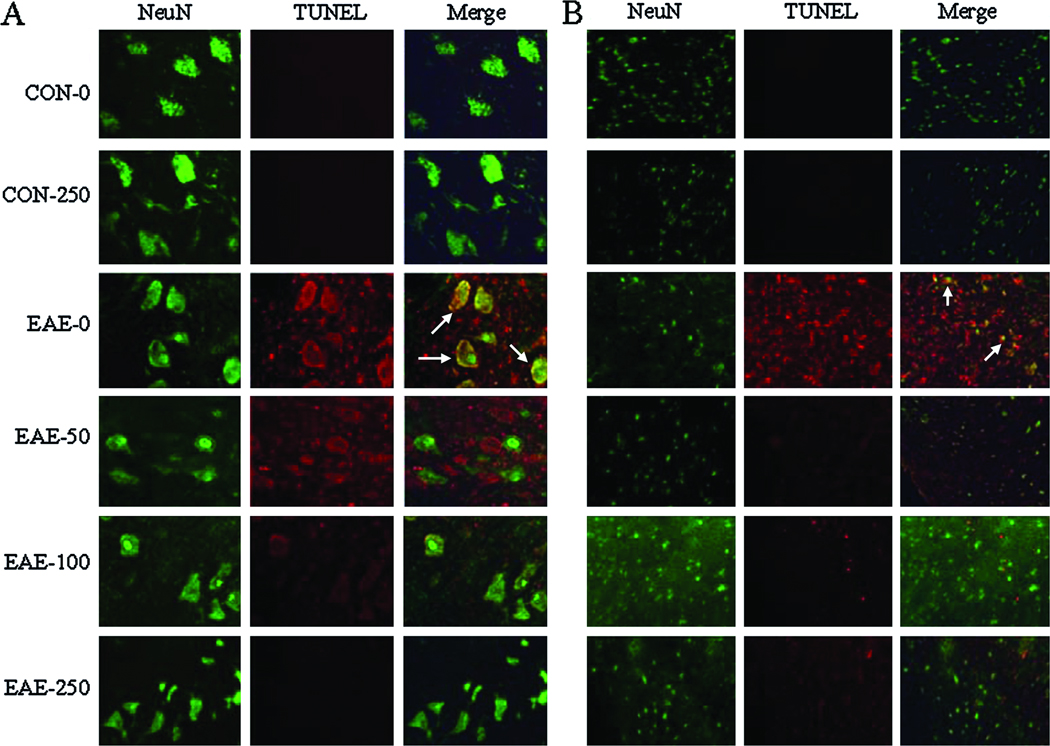
Calpeptin Therapy Attenuated Oligodendrocyte Death and Neuron Death in EAE
In order to further characterize the effects of calpain inhibition on neurodegenerative events during acute EAE, oligodendrocyte and neuronal death were also examined using the antibodies against O-4 and NeuN, which identified mature oligodendrocytes and neurons, respectively (Fig. 6). Hardly any oligodendrocytes stained TUNEL+ in spinal cord tissues from CON animals. However, an increase in TUNEL+ cells was observed in white matter of EAE spinal cord tissues from vehicle-treated animals, with many of these cells co-staining for the O-4 epitope. Treatment with calpeptin at high dose attenuated oligodendrocyte death in EAE animals. Not many TUNEL+ cells were detected in spinal cord grey matter from CON animals (Fig. 7). However, TUNEL staining was markedly increased in both dorsal sensory neurons (Fig. 7A) and ventral motoneurons (Fig. 7B) of EAE-0 group, as detected by morphological features specific for these neurons. While neuronal death was not altered in grey matter from EAE-50 rats; neuronal death was reduced in the EAE-100 and EAE-250 groups to levels seen in CON group.
Fig. 6.
Calpeptin attenuated oligodendrocyte death in acute EAE. Representative images of oligodendrocyte death that was assessed in the spinal cord tissues from CON and EAE Lewis rats after vehicle or calpeptin (50 – 250 µg/kg) therapy (n = 2 for CON groups, n = 4 to 5 for EAE groups, and magnification 200×). Staining with O-4 antibody (green) detected mature oligodendrocytes and TUNEL assay (red) detected nicked DNA. Arrows indicated co-staining.
Fig. 7.
Calpeptin attenuated neuronal death in acute EAE. Death of neurons was assessed by staining with NeuN antibody (green) and TUNEL assay (red) in (A) vental horn and (B) dorsal horn of the spinal cord tissues from CON and acute EAE Lewis rats after vehicle or (50 – 250 µg/kg) therapy (n = 2 for CON groups, n = 4 to 5 for EAE groups, and magnification 200×). Arrows indicated co-staining.
