Abstract
Background
There are no drugs that specifically target the social deficits of autism spectrum disorders (ASD). This may be due to a lack of behavioral paradigms in animal models relevant to ASD. Partner preference formation in the prairie vole represents a social cognitive process involving socially reinforced learning. D-cycloserine (DCS) is a cognitive enhancer that acts at the N-methyl-D-aspartate receptor to promote learning. If DCS enhances socially reinforced learning in the partner preference paradigm, it may be useful in combination with behavioral therapies for enhancing social functioning in ASD.
Methods
Female prairie and meadow voles were given DCS either peripherally or directly into one of three brain regions: nucleus accumbens, amygdala, or caudate putamen. Subjects were then cohabited with a male vole under conditions that do not typically yield a partner preference. The development of a preference for that stimulus male vole over a novel male vole was assessed using a partner preference test.
Results
A low dose of DCS administered peripherally enhanced preference formation in prairie voles but not meadow voles under conditions in which it would not otherwise occur. These effects were replicated in prairie voles by microinfusions of DCS into the nucleus accumbens, which is involved in reinforcement learning, and the amygdala, which is involved in social information processing.
Conclusions
Partner preference in the prairie vole may provide a behavioral paradigm with face, construct, and predictive validity for identifying prosocial pharmacotherapeutics. D-cycloserine may be a viable treatment strategy for social deficits of ASD when paired with social behavioral therapy.
Keywords: Animal model, glutamate, NMDA receptor, prairie vole, social cognition, social impairment
Despite the growing public health concern over autism spectrum disorders (ASD), there have been few advances in the development of pharmacotherapeutic treatment options for these neurodevelopmental disorders. Most existing pharmacotherapies for individuals with ASD are simply relabeled drugs commonly used for the treatment of other neuropsychiatric disorders, which, in ASD, target only peripheral comorbid symptoms rather than key features like social impairment (1). Consequently, there is a significant need for the use of animal models and behavioral paradigms relevant to ASD to gain understanding of the fundamental neurobiology of the core endophenotypes of ASD so that informed pharmacotherapies based on biology can be developed. Given the heterogeneous nature of ASD, targeting individual endophenotypes may be a more viable approach for drug development than targeting the global etiology. For this reason, we have focused on behavioral paradigms that may be useful in screening drugs that enhance social cognitive function in animal models, with the presumption that similar pharmacotherapeutic approaches may enhance social cognition in patients with ASD.
Cognitive enhancers such as D-cycloserine (DCS) have gained considerable attention in recent years for their potential in facilitating selective cognitive processes in the treatment of psychiatric disorders such as phobias, social anxiety, obsessive-compulsive disorder, and posttraumatic stress disorder (2–5). D-cycloserine is a partial agonist of the N-methyl-D-aspartate (NMDA) glutamate receptor that binds to the glycine site of the NMDA receptor, enhancing receptor activation only in the presence of glutamate (6). The NMDA receptor plays a pivotal role in long-term plasticity, the neuronal correlate of memory (7). D-cycloserine enhances many different forms of learning and memory (8–12), which suggests the drug may also be effective in improving social memory and cognition.
Currently, there are two rodent behavioral paradigms that are particularly well suited to the investigation of the neurobiological mechanisms underlying social cognition and for screening compounds that may enhance social cognition: social recognition in the mouse (Mus musculus) and partner preference formation in the socially monogamous prairie vole (Microtus ochrogaster) (13,14).
Social recognition paradigms in mice have revealed an important role for the amygdala (Amyg) in social information processing. Mice discriminate novel from familiar mice using olfactory cues and habituate to a familiar mouse following repeated exposures (15). Mice genetically deficient in oxytocin (OT) fail to habituate to a conspecific after repeated exposure and therefore fail to discriminate familiar from novel conspecifics (16). Silencing OT receptor expression or infusion of OT receptor antagonist into the Amyg disrupts social recognition in wild-type mice, while microinjections of OT directly into the medial Amyg rescues social recognition (17,18). D-serine, a compound related to DCS, increases social recognition in rats at high doses (19). Correspondingly, antagonists of the NMDA receptor prevent the expression of social memory (20).
Social bonding in the monogamous prairie vole, which is assessed in the laboratory using a partner preference paradigm, is a higher order and multidimensional social cognitive process that involves functional circuits for social recognition, social reward and reinforcement, and associative social learning (21). In this paradigm, the social learning phase (e.g., the initial cohabitation) can be manipulated pharmacologically to either accelerate or inhibit the formation of the social bond, consequently enabling the identification of compounds and neural circuits that affect social learning. In female prairie voles, OT and dopamine interact in the nucleus accumbens (NAcc) to promote partner preference formation (22–24). Direct infusion of OT into the brain during the social learning phase accelerates partner preference formation in the absence of mating (25). Infusion of an OT antagonist or a D2 dopamine antagonist directly into the NAcc prevents mating-induced partner preference formation (23). An alternative animal model relevant to ASD is the meadow vole (Microtus pennsylvanicus), which, despite being closely related to the prairie vole, is relatively asocial and does not typically display partner preferences, in part because of a lack of OT receptors in the NAcc (26).
Although a role for glutamate in partner preference formation has not yet been demonstrated, we hypothesize that DCS, acting in the Amyg and NAcc, will facilitate social learning during the initial cohabitation, accelerating partner preference formation. G-protein coupled receptors, like dopamine and OT receptors, can potentiate the action of NMDA receptors in the encoding of long-term behavioral changes (27). The effect of DCS on the enhancement of social learning should therefore be most profound in brain areas that mediate OT-dependent social functions, like the Amyg and NAcc. It is likely, though, that DCS will not facilitate partner preferences in meadow voles because they lack some of the neural substrates essential for socially reinforced learning of olfactory cues.
