Abstract
A sensitive and accurate approach for the determination of CP-31398 (N-{2-[(E)-2-(4-methoxy-phenyl)-vinyl]-quinazolin-4-yl}-N’,N’-dimethyl-propane-1,3-diamine hydrochloride) in rat and dog plasma by LC-MS/MS was validated to support pre-clinical toxicological and pharmacological studies. Based on the results of stability experiments with diluted CP-31398 solutions using NMR, LC-MS/MS and LC-Q-TOF, all sample preparation and handling steps were performed under yellow light to avoid CP-31398 decomposition. CP-31398 was extracted by protein precipitation with acetonitrile and separated using a Phenomenex Luna 3 μm phenyl-hexyl, 100Å, 30 × 2.0 mm column (rat plasma) or a Phenomenex Synergi 4μ Polar-RP, 80Å, 30×2.0 mm column (dog plasma) at a flow rate of 0.30 mL/min. The mobile phase consisted of A: 1% formic acid in water and B: 1% formic acid in methanol or acetonitrile. Total run times for rat and dog samples were 7 and 8 min, respectively, with accompanying retention times of 1.8 for both columns. A turbo ion spray interface was used as the ion source operating in positive mode. Calibration curves were linear from 5 to 1000 ng/mL. Linearity was assessed using the external standard method. Within-run and between-run accuracy was 93 to 109% of the true value for all analytes with precision (SD) of 8% or less for all experiments. The validated method was applied to preclinical toxicology studies in rats and dogs after oral administration of CP-31398.
Keywords: CP-31398, Plasma, Stability, Method Validation, LC-MS/MS
Introduction
CP-31398 (N-{2-[(E)-2-(4-methoxy-phenyl)-vinyl]-quinazolin-4-yl}-N’,N’-dimethyl-propane-1,3-diamine hydrochloride; Figure 1) is a styrylquinazoline synthetic drug under evaluation by NCI’s Division of Cancer Prevention as a candidate for clinical trials. Interest in the agent stems from its reported singular properties to restore and stabilize the tumor suppressor p53 gene functionality [1]. The transcription factor p53 participates in a wide array of key cellular functions, neutralizing various stress signals through the induction of cell cycle arrest, apoptosis and senescence [2]. Mutations affecting p53 are reported to be present in more than half of all human cancers [3], while even the cancers retaining the wild-type p53 often find an alternative mechanism for its inactivation [4]. Thus, restoration or stabilization of p53 function presents an attractive strategy for cancer prevention and therapy. CP-31398 has demonstrated measurable in-vitro effects against several cancer cell lines [5,6,7] as well as chemopreventive efficacy in animal models for skin [4] and colon [3,8] carcinogenesis, and it is often used as an activity benchmark for novel drugs targeting p53 [9]. In this work, we present validated methodology for the determination of CP-31398 in plasma of experimental animals by LC-MS/MS, along with a stability evaluation of the agent. The challenges of CP-31398 degradation in solution over time [9] and the chromatographic interference of low-level impurities were addressed.
Fig. 1.
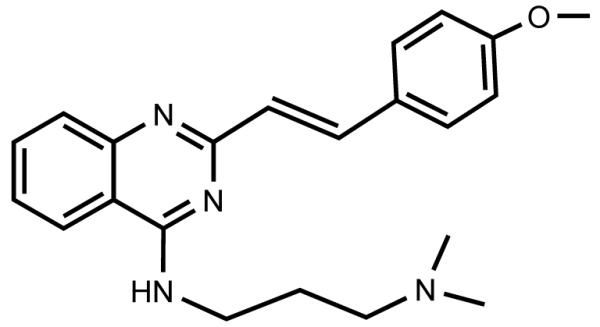
Chemical structure of CP-31398
1. Experimental
1.1. Chemicals and reagents
CP-31398 (Indofine Chemical Company, Hillsborough, NJ ) was obtained from the Chemopreventive Agent repository maintained by the Chemopreventive Agent Development Research Group, Division of Cancer Prevention, National Cancer Institute (Bethesda, MD) and was used both as the standard for the analytical method and as the dosing agent for animal studies. The bulk test article (>99.1% purity) was stored refrigerated (approximately 2-8°C) under nitrogen gas and protected from light. Stability of the bulk test article was confirmed by purity analyses performed at the beginning and end of the animal studies. Acetonitrile, methanol and formic acid (all HPLC grade) were purchased from Fisher Scientific (Pittsburgh, PA). HPLC-grade water was generated using a PURELAB Ultra system from ELGA (Lowell, MA) followed by filtration with a Millipore (Billerica, MA) 0.25 μm filter. Blank plasma (dog and rat) was obtained from Bioreclamation Inc. (Westbury, NY) and kept frozen at −20°C until analysis.