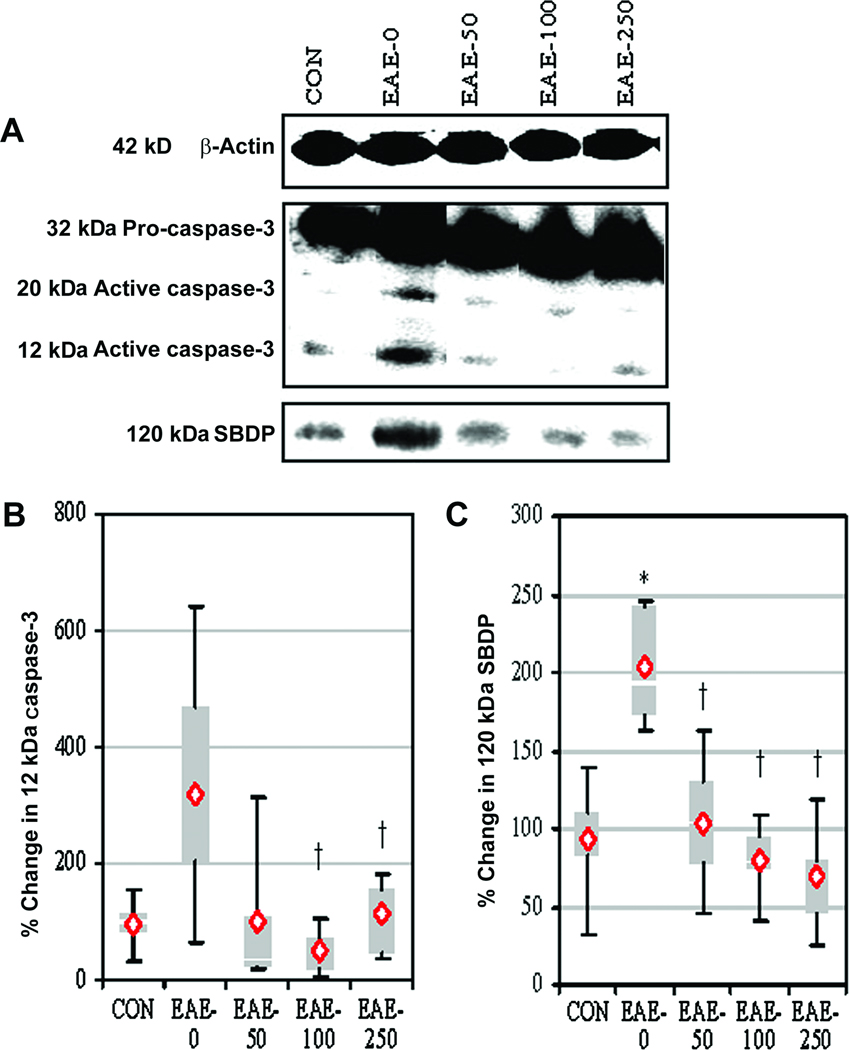
Calpeptin Therapy Decreased Pro-apoptotic Proteins in Acute EAE
The effects of calpain inhibition on proteins involved in receptor-mediated (e.g., caspase-8, tBid) pathway (Supp. Info. Fig. 1) and mitochondrial-mediated (e.g., increased Bax:Bcl-2 ratio) pathway (Supp. Info. Fig. 2) of apoptosis were examined in spinal cord tissues from EAE animals via Western blotting. Caspase-8 activity was significantly increased in spinal cord tissue from EAE-0 animals, compared with CON animals (Supp. Info. Fig. 1A, B). In contrast, calpain inhibition significantly reduced caspase-8 activity after treatment with 100 or 250 µg/kg of calpeptin. The production of the truncated Bid (tBid) by caspase-8 activity was increased in EAE-0 group, compared with CON group (Supp. Info. Fig. 1A, C). Also, Bax:Bcl-2 ratio was increased in the EAE-0 group (Supp. Info. Fig. 2A, B). Calpeptin at all three doses significantly decreased the tBid production and the Bax:Bcl-2 ratio to the levels seen in CON animals.
The effects of calpain inhibition on activation and activity of caspase-3, the final executioner of apoptosis, were also assessed via Western blotting (Fig. 8). The expression of 12-kDa caspase-3 fragment, an indicator of caspase-3 activation, was significantly decreased following treatment with 100 or 250 µg/kg of calpeptin (Fig. 8A, B). Capase-3 activity, as assessed in the production of 120-kDa SBDP, was significantly increased in spinal cord tissues from EAE-0 animals, compared with CON animals (Fig. 8A, C). Calpeptin at all three doses significantly attenuated the production of 120-kDa SBDP in EAE animals to the levels seen in CON animals.
Fig. 8.
Calpeptin decreased caspase-3 activation and activity in EAE. (A) Representative blot and (B) average scanning densitometry values of (B) 12-kDa active caspase-3 and (C) caspase-3 activity as determined in the formation of caspase-3-cleaved 120-kDa SBDP. Results were presented as box-plot of inter-quartile data with the white line denoting the median expression. Diamonds represented mean protein expression (n = 4 to 5 per group, * P ≤ 0.05 compared with CON, and † P ≤ 0.05 for EAE-0 versus EAE-calpeptin).
