Abstract
Cognitive strategies typically involved in regulating negative emotions have recently been shown to also be effective with positive emotions associated with monetary rewards. However, it is less clear how these strategies influence behavior, such as preferences expressed during decision-making under risk, and the underlying neural circuitry. That is, can the effective use of emotion regulation strategies during presentation of a reward-conditioned stimulus influence decision-making under risk and neural structures involved in reward processing such as the striatum? To investigate this question, we asked participants to engage in imagery-focused regulation strategies during the presentation of a cue that preceded a financial decision-making phase. During the decision phase, participants then made a choice between a risky and a safe monetary lottery. Participants who successfully used cognitive regulation, as assessed by subjective ratings about perceived success and facility in implementation of strategies, made fewer risky choices in comparison to trials where decisions were made in the absence of cognitive regulation. Additionally, blood-oxygen-level-dependent (BOLD) responses in the striatum were attenuated during decision-making as a function of successful emotion regulation. These findings suggest that exerting cognitive control over emotional responses can modulate neural responses associated with reward processing (e.g., striatum), and promote more goal-directed decision-making (e.g., less risky choices), illustrating the potential importance of cognitive strategies in curbing risk-seeking behaviors before they become maladaptive (e.g., substance abuse).
Keywords: Reward, emotion regulation, reappraisal, risk, addiction, striatum, insula, dopamine, decision-making, fMRI
INTRODUCTION
The ability to control emotional responses is essential for adaptive function. For instance, an individual unable to cope with sudden urges elicited by a conditioned stimulus (e.g., casino environment) may engage in maladaptive risk-seeking behavior (e.g., gambling) that can potentially turn into a compulsive disorder (Kushner, Abrams, Donahue, Thuras, Frost, & Kim, 2007). One promising intervention is the application of cognitive strategies during the emotion generation process, a practice known as emotion regulation, which results in an alteration in the affective experience of emotional stimuli (Ochsner & Gross, 2005). The use of such cognitive strategies has been shown to decrease physiological and subjective responses associated with the expectation of prospective monetary rewards, which in turn modulate blood oxygenated level dependent (BOLD) responses in the striatum (Delgado, Gillis, & Phelps, 2008; Staudinger, Erk, Abler, & Walter, 2009), a region previously associated with reward-related processing (Delgado, 2007; Haber & Knutson, 2010; O’Doherty, 2004; Rangel, Camerer, & Montague, 2008).
It is unclear, however, if the effects of emotion regulation can extend beyond changes in emotional experience to changes in goal-directed behavior. Affective responses elicited by salient cues are known to influence behavior, for instance cue-induced drug craving is associated with increased drug-seeking (Weiss, 2005). Recently, application of regulation strategies to drug cues has been found to reduce subjective feelings of craving in cigarette smokers (Kober, Kross, Mischel, Hart, & Ochsner, 2009) and in cocaine abusers (Volkow, Fowler, Wang, Telang, Logan, Jayne et al., 2010), and lead to decreased activation in regions such as the ventral striatum. While these studies did not probe shifts in behavior associated with regulation of craving, it is possible that regulation of such conditioned cues can extend to risk-taking behaviors such as drug-seeking. The goal of the current study was to examine the effect of cognitive regulation of a conditioned cue on subsequent behavior in the normative brain. Specifically, this study probed if the successful use of cognitive strategies during presentation of a conditioned stimulus (e.g., slot machine) would influence decision-making under risk (e.g., gambling) and associated neural circuits such as the striatum.
One hypothesis was that emotion regulation would lead to increased risk-seeking behavior, as individuals who successfully regulate tend to make choices that maximize performance (Seo & Barrett, 2007) and place less weight on the outcome of a single decision, in turn leading to a reduction in loss aversion (Sokol-Hessner, Hsu, Curley, Delgado, Camerer, & Phelps, 2009). An alternative hypothesis, however, was that exerting cognitive control over emotional responses would promote more goal-directed decision-making, thus attenuating risky decisions and associated BOLD signals in the striatum. This hypothesis was motivated by previous observations that imagery-focused regulation modulated the expectation of reward and blood-oxygen-level-dependent (BOLD) responses in reward-related areas (Delgado, Gillis, & Phelps, 2008). The multifaceted human striatum is a region often identified during investigations of risky decision-making (Christopoulos, Tobler, Bossaerts, Dolan, & Schultz, 2009; Kuhnen & Knutson, 2005; Matthews, Simmons, Lane, & Paulus, 2004), whose signals correlate with drug specific cravings (Sinha, Lacadie, Skudlarski, Fulbright, Rounsaville, Kosten et al., 2005) and impulsive, risky decisions in substance users (Leland, Arce, Feinstein, & Paulus, 2006). As previously mentioned, neural signals in the striatum have also been reported to be modulated by emotion regulation strategies during expectation of monetary (Delgado, Gillis, & Phelps, 2008; Staudinger, Erk, Abler et al., 2009) and drug (Kober, Kross, Mischel et al., 2009; Volkow, Fowler, Wang et al., 2010) rewards. Thus, the striatum provides an ideal target for potential regulatory influences that may occur during decision-making under risk.
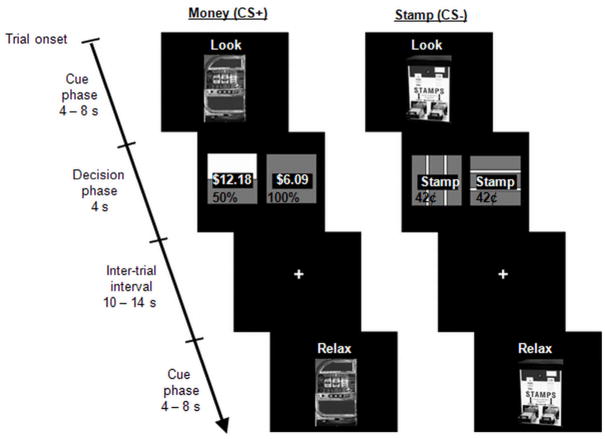
We investigated the effect of cognitive regulation on risk-taking and its neural correlates using a functional magnetic resonance imaging (fMRI) experimental paradigm that included both a cue and a decision phase. The cue phase consisted of the presentation of a conditioned stimulus (CS+ or CS−) and a cognitive instruction (“Look”, “Relax” or “Excite”). The decision phase followed the cue presentation and comprised either a selection between risky and safe monetary lotteries (CS+ trials) or a non-monetary control decision between two different stamps (CS− trials; Fig. 1). Decision-making under risk was quantified as the proportion of trials in which the risky option was chosen for each type of cognitive instruction. Finally, we acquired post-experimental self-assessment of participants’ perceived success in using the cognitive strategies in order to probe how the successful application of emotion regulation modulates decision-making under risk and its associated neural correlates.