1.2. Animals
All animal care and use in this study was performed in accordance with standards set forth in the Guide for Care and Use of Laboratory Animals (National Research Council, 1996), by the U.S. Department of Agriculture through the Animal Welfare Act (7 USC 2131, 1985), and Animal Welfare Standards incorporated in Title 9, Part 3 of the Code of Federal Regulations, 1991. Male and female CD rats ([Crl:CD(SD) IGS BR]; Charles River Laboratories, Portage, MI; approximately six weeks of age) and naïve beagle dogs (Ridglan Farms, Inc., Mount Horeb, WI; approximately 14 months old) were used for this study. Rats and dogs were held in quarantine for at least one or three weeks, respectively, prior to use in the study. During the quarantine period, animals were observed daily for survival and general health status. Prior to randomization into experimental groups, each animal underwent a detailed physical examination to demonstrate its suitability for use as a test animal. Animals were housed individually in stainless steel cages (rats) and pens (dogs) in a windowless room. Animal rooms were held within a temperature range of 22-23°C and a humidity range of 30-50% for rats and 20-22°C and 50-80% for dogs. Fluorescent lighting in the animal rooms was provided for 12 h followed by 12 h of darkness. Rats were permitted free access to Certified Rodent Diet #5002 (PMI Nutrition International, Brentwood, MO); each dog was provided with 400 g of Certified Canine Diet 2021C (Harlan Teklad, Madison, WI) for a minimum of two hours daily. City of Chicago municipal water was available ad libitum by automatic watering systems.
1.3. Preparation of analytical stock and standards solutions
Stock solutions (1 mg/mL) of CP-31398 were prepared in water and stored protected from light at 4°C. Working standards, which were prepared by further diluting the stock solution in methanol-water (20:80, v/v) in a range of 50 to 10000 ng/mL, were stored under the same conditions.
1.4. Sample preparation
Plasma samples (100 μL) were transferred to 2-mL microcentrifuge plastic vials and mixed with 10 μL of methanol-water (20:80, v/v; or a standard or QC solution) and 1 mL of acetonitrile. The samples were vortex-mixed for 1 min, centrifuged at 4 °C in a Sorvall RC 5C Super Speed centrifuge (Thermo Fisher Scientific, Waltham, MA) at 8000 ×g for 10 min to remove precipitated proteins, and the supernatant was transferred to clean tubes and dried under nitrogen flow at room temperature (about 25 °C) for approximately 1 hr. After evaporation was complete, the residue was reconstituted in 500 μL of methanol-water (20:80, v/v), vortex-mixed and centrifuged again. The resulting supernatant was transferred to sample vials for instrumental analysis. To avoid CP-31398 decomposition, all sample preparation and handling steps were performed under yellow light, as further discussed in sections 1.6 (set up ) and 2 (results).
1.5. Instrumentation
Samples were analyzed on an API 3000 MS/MS system (Applied Biosystems/MDS Sciex, Foster City, CA) equipped with an Agilent 1100 HPLC (Agilent Technologies, Wilmington, DE). Analyst™ 1.4.2 was used to control the system and to capture the mass spectrometer data, perform linear regression analysis and calculate sample concentrations.