We propose that DCS will accelerate bonding in female prairie voles by enhancing socially reinforced learning through modulation of NMDA receptors in socially relevant brain regions. If our assertion is correct and DCS accelerates partner preference formation during the learning phase of the paradigm, then DCS may be a useful adjunct to behavioral based therapies currently used to enhance social function in ASD.
Methods and Materials
Subjects
Subjects were adult (60–120 days of age), sexually naive female prairie voles or meadow voles, and stimulus male voles were adult (90–180 days of age), sexually experienced prairie voles or sexually naive meadow voles. All prairie voles were generated from an in-house breeding colony originally derived from wild-caught populations in Illinois (prairie voles) or Pennsylvania (meadow voles). After weaning at 21 days of age, subjects were housed in same sex pairs or trios with water and Purina rabbit chow provided ad libitum. All experiments were done in accordance to the Institutional Animal Care and Use Committee at Emory University.
Peripheral Effects of DCS on Partner Preference in Prairie Voles
Adult, gonadally intact female prairie voles were injected intraperitoneally with either saline or D-cycloserine dissolved in saline (Sigma-Aldrich, St. Louis, Missouri; C6880; 0 mg/kg n = 6; 10 mg/kg n = 7; or 20 mg/kg n = 7). The doses used were based on the functional doses of DCS for appetitive learning in other rodent models (28). Immediately after the injection, female voles were placed into the cage of a novel, sexually experienced stimulus male vole for a 6-hour cohabitation period. As female prairie voles are induced ovulators, the female voles were nonreceptive and should not have mated. Following the cohabitation period, the subjects were tested for partner preference. Partner preference was tested in a three-cage apparatus. The familiar partner male vole and the novel stranger male vole were tethered, one in each of the two end cages. The female vole was then placed in the center nonsocial cage and allowed to freely move through the three cages. The amount of time the female vole spent in social proximity with each male vole was recorded using the VoleTracker (Wang Laboratory, Florida State University) beam-break infrared monitoring system (29). The infrared beams were placed just beyond the reach of each tethered animal, such that a beam break indicated that the female vole entered the social proximity zone in which social contact was possible. The amount of time the female vole spent in the social proximity zone for each stimulus animal was used as a measure of time spent with the partner and the stranger for the determination of a partner preference.
Central Effects of DCS on Partner Preference in Prairie Voles
In an effort to further specify the area of action of DCS, we made some refinements in the partner preference paradigm. All experimental prairie vole female subjects were ovariectomized to ensure nonreceptivity throughout the testing. The pairs were video recorded during the social learning period to verify that they did not mate. The method of quantifying social interaction between the female and male voles was also refined through a move to automated computerized scoring, using SocialScan 2.0 (CleverSys Incorporated, Reston, Virginia) (30). The validity of the automated computerized scoring was previously assessed. SocialScan 2.0 correlated highly with manual scoring of partner preference (R = .904) (30).
Adult, ovariectomized female prairie voles were bilaterally cannulated into the nucleus accumbens, the amygdala, or the caudate-putamen (CP) using stereotaxic methods. Ovariectomy was performed at approximately 60 days and animals were allowed to recover for 14 days before beginning the study. Subjects were anesthetized using isoflurane and 26-gauge bilateral guide cannulae (Plastics One, Roanoke, Virginia) aimed at the NAcc (anterior 1.7 mm, bilateral ±1 mm, ventral −3.5 mm to bregma), Amyg (anterior −1.3 mm, bilateral ±2.7 mm, ventral −6.1 mm), or CP (anterior 1.7 mm, bilateral ±1 mm, ventral −2.5 mm to bregma) were implanted. Location of the cannulae was verified postexperimentally in Nissl-stained brain sections. The coordinates used for the Amyg group targeted specifically the medial amygdala; however, any cannulae that hit within the amygdala were included in the analysis. After 2 to 3 days of recovery, subjects received microinjections with a 33-gauge internal cannula (Plastics One) that extended 1 mm below the guide cannula into the target area. The needle was connected to a Hamilton syringe (Hamilton, Reno, Nevada) via polyethylene-20 tubing (Plastics One), through which the solution was injected slowly over the course of 1 minute. The internal needle was left in place for 2 minutes after the injection to prevent backflow.
The effect of DCS on partner preference was tested in each brain location independently. Animals received either a bilateral control injection of Ringer's solution or 10 μg of DCS dissolved in 500 nL of Ringer's solution (Fisher Scientific, Pittsburg, Pennsylvania) per side into the NAcc (n = 11–12/treatment), Amyg (n = 11/treatment), or CP (n = 12/treatment). The 10 μg dose is based on the effective dose needed for intra-amygdalar infusion in studies examining fear learning in rats (31). Immediately after the injection, the female voles were placed into the cage of a novel sexually experienced male vole for a 6-hour cohabitation period. Subjects were video recorded during the cohabitation to ensure no mating occurred. Mating was not observed in any of these animals. Following the cohabitation period, the subjects were tested for partner preference (Figure 1).
Figure 1.