Fig. 1.
The paradigm consisted of the presentation of a picture of a conditioned stimulus (CS) and a cognitive instruction. The CS was either a slot machine (CS+), which predicted a monetary decision between a safe and a risky lottery, or a stamp machine (CS−), which predicted a non-monetary choice between stamps. Participants applied emotion regulation strategies (Relax or Excite) or acted naturally (Look) during presentation of both CS trials, followed by a decision-making phase.
METHODS
Participants
Thirty-five right-handed volunteers participated in this study (17 female, 18 male). Three participants were excluded due to failure to comply with task requirements (assessed by post-experimental questionnaires), which included not following instructions and using an incorrect strategy. One additional participant was excluded due to indifference during task performance as assessed by behavior (i.e., participant consistently chose one response) and self-report. Finally, data from one MRI session was excluded due to equipment malfunction. Thus, final analysis was conducted on thirty participants (15 female, 15 male; mean age: M = 20.87, SD = 2.99). Participants responded to posted advertisements and gave informed consent according to the Rutgers University Institutional Review Board for the Protection of Human Subjects in Research and the Newark Campus Institutional Review Board of the University of Medicine and Dentistry of New Jersey.
Procedure
The experimental task consisted of 90 trials, divided into 6 blocks of 15 trials. Each trial started with the cue phase, involving the presentation of a conditioned stimulus (CS; a slot machine, CS+ or stamp machine, CS−) and a cognitive instruction (Look, Relax or Excite) for a variable duration of 4, 6 or 8 s (Fig. 1). The conditioned stimulus indicated if the trial presented an opportunity to earn money (CS+) or not (CS−). The cognitive instruction was presented above the CS and directed participants to either a) respond naturally to the slot machine, that is, think about the decision coming up and the chance to win money (“Look”); b) engage in imagery-focused regulation by imagining a calming scene (“Relax”) or c) imagine an exciting scene (“Excite”). The instructions were adapted from previous experiments that used an imagery-focused regulation strategy (Delgado, Gillis, & Phelps, 2008; Delgado, Nearing, LeDoux, & Phelps, 2008), initially based on more traditional emotion regulation techniques (Ochsner, Bunge, Gross, & Gabrieli, 2002; Ochsner, Ray, Cooper, Robertson, Chopra, Gabrieli et al., 2004). The cue phase was followed by the decision phase, where participants were presented with two options for a fixed duration of 4 s. For CS+ trials, participants chose between two monetary options: a gamble (risky option) and a guaranteed amount (safe option) that varied with respect to probability and amount. For CS− trials, the decision carried no affective significance, as participants chose between two different representations of postage stamps with no monetary value. A jittered 10 to 14 s inter-trial interval followed the decision phase.
Participants received no immediate feedback about the outcomes of their decisions. To ensure the perception that each decision was independent and significant, six decisions (lotteries) were realized during the experimental session. These outcome sessions occurred during three specific periods during the experiment. The first outcome session occurred after the initial two task blocks and as a result, reflected the resolution of those two task blocks. That is, two decisions were resolved, with one decision being chosen from each of the two blocks just completed. The second outcome session occurred after task blocks 3 and 4, while the third and final outcome session occurred at the end of the experiment (after task blocks 5 and 6). During each of these three outcome sessions, participants first saw the computer select two decisions from the five possible decision types by spinning a wheel. Next, they watched the experimenter open their data file to identify their choices (risky or safe option) for those decisions. Finally, participants were informed by the experimenter over the intercom of the outcomes of the decisions and how much money they had won. Participants were compensated a base rate of $20, plus whatever money they earned from the six selected decisions. The decisions selected were the same for all participants leading to an average earning of $53.33 (SD = $4.08).
Prior to scanning, participants were extensively trained on the task instructions, especially the application of the emotion regulation techniques. They were informed that pictures of a slot machine and a stamp machine would serve as cues to signal upcoming decisions involving either money or stamps, respectively. They were also informed that a word presented above the picture would serve as the instruction for that trial. There were three such instructions: “Look”, “Relax” and “Excite”. When instructed to “Look” participants were asked to look at the picture while it was presented on the screen and react naturally while contemplating its significance for them in this “game”. More specifically, when the instruction Look was paired with the slot machine, they were asked to think that they would have to make a financial decision and based on their choice, they could potentially win money. In contrast, when the instruction Look was paired with the stamp machine, they would think about a potential decision between two stamps with no financial outcome. When instructed to “Relax”, participants were prompted to imagine a calming scene such as a sunny day in a park. During the training period, each participant generated his/her own image with guidance from the experimenter with the requirements that such imagery would be relaxing and easy to conjure up to facilitate regulation. Participants were instructed to think of the same image each time the word Relax was presented, irrespective of type of trial (CS+, CS−). Finally, participants were also presented with a third instructional cue named “Excite”. For the Excite emotion regulation instruction, participants were to imagine an exciting scene, such as a roller coaster ride, in order to increase their arousal.
There were five different financial decisions in the task (Table 1). Each lottery included a risky option with one of five different levels of probability (0.20, 0.35, 0.50, 0.65, 0.80) and a safe option with an amount equivalent to the expected value of the gamble (e.g. risky: 20% chance of winning $10.35 or safe: 100% chance of winning $2.07). The location (right or left side of screen) of the risky and safe options was counterbalanced. For CS− trials, participants made decisions between two stamps with different patterns, with four types of stamps included overall and presentation location being counterbalanced. Participants used a MRI compatible response unit and used either the index or middle finger of the right hand to make a decision during both CS+ and CS− trials. Thus, the experiment included six different types of trials that varied in respect to cognitive strategy (Look, Relax and Excite) and affective significance of decision (CS+ and CS−). There were 60 CS+ trials with 20 of each instruction and 30 CS− trials with 15 of each instruction. The five different financial decisions were repeated 12 times (four times with each instruction).
Table 1.