DISCUSSION
The current study demonstrated that multiple damaging events were reduced in EAE animals after treatment with the calpain inhibitor calpeptin. Inflammatory events including immune cell infiltration, perivascular cuffing, and microgliosis were reduced. Neurodegenerative events such as astrogliosis, MBP degradation, axonal damage, and neuronal, and oligodendrocyte death were also reduced by calpeptin. Further, calpain:calpastatin ratio, calpain activity, and apoptotic events were decreased in EAE animals following calpain inhibition, indicating that the loss of oligodendrocytes and neurons occurred through multiple cell death pathways linked to calpain activity. Another significant finding of the current study was that treating therapeutically at the onset of EAE induction was as effective at reducing disease severity as treating prophylatically, beginning on day 1 post-EAE induction, at least partially by reducing the number of immune cells infiltration into the spinal cord through leaky vessels. Current MS therapies are immunomodulary and must be given prophylactically to reduce relapse rates primarily by altering activity of autorective T cells. However, reducing neurodegeneration after the onset of a clinical episode is a major goal in developing novel MS therapies. These findings are particularly important because they demonstrate that calpain inhibitor can reduce disability at the onset of EAE.
Targeting calpain as a novel therapy offers great potential in reducing disease severity; however, current calpain inhibitors are limited in that many are peptide-based, which means bioavailability is short and calpain inhibitors tend to be insoluble in aqueous solutions. Calpain inhibitors can also weakly inhibit other proteases, including cathepsins. Calpeptin, a reversible, cell permeable, peptide aldehyde inhibitor of calpain (Tsujinaka et al., 1988). Calpeptin dose range was chosen in the present investigation based on our previous report demonstrating that it entered into the CNS to prevent neurodegeneration (Ray et al., 1999). Calpastatin is the endogenous inhibitor of calpain and upon Ca2+ influx and calpain activation, calpastatin is degraded by calpain (Blomgren et al., 1999); thus an increase in calpain:calpastatin ratio may suggest an increase in calpain activity. The exact mechanism by which calpain expression is increased in CNS disorders is unknown; however, studies demonstrate that calpain mRNA level is not altered indicating a plausible mechanism involving post-ranscriptional regulation (Shields and Banik, 1998). In order to verify that calpeptin inhibited calpain activity in spinal cord tissues from EAE animals, calpain activity was measured indirectly in the production of the calpain-specific 145-kDa SBDP. Calpeptin reduced the production of the 145-kDa SBDP in EAE animals to the levels similar to those seen in CON animals.
In the CNS disorders, microglia and astrocytes become reactive that can be protective or detrimental depending on the local environment (Carson and Sutcliffe, 1999). Previous studies reported that microgliosis and astrogliosis were increased in EAE (Guyton et al., 2005), along with increase in calpain expression. In the current study, we found that calpeptin reduced microgliosis and astrogliosis in spinal cord tissues from acute EAE animals. Further support for a role for calpain in activation of glial cells in the CNS following an insult has been documented in animal models of SCI in which decreases in microgliosis and astrogliosis correlated with diminished calpain expression following therapies with compounds known to inhibit calpain activity, either directly with calpain inhibitors (Ray et al., 2001; Hung et al., 2005) or indirectly with immunomodulatory therapies (Chvatal et al., 2008; Samantaray et al., 2008). However, other reports suggested that microglial activation was not reduced even though both calpain and caspase-3 activities were diminished in a neonatal brain ischemia model (Dingman et al., 2006). These discrepancies whether due to the type of CNS injury, therapy used, or the age of the animal remain unclear, but protease activity may not be as important for microglial activity in neonates following an insult (Derugin et al., 2000). Whether or not calpeptin inhibited microgliosis and/or astrogliosis directly or indirectly in these studies is unknown. Calpeptin is known to block T cell activation (Schaecher et al., 2001a) and migration of immune cells (Butler et al., 2009), which may indirectly reduce the exposure of glial cells to cytokines or other mediators in the CNS. However, in vitro studies demonstrating Ca2+ influx in activated microglia and astrocytes suggest that calpain activation may be increased during activation of glial cells (Abbracchio and Verderio, 2006; Farber and Kettenmann, 2006). Thus, calpeptin could potentially inhibit glial cell reactivity by directly blocking calpain in these cells.
Axonal damage is a key determinant in neurological disability in MS patients (Trapp et al., 1998; Bjartmar et al. 2000). Calpain is known to degrade myelin and other cytoskeletal proteins, including NFP (Schaecher et al., 2001b). Studies in post-mortem tissue from MS patients have indicated that calpain could be co-localized with damaged axons (Diaz-Sanchez et al., 2006) and that the Ca2+-buffering protein parvalbumin was downregulated in lesion areas (Clements et al., 2008), further supporting a significant role for calpain in axonal degeneration. In vitro studies have indicated that proteases secreted from the activated PBMCs cleave myelin into antigenic fragments and degradation was partially blocked by the endogenous calpain-specific inhibitor calpastatin (Deshpande et al., 1995). Subsequent studies where purified MBP was incubated with purified calpain confirmed that calpain cleaved MBP and this degradation was inhibited by calpeptin (Banik et al., 1997). Thus, therapies that reduce calpain activity may attenuate the destruction of myelin directly by reducing cleavage of axonal proteins and MBP.