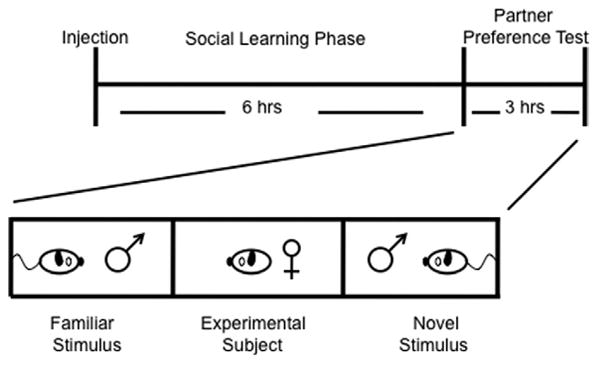
The partner preference paradigm. Drug manipulation occurs immediately before animal pairing. This is followed by the social learning phase in which the experimental female animal is allowed to freely interact with a stimulus male animal for 6 hours during which the animals do not mate. This level of social interaction is typically insufficient to induce a social bond (bonding usually requires 24 hours of cohabitation with mating). This subthreshold paradigm allows for the testing of drugs that accelerate social bonding. The formation of a social bond can be assayed in the laboratory using the partner preference test. In the test phase of the paradigm, the male animal with which the female animal was cohabited is tethered to one end of a three-chambered arena and a novel male animal of equal stimulus value is tethered to the opposite end of the arena. The female animal is placed in the center of the arena and allowed to freely wander for 3 hours. The amount of time the female animal spends in social proximity or huddling with either male animal is recorded.
Partner preference testing was conducted in a three-chamber arena in which a novel male stranger was tethered at one end and the familiar partner male vole was tethered at the other. The amount of time the female vole spent in side-by-side immobile social contact, huddling, with either male vole was measured using SocialScan 2.0. Huddling time is more reflective of partner preference than time in social proximity, as it precludes nonsocial or agonistic behavior in the social proximity zone. Distance traveled by the stimulus animal was also recorded as a measure of general levels of activity to control for possible locomotor effects of the drug.
Peripheral Effects of DCS on Partner Preference in Meadow Voles
Adult, gonadally intact female meadow voles were injected intraperitoneally with either saline or DCS dissolved in saline (0 mg/kg n = 11; 10 mg/kg n = 11). For 2 days before drug injection, the female voles were primed with estradiol benzoate (Sigma-Aldrich BP958; 2 μg/day) dissolved in .1 mL sesame oil via subcutaneous injection. On the day of the experiment, the female voles were injected with an additional dose of estradiol benzoate at the same time as the drug injection. Immediately after the injection, the female voles were placed into the cage of a novel, stimulus male vole for a 24-hour cohabitation period. Mating during the cohabitation period was promoted by hormonal priming to induce sexual receptivity. Receptivity was desired in the meadow vole experiment, as opposed to the prairie vole experiments, to increase the likelihood of detecting an enhancement of partner preference in this asocial species. Following the cohabitation period, the subjects were tested for partner preference as described for the prairie vole. To assess potential effects of DCS on sociability, we divided the testing arena into three zones (partner zone, stranger zone, nonsocial zone), and the amount of time the female vole spent in each zone was measured using SocialScan 2.0. The partner or stranger zone was defined as the area in which the tethered stimulus could engage in social contact with the experimental animal and the nonsocial zone was defined as the area between the other two zones.
Data Analysis of Partner Preference
Time spent in social proximity (peripheral injection experiments) or immobile social contact (central injection experiment) with the partner male vole was compared with that spent with the stranger male vole for each experiment using a two-way analysis of variance (ANOVA) in which stimulus (partner, stranger, or nonsocial) and treatment (control or DCS) were factors. In addition, Student t tests were used to compare time spent with the partner and stranger within each treatment condition. Bonferroni corrections for the level of significance of the Student t tests were made for each experiment to correct for multiple comparisons. For the meadow vole experiment, a Bonferroni post hoc comparison was used to compare time spent in each zone. Significantly more time spent with the partner than the stranger constituted a partner preference (29).
Results
Peripheral DCS Administration in Prairie Voles
We predicted that DCS would accelerate social bonding in prairie voles, by inducing partner preference under conditions in which it would typically not form. A comparison using a two-way ANOVA revealed a significant main effect of stimulus animal [F(1,19) = 11.359, p = .002]; no other significant main effects or interactions were found. To determine which treatments resulted in significantly more time spent with the partner than the stranger, Student t tests were performed with Bonferroni corrections of the p value. Peripheral administration of DCS at a low dose of 10 mg/kg (p < .001; Student t test, Bonferroni level set at p < .01; Figure 2), but not a high dose of 20 mg/kg (p = .359; Student t test, Bonferroni level set at p < .01), resulted in the female voles spending significantly more time with the familiar partner than the novel stranger on a group level. Animals receiving saline did not spend more time with the partner than the stranger (p = .419; Student t test, Bonferroni level set at p < .01).
Figure 2.
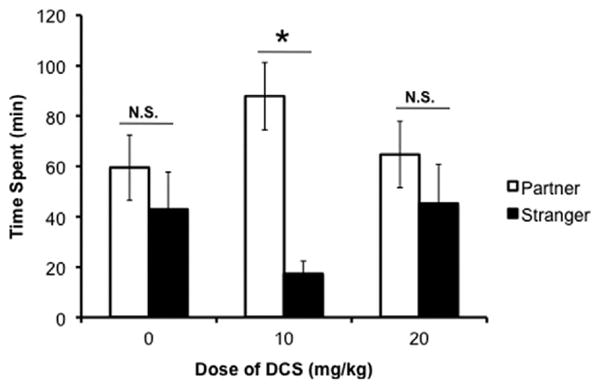
Effect of peripheral D-cycloserine (DCS) administration on partner preference formation in prairie voles. Ten milligrams/kilogram of DCS facilitated partner preference formation, as measured by time in social proximity, in female prairie voles after an abbreviated cohabitation with a male vole in the absence of mating (p < .001). Both the higher dose of 20 mg/kg and the control injection failed to induce a partner preference (20 mg/kg p = .359; saline p = .419). Time spent with stimulus animals was compared using a Student t test with a Bonferroni correction for multiple comparisons. *p < .01. N.S., nonsignificant.