Financial decisions included in experimental paradigm
| Lottery | Risky Option
|
Safe Option
|
||
|---|---|---|---|---|
| Probability | Value | Probability | Value | |
| 1 | 0.20 | $10.35 | 1 | $2.07 |
| 2 | 0.35 | $11.66 | 1 | $4.08 |
| 3 | 0.50 | $12.18 | 1 | $6.09 |
| 4 | 0.65 | $6.28 | 1 | $4.08 |
| 5 | 0.80 | $2.59 | 1 | $2.07 |
At the conclusion of the scanning session, participants completed several questionnaires including a post-experimental questionnaire that assessed compliance with the emotion regulation demands and measured perceived successful use of cognitive strategies. Additional questionnaires that considered potential individual differences included a measure of risk preferences (Holt & Laury, 2002), use of emotion regulation strategies (Emotion Regulation Questionnaire; Gross & John, 2003) and behavioral inhibition and activation (BIS/BAS; Carver & White, 1994). Finally, at least a day after the scanning session, participants were asked to complete a paper questionnaire with the five financial decisions from the scanner task along with two variations where the amounts were either increased or decreased by $0.50. This additional questionnaire allowed for the evaluation of individual’s choice preferences in the absence of any regulation instruction.
fMRI Acquisition & Analysis
Imaging data were acquired using a 3T Siemens Allegra head-only scanner with a standard head coil at Rutgers’ University Heights Center for Advanced Imaging. Structural images were acquired using a T1-weighted sequence (256 × 256 matrix, 176 1 mm sagittal slice). Functional images were acquired using a single-shot gradient echo EPI sequence (TR = 2000 ms, TE = 25 ms, FOV = 192 cm, flip angle = 80°, bandwidth = 2604 Hz/px, echo spacing = 0.29 ms). Thirty-five contiguous (3 × 3 × 3 mm voxels) oblique-axial images were acquired parallel to the AC-PC line. Imaging data analysis was performed with Brain Voyager software (version 1.9: Brain Innovation, Maastricht, The Netherlands). Data were corrected for excessive motion (using a cutoff of 2mm within a run) and slice scan time adjustments were made using sinc interpolation. Spatial filtering was performed using a three-dimensional Gaussian filter (4 mm FWHM) while temporal filtering was used with voxel-wise linear detrending and high-pass filtering of frequencies (three cycles per time-course). Finally, structural and functional data for each participant were transformed into standard Talairach stereotaxic space (Talairach & Tournoux, 1988).
A random-effects analysis was performed on the functional data using a general linear model (GLM) that estimated beta weights for two boxcar predictors (cue phase and decision phase) and one parametric predictor time-locked to the onset of the decision phase that varied in accordance to the five levels of probability (0.20, 0.35, 0.50, 0.65, 0.80). This analysis allowed for the non-biased identification of functionally defined regions of interest (ROIs) involved in decision-making under risk. Previous studies have examined neural coding of expected value of rewards (e.g., Knutson & Cooper, 2005) and how this process is modulated by emotion regulation (Delgado, Gillis, & Phelps, 2008; Staudinger, Erk, Abler et al., 2009); thus a goal of the current study was to extend that research by probing neural coding of risk (i.e., probability) information during decision-making and examining modulation by emotion regulation. Statistical maps were created using the False Discovery Rate (FDR) method with a threshold of q < 0.01 (Genovese, Lazar, & Nichols, 2002), and functional ROIs were extracted based on a peak voxel center and a cluster extent of 10 voxels in all directions. To test for modulation by emotion regulation, mean parameter estimates (i.e., beta weights) were extracted from the functional ROIs defined by the parametric probability predictor using a second GLM that included 18 different predictors that indicated the instruction (Look, Relax, Excite) and subsequent choice (risky, safe, stamp) at the time of the cue phase and the instruction (Look, Relax, Excite) and choice (risky, safe, stamp) at the time of the decision phase. Additionally, missed trials and six motion parameters were included as predictors of no interest. Analysis of variance (ANOVA) tests were then performed on the extracted beta weights to probe the effects of emotion regulation on decision-making under risk during the decision phase.
RESULTS
Behavioral Results
Subjective Ratings
Subjective ratings of excitement experienced during presentation of the CS+ (the slot machine) and the CS− (the stamp machine) cues were acquired throughout the experiment to verify the affective value attributed to CS+ trials. Specifically, these ratings were collected six times during the experimental task, once after each of the six experimental blocks of trials, and were independent of the emotion regulation manipulation (i.e., did not include the instruction words Look, Relax, Excite). Participants rated how excited they felt when they saw the slot machine and the stamp machine using a Likert scale (1 = not at all excited; 7 = extremely excited). Using ratings from all participants a comparison of the averaged ratings was made with a repeated-measures ANOVA with CS type (slot machine, stamp machine) as a within subjects factor. Participants felt significantly more excited about the slot machine (M = 5.32, SD = 0.83) than the stamp machine (M = 2.97, SD = 1.19) during the task [F(1, 29) = 107.07, p < 0.001], suggesting that participants associated the slot machine cue with an opportunity for reward.
After the scanning session, all participants completed a post-experiment questionnaire, which addressed whether they had effectively used the two imagery-focused regulation strategies. Specifically, participants rated how successful they were at visualizing relaxing imagery using a Likert scale in which 1 = not at all successful and 7 = very successful. Participants also completed this rating for the excite visualization. These subjective ratings provide an index of regulation success, and they suggest that participants felt fairly successful at the Relax (M = 5.07, SD = 1.76) and Excite (M = 5.43, SD = 1.48) techniques.
Decision-Making
Decision-making under risk was quantified as the proportion of trials in which the risky option was chosen for each instruction type (Look, Relax, Excite). To examine the effect of regulation (Relax, Excite) on risk-taking, a repeated-measures ANOVA with type of instruction as a within-subjects factor and success ratings for relax and excite regulation as between-subjects factors was estimated. Success ratings were included to account for the observed individual differences in application of the emotion regulation strategies. The ANOVA revealed a significant main effect of instruction [F(2, 32) = 5.47, p < 0.01], suggesting that cognitive strategies can influence decision-making under risk. Moreover, a trend that approached significance for an interaction of instruction and relax success ratings was observed [F(8, 32) = 2.03, p = 0.07]. Specifically, participants who experienced perceived success in applying the relax strategy chose the risky option less often during Relax compared to Look trials. A similar analysis investigating the interaction of instruction and excite success ratings was not significant, however [F(8, 32) = 1.08, p = 0.40]. These results suggest that when presented with a conditioned cue that represents reward, engaging in relax-focused emotion regulation, but not excite-focused emotion regulation, alters subsequent decision-making.