Loss of oligodendrocytes has been well-documented in MS (Dhib-Jalbut, 2007) and EAE (Schaecher et al., 2001b) and is thought to contribute to disability. Oligodendrocyte loss was reduced following calpeptin therapy, indicating that calpain activity correlated well with oligodendrocyte death. This correlation is supported by previous research that showed that the lifespan of oligodendrocytes was much shorter in female rodents and expression and activity of μ-calpain was greater, compared with male rodents (Cerghet et al., 2006). Interestingly, only the highest dose of calpeptin reduced oligodendrocyte death in this study, even though all other neurodegenerative events were prevented with calpeptin at lower doses. These discrepancies may be due to differential expression of calpain in each cell type or other factors (such as cytokines, other proteases, and reactive oxygen species) that regulate apoptosis and damage oligodendrocytes to a larger extent than neurons.
Previously, we demonstrated for the first time that calpain expression was increased in spinal cord neurons in EAE animals (Guyton et al., 2005). Calpain inhibition can protect many neuronal cell types in vitro (Das et al., 2005; Ray et al., 2006). In the current study, calpeptin therapy reduced the loss of both sensory and motoneurons in spinal cord in EAE animals. These findings are important since patients with MS suffer from both sensory and motor deficits and therapies that protect multiple neuronal cell types can be advantageous in treating heterogeneous neurological diseases such as MS (Tekok-Kilic et al., 2006).
Since calpain is known to alter the activation of many proteins involved in regulation of apoptotic events, blocking calpain may reduce cell death. Calpain inhibitor reduced cell death by attenuating the expression and/or activity of multiple proteins involved in apoptosis. However, the reduction in cell death in spinal cord of EAE animals following calpain inhibition may also be partially due to prevention of necrosis since calpain is known to cause necrosis by day 11 post-EAE induction, as DNA fragmentation changes from an apoptotic profile to one of necrosis (Das et al., 2008). Caspase-8 is a death receptor-mediated initiator that is upstream of calpain in certain apoptotic pathways (Frisch, 2008). While very little has been documented on the role of caspase-8 in death following EAE induction, we have recently shown that caspase-8 activity is upregulated in spinal cord of EAE rats (Das et al., 2008). Caspase-8 cleaves Bid to tBid, which translocates to the mitochondria for involvment in mitochondrial pathway of apoptosis by activating the pro-apoptotic protein Bax that binds to the anti-apoptotic protein Bcl-2 under physiological conditions (Cao et al., 2003). In line with the complexity of apoptotic pathways, calpain can also directly activate Bax by cleaving Bcl-2 (Wood et al., 1998). In the present study, Bax:Bcl-2 ratio was reduced in spinal cord of EAE animals following calpeptin therapy. These findings suggest that the increases in tBid production and Bax:Bcl-2 ratios are at least partially due to calpain activity and thus, calpain inhibition can attenuate mitochondrial pathway of apoptosis to protect neurons and oligodendrocytes.
Activity of caspase-3, the final executioner of apoptosis, is also increased in EAE (Das et al., 2008). In the current study, activity of caspase-3 was significantly decreased following calpeptin therapy. Caspase-3 and calpastatin are substrates of calpain; however, calpastatin is a suicide substrate that is degraded by calpain (Blomgren et al., 1999) while caspase-3 is activated by calapin (McGinnis et al., 1999). Caspase-3 can also cleave calpastatin into inactive fragments (Wang et al., 1998), which may also contribute to the reduction in calpain:calpastatin ratio in EAE animals that did not receive calpeptin therapy. Even the lowest doses of calpeptin reduced calpain:calpastatin ratio and calpain activity, but higher doses were required to reduce other damaging events in EAE. Thus, other culprits could also be involved in the pathogenesis of EAE.
A major concern of blocking pro-apoptotic signaling proteins involved in neuronal and oligodendrocyte cell death is that the same proteins may also be blocked in autoreactive T cells. In fact, calpeptin has previously been shown to block apoptosis in the Jurkat T cell line (Diaz and Bourguignon, 2000). However, this cell line was derived from a lymphoma patient, and calpain activity may preferentially induce apoptosis signaling pathways in cancer cells, compared with normal immune cells. A recent report from our laboratory indicated that treating MBP-specific T cells with calpain inhibitor before adoptive transfer of EAE into naïve mice resulted in a significant decrease in clinical signs of disease; however, this decrease was not due to death of these T cells since viability of T cells incubated with calpain inhibitor were similar to viability of T cells that were incubated with vehicle alone before adoptive transfer (Guyton et al., 2009). In line with these findings, studies involving LL-37, a compound known to induce chemotaxis of CD4 T cells and other immune cells, also induced apoptosis in Jurkat T cells (Mader et al., 2009).