Central DCS Administration in Prairie Voles
D-cycloserine was injected site-specifically into two brain regions known to be involved in social learning and reinforcement learning, the NAcc and Amyg. The CP served as an anatomical control site to control for drug diffusion. The effect of DCS microinjection on time spent with partner and stranger was compared using a two-way ANOVA that revealed a significant main effect of stimulus animal in both brain areas [NAcc F(1,22) = 15.923, p < .0001; Amyg F(1,21) = 8.959, p = .005]; no other significant main effects or interactions were found for this comparison. To determine the cause of the effect of stimulus animal, time spent in immobile social contact with the partner and the stranger was directly compared in each of the brain regions. Injection of 10 μg of DCS into the NAcc and Amyg resulted in significantly more time spent with the partner than the stranger (NAcc p = .002; Amyg p = .003; Student t test, Bonferroni level set at p < .01; Figure 3A,B). Animals injected with saline into these areas did not display a partner preference, though there was a trend toward more time spent with the partner when saline was injected into the NAcc (p = .04, failed to meet the corrected Bonferroni level of p < .01). Injection of the same dose of DCS into the CP, however, failed to induce partner preference under these conditions; no significant interactions were seen in a two-way ANOVA comparing stimulus animal and treatment. Direct comparison of time spent with partner and the stranger also failed to show a significant difference (CP p = .287; Student t test, Bonferroni level set at p < .01; Figure 3C). There were no significant differences in locomotion between control and drug injections in any of the three anatomical areas (NAcc p = .04; Amyg p = .544, CP p = .460; Figure 3D,E,F). The location of the canulae for each site was verified post-experimentally for inclusion in results (Figure 3G,H,I).
Figure 3.
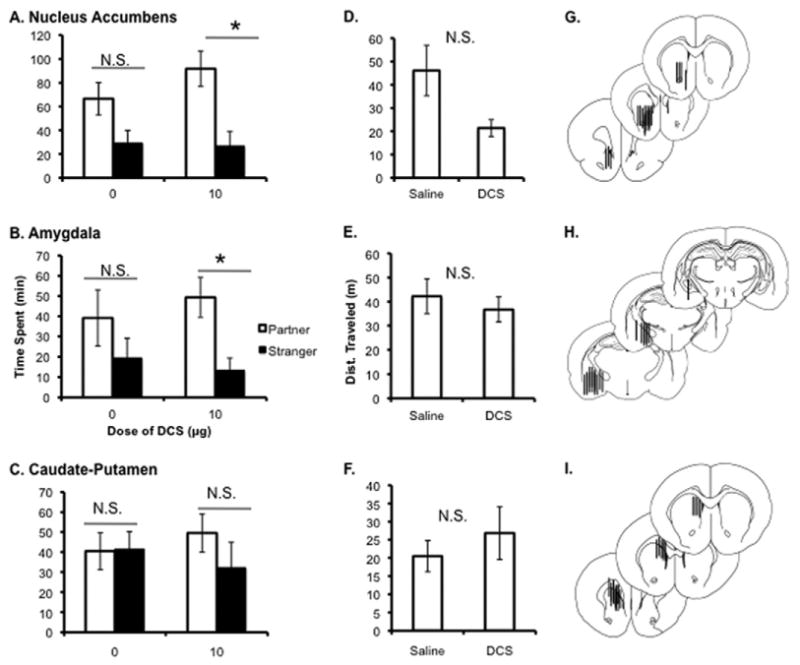
Effect of central D-cycloserine (DCS) administration of partner preference in prairie voles. Injection of 10 μg of DCS bilaterally into both the nucleus accumbens (NAcc) (A) and amygdala (Amyg) (B) accelerated partner preference formation as indicated by significantly more time spent huddling with partner than the stranger (NAcc p = .002; Amyg p = .003; Student t test, Bonferroni level set at p < .01). The same injection into the caudate-putamen (C) failed to induce a preference (p = .287). There were no differences in total locomotion induced by DCS administration in any site tested ([D] NAcc p = .04; [E] Amyg p = .544; [F] caudate-putamen p = .460; Student t test, Bonferroni level set at p < .01). Location of the cannulae placement for each site verified post-experimentally are shown (G,H,I) (reprinted from [59], with permission from Elsevier, copyright 1998). *p < .01. Dist., distance; N.S., nonsignificant.
Peripheral DCS Administration in Meadow Voles
Peripheral DCS had no effect on partner preference or time spent in close social proximity to either stimulus animal in female meadow voles. A comparison using a two-way ANOVA showed a significant main effect of zone [partner, stranger, or nonsocial zone; F(2,33) = 1.100, p = .301; Figure 4]. A post hoc Bonferroni test for multiple comparisons revealed significantly more time spent in the nonsocial zone than time spent in social proximity with either the partner (p < .0001) or the stranger (p < .0001) but no difference in time spent in social proximity of either the partner or the stranger (p = .982). There were no significant main effects of drug [F(1,22) = .312, p = .579]. Meadow voles spent, on average, 70% of the test in the nonsocial zone, while prairie voles, in comparison, only spent, on average, 23% of the test in the nonsocial zone, which highlights the profound species differences in social behavior.
Figure 4.
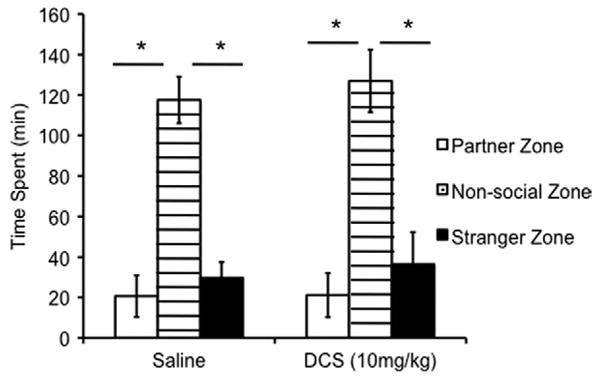
Effect of peripheral DCS administration on partner preference formation in meadow voles. DCS had no effect on partner preference formation in the female meadow vole. Meadow voles, regardless of treatment, spent significantly more time in the nonsocial zone than in social proximity of either the partner (nonsocial vs. partner; p < .0001) or stranger (nonsocial vs. stranger; p < .0001). Time spent in social proximity of either stimulus animal or the nonsocial zone was compared using a two-way analysis of variance followed by a Bonferroni post hoc test for multiple comparisons. *p < .0001. DCS, D-cycloserine.
