Summary
An organized microtubule array is essential for polarized motility of fibroblastic cells. Dynamic microtubules closely interact with focal adhesion sites in migrating cells. Here, we examined the effect of focal adhesions on microtubule dynamics. We observed that the probability of microtubule catastrophes (transitions from growth to shrinkage) was 7 times higher at focal adhesions than elsewhere.
Analysis of dependence between the microtubule growth rate and catastrophe probability throughout the cytoplasm revealed that a non-specific (mechanical or spatial) factor provided a minor contribution to the catastrophe induction by decreasing microtubule growth rate at adhesions. Strikingly, at the same growth rate the probability of catastrophes was significantly higher at adhesions than elsewhere, indicative of a site-specific biochemical trigger. Observed catastrophe induction occurred at adhesion domains containing scaffolding protein paxillin, that was previously shown to interact with tubulin. Furthermore, replacement of full-length paxillin at adhesion sites by microinjected paxillin LIM2/3 domains suppressed microtubule catastrophes exclusively at adhesions. We suggest that paxillin influences microtubule dynamics at focal adhesions by serving as a scaffold for a putative catastrophe factor and/or regulating its exposure to microtubules.
Keywords: microtubules, focal adhesions, microtubule catastrophe, paxillin, cell motility
Introduction
Microtubule dynamics in higher eukaryotic cells is complex. It is tightly regulated by a variety of microtubule-associated proteins. However, the precise mechanisms of this regulation are only partially understood. It was well accepted that microtubules demonstrate random transitions from periods of growth to shortening and back at plus ends (dynamic instability, (Mitchison and Kirschner, 1984)), with most minus ends blocked due to the association to microtubule organizing centers (MTOCs). Plus end dynamics can be described by four parameters: the rates of growth and shortening, catastrophe frequency (transitions from growth to shortening), rescue frequency (transitions from shortening to growth) and time spent in pauses.
The overall pattern of microtubule dynamics differs between different cell types (Shelden and Wadsworth, 1993) and depends on the availability of serum factors (Danowski, 1998), as well as the phase in the cell cycle (Salmon et al., 1984). Most important, regional microtubule dynamics can differ within a single polarized cell. Thus, detyrosinated stable microtubules are found specifically in advancing lamellae of fibroblasts in wound healing assays (Nagasaki et al., 1992). Dynamic microtubules in the rear of motile fibroblasts undergo more catastrophe than in the front, while certain microtubules in lamellae, so called “pioneer” microtubules, are characterized by almost no catastrophes (Wadsworth, 1999; Waterman-Storer and Salmon, 1997).
Microtubule dynamics in vivo can be specifically regulated by diverse protein factors (Amos and Schlieper, 2005; Howard and Hyman, 2007), certain posttranslational tubulin modifications (Tran et al., 2007) or tensile forces (Kaverina et al., 2002). In the last few years, more and more data indicate that interactions with the actin cytoskeleton play a critical role in the local regulation of microtubule dynamics (Etienne-Manneville, 2004; Rodriguez et al., 2003). Indeed, it is the actin system and its regulators that possess region specific differences in motile cells. Noteworthy in this regard, actin anchorage sites, focal adhesions, are able to precisely influence the dynamics of individual microtubules in the course of microtubule targeting of focal adhesions (Kaverina et al., 1998). The mechanism of targeting includes directional growth of the plus end to the adhesion site and a short association of the microtubule tip with the adhesion plaque. Three subsequent scenarios are possible: first, the microtubule continues to grow, second it pauses in the adhesion, and third it undergoes catastrophe and shrinks (Small and Kaverina, 2003).In this study we show that catastrophe events are specifically enriched at focal adhesion sites. Such precise catastrophe activity has not been described before at any specific locus of interphase cells.
The nature of microtubule catastrophes can be diverse. They can be a part of dynamic instability and depend on availability of free tubulin. Additionally, in vitro catastrophe frequency can be enhanced when microtubules polymerize against a stiff obstacle (Janson et al., 2003). Thus, a possibility exists that the catastrophes at adhesion sites are enhanced passively due to mechanical stiffness of adhesions or due to spatially restricted availability of free tubulin at dense adhesion plaques. However, it is known that catastrophes in cells are often induced by specific molecular factors, including stathmin or kinesin-13 family proteins (Cassimeris, 2002; Wordeman, 2005). Our present data suggest that a biochemical trigger locally activated at the focal adhesion plaque is critical for catastrophe stimulation, while the physical influence of a dense adhesion structure makes a minor contribution to this process.
In order to clarify what makes the adhesion plaque a preferred site for catastrophe induction, we investigated focal adhesion molecules potentially involved in interaction with microtubules. In particular, we studied the focal adhesion scaffolding protein paxillin that was previously found to bind tubulin (Herreros et al., 2000).
Paxillin is a 68-kDa scaffold protein that contains many protein-binding modules and interacts with a variety of structural and signaling molecules (Brown and Turner, 2004). Paxillin contains two principle structural domains: five LD domains at the amino-terminus and four LIM domains at the carboxyl terminus. A number of kinases and phosphatases critical for adhesion signaling bind to either LD or LIM domains, making paxillin a central player in several regulatory pathways (Brown and Turner, 2004). The paxillin LIM-domain region was shown to serve as an anchor to a plasma membrane component and thus assure localization to focal adhesion sites (Brown et al., 1996), although the protein that mediates this association has not been yet identified. Paxillin LIM2/3 domains are also capable of binding tubulin (Brown and Turner, 2002; Herreros et al., 2000).
In the present study we show that the same two LIM domains of paxillin significantly change microtubule dynamics at adhesion sites when microinjected into fibroblastic cells. Substituting full-length paxillin at focal adhesions, LIM2/3 protein reduces catastrophic frequency exclusively at these locations, implicating paxillin in the local regulation of microtubule catastrophes.
Results
The frequency of catastrophes is higher at focal adhesion sites
In order to clearly visualize microtubule dynamics in relation to focal adhesions we applied TIRF microscopy to illuminate only the ventral layer of cells attached to a glass substrate (Axelrod, 1989). Fish fibroblasts were transfected with mCherry-tubulin or 3xGFP-EMTB to visualize microtubules and GFP-Paxillin or mCherry-Paxillin to mark adhesion sites (Fig 1A-C). Under TIRF conditions, we assumed that co-localization of a microtubule with an adhesion site indicates their close physical interaction. In live cell time-lapse sequences, we observed that when growing microtubules reached an adhesion site they frequently underwent catastrophe and shrank. Individual microtubules often underwent multiple catastrophe events at a focal adhesion followed by subsequent rescues (Fig 1A) whereas only few catastrophes occurred in adhesion-free areas (Fig. 1B). Analysis of overall adhesion numbers showed that approximately 40% of catastrophes occurred at adhesion sites, 12% at the cell edge and 48% elsewhere (Fig. 1D). Since overall adhesion area is considerably smaller than the adhesion-free ventral cell surface, these numbers indicate that adhesions serve as preferential sites for catastrophes.
Fig. 1. Microtubule catastrophes at focal adhesions (FA) are specific events.
A-B. Kymographs of microtubule dynamics at focal adhesion (A) and in adhesion-free cytoplasm (B). Upper panels show 3xGFP-EMTB (green), mCherry-paxillin (red), microtubule life history plot (white line), catastrophes at adhesion (arrows) catastrophe in adhesion-free area (arrowhead). Lower panels, microtubule images only. Microtubule shrinkage of 0.5 μm or more is considered as a catastrophe. C. A frame from TIRF video sequence of fish fibroblast cell co-transfected with 3xGFPEMTB (green) to visualize microtubules and mCherry-paxillin (red) to mark FA. Bar is 10μm. Boxed region is presented in kymograph in A. D. Microtubule catastrophe distribution in the ventral cell layer. Total of 292 catastrophes in 5 3xGFP-EMTB and mCherry-paxillin co-transfected cells within 12 minutes quantified. E. Average microtubule elongation per catastrophe is 7 times shorter at focal adhesions (blue, 0.71 μm/catastrophe) that elsewhere (red, 4.91 μm/catastrophe). Total of 24 microtubules in 5 3xGFP-EMTB and mCherry-paxillin cells within 12 minutes were quantified.