Chromatographic separation of the agent from rat plasma components was performed using a Luna 3 μm phenyl-hexyl, 100Å, 30 × 2.0 mm column (Phenomenex, Torrance, CA). The column was maintained at room temperature, the flow rate was 0.30 mL/min, and the injection volume was 10 μL. The mobile phase consisted of A: 1% formic acid in water and B: 1% formic acid in methanol with a gradient as follows: after injection, initial conditions with Solvent A at 65% were held for 0.5 min, decreased to 5% in 0.5 min and held constant for 3 min, returning to initial conditions for another 3 min of reequilibration time. CP-31398 retention time was approximately 1.8 min; total run time was 7 min.
For dog plasma samples, a Synergi 4μ Polar-RP, 80Å, 30×2.0 mm (Phenomenex) was used under the same conditions and with the same mobile phase as for rat samples, but using acetonitrile instead of methanol and with a modified gradient: initial conditions with Solvent A at 80% were held for 0.5 min, decreased to 5% in 1.5 min and held constant for 3 min, returning to initial conditions for another 3 min of reequilibration time. CP-31398 retention time was approximately 1.8 min; total run time was 8 min.
A turbo ion spray interface was used as the ion source operating in positive mode. Acquisition was performed at unit resolution in MRM mode using m/z 363.4 (protonated molecule, [M + H]+) → 318.2 ([M+H-NH(CH3)2 ]+). Ion spray voltage and collision energy were 4500 and 35V, respectively. The collision gas was nitrogen; the ion spray temperature was 450 °C; and dwell time was 300 ms.
1.6. Stability study of CP-31398 in solution and exposed to light
CP-31398 decomposes rapidly in diluted solutions when exposed to light. To examine this further, we performed experiments using NMR; LC-MS/MS and LC-Q-TOF. CP-31398 was dissolved in deuterium oxide (D2O) under yellow light and analyzed using a Bruker (Billerica, MA) 300 MHz instrument. The spectrum was also recorded after exposure to laboratory fluorescent light for 24 h and the NMR spectra were compared. Similarly, a freshly prepared CP-31398 stock solution in water (10 μg/m) was stored according to the following conditions: a) refrigerated and protected from light; or b) refrigerated without light protection. After five days in storage, both samples were further diluted to 200 ng/mL with 20% methanol in water and analyzed using LC-MS/MS as described above. The samples were also analyzed using a 6520 Q-TOF LC/MS system equipped with a 1200 Series HPLC (Agilent Technologies) in an attempt to identify the main degradation products.
1.7. Stability study of CP-31398 in water and plasma
Experiments to evaluate the stability of CP-31398 in water (solvent of choice and vehicle for the toxicological studies) and plasma [human (three individual lots), rat and dog] samples protected from light at various time points under different temperature conditions were performed. CP-31398 solutions at a target agent concentration of 200 ng/mL (0.5 μM) in water and in each plasma matrix were incubated at 37°C, 4°C, −20°C and −70°C and were tested for concentration at time points of 0 (room temperature baseline measurement); 1, 4, 24 and 168 h; and 1 month. Plasma and water samples were analyzed for the agent’s concentration by LC-MS/MS as described above.