Given the effectiveness of the relax-focused regulation and the lack of excite-focused regulation effects, all subsequent analyses excluded the excite condition. To further probe the observed effect of the relax emotion regulation strategy on risk-taking, we divided participants into two groups based on their relax visualization success rating. Participants who rated themselves as successful (ratings of 5 to 7) were considered to be effective regulators (n = 20), while those that rated their performance as neutral or unsuccessful (ratings of 1 to 4) were considered to be non-regulators (n = 10). Notably, participants in the regulators group rated the relax strategy as significantly easier to implement than participants in the non-regulators group [regulators: M = 6.3, SD = 0.73, non-regulators: M = 4.5, SD = 1.84; t(28) = 3.85, p < 0.001].
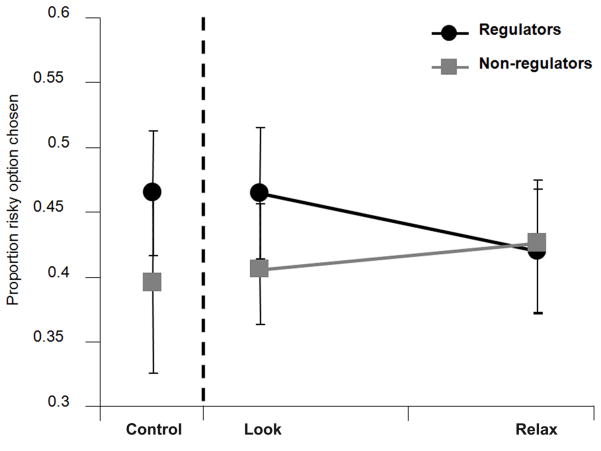
Using these two groups, the effect of emotion regulation on decision-making was probed with a repeated-measures ANOVA using type of instruction (Look, Relax) as a within-subjects factor and group (regulator, non-regulator) as a between-subjects factor. A significant interaction of type of instruction and group was found [F(1, 28) = 4.20, p < 0.05], suggesting that regulator status influenced the effect of the relax emotion regulation strategy on decision-making. We then compared the proportion the risky option was chosen across each instruction type (Look, Relax) for both the regulator and non-regulator groups separately (Fig. 2). In the regulators, the proportion that the risky option was chosen was lower during Relax compared to Look trials [t(19) = 2.19, p < 0.05], suggesting that the successful use of emotion regulation strategies can modulate decision-making under risk. This difference in decision-making across instruction was not observed in the non-regulators [t(9) = 1.11, p = 0.30].
Fig. 2.
Decrease in risky behavior as a function of successful regulation is displayed for the regulator group (±s.e.m.).
To ensure the observed change in risk-taking in the regulator group was due to decreases in risk-taking associated with successful use of the relax emotion regulation strategy and not increases in risk-taking associated with the Look condition we assessed decision-making in the absence of any instruction cues. Specifically, participants were asked to complete a questionnaire with 15 financial decisions, which consisted of the five financial decisions from the scanner task and two variations (the amounts plus and minus $0.50). This questionnaire was completed at least one day after the scanning session and was administered without the use of any explicit cognitive strategy. Participants’ choices in this follow-up decision-making questionnaire did not differ from those observed in the Look condition for either group of participants, supporting the main result of decreases in risky behavior after successful use of the relax emotion regulation strategy.
Decision-Making: Reaction Time
An ANOVA was performed to probe differences in reaction time using instruction (Look, Relax) and choice (risky, safe) as within-subjects variables and group (regulator, non-regulator) as a between-subjects variable. No significant effects were observed for any of the contrasts, suggesting that reaction time did not differ as a function of instruction or choice, or across regulators and non-regulators.
Comparison of Regulators and Non-regulators on Individual Differences Measures
All participants completed a series of questionnaires to probe potential individual differences. As previously described, the post-experimental questionnaire divided participants into regulators and non-regulators based on their perceived success in using the imagery-focused regulation strategy. While these groups differed with respect to how emotion regulation influenced their decision-making, we did not find differences between the groups on any of the individual difference measures we obtained. Regulators and non-regulators did not show different levels of risk aversion as assessed by the Holt & Laury (2002) questionnaire, suggesting that the different patterns of decision-making observed in these groups were not due to different risk preferences. These groups also did not differ on the subscale scores of the Emotion Regulation Questionnaire, which assesses use of emotion regulation in daily life, (Gross & John, 2003). Finally, the groups showed no differences in approach- and avoidance-focused motivation as measured by the Behavioral Inhibition and Activation Scales (Carver & White, 1994). Although it is possible that these groups may differ in ways not probed by these selected questionnaires, the results highlight the major difference between the two groups as their success at visualizing the relaxing imagery.
Neuroimaging Results
Neuroimaging analysis focused on the decision phase and sought to indentify brain regions recruited during decision-making that were modulated by emotion regulation. Regions of the brain involved in processing risk and reward were identified using a GLM in which the probability of winning each risky lottery (0.20, 0.35, 0.50, 0.65, 0.80) was included as a parametric regressor time-locked to the onset of the decision phase. This GLM revealed brain regions whose BOLD signals correlated with increasing probability of reward (Table 2), including various structures that have been previously associated with risky decision-making in humans: the striatum with a loci of activation that extended ventrally (Christopoulos, Tobler, Bossaerts et al., 2009; Hsu, Krajbich, Zhao, & Camerer, 2009; Kuhnen & Knutson, 2005; Matthews, Simmons, Lane et al., 2004; Tom, Fox, Trepel, & Poldrack, 2007), the midbrain (Tom, Fox, Trepel et al., 2007), the insula (Kuhnen & Knutson, 2005), and the medial frontal cortex (Christopoulos, Tobler, Bossaerts et al., 2009; Engelmann & Tamir, 2009).
Table 2.