A simple quantification of catastrophe events within a large area is insufficient for the analysis of microtubule catastrophe induction at local sites. The reason is that since catastrophe is an event in which a microtubule switches from growth to shrinkage it can occur only to a growing but not to a shortening plus end. Thus, only those sites where growing microtubule ends were present were taken into consideration. Accordingly, we measured the length of elongation for microtubules while growing through adhesions or through an adhesion-free area. To obtain average persistent microtubule elongation, we normalized the average microtubule elongation by the average number of catastrophe events for each group of data. Strikingly, persistent microtubule elongation without catastrophes was 7 times lower at adhesion sites (Fig. 1E) than elsewhere. These data suggest a specific mechanism that triggers microtubule catastrophes at adhesion sites.
Adhesions trigger catastrophes by combined mechanical and biochemical factors
A critical question is whether catastrophes at adhesions are triggered by a specific biochemical mechanism or as a consequence of increased actin filament density at the adhesion plaque. Such non-specific mechanisms may include mechanical restraint or spatial restriction for free tubulin penetration. In order to answer this question we analyzed dynamics of mCherry-EB3-marked plus tips of microtubules in the vicinity of GFP-paxillin-containing focal adhesion sites (Fig. 2).
Fig. 2. Microtubule tips dynamics at focal adhesions.
A. Kymograph of a microtubule catastrophe event at focal adhesion (FA). Microtubule marked with mCherry-EB3 (red), focal adhesion marked with GFP-paxillin (green). B. Kymograph of a microtubule tip (mCherry-EB3, red) which does not undergo catastrophe at the adhesion (GFP-paxillin, green). Microtubule tip changes its growth dynamic at focal adhesion. C. An example of assigned area zones for microtubule growth path near focal adhesion (GFP-paxillin, green). Cytoplasmic zones close to the cell center encoded as zones -2 and -1. Zone adjacent to focal adhesion is encoded as zone 0. Focal adhesion contains zones 1–3, according to its length. Cytoplasmic zones towards the cell periphery are encoded as zones 1out and 2out. Each zone is 1 μm in size. D. Distribution between fast (growth rate >0.1 μm/s) and slow (growth rate <0.1 μm/s) microtubules for each zone. In cytoplasm (yellow background) ~ 70% of microtubules grow with the speed 0.1μm/second or faster. At focal adhesion (green background) percentage of fast microtubule reduces down to ~50%. E. Dependence of microtubule catastrophe ratio (percent of approaching microtubules undergo catastrophe) of time microtubule spends in cytoplasm zone (zone -1, green), at adhesion base (zone 0, blue) and in adhesion (zone 1, red). F. Total microtubule catastrophe ratio for each zone. Percent of approaching microtubules undergo catastrophe increases from 2% in cytoplasm to 25% at focal adhesion. Total of 139 microtubules in 3 3xGFP-EMTB and mCherry-paxillin cells within 15 minutes were quantified. Scale bar 1μm.
Kymographic analysis of microtubule tip dynamics revealed two scenarios (Fig. 2A,B): either EB3 was released from a microtubule at an adhesion site, indicative of a catastrophe (Fig. 2A) or a growing tip proceeded through an adhesion (Fig. 2B). In the second case, the velocity of microtubule tips was notably reduced while moving through the adhesion site (Fig. 2B). In adhesion-free cytoplasm ~70% of microtubules grew at a rate of 0.1μm/second or more (fast microtubules) and ~30% of microtubules grew slower (slow microtubules). At focal adhesions, the proportion of slow microtubules increased up to 50% within the same microtubule population (Fig. 2D).
At the same time, the catastrophe ratio dramatically increased at adhesion sites. For microtubules approaching adhesions, the catastrophe probability increased from less than 5% on the approach route to around 25% at the adhesion (Fig. 2F).
Combined, these data reveal a correlation between microtubule tip speed and catastrophe ratio. The percentage of “slow MTs” goes up (Fig. 2D, red line) at the same location at inner adhesion (zones 0–1) where catastrophes are induced (Fig.2F, blue line). These data suggest that dense focal adhesion structure suppresses microtubule growth and thus increases the probability of catastrophe. This mechanism, however, is not specific for adhesions. We found that the catastrophe ratio of individual microtubules reversibly correlated with their plus tip velocity both for microtubules growing through adhesions and through adhesion-free area (Fig. 2E) suggesting that microtubule catastrophe rate can be increased by cytoplasmic rigidity or spatially restricted concentration of tubulin dimers equally at adhesion plaques and at other locations. Strikingly, for microtubules with the same growth rate, the catastrophe ratio was higher at adhesion sites than elsewhere (Fig. 2E).
We have further tested whether softening of the adhesion structure decreases an adhesions ability for catastrophe induction. For this purpose, we have plated cells on a soft gelatin gel cushion (Seals et al., 2005) where strong tensile forces cannot be developed (Discher et al., 2005). Adhesions formed under these conditions were smaller than on glass substrate (<2μm versus <4 μm in length) but catastrophe ratio increase at adhesions was similar (23% on glass and 32% on gelatin gel). Together, these data indicate that a specific biochemical catastrophe trigger acts at adhesion sites.
In line with this finding, some microtubules continued to polymerize when their plus ends were apparently prevented from advancing by an obstacle. The polymerizing plus ends of such microtubules remained immobile while the length of the microtubule increased, resulting in loop formation (Supplementary Fig. S1). Thus, mechanical obstacles do not necessarily cause microtubule catastrophe. Combined, our results strongly suggest that a biochemical rather than a mechanical mechanism or spatially restricted concentration of tubulin dimers drives microtubule catastrophe induction at focal adhesions sites.
Catastrophe induction does not depend on the maturation stage of adhesions
Having determined that a specific biochemical mechanism at the adhesion sites enhances microtubule catastrophe activity, we aimed to identify focal adhesion proteins potentially involved in the process. As focal adhesion composition and signaling changes in their life course, we first investigated whether catastrophe induction depends of the adhesion maturation stage. To distinguish initial (early) focal adhesions from mature (late) adhesion sites, we used two adhesion markers: paxillin that is present in the majority of adhesion sites and zyxin that has been shown to incorporate into adhesions at later stage of maturation (Zaidel-Bar et al., 2003). Simultaneous transfection of a microtubule marker GFP-EMTB, mCherry-paxillin and Cerulean-zyxin allowed us to compare microtubule dynamics at early (paxillin-only) versus late (paxillin–and-zyxin) adhesions (Fig. 3A,B).
Fig. 3. Microtubule catastrophes do not depend on maturation stage of focal adhesion.
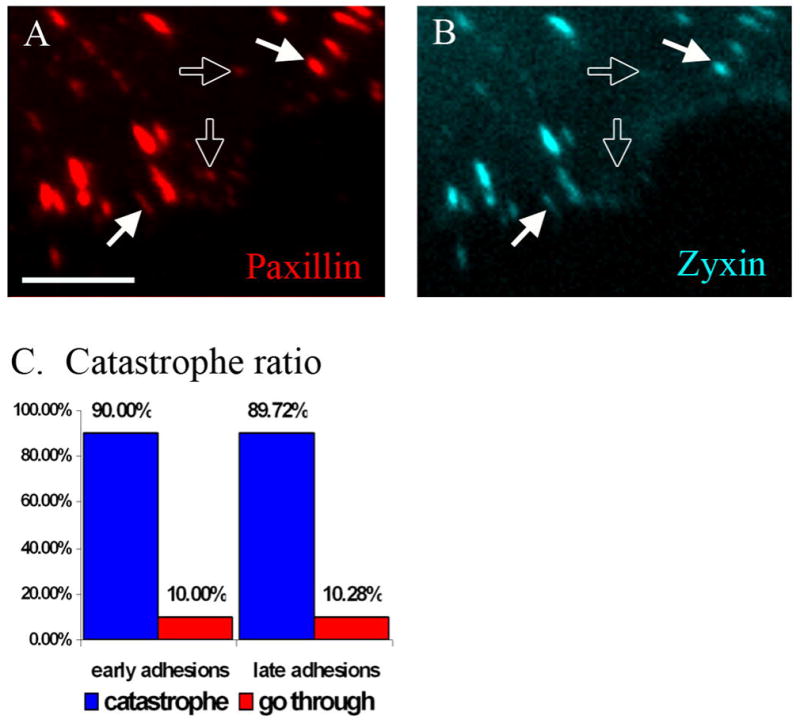
A.-B. A frame from video sequence of cell co-transfected with mCherry-paxillin (A. red) and Cerulean-Zyxin (B. cyan). Paxillin marks both early (hollow arrows) and late (filled arrows) focal adhesions. Zyxin is marker for late focal adhesions only (filled arrows). Scale bar 5 μm. C. Microtubule catastrophe ratio. ~90% of approaching microtubules undergo catastrophe both at early and late adhesions. Total 117 catastrophe events in 4 cells were quantified.