Discussion
D-cycloserine Facilitates Partner Preference Formation in Prairie Voles
We demonstrated for the first time that when administered immediately before a social cohabitation, DCS enhances the social cognitive processes involved in the development of a partner preference in female prairie voles. The effects were dose-dependent, as the low dose of DCS (10 mg/kg) accelerated partner preference formation, while the higher dose (20 mg/kg) did not. This dose effect may be due to the mixed agonist/antagonist properties of the drug. D-cycloserine binds to the strychnine insensitive glycine site of the NMDA receptor. At low doses, the drug increases occupancy of this binding site, thereby increasing glutamate neurotransmission. However, at high doses, DCS outcompetes the endogenous ligand at the site but only yields 40% to 50% of the maximal receptor activation achieved with glycine saturation (6).
To better understand the neural processes through which peripheral DCS may be modulating to facilitate partner preference formation, we infused DCS into two candidate brain sites likely to be involved in social bond formation, the Amyg and the NAcc. These sites were chosen because of their known role in OT-mediated social learning in mice and prairie voles and our hypothesis that the OT and glutamate systems interact to facilitate social learning processes at the level of the synapse. Partner preference formation in prairie voles likely involves at least two distinct processes: 1) processing the olfactory signature of the partner to form a social memory (e.g., social recognition) (32), and 2) linking that recognition memory to the reinforcing nature of the social interaction (22). In mice, the first process involves OT acting in the Amyg (18), and in prairie voles, the second process involves OT acting in the NAcc (23). We hypothesized that infusion of DCS into either of these regions, but not into the CP (which contains OT receptors but where OT does not promote partner preferences), would promote the bond formation despite an abbreviated social learning period. Our hypotheses were supported by our findings in this study; DCS infused into the Amyg or NAcc, but not the CP, accelerated partner preference formation.
These findings suggest the glutamate system, along with the OT and dopamine systems, converge in the NAcc and other social relevant brain regions to facilitate the cellular and behavioral changes associated with bonding in female prairie voles, adding to our basic understanding of the underlying neurobiological events mediating social bonding.
Partner Preference as a Drug Discovery Paradigm for Social Cognition Enhancers
The partner preference paradigm in prairie voles has face, construct, and predictive validity as a potential drug screen for compounds that accentuate the beneficial effects of behavioral therapies aimed at reducing social deficits. Regarding face validity, one may consider the social learning phase, in which the prairie vole pair cohabitates and interacts, to be analogous to the acquisition of social information either in a spontaneous social interaction or in the context of a social behavioral therapy session in humans. In both situations, socially relevant information must be identified and encoded, so that it can be retrieved and utilized appropriately in future social encounters. Regarding the construct validity, converging lines of evidence suggest there is a considerable amount of evolutionary conservation in the systems that regulate social behavior and social cognition from rodent to man (33). Elucidation of the neurochemical systems that mediate social bonding in the prairie vole, including OT, dopamine, and as we have demonstrated here, glutamate, has provided direction for identification of the neural systems underlying human social behavior and those that may be compromised in disorders of social behavior. Genetic and epigenetic modifications of the oxytocin, glutamate, and dopamine systems have all been linked with ASD (34–41). Oxytocin has also been implicated in several recent studies in a number of human social cognitive processes (42–44), including socially reinforced learning (45).
Finally, there is preliminary evidence for the predictive validity of the partner preference test. Three independent studies have now reported some improvement in social cognition in ASD subjects following intranasal OT administration (46–48). In addition, a preliminary study has shown that DCS decreases social withdrawal in individuals with ASD as measured by the Aberrant Behavior Checklist (49). Therefore, we propose that the partner preference paradigm in prairie voles may have widespread potential in identifying multiple classes of drugs for the treatment of social impairments based on its face, construct, and predictive validity.
Selection of Animal Model
Based on the demographics and impairments associated with ASD, one might argue that male meadow voles are most suited to identifying drugs to treat the social impairments of the disorder. However, it should be noted the goal of the present study was to use a behavioral paradigm that was not a model of autism but a predictive model for screening prosocial drugs. Female prairie voles were used in this paradigm because partner preference in female voles is well established as an OT-dependent process (23,25), and because OT enhances social cognitive processes in humans, we felt that partner preference formation in female voles should have better predictive validity for screening drugs to enhance social cognition than partner preference formation in male voles, which has not been shown to be dependent on OT.
Based on our results, prairie voles appear to be better suited than meadow voles for use in the partner preference paradigm to identify drugs that enhance social cognition. This is likely because meadow voles lack some of the neural substrates essential for socially reinforced learning of olfactory cues that are involved in partner preference formation, like OT receptors in the NAcc (50). However, their brain is not dysfunctional, but rather evolutionarily adapted for an asocial, sexually promiscuous mating strategy. The brain differences between typical and ASD individuals, though, are likely far more subtle than the species differences between meadow and prairie voles. Evidence suggests many ASD subjects benefit from social skills therapies (51), thus demonstrating the presence of some of the neural mechanisms necessary for the acquisition of social information and their viability as a target for enhancement by potential pharmacological therapies.