Regions that correlated with increasing probability of reward in the regulator group; q(FDR) < 0.01
| Region of Activation | Laterality | Talairach Coordinates | Voxels | t-stat | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Superior frontal gyrus (BA 6) | L | −3 | −16 | 55 | 161 | 6.39 |
| Medial frontal gyrus (BA 32/6) | R | 6 | 2 | 49 | 347 | 6.00 |
| Medial frontal gyrus (BA 32/6) | L | −3 | 0 | 49 | 365 | 5.35 |
| Inferior frontal gyrus (BA 44) | L | −39 | 8 | 31 | 119 | 6.69 |
| Insula | L | −36 | 5 | 13 | 132 | 5.73 |
| Ventral striatum | L | −15 | 5 | 4 | 340 | 7.38 |
| Thalamus | L | −6 | −10 | 4 | 342 | 6.86 |
| Ventral striatum | R | 12 | 2 | 1 | 284 | 6.61 |
| Thalamus | R | 6 | −16 | 1 | 283 | 7.12 |
| Insula | L | −36 | 11 | −2 | 300 | 8.79 |
| Hippocampus | R | 21 | −28 | −2 | 194 | 7.48 |
| Midbrain | R | 6 | −22 | −8 | 889 | 11.40 |
| Midbrain | L | −6 | −16 | −8 | 747 | 9.54 |
| Midbrain | L | −6 | −25 | −8 | 850 | 8.72 |
| Lingual gyrus (BA 17) | R | 18 | −91 | −8 | 804 | 8.81 |
| Lingual gyrus (BA 17) | L | −18 | −94 | −8 | 344 | 7.46 |
| Occipital Lobe (BA 18) | R | 24 | −85 | −14 | 544 | 8.08 |
| Cerebellum | L | −1 | −49 | −28 | 200 | 5.76 |
BA, Brodmann area; L, left; R, right
Modulation of ventral striatum activity by emotion regulation was an a priori prediction. To test for effects of emotion regulation, a second GLM was applied to the left ventral striatum ROI to extract mean beta weights. This GLM included cue phase and decision phase predictors that each specified the type of instruction (Look, Relax, Excite) and option chosen (risky, safe). The cue and decision phase predictors were matched in starting time and duration to their task events. The decision phase beta weights were input into a repeated-measures ANOVA with instruction and choice as within-subjects factors and success ratings for relax and excite regulation as between-subjects factors. The ANOVA demonstrated a significant interaction of instruction and choice [F(2, 30) = 4.70, p <0.05] and a trend for an interaction of instruction, choice and relax success rating [F(8, 30) = 1.94, p = 0.09] in the left ventral striatum. Echoing the behavioral analysis, there were no interactions involving excite success ratings [F(8, 30) = 1.25, p = 0.30]. Post hoc paired t tests showed that in trials without emotion regulation (Look), the BOLD response was significantly greater when participants made risky choices compared to safe ones [t(29) = 2.49, p < 0.05]. This heightened natural response to risky choices was diminished in the relax regulation trials [t(29) = 0.81, p = 0.42], suggesting that regulation modulated activity associated with risky choices.
Regulators: Emotion Regulation of Decision-making under Risk
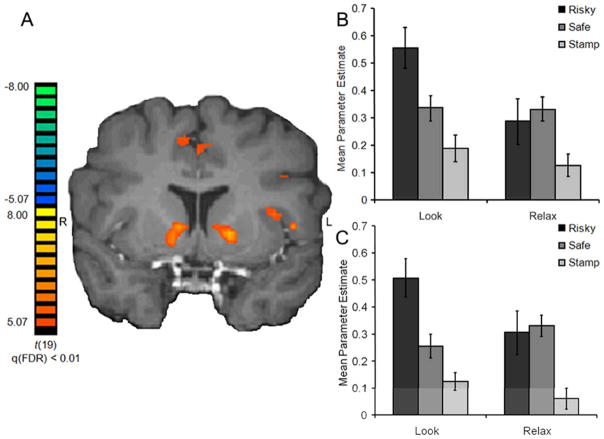
Given the influence of the relax emotion regulation strategy on risk-taking observed in the behavioral results, along with the lack of behavioral or neural effects with the excite regulation strategy, additional neuroimaging analyses were conducted focusing specifically on the regulators group defined by relax success ratings. Using the 20 regulator participants, regions whose BOLD signals correlated with increasing probability of reward were identified with the parametric GLM described above. Of particular interest are results highlighting the modulation of both left and right ventral striatum BOLD signals during risky decision-making by the relax emotion regulation strategy (Fig. 3A). In the left ventral striatum (Fig. 3B), a main effect of instruction [F(1, 19) = 6.85, p < 0.05], a trend for a main effect of choice approaching significance [F(1, 19) = 4.25, p = 0.05] and an interaction of instruction and choice [F(1, 19) = 6.76, p < 0.05] were observed. Specifically, greater BOLD signals in the left ventral striatum were observed when participants chose the risky option, compared to when they chose the safe option during trials where they were acting naturally [Look condition; t(19) = 3.51, p < 0.005], but not after they used emotion regulation strategies [Relax condition; t(19) = 0.63, p = 0.54] as assessed by post hoc paired t tests. Across types of instruction, BOLD signals were lower during Relax than Look when the choice was the risky option [t(19) = 2.90, p < 0.01], while no significant effects of instruction were found when the decision was to take the safe option [t(19) = 0.08, p = 0.94]. Finally, beta weights associated with control decisions (CS−), a choice between two postage stamps, were also obtained. While both Look and Relax instructions were used in the control trials, no modulation was expected in the ventral striatum as the control decisions did not involve risky propositions or rewards. As expected, no significant differences between Look and Relax beta weights for the control condition were seen, suggesting that emotion regulation effects were particular to trials where a risky decision was presented.
Fig. 3.
A) Bilateral striatum correlated with increasing probability of reward during decision-making under risk. B) Mean parameter estimates for left ventral striatum reveal an interaction between instruction (Look, Relax) and choice (risky, safe). C) A similar result is observed in the right ventral striatum (±s.e.m.).