Overall quantification based on time-lapse live image sequences revealed that ~90% of adhesion-associated catastrophes occur at late adhesions, and only ~10% at early adhesions (Fig. 3C). Nevertheless, taking into account that late adhesions are approached by a significantly higher number of microtubules, the final analysis showed that both types of adhesions were equally effective in catastrophe induction: 90% of approaching microtubules underwent catastrophe at adhesions (Fig. 3D) regardless of their maturation stage.
Paxillin-associated adhesion structures are sufficient for microtubule catastrophe induction
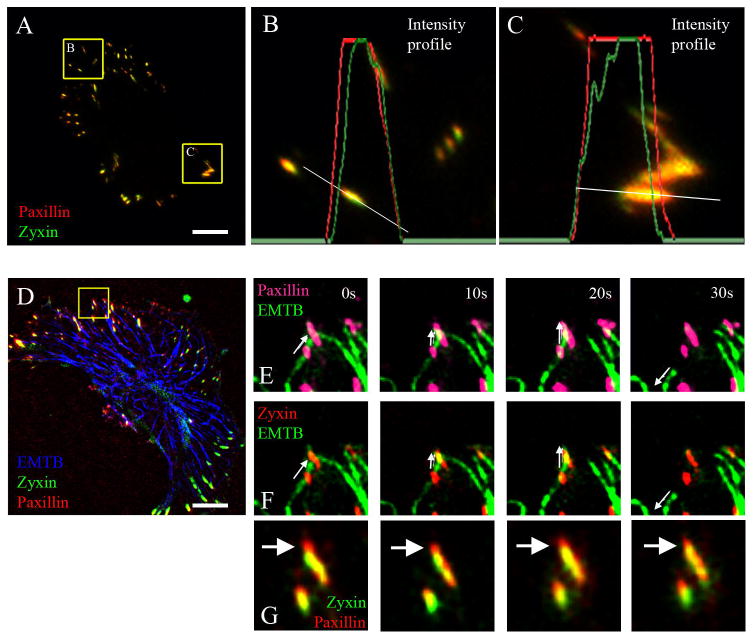
Though both zyxin and paxillin are present in late adhesions, we found that these adhesion proteins are distributed unevenly within single elongated mature adhesion sites: paxillin extends more distantly toward the cell edge than zyxin (Fig. 4). Such uneven distribution was found both at adhesions behind the leading edge (Fig. 4B) and sliding adhesions at the trailing edge (Fig. 4C) of the cell. We observed that a considerable number of microtubules underwent catastrophes at the distal ends of late adhesions (Fig. 1B). Detailed analysis of these catastrophes showed that they occurred at paxillin-rich adhesion domains devoid of zyxin (Fig. 4D-G). These data suggest that paxillin-associated adhesion structures are sufficient for microtubule catastrophe induction.
Fig. 4. Catastrophes can occur in the zyxin free zone of focal adhesions.
A. A frame from a video sequence of a cell transfected with mCherry-paxillin (red) and GFP-zyxin (green). Scale bar 10μm. B-C. Enlarge areas at the leading edge (B) and trailing edge (C) of the cell (paxillin – red, zyxin – green). The intensity profiles of paxillin (red lines) and zyxin (green lines) along a lines (white) one-pixel wide are shown. Paxillin extends more distantly towards the cell edge. A representative example from 15 cells. D. A frame from a video sequence of a cell transfected with GFP-EMTB (blue) to visualize microtubules, Cerulean-zyxin (green) to mark late adhesions and mCherry-paxillin (red) to mark both early and late adhesions. Scale bar 10μm. Box in enlarged to the right (E-F). E. Enlarged frame sequence of microtubule (green) and paxillin (pink). F. Enlarged frame sequence of microtubule (green) and zyxin (red). Catastrophe happened at time point 20s at the distal zyxin-free end of focal adhesion. Time, seconds. Arrows show direction of microtubule movement. G. Enlarged frame sequence of focal adhesion from 4E,F. Zyxin (green), paxillin (red). Arrows point at zyxin-free distal end of focal adhesion.
Displacement of paxillin from focal adhesions by LIM2/3 domain microinjection decreases the number of catastrophes specifically at adhesion sites
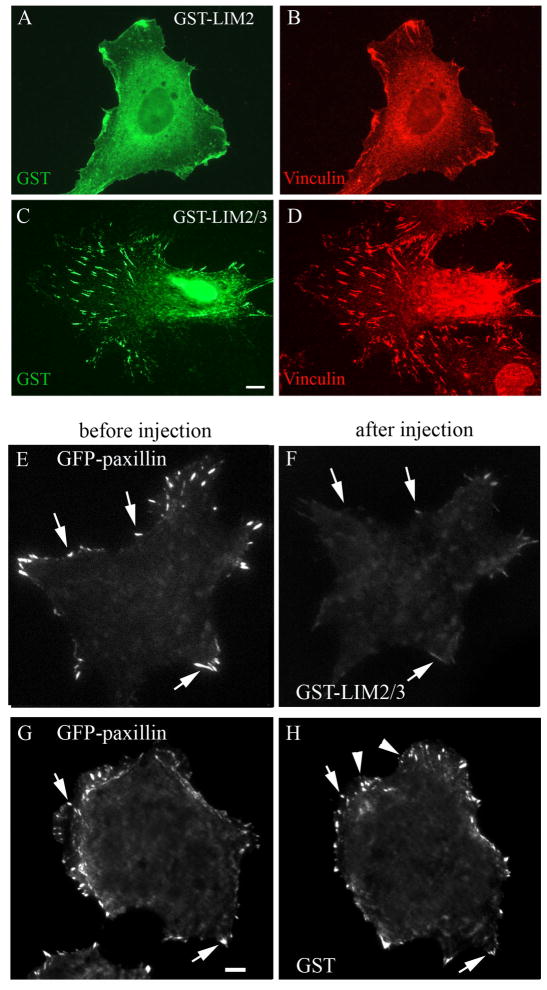
We further examined paxillin involvement in catastrophe regulation by displacement of paxillin from adhesion sites. For this purpose, individual LIM domains of paxillin, including LIM2, LIM3 and tandem LIM2/3 domains were produced as GST-fusion proteins (see Materials and Methods) and microinjected into cells. One hour after injection, cells were fixed and processed for immunostaining. Single GST-LIM2 (Fig.5A,B) and GST-LIM3 (not shown) showed mostly diffuse staining in the cytoplasm with only occasional weak focal adhesion localization, whereas GST-LIM2/3 was localized robustly to focal adhesions (Fig. 5C,D). Moreover, live cell imaging of GFP-paxillin-expressing cells showed that injected LIM2/3 domains caused displacement of GFP-paxillin from adhesion sites within 45 minutes (Fig. 5E,F). Immunostaining of injected cells confirmed paxillin displacement (not shown) while another core adhesion component vinculin remained in the adhesions (Fig. 5D).
Fig. 5. LIM2/3 displaces full length GFP-paxillin from focal adhesions.