1.8. Method validation
The following factors were used to assess assay performance in both plasma matrices: selectivity, linearity, precision, accuracy, recovery and stability, following the FDA’s Guidance for Industry: Bioanalytical Method Validation [10]. The selectivity of the method was assessed by analyzing extract from six individual animals for the presence of analytical interferences and comparing the results to those obtained from spiking blank plasma sources with the analyte at the LLOQ (5 ng/mL). Linearity was assessed using the external standard method and up to eight calibrators prepared with blank animal plasma and analyte concentrations in the 5 to 1000 ng/mL range. The curves were built from peak areas using least-squares linear regression with a (1/x2) weighting factor. The weighting factor was chosen based on goodness-of-fit criteria including coefficient of determination (r2), the back-calculated concentration of individual calibrators, and minimization of the intercept value. Precision and accuracy of the method were determined from three validation runs with dog plasma QC samples (n = 6) spiked at the LLOQ (5 ng/mL) and at low (12 ng/mL), mid (400 ng/mL) and high (800 ng/mL) concentrations. Within-run precision and accuracy were assessed from the results from a single day, while between-run precision and accuracy were determined from the results from the three validation runs on different days. Plasma extraction recovery of CP-31398 was determined by comparison of peak area results of plasma QC samples ( n= 6) to peak area results of extracted blank plasma spiked post extraction with analyte concentrations at the same levels. Bench-top stability was determined by analyzing low and high level QC samples for the analyte concentrations after 4 and 24 hours of storage at ambient temperature and comparing the results to those obtained from freshly prepared samples. Freeze-thaw stability of the low and high level QC samples was determined over three freeze-thaw cycles. Stability was also determined for low and high level QC samples that were extracted and stored in the instrument autosampler under refrigerated conditions (approximately 4 °C); sample extracts were injected on the LC-MS/MS instrument and the concentrations determined after 1 or 3 days of storage. To evaluate the impact of sample dilution on the method’s accuracy and precision, six replicate QC samples were prepared in blank animal plasma at 4000 ng/mL and diluted 5-fold with additional blank plasma prior to analysis.
1.9 Application to pre-clinical toxicological studies
CP-31398 was administered to CD rats and beagle dogs to evaluate the toxicity and pharmacokinetics of the agent following daily oral (gavage; using ASTM Type 1 water as vehicle) administration for 28 consecutive days. Animals were randomly assigned to control or treatment groups at the end of the quarantine period using a computerized body weight stratification procedure that produced similar group mean body weight values. The experimental design called for once daily administration of CP-31398 for 28 consecutive days to 4 cohorts at dose levels of 0, 40, 80 or 160 mg/kg/day for rats (twelve animals/sex/group) or 0, 10, 20 or 40 mg/kg/day for dogs (five animals/sex/group).
Blood samples were collected via retro-orbital sinus puncture (rats) or from the jugular vein (dogs) into Vacutainer tubes (Fisher Scientific, Pittsburgh, PA) containing EDTA at 9 time points (0, 0.25, 0.5, 1, 2, 4, 8, 12 and 24 hours post-dose) on Day 1 or during Week 4. Two additional time points at 48 and 72 h were used for the rat study on Day 1. Tubes were inverted several times to mix and placed on ice until centrifuged to separate plasma (within 1 h). After centrifugation, plasma was transferred into storage tubes (0.5 mL) and stored frozen (approximately °70°C) until analyzed. Plasma samples were analyzed for levels of CP-31398 using the analytical method presented above.
2. Stability Results – CP-31398 in solution and exposed to light
The NMR spectrum of a CP-31309 sample prepared under yellow light and kept in the dark until analysis was consistent with the trans (E)conformation of the agent, as shown by the olefinic protons with chemical shift of 6.25 and 7.45 ppm and coupling constant (J) of approximately 17 Hertz. After exposure to light, other peaks appeared in the NMR spectrum, indicating degradation of the agent. In particular, new peaks at 6.60 and 7.21 ppm and J value of 8 Hz suggest isomerization of the molecule to the cis (Z) conformation. Prolonged exposure to light resulted in the complete degradation of the agent judging for the complexity of the resulting NMR spectrum.