Similar patterns emerged in the right ventral striatum (Fig. 3C), depicted by a trend approaching significance for a main effect of instruction [F(1, 19) = 3.70, p = 0.07], a significant main effect of choice [F(1, 19) = 4.88, p < 0.05] and an interaction of instruction and choice [F(1, 19) = 7.06, p < 0.05]. Greater activity in the right ventral striatum was observed when participants chose the risky option, compared to when they chose the safe option during trials in which they acted naturally [Look condition; t(19) = 4.00, p < 0.001], but not after using emotion regulation [Relax condition; t(19) = 0.31, p = 0.76]. Additionally, BOLD signals were influenced by instruction; specifically, activity was lower during Relax than Look when the choice was risky [t(19) = 2.53, p < 0.05], as observed on the left striatum ROI. Interestingly, when the choice was the safe option, in this ROI only, BOLD signals were greater during Relax than Look [t(19) = 2.18, p < 0.05]. There were no emotion regulation effects on the BOLD response for control decisions (CS− trials). Taken together, these results suggest that the relax emotion regulation strategy modulated brain activity in the striatum associated with decision-making under risk, particularly decreasing BOLD responses when choosing a risky option.
An additional analysis was performed to test if a specific level of probability (e.g., 0.50) was driving the observed pattern of BOLD signals in the striatum. Mean beta weights were extracted from the left ventral striatum region previously defined by the parametric analysis of probability of reward using a model that included predictors for instruction (Look, Relax) and level of probability (.0.20, 0.35, 0.50, 0.65, 0.80). These beta weights were entered into a repeated measures ANOVA which revealed a main effect of instruction [F(1, 19) = 5.46, p < 0.05] and a trend for a main effect of probability [F(4, 76) = 2.07, p = 0.09]. Importantly, this region did not show a significant interaction of instruction and probability [F(4, 76) = 0.27, p = 0.89]. The lack of interaction between instruction and level of probability coupled with the significant main effect of instruction suggests that the decreased activity associated with risky choices observed in the Relax condition is not primarily driven by one particular level of probability in this paradigm.
To probe potential interactions between the ventral striatum and other regions, an exploratory correlation analysis was performed using the left ventral striatum. Specifically, a whole brain correlation was conducted using the left ventral striatum ROI as the seed region, which served to identify regions that may be functionally connected with the striatum. The resulting statistical parametric map was thresholded at p < 0.01 using conservative Bonferroni corrections for multiple comparisons. A cluster in the dorsal medial pFC, located in the dorsal cingulate cortex (x, y, z, = 2, 7, 42), was observed to correlate with BOLD signals in the left ventral striatum. A post hoc test of this region during the use of emotion regulation strategies was further conducted by extracting beta weights using a simplified GLM with instruction predictors (e.g., Look, Relax) during the cue phase. This post hoc paired t test revealed that beta weights for Relax (regulation condition) trials tended to be greater than those for Look (no regulation condition [t(19) = 1.81, p = 0.09]. While these results are deemed exploratory, they suggest that one potential region engaged in emotion regulation that is mediating control over the striatum during decision-making is the dorsomedial pFC cortex, particularly the dorsal cingulate cortex – a topic for future research.
Additional Regions Showing Modulation by Emotion Regulation in the Regulators Group
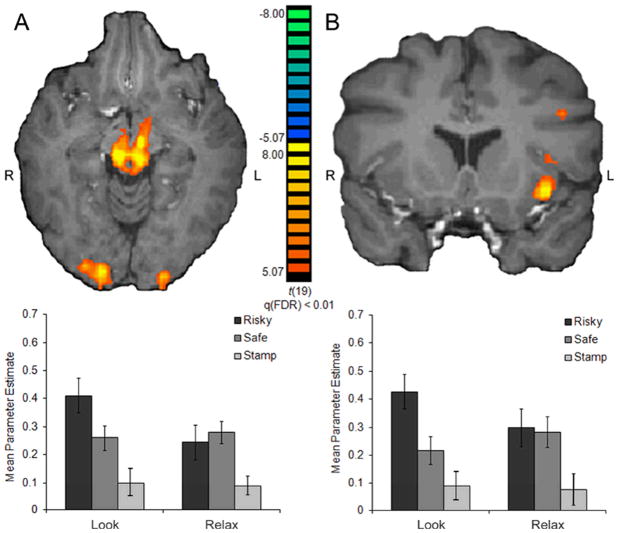
Within other regions that correlated with increasing probability of reward during decision-making, only regions in the midbrain, insula and superior frontal gyrus (BA 6; encompassing premotor cortex and supplementary motor area) were found to be modulated by instruction and/or choice in the regulators group. An ANOVA performed with beta weights extracted from the left midbrain, for instance, showed a significant interaction of instruction and choice [F(1, 19) = 4.60, p < 0.05], with a pattern of results resembling the striatum (Fig. 4A). Specifically, when participants chose the risky option, a paired t test revealed that activity in the regulation condition was significantly lower than that in the Look condition [t(19) = 2.32, p < 0.05], while no differences for safe choices were seen [t(19) = 0.38, p = 0.71]. In the right midbrain region, a main effect of instruction was observed [F(1, 19) = 5.16, p < 0.05]. During the CS− decisions, BOLD signals in the left and right midbrain region did not vary as a function of instruction.
Fig. 4.
The effect of instruction and choice in (A) the midbrain and (B) the insula. The left midbrain BOLD responses demonstrated an interaction of instruction and choice such that activity to risky choices was significantly reduced after regulation (Relax) compared to after responding naturally (Look). A main effect of choice was observed in the left anterior insula, with greater responses to risky compared to safe choices (±s.e.m.).
A trend for an interaction of instruction and choice and was also observed in the left anterior insula [F(1, 19) = 3.92, p = 0.06], although a main effect of choice was the primary effect in this ROI [F(1, 19) = 5.01, p < 0.05; Fig. 4B]. Interestingly, a different pattern was apparent in a smaller, more dorsal anterior insula ROI in the left hemisphere, where a main effect of instruction was observed [F(1, 19) = 5.76, p < 0.05], characterized by decreased activation during regulation. In both insula ROIs, activity during CS− decisions was not affected by instruction. Finally, activity in the left superior frontal gyrus (BA 6) during financial (CS+), but not control (CS−), decisions demonstrated a main effect of instruction [F(1, 19) = 12.23, p < 0.01] such that activity was decreased after regulation compared to after natural responding.