Cells microinjected with GST-LIM2 (A,B) and GST-LIM2/3 (C,D) proteins were stained with anti-GST (A,C) and anti-vinculin (B,D) antibodies. Only GST-LIM2/3 (C,D) localizes to focal adhesions. Scale bar 10 μm. Cells expressing GFP-Paxillin (E,G) were microinjected with either GST-LIM2/3 (F) or GST only (H). Within 45 minutes after injection of GST-LIM2/3 paxillin is displaced from existing focal adhesions (F, arrows) and do not appears in new adhesion sites. Microinjection of GST only changed neither paxillin intensity level in old adhesions (H, arrows) nor its ability to incorporate into new adhesion sites (H, arrowheads).
Notably, although LIM2/3 domains have been shown to bind tubulin (Brown and Turner, 2002; Herreros et al., 2000), no detectable localization to microtubules has been observed for injected protein (Fig. 5E). Thus, if LIM2/3 domains of paxillin interact with microtubules in vivo it can only occur in the vicinity of focal adhesions.
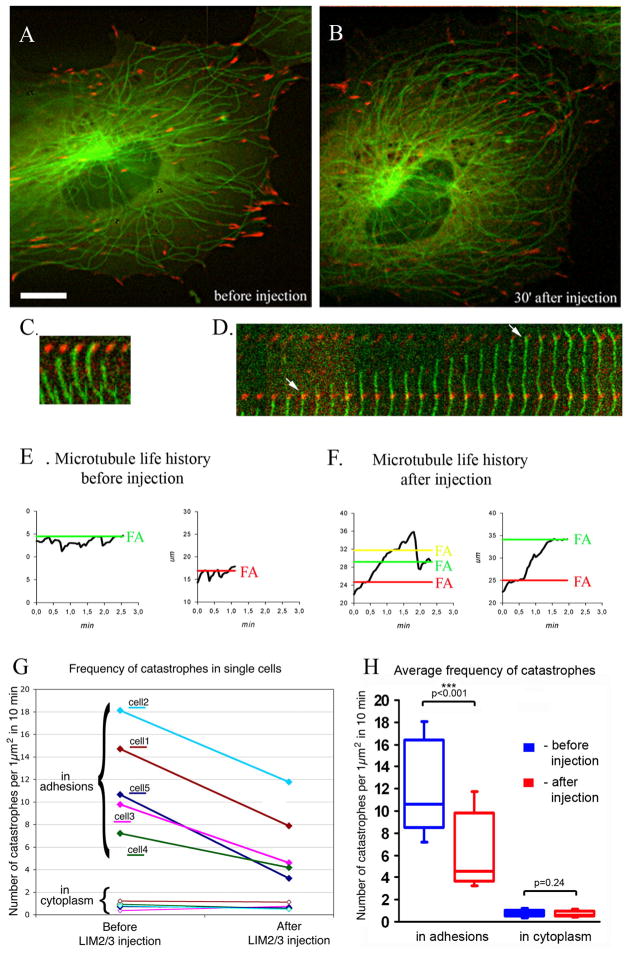
In order to determine whether substitution of paxillin by LIM2/3 protein at adhesion sites modulates microtubules at this location, we microinjected LIM2/3 into fish fibroblasts co-transfected with GFP-beta tubulin and dsRed-zyxin. Time-lapse live image sequences were recorded prior to the injection as well as 5 minutes after the injection. We found first that microtubules more often exhibited persistent growth resulting in an increase of microtubule length and density at the cell periphery (Fig. 6A-B). Analysis of microtubule dynamics revealed that for each cell, the percentage of catastrophe events in the adhesion sites was 40% lower than in the same cell before injection (Fig. 6G,H). Typically, microtubules grew processively through 3-4 focal adhesions during a single growth phase as compared with 1–2 adhesions in the same cell prior to injection (Fig. 6C-F). Along with a statistically significant decrease of catastrophe frequency at adhesions, no changes were observed in microtubule catastrophes in adhesion-free cytoplasm (Fig. 6G,H). This effect appeared to be specific for LIM2/3 domains since microinjections of paxillin LIM1 domain alone and either LIM1/2 or LIM3/4 domains had no influence on microtubule dynamics (not shown).
Fig 6. GST-LIM2/3 inhibits microtubule catastrophe at focal adhesions.
A-B. Cell expressing GFP-beta-tubulin (green) and mRFP-Zyxin (red) before (A) and after (B) microinjection of GST-LIM2/3 protein. After injection, microtubule density increases as a result of catastrophe suppression at adhesion sites. Scale bar 10 μm. C–D. Kymographs of microtubule dynamics at focal adhesions. Microtubule undergoes catastrophe at adhesion before injection (C) but grows through adhesions after injection (D, arrows). E–F. Examples of microtubule life history plots in cells before (E) and 30 minutes after (F) injecting GST-LIM2/3 construct. Black line indicates tracking position of individual microtubule tips in relation to focal adhesions (FA) marked with red, green and yellow lines. Note multiple catastrophes at adhesions in (E) while microtubules grow through several adhesions without catastrophe in (F). G. Frequency of catastrophes per 1μm2 before and after microinjection of GST-LIM2/3 protein. Upon injection, frequency of catastrophes reduces in focal adhesions (upper bracket) but stays the same in cytoplasm (lower bracket). Data for 271 catastrophes in 5 individual cells recorded both prior to injection and 30 minutes after injection are shown. H. The difference in microtubule catastrophe before (blue boxes) and after (red boxes) injection of GST-LIM2/3 shown in box-and-whisker plot. The P-value was calculated by t-test (Microsoft Excel). Frequency of catastrophes at focal adhesions statistically significantly reduced (p-value <0.001) after injection GST-LIM2/3. At the same time frequency of catastrophes in cytoplasm statistically did not change (p-value=0.24).
We further found that injection of LIM2/3 paxillin domains in GFP-EB1-expressing cell resulted in an increased number of growing microtubules, but no effect on the velocity of microtubule growth. Namely, the number of EB1 comets indicating polymerizing microtubule plus tips was increased by 30% 5 minutes after LIM2/3 microinjection (Supplemental Fig. S2). However, the rate of microtubule growth did not change significantly (Supplemental Fig. S2), indicating that besides decreasing catastrophe rate at the adhesion site, LIM2/3 protein injection had no detectable effect on microtubule polymerization.
Discussion
Substrate adhesion turnover in fibroblasts and other cells is influenced by changes in microtubule dynamics (Small and Kaverina, 2003; Wittmann and Waterman-Storer, 2001). Our previous findings suggested that the origin of this interdependence is a direct cross-talk between microtubules and focal adhesions, involving their mutual interaction during dynamic targeting events (Kaverina et al., 2002; Kaverina et al., 1999). However, the molecular players involved in microtubule–focal adhesion interactions remained unclear.
Here, we show that microtubule catastrophes are specifically enriched at adhesion sites. In adhesion-free cytoplasm, a microtubule can grew for 4.9 μm on average without catastrophes, while at adhesion sites catastrophes occur already after only 0.7 μm of microtubule extension.
The accumulation of catastrophe events at the focal adhesion sites is notable for two reasons. First, focal adhesions serve as platforms where diverse signalling pathways are initiated, including those controlling cell motility and morphology, as well as proliferation and differentiation (DeMali et al., 2003; Martin, 2003). Second, microtubule catastrophe leads to a local release of a large group of microtubule-associated regulatory proteins. For example, microtubule catastrophe and disassembly results in the release of the microtubule-binding factor Rho GEF-H1 (Krendel et al., 2002) which mediates cross-talk between microtubules and the actin cytoskeleton through activation of Rho. Release of this factor from microtubules drives cell movements during convergent extension of Xenopus laevis embryos (Kwan and Kirschner, 2005) making catastrophes critical regulatory events in Xenopus development. Additionally, a set of plus tip-binding proteins that are concentrated at the growing microtubule plus ends is released at catastrophe sites. These proteins include APC (adenomatous polyposis coli) that is able to bind members of Rac and Cdc42 regulatory pathways ASEF and IQGAP1 (Kawasaki et al., 2000; Watanabe et al., 2004) . Thus, catastrophes may be important for modulation of Rac and Cdc42 signalling that control actin cytoskeleton and cell polarity. Moreover, release of APC can induce degradation of β-catenin and thus cause Wnt signalling silencing (Polakis, 2007) suggesting that microtubule catastrophes may be involved in the regulation of cell proliferation and differentiation.