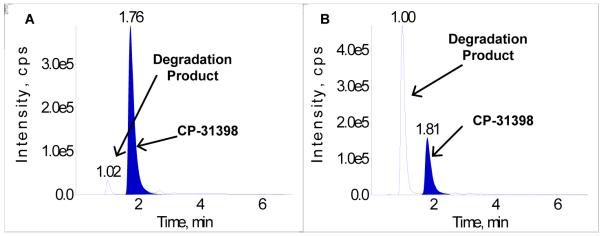
Figure 2A and 2B present the extracted ion chromatograms (EIC) [ions monitored (Q1→Q3) m/z 363.4→318.2] for the sample stored in the dark and exposed to light during storage, respectively. The results illustrate the degradation of the agent and extensive conversion to the degradation product under the experiment conditions. The Q-TOF product ion spectra for CP-31398 and major impurity are virtually identical , with ions at 363.22 (M+H)+, 318.16, 290.13, 263.12, 86.10 and 58.07 m/z, most likely indicating an isomerization transformation, as suspected from the NMR experimental results.
Fig. 2.
A. CP-31398 extracted ion chromatogram: dark storage; B. CP-31398 extracted ion chromatogram: exposed to light
3. Stability Results – CP-31398 in water and plasma
Results indicate that CP-31398 is stable in water at 200 ng/mL for at least one month (period tested) when stored in the dark at 4, −20 and −70°C. However, at 37°C and otherwise identical conditions, the solutions degraded to an average result of 81% and 67% of the initial value after 168 hours and 1 month, respectively. Similarly, CP-31398 in human, rat and dog plasma samples was stable for at least one month when stored at 4, −20 and −70°C. However, at 37°C, the samples degraded to an average result as low as 40% (rat plasma experiment; range 40 to 72% for all matrices) and 2% (rat plasma experiment; range 2 to 36% for all matrices) of the target values after 168 hours and 1 month, respectively.
4. Method validation results
4.1. Specificity
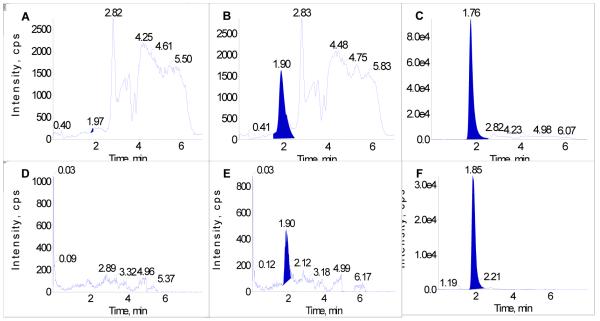
No significant chromatographic peaks interfering with the determination of CP-31398 were detected in the chromatograms of blank rat or dog plasma at the method’s LLOQ. Figures 3A-F show respective representative chromatograms of CP-31398 in blank rat and dog plasma extract, at the LLOQ (5 ng/mL) for each matrix, and a 200 ng/mL calibrator for each matrix.
Fig. 3.
A. CP-31398 – blank rat plasma; B. CP-31398 – calibrator at the LLOQ (5 ng/mL) in rat plasma; C. CP-31398 – calibrator at 200 ng/mL in rat plasma; D. CP-31398 – blank dog plasma; E. CP-31398 – calibrator at the LLOQ (5 ng/mL) in dog plasma; F. CP-31398 – calibrator at 200 ng/mL in dog plasma
4.2. Calibration curves
Calibration curves were linear from 5 to 1000 ng/mL with r2 values of 0.995 or greater for all curves. Calibrators that fell outside the acceptable accuracy range of 85 to 115% were not used in the statistics of the standard curve. Representative regression equations for the calibration curves were y = 5290 x + 5740 and y = 1690 x - 1830 for rat and dog plasma, respectively. The mean accuracy (% of true value) of individual calibrators used to determine the calibration curves ranged from 91 to 106% and 92 to 109% for rat and dog plasma, respectively. Between-run precision (SD) for the back-calculated calibrator concentrations ranged from 0 to 8% for both plasma matrices.
4.3. Precision and accuracy
For the low, mid and high QC levels, within-run and between-run precision ranged from 1 to 8 and 4 to 5% for rat plasma and 2 to 6 and 3 to 8% for dog plasma. Within-run and between-run accuracy ranged from 94 to 105 and 99 to 101% for rat plasma and 93 to 109 and 98 to 100% for dog plasma. Precision and accuracy results are presented in Table 1.