In conclusion, the present study demonstrates that calpain is a promising target for treating the inflammatory and neurodegenerative events associated with disability in EAE and MS. In the current investigation, EAE rats were daily treated with the calpain inhibitor calpeptin, which would target the inflammatory arm of EAE by reducing calpain activity in immune cells in the periphery to potentially block T cell activity and immune cell migration and/or by preventing neurodegeneration in the spinal cord when clinical signs of paralysis first appear. Whether or not calpeptin reduced gliosis, axonal damage, myelin degradation, and cell death directly in the CNS or indirectly by inhibiting immune cells before they can enter the spinal cord and induce damage is unknown. Studies to address these issues, either by blocking calpain in encephalogenic T cells before adoptive transfer or by treating EAE animals after onset of clinical signs of disease (indicating neurodegeneration), will help dissect which attenuating events following calpain inhibition are direct or indirect.
Supplementary Material
Acknowledgements
The assistance of Denise Matzelle in the preparation of the manuscript is gratefully acknowledged.
Contract Grant sponsor: NIH; Contract grant numbers: NS-38146, NS-41088, NS-56176, NS-57811, CA-91460, and C06RR015455.
Footnotes
Disclosures
The authors have no financial conflicts of interest.
REFERENCES
- Abbracchio MP, Verderio C. Pathophysiological roles of P2 receptors in glial cells. Novartis Foundation symposium. 2006;276:91–103. [PubMed] [Google Scholar]
- Banik NL, Chou CH, Deibler GE, Krutzch HC, Hogan EL. Peptide bond specificity of calpain: proteolysis of human myelin basic protein. J Neurosci Res. 1994;37:489–496. doi: 10.1002/jnr.490370408. [DOI] [PubMed] [Google Scholar]
- Banik NL, Matzelle D, Terry E, Hogan EL. A new mechanism of methylprednisolone and other corticosteroids action demonstrated in vitro: inhibition of a proteinase (calpain) prevents myelin and cytoskeletal protein degradation. Brain Res. 1997;748:205–210. doi: 10.1016/s0006-8993(96)01302-9. [DOI] [PubMed] [Google Scholar]
- Banik NL, Shields DC. A putative role for calpain in demyelination associated with optic neuritis. Histol Histopathol. 1999;14:649–656. doi: 10.14670/HH-14.649. [DOI] [PubMed] [Google Scholar]
- Bjartmar C, Kidd G, Mork S, Rudick R, Trapp BD. Neurological disability correlates with spinal cord axonal loss and reduced N-acetyl aspartate in chronic multiple sclerosis patients. Ann Neurol. 2000;48:893–901. [PubMed] [Google Scholar]
- Blomgren K, Hallin U, Andersson AL, Puka-Sundvall M, Bahr BA, McRae A, Saido TC, Kawashima S, Hagberg H. Calpastatin is up-regulated in response to hypoxia and is a suicide substrate to calpain after neonatal cerebral hypoxia-ischemia. J Biol Chem. 1999;274:14046–14052. doi: 10.1074/jbc.274.20.14046. [DOI] [PubMed] [Google Scholar]
- Butler JT, Samantaray S, Beeson CC, Ray SK, Banik NL. The involvement of calpain in the process of Jurkat T cell chemotaxis. J Neurosci Res. 2009;87:626–635. doi: 10.1002/jnr.21882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Deng X, May WS. Cleavage of Bax to p18 Bax accelerates stress-induced apoptosis, and a cathepsin-like protease may rapidly degrade p18 Bax. Blood. 2003;102:2605–2614. doi: 10.1182/blood-2003-01-0211. [DOI] [PubMed] [Google Scholar]
- Carson MJ, Sutcliffe JG. Balancing function vs. self defense: the CNS as an active regulator of immune responses. J Neurosci Res. 1999;55:1–8. doi: 10.1002/(SICI)1097-4547(19990101)55:1<1::AID-JNR1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Cerghet M, Skoff RP, Bessert D, Zhang Z, Mullins C, Ghandour MS. Proliferation and death of oligodendrocytes and myelin proteins are differentially regulated in male and female rodents. J Neurosci. 2006;26:1439–1447. doi: 10.1523/JNEUROSCI.2219-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chvatal SA, Kim YT, Bratt-Leal AM, Lee H, Bellamkonda RV. Spatial distribution and acute anti-inflammatory effects of Methylprednisolone after sustained local delivery to the contused spinal cord. Biomaterials. 2008;29:1967–1975. doi: 10.1016/j.biomaterials.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements RJ, McDonough J, Freeman EJ. Distribution of parvalbumin and calretinin immunoreactive interneurons in motor cortex from multiple sclerosis post-mortem tissue. Exp Brain Res. 2008;187:459–465. doi: 10.1007/s00221-008-1317-9. [DOI] [PubMed] [Google Scholar]