Non-Regulators: Emotion Regulation of Decision-Making Under Risk
Two exploratory analyses were conducted to test for effects of instruction and choice in the non-regulator sample (n = 10). First, using the left ventral striatum ROI defined by the regulator group parametric risk analysis, we extracted mean beta weights for the non-regulators with the model that included instruction and choice predictors. An ANOVA found no significant effects of instruction or choice. Similar results were found with the right ventral striatum ROI defined by the regulator group risk analysis. Second, a parametric risk analysis was conducted in the non-regulator group only, leading to the identification of a left ventral striatum ROI defined by this set of participants. A follow-up ANOVA on beta weights extracted for this ROI did not show any significant effects of instruction or choice. While these null findings should be interpreted with caution given the nature of null findings in fMRI analysis and the small sample size of the non-regulator group, the observations are in line with the non-regulator group’s self-reports and behavioral results.
DISCUSSION
Previous studies have highlighted how an array of emotion regulation strategies can be used to alter the intensity of emotional experience (for review, see Green & Malhi, 2006; Ochsner & Gross, 2008). The present study suggests that cognitive emotion regulation strategies influence subsequent decision-making. Specifically, participants who were successful in their application of an imagery-focused relax regulation strategy (i.e., regulator group) showed a decrease in risky behaviors; in particular selecting a safe, compared to a risky monetary lottery more often. This shift in behavior during decision-making under risk was accompanied by attenuation in BOLD signals in the striatum, a structure previously linked with reward-related processing (Delgado, 2007; Haber & Knutson, 2010; O’Doherty, 2004; Rangel, Camerer, & Montague, 2008). In contrast, participants who did not effectively use emotion regulation strategies (i.e., non-regulator group) failed to show behavioral or neural differences during decision-making. While further research is necessary to fully understand the conditions in which regulation can exert its effect (e.g., individual differences), these findings represent a potential approach to control decision-making under risk that may become compulsive.
The observed relax regulation results support the idea that successful use of cognitive strategies can foster more goal-directed behavior and promote safer, compared to riskier decision-making. This is in slight contrast with recent studies that suggest successful use of emotion regulation can lead one to reduce loss aversion (Sokol-Hessner, Hsu, Curley et al., 2009) and maximization of rewards (Seo & Barrett, 2007). One potential difference between these studies is the type of strategy employed. For instance, the strategy used by Sokol-Hessner and colleagues (2009) was focused to the particular task at hand, asking participants to place less weight on the outcome of a single decision, rather thinking of it as a series of decisions (e.g., an investor’s portfolio). In the current experiment, we used a more general imagery-based strategy previously shown to be successful in attenuating the physiological and neural correlates of conditioned fear (Delgado, Nearing, LeDoux et al., 2008) and the expectation of reward (Delgado, Gillis, & Phelps, 2008). While both strategies can be considered a form of cognitive control, they might exert different influences in the underlying neural circuitry, as observed in studies comparing reappraisal and distraction strategies during negative emotions (Kalisch, Wiech, Herrmann, & Dolan, 2006; McRae, Hughes, Chopra, Gabrieli, Gross, & Ochsner, 2010), that could cause different effects in behavior. This is an interesting question for future research examining a) the effect of different cognitive strategies on subsequent affective behaviors exerted during decision-making and b) how specific strategies may better suit specific individual differences to have the desired effect on behavior (e.g., promote improved decision-making depending on the context).
Indeed, individual differences with respect to the effective use of the imagery-based strategy were observed in the current experiment as measured by post-experimental ratings. A regulator group was defined by perceived success in applying the Relax strategy, while a non-regulator group comprised participants who felt they were unable to successfully implement the Relax strategy. Differences between these two groups were apparent in subjective ratings (how easy was it to implement strategy), behavioral responses (picking between safe and risky options) and neural signals (striatum responses during decision-making under risk). Of particular interest, the regulator group made fewer risky choices than their counterparts. This behavior was not due to an inherent risk aversion, as both groups risk preferences did not differ according to a paper test assessment (Holt & Laury, 2002). Instead, this shift in behavior could be attributed to the successful use of cognitive strategies.
This behavioral modulation due to the application of cognitive strategies was not observed in the group of self-assessed non-regulators. Neither was the modulation of striatum activation by cognitive strategies, consistent with previous studies suggesting that striatum signals during decision-making can correlate with success in task performance (Schonberg, Daw, Joel, & O’Doherty, 2007). It is possible that fatigue contributed to the non-regulators’ lack of success at using the imagery regulation, as the task duration was about 40 minutes. While the two groups did not differ in any individual measures used in our study, additional research may probe potential differences that allow some to exert better control over their decisions. For instance, are there specific traits, or perhaps more likely, do certain situational factors (e.g. type of strategy attempted, amount of effort applied) determine whether a person will be able to successfully employ regulation? The topic of individual differences in the use of cognitive strategies for regulatory purposes is of great interest currently (e.g., Canli, Ferri, & Duman, 2009; Drabant, McRae, Manuck, Hariri, & Gross, 2009; Hariri & Holmes, 2006; John & Gross, 2004; Modinos, Ormel, & Aleman, 2010; Ray, Ochsner, Cooper, Robertson, Gabrieli, & Gross, 2005), as research attempts to identify key neural differences between those who exhibit self-control and regulation in their behavior and those who do not. When successful self-controllers (dieters) make food choices, for example, activity in a brain region involved in valuation, namely the ventromedial pFC (Rangel, Camerer, & Montague, 2008), reflects both taste and health ratings, while in non-self-controllers this region only reflects taste information (Hare, Camerer, & Rangel, 2009), highlighting how the ability to exert cognitive control can promote better decision-making (e.g., eating healthy).
The current study found that activity in the ventral striatum of regulators was influenced by the use of cognitive regulation, in accordance with previous research (Delgado, Gillis, & Phelps, 2008; Staudinger, Erk, Abler et al., 2009). Yet, such studies focused mostly on reward expectations and learning, while the current paradigm focuses on the role of emotion regulation on decision-making under risk. The human striatum is often identified during investigations of reward and risky decision-making (Christopoulos, Tobler, Bossaerts et al., 2009; Kuhnen & Knutson, 2005; Matthews, Simmons, Lane et al., 2004), showing greater responses as expected reward values increase (Knutson & Cooper, 2005; Tom, Fox, Trepel et al., 2007; Yacubian, Glascher, Schroeder, Sommer, Braus, & Buchel, 2006) and patterns of activity that suggest processing of reward probabilities (Abler, Walter, Erk, Kammerer, & Spitzer, 2006; Hsu, Krajbich, Zhao et al., 2009; Yacubian, Sommer, Schroeder, Glascher, Braus, & Buchel, 2007). Building on previous research that suggests expected value-related reward activity in the striatum is modulated by emotion regulation (Delgado, Gillis, & Phelps, 2008; Staudinger, Erk, Abler et al., 2009), we chose to model reward probability in our analyses to probe the role of the striatum in probability (risk) coding during decision-making and the effects of emotion regulation on this process. In our experiment, the ventral striatum activity during the decision phase was decreased overall for regulators, especially during trials where a risky choice was made, suggesting that effective regulation can dampen the natural heightened response to decisions under risk.