Table 1.
Within-run and between-run precision and accuracy for QC plasma samples
| Nominal CP-31398 Concentration (ng/mL) |
Mean Method Precision and Accuracy ( %)a |
||
|---|---|---|---|
| Rat Plasma | Dog Plasma | ||
| 5 (LLOQ) |
Within-Run SD |
103, 95.7, 100 15, 5.5, 6.9 |
101, 102, 99.9 8.7, 6.2, 2.8 |
| Between-Run SD |
99.5 9.5 |
101 6.4 |
|
|
| |||
| 12 (Low QC) |
Within-Run SD |
93.7, 95.2, 96.0 3.5, 7.7, 3.7 |
96.8, 97.5, 99.6 4.3, 5.7,3.2 |
| Between-Run SD |
95.0 5.1 |
97.8 4.4 |
|
|
| |||
| 400 (Mid QC) |
Within-Run SD |
101, 105, 97.4 3.8, 3.2, 1.9 |
101, 101, 98.9 2.5, 3.6, 2.3 |
| Between-Run SD |
101 4.2 |
100 2.8 |
|
|
| |||
| 800 (High QC) |
Within-Run SD |
98.6, 103, 94.9 1.9, 1.1, 2.6 |
109, 100, 92.5 2.0, 5.2, 1.9 |
| Between-Run SD |
98.9 3.9 |
100 7.6 |
|
n = 6 for individual validation runs (within-run); 18 for between-run. Accuracy expressed as % of true value, precision as SD.
4.4. Recovery
Average recovery for low, mid and high QC samples prepared in rat plasma was 58, 89 and 89%, respectively. Recovery results for similar experiments performed in dog plasma were 96, 98 and 100%, respectively.
4.5. Lower limit of quantification
For samples prepared at LLOQ levels, within-run and between-run precision was 6 to15 and 10% for rat plasma and 3 to 9 and 6% for dog plasma. Within-run and between-run accuracy was 96 to 103 and 100% for rat plasma and 100 to 102 and 100% for dog plasma. Results for the LLOQ determinations are included in Table 1.
4.6. Plasma bench-top, refrigerated and freeze-thaw and extract autosampler stability
The experiments designed to evaluate the bench-top stability of CP-31398 in low and high QC plasma samples after 4 or 24 h (dog) or 24 h (rat) at room temperature had recoveries within ±10% of nominal concentration for both matrices and concentrations, with the exception of dog plasma after 24 h (low and high QC recovery was 89 and 87%, respectively, of initial concentration). No significant changes for the low and high QC samples were observed for either plasma matrix after three freeze-thaw cycles. Changes for the QC sample extracts after three days of storage in the refrigerated instrument autosampler were within ±10% of nominal concentrations, with one exception (dog plasma, high QC after 72 h; 89% recovery). Results for all stability experiments performed are presented in Table 2.
Table 2.
Stability assessment in plasma and plasma extracts
| Nominal CP-31398 Concentration (ng/mL) - Time |
Mean Measured Recovery (SD)a (% of True Value) |
|
|---|---|---|
| Rat Plasma | Dog Plasma | |
| Plasma Bench-Top Stability (Room Temperature) |
||
| 12 (Low QC) - 4 h | _b | 96.0 (4.4) |
| 12 (Low QC) - 24 h | 101 (3.6) | 89.0 (0.29) |
| 800 (High QC) - 4 h | _b | 90.2 (1.3) |
| 800 (High QC) - 24 h | 90.4 (3.1) | 86.6 (1.5) |
|
| ||
| Plasma Freeze-Thaw Stability (3 Cycles) |
||
| 12 (Low QC) | 110 (6.5) | 105 (7.4) |
| 800 (High QC) | 96.4 (0.97) | 103 (0.53) |
|
| ||
| Plasma Extract Autosampler Stability (4 °C) |
||
| 12 (Low QC) - 24 h | 97.1 (3.1) | 91.0 (3.3) |
| 12 (Low QC) - 72 h | 106 (2.3) | 99.9 (1.3) |
| 800 (High QC) - 24 h | 92.2 (1.5) | 96.0 (3.4) |
| 800 (High QC) - 72 h | 90.3 (0.37) | 89.2 (1.8) |
n = 3
experiment not performed for the indicated species
4.7. Sample dilution
For the six-replicate set of QC plasma samples prepared at 4000 ng/mL and diluted 5-fold before analysis, accuracy was 99 and 101% of the target value for rat and dog plasma, respectively, with associated precision of 2 and 2%, respectively.