- Das A, Guyton MK, Matzelle DD, Ray SK, Banik NL. Time-dependent increases in protease activities for neuronal apoptosis in spinal cords of Lewis rats during development of acute experimental autoimmune encephalomyelitis. J Neurosci Res. 2008;86:2002–3001. doi: 10.1002/jnr.21737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Sribnick EA, Wingrave JM, Del Re AM, Woodward JJ, Appel SH, Banik NL, Ray SK. Calpain activation in apoptosis of ventral spinal cord 4.1 (VSC4.1) motoneurons exposed to glutamate: calpain inhibition provides functional neuroprotection. J Neurosci Res. 2005;81:551–562. doi: 10.1002/jnr.20581. [DOI] [PubMed] [Google Scholar]
- Derugin N, Wendland M, Muramatsu K, Roberts TP, Gregory G, Ferriero DM, Vexler ZS. Evolution of brain injury after transient middle cerebral artery occlusion in neonatal rats. Stroke. 2000;31:1752–1761. doi: 10.1161/01.str.31.7.1752. [DOI] [PubMed] [Google Scholar]
- Deshpande RV, Goust JM, Hogan EL, Banik NL. Calpain secreted by activated human lymphoid cells degrades myelin. J Neurosci Res. 1995;42:259–265. doi: 10.1002/jnr.490420214. [DOI] [PubMed] [Google Scholar]
- Dhib-Jalbut S. Pathogenesis of myelin/oligodendrocyte damage in multiple sclerosis. Neurology. 2007;68:S13–S21. doi: 10.1212/01.wnl.0000275228.13012.7b. [DOI] [PubMed] [Google Scholar]
- Diaz F, Bourguignon LY. Selective down-regulation of IP3 receptor subtypes by caspases and calpain during TNF-α-induced apoptosis of human T-lymphoma cells. Cell Calcium. 2000;27:315–328. doi: 10.1054/ceca.2000.0126. [DOI] [PubMed] [Google Scholar]
- Diaz-Sanchez M, Williams K, DeLuca GC, Esiri MM. Protein co-expression with axonal injury in multiple sclerosis plaques. Acta Neuropathol. 2006;111:289–299. doi: 10.1007/s00401-006-0045-0. [DOI] [PubMed] [Google Scholar]
- Dingman A, Lee SY, Derugin N, Wendland MF, Vexler ZS. Aminoguanidine inhibits caspase-3 and calpain activation without affecting microglial activation following neonatal transient cerebral ischemia. J Neurochem. 2006;96:1467–1479. doi: 10.1111/j.1471-4159.2006.03672.x. [DOI] [PubMed] [Google Scholar]
- Farber K, Kettenmann H. Functional role of calcium signals for microglial function. Glia. 2006;54:656–665. doi: 10.1002/glia.20412. [DOI] [PubMed] [Google Scholar]
- Fleming KK, Bovaird JA, Mosier MC, Emerson MR, LeVine SM, Marquis JG. Statistical analysis of data from studies on experimental autoimmune encephalomyelitis. J Neuroimmunol. 2005;170:71–84. doi: 10.1016/j.jneuroim.2005.08.020. [DOI] [PubMed] [Google Scholar]
- Frisch SM. Caspase-8: fly or die. Cancer Res. 2008;68:4491–4493. doi: 10.1158/0008-5472.CAN-08-0952. [DOI] [PubMed] [Google Scholar]
- Govindarajan KR, Rauch HC, Clausen J, Einstein ER. Changes in cathepsins B-1 and D, neutral proteinase and 2',3'-cyclic nucleotide-3'-phosphohydrolase activities in monkey brain with experimental allergic encephalomyelitis. J Neurol Sci. 1974;23:295–306. doi: 10.1016/0022-510x(74)90232-9. [DOI] [PubMed] [Google Scholar]
- Guyton MK, Brahmachari S, Das A, Samantaray S, Inoue J, Azuma M, Ray SK, Banik NL. Inhibition of calpain attenuates encephalitogenicity of MBP-specific T cells. J Neurochem. 2009;110:1895–1907. doi: 10.1111/j.1471-4159.2009.06287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyton MK, Das A, Matzelle DD, Samantaray S, Azuma M, Inoue J, Ray SK, Banik NL. In: SJA6017 Attenuates Immune Cell Infiltration and Neurodegeneration in EAE. Tabira T, Yamamura T, Kira J, editors. Nagoya, Japan: Medimond; 2006. Oct 15–19, pp. 107–112. [Google Scholar]
- Guyton MK, Wingrave JM, Yallapragada AV, Wilford GG, Sribnick EA, Matzelle DD, Tyor WR, Ray SK, Banik NL. Upregulation of calpain correlates with increased neurodegeneration in acute experimental auto-immune encephalomyelitis. J Neurosci Res. 2005;81:53–61. doi: 10.1002/jnr.20470. [DOI] [PubMed] [Google Scholar]