Pharmacological Adjuncts to Behavioral Therapies
The limited effects of compounds identified as prosocial therapeutics by the partner preference paradigm may be enhanced by combination with social behavioral therapies. D-cycloserine has been successfully used in the psychiatric setting for the treatment of several disorders (3,5,52–55). The crux of the successful use of DCS in the treatment of these disorders is the combination of pharmacotherapy with behavioral therapies (53,54,56). This treatment model is reflected in the design of the partner preference paradigm, in that potential prosocial or cognitive-enhancing drugs are administered before the social learning phase, so the animals are receiving both pharmacological and behavioral stimulation simultaneously. Based on successful use of the drug in the psychiatric setting and the effect of the drug on social bonding in this study, we propose that DCS could be used in combination with applied behavioral analysis for the treatment of the social impairments associated with ASD. D-cycloserine may have a particularly profound effect on social skills training because of its potential interaction with the other neurochemical systems mediating functional social behavior. Many of the subskills taught in social skills training, like maintaining eye contact and understanding facial expressions, are modulated by OT (42,57,58). D-cycloserine could interact with the endogenous OT system activated during these processes in subjects to produce accelerated acquisition of these skills.
Conclusions
Through the findings in this study, we have identified a role for glutamate neurotransmission in the formation of social bonds in prairie voles. Enhancement of the glutamate system, through the use of the NMDA receptor agonist DCS, accelerates the acquisition of social information in the prairie voles. D-cycloserine may, therefore, be a promising candidate for the treatment of the social impairments associated with disorders of social behavior, particularly if combined with social behavioral therapy. The partner preference paradigm has face, construct, and predictive validity for the identification of drugs that enhance social cognition and should be utilized in the generation of novel pharmacotherapies for the treatment of ASD and psychiatric diseases characterized by social impairment.
Acknowledgments
This research was supported by Autism Speaks-Predoctoral Fellowship (MEM) and National Institutes of Health MH064692 (LJY) and RR00165 to Yerkes National Primate Research Center.
At Emory University, we thank Lorra Mathews for her support of the vole colony and Dr. Mike Davis for his pioneer work on D-cycloserine and his advice and guidance over the course of these experiments.
Footnotes
The authors reported no biomedical financial interests or potential conflicts of interest.
References
- 1.Bartz JA, Hollander E. Oxytocin and experimental therapeutics in autism spectrum disorders. Prog Brain Res. 2008;170:451–462. doi: 10.1016/S0079-6123(08)00435-4. [DOI] [PubMed] [Google Scholar]
- 2.Heresco-Levy U, Kremer I, Javitt D, Goichman R, Reschef A, Blanaru M, Cohen T. Pilot-controlled trail of D-cycloserine for the treatment of post-traumatic stress disorder. Int J Neuropsychopharmacol. 2002;5:301–307. doi: 10.1017/S1461145702003061. [DOI] [PubMed] [Google Scholar]
- 3.Guastella A, Richardson R, Lovibond P, Rapee R, Gaston J, Mitchell P, Dadds MR. A randomized controlled trial of D-cycloserine enhancement of exposure therapy for social anxiety disorder. Biol Psychiatry. 2008;63:544–549. doi: 10.1016/j.biopsych.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 4.Jorstad-Stein E, Heimberg R. Social phobia: An update on treatment. Psychiatr Clin North Am. 2009;32:641–663. doi: 10.1016/j.psc.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Wilhelm S, Buhlmann U, Tolin D, Meunier S, Pearlson G, Reese H, et al. Augmentation of behavior therapy with D-cycloserine for obsessive-compulsive disorder. Am J Psychiatry. 2008;165:335–341. doi: 10.1176/appi.ajp.2007.07050776. [DOI] [PubMed] [Google Scholar]
- 6.Watson G, Bolanowski M, Baganoff M, Deppeler C, Lanthorn T. D-cycloserine acts as a partial agonist at the glycine modulatory site of the NMDA receptor expressed in Xenopus oocytes. Brain Res. 1990;510:158–160. doi: 10.1016/0006-8993(90)90745-w. [DOI] [PubMed] [Google Scholar]
- 7.Kauer J, Malenka R, Nicoll R. NMDA application potentiates synaptic transmission in the hippocampus. Nature. 1988;334:250–252. doi: 10.1038/334250a0. [DOI] [PubMed] [Google Scholar]
- 8.Win-Shwe T, Kageyama S, Tsukahara S, Nakajima D, Fujimaki H. Effect of D-cyloserine on spatial learning performance and memory function-related gene expression in mice following toluene exposure. J UOEH. 2010;32:127–140. doi: 10.7888/juoeh.32.127. [DOI] [PubMed] [Google Scholar]
- 9.Davis M, Ressler K, Rothbaum B, Richardson R. Effects of D-cycloserine on extinction: Translation from preclinical to clinical work. Biol Psychiatry. 2006;60:369–375. doi: 10.1016/j.biopsych.2006.03.084. [DOI] [PubMed] [Google Scholar]
- 10.Lee J, Gardner R, Butler V, Everitt B. D-cycloserine potentiates the reconsolidation of cocaine-associated memories. Learn Mem. 2009;16:82–85. doi: 10.1101/lm.1186609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Onur O, Schlaepfer T, Kuolja J, Bauer A, Jeung H, Patin A, et al. The N-methyl-d-aspartate receptor co-agonist D-cycloserine facilitates declarative learning and hippocampal activity in humans. Biol Psychiatry. 2010;67:1205–1211. doi: 10.1016/j.biopsych.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 12.Zlomuzica A, De Souza Silva M, Huston J, Dere E. NMDA receptor modulation by D-cycloserine promotes episodic-like memory in mice. Psychopharmacology (Berl) 2007;193:503–509. doi: 10.1007/s00213-007-0816-x. [DOI] [PubMed] [Google Scholar]