5. Animal study results
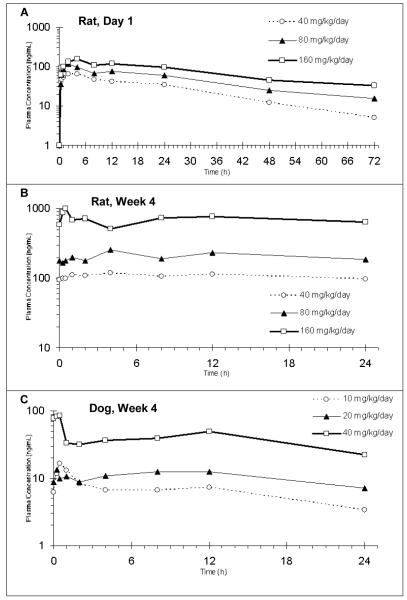
Representative CP-31398 mean animal plasma concentrations time profiles for the studies performed are presented in Figure 4A (male rats, single dose), 4B (male rats, Week 4) and 4C (male dogs, Week 4). Average male rat plasma concentrations were as high as 150 ng/mL for a single dose and in the 95 to 1000 ng/mL range on Week 4. Average dog plasma concentrations were in the 3 to 85 ng/mL range.
Fig 4.
A. Representative CP-31398 plasma concentration-time curve – male rats, single dose; B. Representative CP-31398 plasma concentration-time curve – male rats, Week 4; C. Representative CP-31398 plasma concentration-time curve – male dogs, Week 4.
6. Discussion
6.1. Method development
Analytical work with the agent was initially challenging due to the inherent instability of CP-31398 in solution when exposed to light. Once identified, however, this issue was effectively addressed by performing all experimental procedures under yellow light. In addition, different batches of the agent presented low levels of impurities and degradation products. One of the impurities, eluting after the agent in our method, constitutes an analytical interference if chromatographic resolution is not achieved, affecting the observable LLOQ of the method. Finding a chromatographic column that could achieve this separation was a challenge, but after trying multiple phases and vendors, we found that the Luna phenyl-hexyl column from Phenomenex performed acceptably. However, different lots of this same column lacked performance consistency. The Synergi Polar-RP column from the same vendor proved to be a good alternative. Validations using both columns are presented in this work.
6.2. Assay performance
Assay execution and performance for the preclinical studies in rat and dogs were as expected and without issues. A more sensitive method in dog plasma was also validated for the dog study; the method was fundamentally the same, although the injection volume was 20 μL. For the modified method, calibration curves were linear and in the 1 to 25 ng/mL range with r2 values of 0.99 or greater for all curves. A representative regression equation for the calibration curves was y = 6660 x – 1870. The mean accuracy of individual calibrators used to determine the calibration curves ranged from 91 to 103%, with between-run precision for the back-calculated calibrator concentrations less than 9%. For samples prepared at the LLOQ level (1 ng/mL), accuracy was 100% with precision of 7%.
Utilization of a deuterated analog of CP-31398 as the internal standard, not available at the time of this research, could further improve the method’s performance for more complex matrices such as tissues.