If signalling molecules are released from microtubules at adhesions, they may be brought into direct association with molecular factors concentrated at the adhesion plaques, including key players of the Src signalling pathway (Hanks et al., 2003; Parsons, 2003), molecules controlling actin polymerization (PAK (Brown et al., 2002), Arp2/3 (Kaverina et al., 2003)) and others. The aim of this study was to understand the mechanisms whereby adhesions increase the probability of microtubule catastrophes. We addressed adhesion properties that may be responsible for catastrophe enforcement.
Focal adhesions in cultured cells can be distinguished according to their molecular composition, dynamics and function (Zamir and Geiger, 2001). Early focal adhesions (also called focal complexes) are dot-like adhesions that assemble under the lamellipodium (Nobes and Hall, 1995; Rinnerthaler et al., 1988; Rottner et al., 1999). Within less than a minute of their formation, early adhesions either turn over, or undergo a force-dependent transformation into late, or mature, focal adhesions (Zaidel-Bar et al., 2003). Late adhesions are considerably larger structures that are associated with acto-myosin stress fibers and require contractility for their maintenance (Bershadsky et al., 2003; Galbraith et al., 2002). Protein composition of early and late adhesions is similar though not identical. Paxillin, the major scaffolding adhesion factor, is one of the first proteins incorporated into early adhesions and remains associated during their transformation into mature late adhesions (Zaidel-Bar et al., 2003), and the majority of adhesion proteins co-localize with paxillin throughout the cell. In contrast, zyxin was shown to be consistently absent from early adhesions and only associated with late adhesions (Rottner et al., 2001; Zaidel-Bar et al., 2003). Zyxin is also one of the first proteins dissociated from disassembling adhesions (Rottner et al., 2001). Based on these facts, we assumed that a paxillin-associated adhesion core may be considered distinct from zyxin-containing structures, and used paxillin only and zyxin/paxillin combination as markers for early and late adhesions, respectively.
Early adhesions are associated with poorly developed actin arrays and their rigidity is low compared to stress fiber-bound late adhesions (Bershadsky et al., 2003; Chen et al., 2004). However, microtubules undergo catastrophes both at early and late adhesions with the same efficiency (Fig. 3D). Decreasing of adhesion rigidity by growing cells on gelatin cushion also did not decrease their ability to cause microtubule catastrophes. Additionally, a microtubule tip hitting an intracellular obstacle often does not result in a catastrophe. Combined, these observations suggest that mechanical factors likely play a minor role in promoting catastrophes. Another option for passive induction of catastrophes is spatially decreased availability of free tubulin at dense adhesion plagues. However, such density likely differs similarly to rigidity between early and late adhesions as well as in adhesions under low tension on gelatin gel substrate. Thus, our observations indicate that a mechanical factor does not significantly contribute to catastrophe induction at adhesions. The same line of evidence suggests that spatial restriction of tubulin concentration at dense adhesions does not strongly influence catastrophe induction. We conclude that the influence of adhesions on microtubule catastrophes is a specific biochemical signal.
Catastrophes frequently occur at zyxin-free early adhesions or at the distal end of late adhesions rich in paxillin but devoid of zyxin. This finding suggests that the catastrophe factor is rather coupled with paxillin-associated than with zyxin-associated structures. Zyxin association with adhesions is promoted by acto-myosin contractility. Zyxin not only localizes to mature stress fiber-connected adhesions but it can bind stress fibers themselves under tension (Yoshigi et al., 2005). Since catastrophes tend to occur in zyxin-free loci, the presence of a putative microtubule catastrophe factor at adhesions is likely neither actin-associated nor tension-dependent. Most likely paxillin itself or paxillin-associated factors are involved in microtubule catastrophe induction at adhesion sites. Strikingly, a recent study showed that early adhesions and the distal part of late adhesions are enriched in phosphorylated paxillin (Zaidel-Bar et al., 2007). Since we describe the same adhesion domains as areas of preferential catastrophe induction, it is plausible to speculate that catastrophes could be associated with phosphorylated paxillin–based protein complexes.
It was shown previously that paxillin-tubulin binding occurs through the LIM2 and LIM3 paxillin domains and requires intact LIM zinc-finger structures (Brown et al., 2002). We found that paxillin LIM2/3 domains microinjected into fibroblastic cells localized specifically to adhesion sites replacing full-length paxillin. This led to decreased frequency of microtubule catastrophes at this location. Based on these data, we suggest a model that acknowledges the role of paxillin as a scaffolding protein at the adhesion sites (Fig. 7). According to this model, paxillin serves as a docking site for the catastrophe factor, possibly via phospho-tyrosine containing domains in the amino terminus (Fig. 7-1). When a microtubule approaches an adhesion site, a paxillin-associated factor can trigger its catastrophe and depolymerization directly (Fig. 7-2A) or via activation of a microtubule-associated catastrophe-inducing protein (Fig. 7-2B). Injected LIM2/3 domains replace full-length paxillin at adhesion sites, thereby potentially displacing the putative catastrophe factor (Fig. 7-3). As a result, microtubules do not undergo catastrophe at adhesion sites any more (Fig. 7-4). If during adhesion targeting a microtubule directly binds paxillin via its LIM2/3 domain (Brown and Turner, 2002) it may additionally promote catastrophe by bringing the microtubule and catastrophe factor in close proximity. However, such an interaction may not be necessary for catastrophe induction. Similarly, the putative catastrophe factor may not bind paxillin directly but be part of a paxillin-organized adhesion core.
Fig 7. Model of paxillin involvement in microtubule catastrophe induction at adhesion sites.
In control cells: 1) Paxillin (light blue) binds to adhesion sites (blue) through LIM2/3 domains. Catastrophe factor (Cat F, red box) binds to paxillin through sites other than LIM2/3 domains and in this way enriched at adhesion site. 2A) When microtubule (green) approaches focal adhesion catastrophe factor associated with paxillin induces microtubule catastrophe (red star) and depolymerization (green arrow). Alternatively 2B) When microtubule (green) approaches focal adhesion catastrophe factor associated with paxillin activates microtubule-associated catastrophe-inducing factor (CIF, magenta box) to induce microtubule catastrophe (magenta star) and depolymerization (green arrow).
In LIM2/3 injected cells: 3) Exogenous LIM2/3 (light blue) binds to adhesion site and replaces full-length paxillin. Catastrophe factor cannot bind to LIM2/3 mutant and is excluded from adhesion sites. 4) When microtubule approaches focal adhesion it does not undergo catastrophe and continues to polymerize (green arrow) because catastrophe factor is absent or not activated at adhesion.
The nature of the catastrophe factor involved in microtubule depolymerization at the adhesion sites remains to be clarified. One of the well-known catastrophe-inducing molecules like stathmin (Cassimeris, 2002) or kinesin-13 family, for example MCAK (Wordeman, 2005), could be engaged in the process. However, none of these proteins have been found concentrated at focal adhesions. Thus, it is more likely that upstream regulators of these proteins but not themselves are specifically recruited to the adhesion plaques and play the role of “catastrophe factors” described in our model (Fig.7-2B). As both stathmin (Larsson et al., 1997) and kinesin 13 (Andrews et al., 2004; Ohi et al., 2004) are active in de-phosphorylated form, an adhesion-associated phosphatase could serve such a role. Alternatively, a putative catastrophe factor at adhesions could act via removal of certain stabilizing factors from the microtubule tip that allows catastrophe-inducing molecules to complete their function. In this case, catastrophes may be induced by MCAK that is found at polymerizing microtubule tips in already activated form (Moore et al., 2005) and can be delivered to adhesion sites in the course of microtubule targeting of focal adhesions.