- Hassen GW, Feliberti J, Kesner L, Stracher A, Mokhtarian F. A novel calpain inhibitor for the treatment of acute experimental autoimmune encephalomyelitis. J Neuroimmunol. 2006;180:135–146. doi: 10.1016/j.jneuroim.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Higuchi M, Tomioka M, Takano J, Shirotani K, Iwata N, Masumoto H, Maki M, Itohara S, Saido TC. Distinct mechanistic roles of calpain and caspase activation in neurodegeneration as revealed in mice overexpressing their specific inhibitors. J Biol Chem. 2005;280:15229–15237. doi: 10.1074/jbc.M500939200. [DOI] [PubMed] [Google Scholar]
- Hung KS, Hwang SL, Liang CL, Chen YJ, Lee TH, Liu JK, Howng SL, Wang CH. Calpain inhibitor inhibits p35-p25-Cdk5 activation, decreases tau hyperphosphorylation, and improves neurological function after spinal cord hemisection in rats. J Neuropathol Exp Neurol. 2005;64:15–26. doi: 10.1093/jnen/64.1.15. [DOI] [PubMed] [Google Scholar]
- Imam SA, Guyton MK, Haque A, Vandenbark A, Tyor WR, Ray SK, Banik NL. Increased calpain correlates with Th1 cytokine profile in PBMCs from MS patients. J Neuroimmunol. 2007;190:139–145. doi: 10.1016/j.jneuroim.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keegan BM, Noseworthy JH. Multiple sclerosis. Annu Rev Med. 2002;53:285–302. doi: 10.1146/annurev.med.53.082901.103909. [DOI] [PubMed] [Google Scholar]
- Mader JS, Mookherjee N, Hancock RE, Bleackley RC. The human host defense peptide LL-37 induces apoptosis in a calpain- and apoptosis-inducing factor-dependent manner involving Bax activity. Mol Cancer Res. 2009;7:689–702. doi: 10.1158/1541-7786.MCR-08-0274. [DOI] [PubMed] [Google Scholar]
- Marks N, Benuck M, Hashim G. Hydrolysis of myelin basic protein with brain acid proteinase. Biochem Biophys Res Commun. 1974;56:68–74. doi: 10.1016/s0006-291x(74)80316-5. [DOI] [PubMed] [Google Scholar]
- McGinnis KM, Gnegy ME, Park YH, Mukherjee N, Wang KK. Procaspase-3 and poly(ADP)ribose polymerase (PARP) are calpain substrates. Biochem Biophys Res Commun. 1999;263:94–99. doi: 10.1006/bbrc.1999.1315. [DOI] [PubMed] [Google Scholar]
- Noonan CW, Kathman SJ, White MC. Prevalence estimates for MS in the United States and evidence of an increasing trend for women. Neurology. 2002;58:136–138. doi: 10.1212/wnl.58.1.136. [DOI] [PubMed] [Google Scholar]
- Ray SK, Banik NL. Calpain and its involvement in the pathophysiology of CNS injuries and diseases: therapeutic potential of calpain inhibitors for prevention of neurodegeneration. Curr Drug Targets CNS Neurol Disord. 2003;2:173–189. doi: 10.2174/1568007033482887. [DOI] [PubMed] [Google Scholar]
- Ray SK, Karmakar S, Nowak MW, Banik NL. Inhibition of calpain and caspase-3 prevented apoptosis and preserved electrophysiological properties of voltage-gated and ligand-gated ion channels in rat primary cortical neurons exposed to glutamate. Neuroscience. 2006;139:577–595. doi: 10.1016/j.neuroscience.2005.12.057. [DOI] [PubMed] [Google Scholar]
- Ray SK, Matzelle DD, Wilford GG, Hogan EL, Banik NL. Inhibition of calpain-mediated apoptosis by E-64 d-reduced immediate early gene (IEG) expression and reactive astrogliosis in the lesion and penumbra following spinal cord injury in rats. Brain Res. 2001;916:115–126. doi: 10.1016/s0006-8993(01)02874-8. [DOI] [PubMed] [Google Scholar]
- Ray SK, Schaecher KE, Shields DC, Hogan EL, Banik NL. Combined TUNEL and double immunofluorescent labeling for detection of apoptotic mononuclear phagocytes in autoimmune demyelinating disease. Brain Res Protoc. 2000;5:305–311. doi: 10.1016/s1385-299x(00)00027-1. [DOI] [PubMed] [Google Scholar]
- Ray SK, Wilford GG, Matzelle DC, Hogan EL, Banik NL. Calpeptin and methylprednisolone inhibit apoptosis in rat spinal cord injury. Ann N Y Acad Sci. 1999;890:261–269. doi: 10.1111/j.1749-6632.1999.tb08001.x. [DOI] [PubMed] [Google Scholar]