7. Conclusion
A method for the determination of CP-31398 in plasma samples by LC-MS/MS was validated and applied to preclinical toxicology studies in rats and dogs. After resolving the previously problematic issues of agent instability and chromatographic interference, this method has been shown to be sensitive, accurate and simple to execute.
Acknowledgements
This research was supported by contract N01-CN-43304 (HHSN261200433004C) from the Division of Cancer Prevention, National Cancer Institute. The authors thank Heidi Kreuzer for assistance with the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Foster BA, Coffey HA, Morin MJ, Rastinejad F. Pharmacological Rescue of Mutant p53 Conformation and Function. Science. 1999;286:2507–2510. doi: 10.1126/science.286.5449.2507. [DOI] [PubMed] [Google Scholar]
- [2].Brown CJ, Cheok CF, Verma CS, Lane DP. Reactivation of p53: from peptides to small molecules. Trends in Pharmacological Sciences. 2011;32:53–62. doi: 10.1016/j.tips.2010.11.004. [DOI] [PubMed] [Google Scholar]
- [3].Rao CV, Steele VE, Swamy MV, Patlolla JMR. Inhibition of Azoxymethane-Induced Colorectal Cancer by CP-31398, a TP53 Modulator, Alone or in Combination with Low Doses of Celecoxib in Male F344 Rats. Cancer Res. 2009;69:8175–8182. doi: 10.1158/0008-5472.CAN-09-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Tang X, Zhu Y, Han L, Kim AL, Kopelovich L, Bickers DR, Athar M. CP-31398 restores mutant p53 tumor suppressor function and inhibits UVB-induced skin carcinogenesis in mice. J Clin Invest. 2007;117:3753–3764. doi: 10.1172/JCI32481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wischhusen J, Naumann U, Ohgaki H, Rastinejad F, Weller M. CP-31398, a novel p53-stabilizing agent, induces p53-dependent and p53-independent glioma cell death. Oncogene. 2003;22:8233–8245. doi: 10.1038/sj.onc.1207198. [DOI] [PubMed] [Google Scholar]
- [6].Xu J, Timares L, Heilpern C, Weng Z, Li C, Xu H, Pressey JG, Elmets CA, Kopelovich L, Athar M. Targeting Wild-Type and Mutant p53 with Small Molecule CP-31398 Blocks the Growth of Rhabdomyosarcoma by Inducing Reactive Oxygen Species–Dependent Apoptosis. Cancer Res. 2010;70:6566–6576. doi: 10.1158/0008-5472.CAN-10-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Roh J-L, Kang SK, Minn IL, Califano JA, Sidransky D, Koch WM. p53-Reactivating small molecules induce apoptosis and enhance chemotherapeutic cytotoxicity in head and neck squamous cell carcinoma. Oral Oncol. 2011;47:8–15. doi: 10.1016/j.oraloncology.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Rao CV, Swamy MV, Patlolla JMR, Kopelovich L. Suppression of Familial Adenomatous Polyposis by CP-31398, a TP53 Modulator, in APCmin/+ Mice. Cancer Res. 2008;68:7670–7675. doi: 10.1158/0008-5472.CAN-08-1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zachea N, Lambertac JMR, Rökaeusa N, Shen J, Hainaut P, Bergman J, Wimana KG, Bykova JN. Mutant p53 targeting by the low molecular weight compound STIMA-1. Mol Oncol. 2008;2:70–80. doi: 10.1016/j.molonc.2008.02.004. Vl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Rippin TM, Bykov VJ, Freund SM, Selivanova G, Wiman KG, Fersht AR. Characterization of the p53-rescue drug CP-31398 in vitro and in living cells. Oncogene. 2002;28:2119–29. doi: 10.1038/sj.onc.1205362. [DOI] [PubMed] [Google Scholar]
- [10].Guidance for Industry: Bioanalytical Method Validation. U.S. Department of Health and Human Services. Food and Drug Administration. Center for Drug Evaluation and Research (CDER) Center for Veterinary Medicine (CVM) 2001 May; http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm070107.pdf.