In conclusion, our findings attribute to focal adhesions a decisive role in local regulation of microtubule catastrophes. Paxillin is likely a major upstream regulator of catastrophe induction at focal adhesion sites while the actual catastrophe factor remains to be identified in our future studies.
Materials and Methods
Cells
Goldfish fin fibroblasts (line CAR, no. CCL71; American Type Culture Collection) were maintained in DMEM with nonessential amino acids, 25mM Hepes and with 10% FBS at 27°C. For experiments, cells were plated onto coverslips or glass bottom dishes (MatTek Corp.) coated with human serum fibronectin (BD Biosciences) for at least 48h. Fibronectin was coated onto coverslips or glass bottom dishes (50 μg/ml in PBS for 30 minutes at RT). Gelatin gel cushion was prepared essentially as described in (Seals et al., 2005). In brief, 2.5% gelatin in PBS containing 2.5% sucrose was allowed to polymerize for 30 minutes on glass bottom dishes (300μl per dish). Then, gels were cross-linked by 0.5% Glutar aldehyde for 45 minutes . Free aldehyde groups were blocked by 1 mg/ml NaBH4. Then, gels were coated by fibronectin as described above.
DNA constructs and transfection
mCherry-EB3 and Cerulean-EB3, made by replacing GFP in pEGFP-EB3 construct (kind gift of A. Akhmanova, Rotterdam) by Cerulean (kind gift of Dr. Piston, Nashville) or mCherry (kind gift of Dr Tsien, San Diego), EGFP-EB1 (kind gift of A. Akhmanova, Rotterdam), pEGFP-tubulin (kind gift of J. Wehland, Germany), mCherry-tubulin (kind gift of Dr. R. Tsien, San Diego) and 3xGFP-EMTB (microtubule-binding domain of E-MAP-115 (ensconsin) fused to 3 copied of EGFP (Bulinski et al., 1999), kind gift of Dr. J.C. Bulinski, New York) was used for microtubule plus tips and microtubule visualization. Neither tubulin fusion proteins (Rusan et al., 2001) nor EMTB (Faire et al., 1999) change microtubule dynamics and are routinely used for in vivo studies. GFP-paxillin (West et al., 2001), mCherry-paxillin (kind gift of Dr. S. Hanks, Nashville), mCherry-Zyxin made by replacing GFP in pEGFP-zyxin construct (kind gift of J. Wehland, Germany) by mCherry and Cerulean-Zyxin was constructed by cloning of zyxin from mCherry-Zyxin into mCerulean-C1 vector, were used for adhesion sites visualization. Sub-confluent monolayer cultures on 30mm Petri dishes were used for transfection. For each dish, the transfection mixture was prepared as follows: 1,5-2μg total DNA, 12 μl of Superfect lipofection agent (Qiagen) were mixed in 200μl of serum-free medium. After 30 minute incubation at RT a further 1.3 ml of medium containing 5% serum was added. Cells were incubated in this mixture for 4h at 27°C and the medium then replaced by normal medium containing 10% serum. After 24h, cells were replated at a desired dilution onto coverslips or glass-bottom dishes for microscopy.
Microinjections
Injections were performed with sterile Femtotips (Eppendorf) held in a Leitz Micromanipulator with a pressure supply from an Eppendorf Microinjector 5242. Cells were injected with a continuous outflow mode from the needle under a constant pressure of between 20 and 40hPa.
Proteins for microinjection
Cy3-tubulin was kindly provided by F. Severin (Max Plan Institute, Dresden). It was stored at a concentration of 20 mg/ml in aliquots at −70°C. For microinjections, Cy3-tubulin aliquots were diluted 1:3 with Tris-acetate injection buffer (2mM Tris-acetate, pH 7.0, 50mM KCl and 0.1 mM DTE) and used on the same day. GST only, GST-LIM1, GST-LIM2, GST-LIM3 and GST-LIM2/3 fusion recombinant proteins were expressed in E. coli and purified as described elsewhere (Brown MC et al., 1998). GST, GST-LIM1, GST-LIM2, GST-LIM3 and GST-LIM2/3 were stored at a concentration 1.6 mg/ml at −70°C.
Video microscopy of transfected cells
TIRF live cell videos were acquired on Nikon TE2000E microscope equipped with Perfect Focus System, Nikon TIRF2 System for TE2000 using TIRF 100x NA 1.49 oil lens and back-illuminated EM-CCD camera Cascade 512B (Photometrics) driven by IPLab software (Scanalytics). 40mW Argon laser (Melles Griot) and 10 mW DPSS laser 85YCA010 (Melles Griot) were used for excitation. Custom double dichroic TIRF mirror and emission filters (Chroma) in filter wheel (Ludl) were used. Three-channel movies were made with the TIRF setting as described above for 2 channels and wide field fluorescence using BrightLine-CFP 2432A filter cube (Semrock) for the third channel.
Video microscopy of injected cells
Cells were injected and observed in an open chamber at RT on an inverted microscope (Axiovert 135TV; Zeiss) equipped for epifluorescence and phase contrast microscopy. Injections were performed at an objective magnification of 40X (NA 1.3 Plan Neofluar) and video microscopy with a 100X/NA 1.4 Plan-Apochroma objective. Double fluorescence was achieved by GFP/RFP Pinkel filter set (Chroma) in a custom-made filter wheel. Tungsten lamps (100W) were used for both transmitted and epiillumination. Data were acquired with a back-illuminated, cooled CCD camera from Princeton Research Instruments driven by IPLabs software. Paxillin displacement from focal adhesions where observed by live TIRF imaging of GFP-paxillin expressing cells as described above.
Photobleaching
Cells co-expressing mCherry-alpha-tubulin and GFP-paxillin where photobleached for 10s with 10 mW DPSS laser 85YCA010 (Melles Griot) by focusing laser light in the focal plane with custom-made lens (Nikon) placed in position of filter cube. 2.5 minutes two channel movies were recorded after bleaching using TIRF as described above.
Immunofluorescence Microscopy
For immunostaining, cells were extracted for 1 minute in 0.25% Triton X-100 in cytoskeleton buffer (CB: 10mM MES, 150mM NaCL, 5mM EGTA, 5mM glucose, and 5mM MgCl2, pH 6.1) and fixed for 20 minutes in 3% paraformaldehyde in CB. Immunostaining was performed using polyclonal goat-anti-GST- and mouse-anti-vinculin antibodies.
Quantitative Analysis
Microtubule catastrophe
time-lapse movies of cells expressing 3xGFP-EMTB to label microtubules and mCherry-paxillin to mark adhesion sites (5 seconds/frame) were processed by background subtraction and intensity adjustment using ImageJ software. Coordinates of each catastrophe event were recorded and then checked either of not there is the adhesion site with the same coordinates.
Average microtubule elongation
time-lapse movies of cells expressing mCherry-tubulin and GFP-zyxin (5 seconds/frame) were processed by background subtraction and intensity adjustment using ImageJ software. Trajectories of microtubule plus end movement were followed using a plugin of ImageJ (“manual tracking”). Microtubule elongation in the cytoplasm or at adhesion sites was normalized by the number of catastrophes in each location for each microtubule. Then the average elongation distance was calculated for all microtubules. Statistics was performed in Microsoft Excel.
Tip dynamics at adhesion sites
time-lapse movies of cells expressing mCherry-EB3 and GFP-paxillin (5 seconds/frame) were processed for intensity adjustment using ImageJ. For uniform analysis, only most common cases when microtubule tips approached adhesions from their proximal ends were considered. Narrow areas around adhesion sites were delineated and combined into kymographs. The area was divided into zones of 1μm each (Fig. 2C). For each microtubule tip, the time it spends in each zone and place of catastrophe were manually calculated. Microtubules which pass through the zone in 1–2 frames (10 seconds or less) were considered as fast microtubules and in 3 frames or more (15 seconds and more) as slow microtubules. For each zone the number of approaching microtubule tips and disappearing tips was calculated. For zones −1, 0 and 1 the probability of microtubule undergoing catastrophe versus the time the microtubule tip spent in the zone was calculated. Statistics was performed in Microsoft Excel.