- Samantaray S, Sribnick EA, Das A, Knaryan VH, Matzelle DD, Yallapragada AV, Reiter RJ, Ray SK, Banik NL. Melatonin attenuates calpain upregulation, axonal damage and neuronal death in spinal cord injury in rats. J Pineal Res. 2008;44:348–357. doi: 10.1111/j.1600-079X.2007.00534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaecher K, Rocchini A, Dinkins J, Matzelle DD, Banik NL. Calpain expression and infiltration of activated T cells in experimental allergic encephalomyelitis over time: increased calpain activity begins with onset of disease. J Neuroimmunol. 2002;129:1–9. doi: 10.1016/s0165-5728(02)00142-x. [DOI] [PubMed] [Google Scholar]
- Schaecher KE, Goust JM, Banik NL. The effects of calpain inhibition upon IL-2 and CD25 expression in human peripheral blood mononuclear cells. J Neuroimmunol. 2001a;119:333–342. doi: 10.1016/s0165-5728(01)00367-8. [DOI] [PubMed] [Google Scholar]
- Schaecher KE, Shields DC, Banik NL. Mechanism of myelin breakdown in experimental demyelination: a putative role for calpain. Neurochem Res. 2001b;26:731–737. doi: 10.1023/a:1010903823668. [DOI] [PubMed] [Google Scholar]
- Shields DC, Banik NL. Upregulation of calpain activity and expression in experimental allergic encephalomyelitis: a putative role for calpain in demyelination. Brain Res. 1998;794:68–74. doi: 10.1016/s0006-8993(98)00193-0. [DOI] [PubMed] [Google Scholar]
- Shields DC, Banik NL. Pathophysiological role of calpain in experimental demyelination. J Neurosci Res. 1999;55:533–541. doi: 10.1002/(SICI)1097-4547(19990301)55:5<533::AID-JNR1>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Shields DC, Schaecher KE, Goust JM, Banik NL. Calpain activity and expression are increased in splenic inflammatory cells associated with experimental allergic encephalomyelitis. J Neuroimmunol. 1999a;99:1–12. doi: 10.1016/s0165-5728(99)00043-0. [DOI] [PubMed] [Google Scholar]
- Shields DC, Schaecher KE, Saido TC, Banik NL. A putative mechanism of demyelination in multiple sclerosis by a proteolytic enzyme, calpain. Proc Natl Acad Sci U S A. 1999b;96:11486–11491. doi: 10.1073/pnas.96.20.11486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields DC, Tyor WR, Deibler GE, Hogan EL, Banik NL. Increased calpain expression in activated glial and inflammatory cells in experimental allergic encephalomyelitis. Proc Natl Acad Sci U S A. 1998;95:5768–5772. doi: 10.1073/pnas.95.10.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soede RD, Driessens MH, Ruuls-Van Stalle L, Van Hulten PE, Brink A, Roos E. LFA-1 to LFA-1 signals involve zeta-associated protein-70 (ZAP-70) tyrosine kinase: relevance for invasion and migration of a T cell hybridoma. J Immunol. 1999;163:4253–4261. [PubMed] [Google Scholar]
- Tekok-Kilic A, Benedict RH, Zivadinov R. Update on the relationships between neuropsychological dysfunction and structural MRI in multiple sclerosis. Expert Rev Neurother. 2006;6:323–331. doi: 10.1586/14737175.6.3.323. [DOI] [PubMed] [Google Scholar]
- Trapp BD, Nave KA. Multiple sclerosis: an immune or neurodegenerative disorder? Annu Rev Neurosci. 2008;31:247–269. doi: 10.1146/annurev.neuro.30.051606.094313. [DOI] [PubMed] [Google Scholar]
- Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mork S, Bo L. Axonal transection in the lesions of multiple sclerosis. N Engl J Med. 1998;338:278–285. doi: 10.1056/NEJM199801293380502. [DOI] [PubMed] [Google Scholar]
- Tsujinaka T, Kajiwara Y, Kambayashi J, Sakon M, Higuchi N, Tanaka T, Mori T. Synthesis of a new cell penetrating calpain inhibitor (calpeptin) Biochem Biophys Res Commun. 1988;153:1201–1208. doi: 10.1016/s0006-291x(88)81355-x. [DOI] [PubMed] [Google Scholar]
- Wang KK, Posmantur R, Nadimpalli R, Nath R, Mohan P, Nixon RA, Talanian RV, Keegan M, Herzog L, Allen H. Caspase-mediated fragmentation of calpain inhibitor protein calpastatin during apoptosis. Arch Biochem Biophys. 1998;356:187–196. doi: 10.1006/abbi.1998.0748. [DOI] [PubMed] [Google Scholar]
- Wood DE, Thomas A, Devi LA, Berman Y, Beavis RC, Reed JC, Newcomb EW. Bax cleavage is mediated by calpain during drug-induced apoptosis. Oncogene. 1998;17:1069–1078. doi: 10.1038/sj.onc.1202034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.