- 13.Hammock EAD, Young LJ. Oxytocin, vasopressin and pair bonding: Implications for autism. Philos Trans R Soc Lond B Biol Sci. 2006;361:2187–2198. doi: 10.1098/rstb.2006.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silverman J, Yang M, Lord C, Crawley J. Behavioural phenotyping assays for mouse models of autism. Nat Rev Neurosci. 2010;11:490–502. doi: 10.1038/nrn2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Macbeth A, Edds J, Young Wr. Housing conditions and stimulus females: A robust social discrimination task for studying male rodent social recognition. Nat Protoc. 2009;4:1574–1581. doi: 10.1038/nprot.2009.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferguson J, Young L, Hearn E, Matzuk M, Insel T, Winslow J. Social amnesia in mice lacking the oxytocin gene. Nat Genet. 2000;25:284–288. doi: 10.1038/77040. [DOI] [PubMed] [Google Scholar]
- 17.Choleris E, Little S, Mong J, Puram S, Langer R, Pfaff D. Microparticle-based delivery of oxytocin receptor antisense DNA in the medial amygdala blocks social recognition in female mice. Proc Natl Acad Sci U S A. 2007;104:4670–4675. doi: 10.1073/pnas.0700670104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferguson J, Aldag J, Insel T, Young L. Oxytocin in the medial amygdala is essential for social recognition in the mouse. J Neurosci. 2001;21:8278–8285. doi: 10.1523/JNEUROSCI.21-20-08278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimazaki T, Kaku A, Chaki S. D-Serine and a glycine transporter-1 inhibitor enhance social memory in rats. Psychopharmacology (Berl) 2010;209:263–270. doi: 10.1007/s00213-010-1794-y. [DOI] [PubMed] [Google Scholar]
- 20.Hlinak Z, Krejci I. Effects of excitatory amino acid antagonists on social recognition of male rats. Behav Pharmacol. 1994;5:239–244. doi: 10.1097/00008877-199406000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Lim M, Young L. Neuropeptidergic regulation of affiliative behavior and social bonding in animals. Horm Behav. 2006;50:506–517. doi: 10.1016/j.yhbeh.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 22.Young L, Lim M, Gingrich B, Insel T. Cellular mechanisms of social attachment. Horm Behav. 2001;40:133–138. doi: 10.1006/hbeh.2001.1691. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, Wang Z. Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience. 2003;121:537–544. doi: 10.1016/s0306-4522(03)00555-4. [DOI] [PubMed] [Google Scholar]
- 24.Ross HE, Freeman SM, Speigel LL, Ren X, Terwilliger EF, Young LJ. Variation in oxytocin receptor density in the nucleus accumbens has differential effects on affiliative behavior in monogamous and polygamous voles. J Neurosci. 2009;29:1312–1318. doi: 10.1523/JNEUROSCI.5039-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams JR, Harbough CR, Carter CS. Oxytocin administered centrally facilitates formation of a partner preference in prairie voles. J Neuroendocrinol. 1994;6:247–250. doi: 10.1111/j.1365-2826.1994.tb00579.x. [DOI] [PubMed] [Google Scholar]
- 26.Young L, Wang Z. The neurobiology of pair bonding. Nat Neurosci. 2004;7:1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]
- 27.Wolf M. Addiction and glutamate-dependent plasticity. In: Herman B, editor. Glutamate and Addiction. Totowa, NJ: Humana Press; 2003. pp. 143–156. [Google Scholar]
- 28.Pussinen R, Sirvio J. Effects of D-cycloserine, a positive modulator of N-methyl-d-aspartate receptors, and ST 587, a putative alpha 1 adrenergic agonist, individually and in combination, on the non-delayed foraging behaviour of rats assessed in the radial arm maze. J Psychopharmacology. 1999;13:171–179. doi: 10.1177/026988119901300210. [DOI] [PubMed] [Google Scholar]
- 29.Aragona B, Wang Z. The prairie vole (Microtus ochrogaster): An animal model of behavioral neuroendocrinology. ILAR J. 2004;45:35–45. doi: 10.1093/ilar.45.1.35. [DOI] [PubMed] [Google Scholar]
- 30.Ahern T, Modi M, Burkett J, Young L. Evaluation of two automated metrics for analyzing partner preference tests. J Neurosci Methods. 2009;182:180–188. doi: 10.1016/j.jneumeth.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walker D, Ressler K, Lu K, Davis M. Facilitation of condition fear extinction by systemic administration of intra-amygdala infusions of D-cycloserine as assessed with fear-potential startle in rats. J Neurosci. 2002;22:2342–2351. doi: 10.1523/JNEUROSCI.22-06-02343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Curtis J, Liu Y, Wang Z. Lesions of the vomeronasal organ disrupt mating-induced pair bonding in female prairie voles (Microtus ochrogaster) Brain Res. 2001;901:167–174. doi: 10.1016/s0006-8993(01)02343-5. [DOI] [PubMed] [Google Scholar]
- 33.Donaldson Z, Young L. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322:900–904. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- 34.Liu X, Kaamura Y, Shimada T, Otowa T, Koishi S, Sugiyama T, et al. Association of oxytocin receptor (OXTR) gene polymorphism with autism spectrum (ASD) in the Japanese population. J Hum Genet. 2010;55:137–141. doi: 10.1038/jhg.2009.140. [DOI] [PubMed] [Google Scholar]
- 35.Lerer E, Levi S, Salomon S, Darvasi A, Yirmiya N, Ebstein RP. Association between the oxytocin receptor (OXTR) gene and autism: Relationship to Vineland Adaptive Behavior Scales and cognition. Mol Psychiatry. 2008;13:980–988. doi: 10.1038/sj.mp.4002087. [DOI] [PubMed] [Google Scholar]