Catastrophes at early and late adhesions
time-lapse movies of cells expressing 3xGFP-EMTB, mCherry-paxillin and Cerulean-zyxin (5sec/frame) were used. Trajectories of microtubule plus end movement were followed using the plugin (“manual tracking”) of ImageJ software. Coordinates of each catastrophe were recorded and then checked with localization of paxillin and zyxin using ImageJ software. Statistics was performed in Microsoft Excel.
Microtubule dynamics analysis after microinjection
The calculation of microtubule growth velocity and catastrophe events were made using MetaMorph software, statistics was performed in Microsoft Excel.
Supplementary Material
Acknowledgments
We thank Dr. Steve Hanks and Dr. Ethan Lee for helpful discussion and advice. This study was funded by Development funds of Department of Cell and Developmental Biology, Vanderbilt University Medical Center and by pilot project within ACS IRG-58-009-49 grant to I.K., by Austrian Science Fund grant #P16743-B09P to JVS and NIH grant RO1 GM47607 to C.E.T.
References
- Amos LA, Schlieper D. Microtubules and maps. Adv Protein Chem. 2005;71:257–98. doi: 10.1016/S0065-3233(04)71007-4. [DOI] [PubMed] [Google Scholar]
- Andrews PD, Ovechkina Y, Morrice N, Wagenbach M, Duncan K, Wordeman L, Swedlow JR. Aurora B regulates MCAK at the mitotic centromere. Dev Cell. 2004;6:253–68. doi: 10.1016/s1534-5807(04)00025-5. [DOI] [PubMed] [Google Scholar]
- Axelrod D. Total internal reflection fluorescence microscopy. Methods Cell Biol. 1989;30 :245–70. doi: 10.1016/s0091-679x(08)60982-6. [DOI] [PubMed] [Google Scholar]
- Bershadsky AD, Balaban NQ, Geiger B. Adhesion-dependent cell mechanosensitivity. Annu Rev Cell Dev Biol. 2003;19:677–95. doi: 10.1146/annurev.cellbio.19.111301.153011. [DOI] [PubMed] [Google Scholar]
- Brown MC, Perrotta JA, Turner CE. Identification of LIM3 as the principal determinant of paxillin focal adhesion localization and characterization of a novel motif on paxillin directing vinculin and focal adhesion kinase binding. J Cell Biol. 1996;135:1109–23. doi: 10.1083/jcb.135.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MC, Turner CE. Roles for the tubulin- and PTP-PEST-binding paxillin LIM domains in cell adhesion and motility. Int J Biochem Cell Biol. 2002;34:855–63. doi: 10.1016/s1357-2725(01)00154-6. [DOI] [PubMed] [Google Scholar]
- Brown MC, Turner CE. Paxillin: adapting to change. Physiol Rev. 2004;84:1315–39. doi: 10.1152/physrev.00002.2004. [DOI] [PubMed] [Google Scholar]
- Brown MC, West KA, Turner CE. Paxillin-dependent paxillin kinase linker and p21-activated kinase localization to focal adhesions involves a multistep activation pathway. Mol Biol Cell. 2002;13:1550–65. doi: 10.1091/mbc.02-02-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulinski JC, Gruber D, Faire K, Prasad P, Chang W. GFP chimeras of E-MAP-115 (ensconsin) domains mimic behavior of the endogenous protein in vitro and in vivo. Cell Struct Funct. 1999;24:313–20. doi: 10.1247/csf.24.313. [DOI] [PubMed] [Google Scholar]
- Cassimeris L. The oncoprotein 18/stathmin family of microtubule destabilizers. Curr Opin Cell Biol. 2002;14:18–24. doi: 10.1016/s0955-0674(01)00289-7. [DOI] [PubMed] [Google Scholar]
- Chen CS, Tan J, Tien J. Mechanotransduction at cell-matrix and cell-cell contacts. Annu Rev Biomed Eng. 2004;6:275–302. doi: 10.1146/annurev.bioeng.6.040803.140040. [DOI] [PubMed] [Google Scholar]
- Danowski BA. Microtubule dynamics in serum-starved and serum-stimulated Swiss 3T3 mouse fibroblasts: implications for the relationship between serum-induced contractility and microtubules. Cell Motil Cytoskeleton. 1998;40:1–12. doi: 10.1002/(SICI)1097-0169(1998)40:1<1::AID-CM1>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- DeMali KA, Wennerberg K, Burridge K. Integrin signaling to the actin cytoskeleton. Curr Opin Cell Biol. 2003;15:572–82. doi: 10.1016/s0955-0674(03)00109-1. [DOI] [PubMed] [Google Scholar]
- Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–43. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S. Actin and microtubules in cell motility: which one is in control? Traffic. 2004;5:470–7. doi: 10.1111/j.1600-0854.2004.00196.x. [DOI] [PubMed] [Google Scholar]
- Faire K, Waterman-Storer CM, Gruber D, Masson D, Salmon ED, Bulinski JC. E-MAP-115 (ensconsin) associates dynamically with microtubules in vivo and is not a physiological modulator of microtubule dynamics. J Cell Sci. 1999;112 (Pt 23):4243–55. doi: 10.1242/jcs.112.23.4243. [DOI] [PubMed] [Google Scholar]
- Galbraith CG, Yamada KM, Sheetz MP. The relationship between force and focal complex development. J Cell Biol. 2002;159:695–705. doi: 10.1083/jcb.200204153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks SK, Ryzhova L, Shin NY, Brabek J. Focal adhesion kinase signaling activities and their implications in the control of cell survival and motility. Front Biosci. 2003;8:d982–96. doi: 10.2741/1114. [DOI] [PubMed] [Google Scholar]
- Herreros L, Rodriguez-Fernandez JL, Brown MC, Alonso-Lebrero JL, Cabanas C, Sanchez-Madrid F, Longo N, Turner CE, Sanchez-Mateos P. Paxillin localizes to the lymphocyte microtubule organizing center and associates with the microtubule cytoskeleton. J Biol Chem. 2000;275:26436–40. doi: 10.1074/jbc.M003970200. [DOI] [PubMed] [Google Scholar]
- Howard J, Hyman AA. Microtubule polymerases and depolymerases. Curr Opin Cell Biol. 2007;19:31–5. doi: 10.1016/j.ceb.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Janson ME, de Dood ME, Dogterom M. Dynamic instability of microtubules is regulated by force. J Cell Biol. 2003;161:1029–34. doi: 10.1083/jcb.200301147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaverina I, Krylyshkina O, Beningo K, Anderson K, Wang YL, Small JV. Tensile stress stimulates microtubule outgrowth in living cells. J Cell Sci. 2002;115:2283–91. doi: 10.1242/jcs.115.11.2283. [DOI] [PubMed] [Google Scholar]
- Kaverina I, Krylyshkina O, Small JV. Microtubule targeting of substrate contacts promotes their relaxation and dissociation. J Cell Biol. 1999;146:1033–44. doi: 10.1083/jcb.146.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaverina I, Rottner K, Small JV. Targeting, capture, and stabilization of microtubules at early focal adhesions. J Cell Biol. 1998;142:181–90. doi: 10.1083/jcb.142.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaverina I, Stradal TE, Gimona M. Podosome formation in cultured A7r5 vascular smooth muscle cells requires Arp2/3-dependent de-novo actin polymerization at discrete microdomains. J Cell Sci. 2003;116:4915–24. doi: 10.1242/jcs.00818. [DOI] [PubMed] [Google Scholar]
- Kawasaki Y, Senda T, Ishidate T, Koyama R, Morishita T, Iwayama Y, Higuchi O, Akiyama T. Asef, a link between the tumor suppressor APC and G-protein signaling. Science. 2000;289:1194–7. doi: 10.1126/science.289.5482.1194. [DOI] [PubMed] [Google Scholar]
- Krendel M, Zenke FT, Bokoch GM. Nucleotide exchange factor GEF-H1 mediates cross-talk between microtubules and the actin cytoskeleton. Nat Cell Biol. 2002;4:294–301. doi: 10.1038/ncb773. [DOI] [PubMed] [Google Scholar]