- 36.Jacob S, Brune CW, Carter CS, Leventhal BL, Lord C, Cook EHJ. Association of the oxytocin receptor gene (OXTR) in Caucasian children and adolescents with autism. Neurosci Lett. 2007;417:6–9. doi: 10.1016/j.neulet.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Krom M, Staal W, Ophoff R, Hendriks J, Buitelaar J, Franke B, et al. A common variant in DRD3 receptor is associated with autism spectrum disorder. Biol Psychiatry. 2009;65:625–630. doi: 10.1016/j.biopsych.2008.09.035. [DOI] [PubMed] [Google Scholar]
- 38.Hettinger J, Liu X, Schwartz C, Michaelis R, Holden J. A DRD1 haplotype is associated with risk for autism spectrum disorders in male-only affected sib-pair families. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:628–636. doi: 10.1002/ajmg.b.30655. [DOI] [PubMed] [Google Scholar]
- 39.Gunter C, Warren S. Polymorphism in the FMR1 gene. Hum Genet. 1998;103:365–366. doi: 10.1007/s004390050830. [DOI] [PubMed] [Google Scholar]
- 40.Blundell J, Blaiss C, Etherton M, Espinosa F, Tabuchi K, Walz C, et al. Neuroligin-1 deletion results in impaired spatial memory and increased repetitive behavior. J Neurosci. 2010;30:2115–2129. doi: 10.1523/JNEUROSCI.4517-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gregory S, Connelly J, Towers A, Johnson J, Biscocho D, Markunas C, et al. Genomic and epigenetic evidence for oxytocin receptor deficiency in autism. BMC Med. 2009;7:62. doi: 10.1186/1741-7015-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Domes G, Heinrichs M, Michel A, Berger C, Herpertz SC. Oxytocin improves “mind-reading” in humans. Biol Psychiatry. 2007;61:731–733. doi: 10.1016/j.biopsych.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 43.Rimmele U, Hediger K, Heinrichs M, Klaver P. Oxytocin makes a face in memory familiar. J Neurosci. 2009;29:38–42. doi: 10.1523/JNEUROSCI.4260-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kosfeld M, Heinrichs M, Zak P, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- 45.Hurlemann R, Patin A, Onur O, Cohen M, Baumgartner T, Metzler S, et al. Oxytocin enhances amygdala-dependent, socially reinforced learning and emotional empathy in humans. J Neurosci. 2010;30:4999–5007. doi: 10.1523/JNEUROSCI.5538-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guastella AJ, Einfeld SL, Gray KM, Rinehart NJ, Tonge BJ, Lambert TJ, Hickie IB. Intranasal oxytocin improves emotion recognition for youth with autism spectrum disorders. Biol Psychiatry. 2010;67:692–694. doi: 10.1016/j.biopsych.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 47.Bartz J, Zaki J, Bolger N, Hollander E, Ludwig N, Kolevzon A, Ochsner KN. Oxytocin selectively improves empathic accuracy. Psychol Sci. 2010;21:1426–1428. doi: 10.1177/0956797610383439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Andari E, Duhamel J, Zalla T, Herbrecht E, Leboyer M, Sirigu A. Promoting social behavior with oxytocin in high-functioning autism spectrum disorders. Proc Natl Acad Sci U S A. 2010;107:4389–4394. doi: 10.1073/pnas.0910249107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Posey D, Kem D, Swiezy N, Sweeten T, Wiegand R, McDougle C. A pilot study of D-cycloserine in subjects with autistic disorders. Am J Psychiatry. 2004;161:2115–2117. doi: 10.1176/appi.ajp.161.11.2115. [DOI] [PubMed] [Google Scholar]
- 50.Insel T, Shapiro L. Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles. Proc Natl Acad Sci U S A. 1992;89:5981–5985. doi: 10.1073/pnas.89.13.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reichow B, Volkmar FR. Social skills interventions for individuals with autism: evaluation for evidence-based practices within a best evidence synthesis framework. J Autism Dev Disord. 2010;40:149–166. doi: 10.1007/s10803-009-0842-0. [DOI] [PubMed] [Google Scholar]
- 52.Grillon C. D-cycloserine facilitation of fear extinction and exposure-based therapy might rely on lower-level, automatic mechanisms. Biol Psychiatry. 2009;66:636–641. doi: 10.1016/j.biopsych.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Storch E, Mariaksin A, Murphy T. Psychotherapy for obsessive-compulsive disorder. Curr Psychiatry Rep. 2009;11:296–301. doi: 10.1007/s11920-009-0043-8. [DOI] [PubMed] [Google Scholar]
- 54.Santa Ana E, Rounsaville B, Frankforter T, Nich C, Babuscio T, Polling J, et al. D-cycloserine attenuates reactivity to smoking cues in nicotine dependent smokers: A pilot investigation. Drug Alcohol Depend. 2009;104:220–227. doi: 10.1016/j.drugalcdep.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tsai G, Lin P. Strategies to enhance N-methyl-d-aspartate receptor-mediated neurotransmission in schizophrenia, a critical review and meta-analysis. Curr Pharm Des. 2010;16:522–537. doi: 10.2174/138161210790361452. [DOI] [PubMed] [Google Scholar]
- 56.Davis M, Barad M, Otto M, Southwick S. Combining pharmacotherapy with cognitive behavioral therapy: Traditional and new approaches. J Trauma Stress. 2006;19:571–581. doi: 10.1002/jts.20149. [DOI] [PubMed] [Google Scholar]
- 57.Weiss M, Harris S. Teaching social skills to people with autism. Behav Modif. 2001;25:785–802. doi: 10.1177/0145445501255007. [DOI] [PubMed] [Google Scholar]
- 58.Guastella A, Mitchell P, Dadds M. Oxytocin increases gaze to the eye region of human faces. Biol Psychiatry. 2008;63:3–5. doi: 10.1016/j.biopsych.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 59.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4th. San Diego: Academic Press; 1998. [Google Scholar]