- Kwan KM, Kirschner MW. A microtubule-binding Rho-GEF controls cell morphology during convergent extension of Xenopus laevis. Development. 2005;132:4599–610. doi: 10.1242/dev.02041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson N, Marklund U, Gradin HM, Brattsand G, Gullberg M. Control of microtubule dynamics by oncoprotein 18: dissection of the regulatory role of multisite phosphorylation during mitosis. Mol Cell Biol. 1997;17:5530–9. doi: 10.1128/mcb.17.9.5530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GS. Cell signaling and cancer. Cancer Cell. 2003;4:167–74. doi: 10.1016/s1535-6108(03)00216-2. [DOI] [PubMed] [Google Scholar]
- Mitchison T, Kirschner M. Dynamic instability of microtubule growth. Nature. 1984;312:237–42. doi: 10.1038/312237a0. [DOI] [PubMed] [Google Scholar]
- Moore AT, Rankin KE, von Dassow G, Peris L, Wagenbach M, Ovechkina Y, Andrieux A, Job D, Wordeman L. MCAK associates with the tips of polymerizing microtubules. J Cell Biol. 2005;169:391–7. doi: 10.1083/jcb.200411089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasaki T, Chapin CJ, Gundersen GG. Distribution of detyrosinated microtubules in motile NRK fibroblasts is rapidly altered upon cell-cell contact: implications for contact inhibition of locomotion. Cell Motil Cytoskeleton. 1992;23:45–60. doi: 10.1002/cm.970230106. [DOI] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- Ohi R, Sapra T, Howard J, Mitchison TJ. Differentiation of cytoplasmic and meiotic spindle assembly MCAK functions by Aurora B-dependent phosphorylation. Mol Biol Cell. 2004;15:2895–906. doi: 10.1091/mbc.E04-02-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons JT. Focal adhesion kinase: the first ten years. J Cell Sci. 2003;116:1409–16. doi: 10.1242/jcs.00373. [DOI] [PubMed] [Google Scholar]
- Polakis P. The many ways of Wnt in cancer. Curr Opin Genet Dev. 2007;17:45–51. doi: 10.1016/j.gde.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Rinnerthaler G, Geiger B, Small JV. Contact formation during fibroblast locomotion: involvement of membrane ruffles and microtubules. J Cell Biol. 1988;106:747–60. doi: 10.1083/jcb.106.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez OC, Schaefer AW, Mandato CA, Forscher P, Bement WM, Waterman-Storer CM. Conserved microtubule-actin interactions in cell movement and morphogenesis. Nat Cell Biol. 2003;5:599–609. doi: 10.1038/ncb0703-599. [DOI] [PubMed] [Google Scholar]
- Rottner K, Hall A, Small JV. Interplay between Rac and Rho in the control of substrate contact dynamics. Curr Biol. 1999;9:640–8. doi: 10.1016/s0960-9822(99)80286-3. [DOI] [PubMed] [Google Scholar]
- Rottner K, Krause M, Gimona M, Small JV, Wehland J. Zyxin is not colocalized with vasodilator-stimulated phosphoprotein (VASP) at lamellipodial tips and exhibits different dynamics to vinculin, paxillin, and VASP in focal adhesions. Mol Biol Cell. 2001;12:3103–13. doi: 10.1091/mbc.12.10.3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusan NM, Fagerstrom CJ, Yvon AM, Wadsworth P. Cell cycle-dependent changes in microtubule dynamics in living cells expressing green fluorescent protein-alpha tubulin. Mol Biol Cell. 2001;12:971–80. doi: 10.1091/mbc.12.4.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon ED, Leslie RJ, Saxton WM, Karow ML, McIntosh JR. Spindle microtubule dynamics in sea urchin embryos: analysis using a fluorescein-labeled tubulin and measurements of fluorescence redistribution after laser photobleaching. J Cell Biol. 1984;99:2165–74. doi: 10.1083/jcb.99.6.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals DF, Azucena EF, Jr, Pass I, Tesfay L, Gordon R, Woodrow M, Resau JH, Courtneidge SA. The adaptor protein Tks5/Fish is required for podosome formation and function, and for the protease-driven invasion of cancer cells. Cancer Cell. 2005;7:155–65. doi: 10.1016/j.ccr.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Shelden E, Wadsworth P. Observation and quantification of individual microtubule behavior in vivo: microtubule dynamics are cell-type specific. J Cell Biol. 1993;120:935–45. doi: 10.1083/jcb.120.4.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small JV, Kaverina I. Microtubules meet substrate adhesions to arrange cell polarity. Curr Opin Cell Biol. 2003;15:40–7. doi: 10.1016/s0955-0674(02)00008-x. [DOI] [PubMed] [Google Scholar]
- Tran AD, Marmo TP, Salam AA, Che S, Finkelstein E, Kabarriti R, Xenias HS, Mazitschek R, Hubbert C, Kawaguchi Y, et al. HDAC6 deacetylation of tubulin modulates dynamics of cellular adhesions. J Cell Sci. 2007;120:1469–79. doi: 10.1242/jcs.03431. [DOI] [PubMed] [Google Scholar]
- Wadsworth P. Regional regulation of microtubule dynamics in polarized, motile cells. Cell Motil Cytoskeleton. 1999;42:48–59. doi: 10.1002/(SICI)1097-0169(1999)42:1<48::AID-CM5>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Wang S, Noritake J, Sato K, Fukata M, Takefuji M, Nakagawa M, Izumi N, Akiyama T, Kaibuchi K. Interaction with IQGAP1 links APC to Rac1, Cdc42, and actin filaments during cell polarization and migration. Dev Cell. 2004;7:871–83. doi: 10.1016/j.devcel.2004.10.017. [DOI] [PubMed] [Google Scholar]
- Waterman-Storer CM, Salmon ED. Actomyosin-based retrograde flow of microtubules in the lamella of migrating epithelial cells influences microtubule dynamic instability and turnover and is associated with microtubule breakage and treadmilling. J Cell Biol. 1997;139:417–34. doi: 10.1083/jcb.139.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West KA, Zhang H, Brown MC, Nikolopoulos SN, Riedy MC, Horwitz AF, Turner CE. The LD4 motif of paxillin regulates cell spreading and motility through an interaction with paxillin kinase linker (PKL) J Cell Biol. 2001;154:161–76. doi: 10.1083/jcb.200101039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann T, Waterman-Storer CM. Cell motility: can Rho GTPases and microtubules point the way? J Cell Sci. 2001;114:3795–803. doi: 10.1242/jcs.114.21.3795. [DOI] [PubMed] [Google Scholar]
- Wordeman L. Microtubule-depolymerizing kinesins. Curr Opin Cell Biol. 2005;17:82–8. doi: 10.1016/j.ceb.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Yoshigi M, Hoffman LM, Jensen CC, Yost HJ, Beckerle MC. Mechanical force mobilizes zyxin from focal adhesions to actin filaments and regulates cytoskeletal reinforcement. J Cell Biol. 2005;171:209–15. doi: 10.1083/jcb.200505018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidel-Bar R, Ballestrem C, Kam Z, Geiger B. Early molecular events in the assembly of matrix adhesions at the leading edge of migrating cells. J Cell Sci. 2003;116:4605–13. doi: 10.1242/jcs.00792. [DOI] [PubMed] [Google Scholar]
- Zaidel-Bar R, Milo R, Kam Z, Geiger B. A paxillin tyrosine phosphorylation switch regulates the assembly and form of cell-matrix adhesions. J Cell Sci. 2007;120:137–48. doi: 10.1242/jcs.03314. [DOI] [PubMed] [Google Scholar]
- Zamir E, Geiger B. Molecular complexity and dynamics of cell-matrix adhesions. J Cell Sci. 2001;114:3583–90. doi: 10.1242/jcs.114.20.3583. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.