The BOLD signal observed in the striatum during the decision-phase may reflect deliberation with respect to the two options, the choice itself and a reaction to the choice made. While our model accounted for increasing probability of reward, the magnitude of the options might have influenced neural activity. The expected value of the risky and safe options was equated, but the magnitude of the risky option was always higher. Thus, the increased activity observed in ventral striatum for risky relative to safe choices during Look trials could perhaps be explained by the greater magnitude of the risky option, in turn suggesting that the lack of differentiation between risky and safe choices by the ventral striatum during regulation may indicate disruption of the ability to code reward magnitude. This interpretation is in line with a recent paper that found that distance-focused regulation disrupted expected-value coding in the ventral striatum such that during regulation trials ventral striatum activity failed to differentiate high and low magnitude cues (Staudinger, Erk, Abler et al., 2009). Nevertheless, participants who successfully employed cognitive strategies prior to making financial decisions made fewer risky choices when deliberating between lotteries under risk and showed attenuated BOLD signals in the striatum. Whether emotion regulation specifically affects the coding of the magnitude of potential rewards or the perception of probability (or risk) inherent in the decision process is a topic for further exploration that will continue to advance our understanding of the ability to control emotional responses for adaptive function.
The ventral striatum is an integral component of a corticostriatal circuit involved in motivated behaviors (Alexander & Crutcher, 1990; Balleine, Delgado, & Hikosaka, 2007; Haber & Knutson, 2010; Middleton & Strick, 2002), with important connections with cortical regions, such as orbitofrontal cortex and the anterior cingulate cortex (Haber & Knutson, 2010). Given the connectivity of the ventral striatum, it is plausible that the observed decrease in ventral striatum activity in the regulator group during decision-making after using emotion regulation may have been driven in part by cortical signals. An exploratory analysis revealed that BOLD signals in dorsal cingulate cortex (BA 24) correlated with those from the left ventral striatum, suggesting a potential functional connectivity that may underlie the control of striatum responses during decision-making under risk. Further analysis of the BOLD response within this cingulate region revealed a trend for greater recruitment during the use of regulation strategies than natural responding, which is consistent with findings from previous emotion regulation studies (Eippert, Veit, Weiskopf, Erb, Birbaumer, & Anders, 2007; Kim & Hamann, 2007; Ochsner, Bunge, Gross et al., 2002; Phan, Fitzgerald, Nathan, Moore, Uhde, & Tancer, 2005; Staudinger, Erk, Abler et al., 2009). Although this analysis is exploratory and thus results should be interpreted with caution, the findings point to an enhancement of cortical regions such as the dorsal cingulate cortex during regulation as a potential modulator of striatal responses during decision-making – a topic that will be explored further in future studies.
In addition to the striatum, the effective use of regulation led to attenuation of BOLD signals in the midbrain and insula. The midbrain results are particularly interesting given that it includes dopaminergic centers that project to the striatum (Haber & Knutson, 2010), and much like the striatum, BOLD signals in the midbrain increase in conjunction with increasing reward values during decision-making under risk (Tom et al., 2007), suggesting that cognitive strategies can have a global impact in the neurocircuitry involved in reward and decision-making. Regulation strategies also had an effect in the insula, a region implicated in risky decision-making (Clark, Bechara, Damasio, Aitken, Sahakian, & Robbins, 2008; Kuhnen & Knutson, 2005) perhaps coding different levels of risk (Preuschoff, Quartz, & Bossaerts, 2008). Specifically, a marginal interaction of instruction and choice was seen in the anterior insula, while an instruction effect was observed in a more dorsal anterior insula ROI. Future studies may probe anatomical and functional dissociations within the insula as a function of emotion regulation during decision-making.
This study employed two opposite cognitive, imagery-focused regulation strategies, Relax and Excite. No significant shifts in risk-taking or neural activity were associated with the Excite strategy. There are several possible explanations for why we did not observe an effect of excite regulation on decision-making, given that relax regulation did influence decision-making. Although the majority of participants rated themselves as successful at visualizing the exciting imagery, they also reported the need to periodically update the exciting images that they thought about as over time these images lost their potency. Additionally, there were some conflicts between what participants wanted to imagine (e.g., Las Vegas casinos) and the instruction to think of something non-task specific (e.g., a roller coaster). It is possible that the level of excitement achieved with the Excite strategy may have been comparable to that which participants experienced when naturally responding to the slot machine cue. If similar affect was associated with the Excite and Look condition, that could underlie the lack of observed differences in risk-taking between these conditions. Future work could address this question by including affect ratings during the task.
Emotion regulation strategies have been traditionally used to control emotional responses induced by stimuli such as pictures, movies or narratives that evoke negative affect (for review, see Ochsner & Gross, 2008). More recently, such strategies have also been applied to positive emotions evoked by pictures, food stimuli or cues that predict reward (Delgado, Gillis, & Phelps, 2008; Hare, Camerer, & Rangel, 2009; Kim & Hamann, 2007; Staudinger, Erk, Abler et al., 2009; Wang, Volkow, Telang, Jayne, Ma, Pradhan et al., 2009). Here, we extend these findings by focusing on the influence of emotion regulation strategies on decision-making processes and associated neural circuits. This research has applications ranging from simple decisions such as dieting (e.g., Hare, Camerer, & Rangel, 2009) to more complex decisions where goal-directed and habit learning systems may be at conflict, such as substance abuse (e.g., Balleine & O’Doherty, 2010; Everitt, Belin, Economidou, Pelloux, Dalley, & Robbins, 2008; Nelson & Killcross, 2006).
Acknowledgments
This work was supported by National Institute on Drug Abuse grants to M.R.D. (DA022998 & DA027764) and a predoctoral fellowship to L.N.M. (DA025426). The authors wish to acknowledge Michael Niznikiewicz for assistance with figures and Elizabeth Tricomi for helpful discussion.
Contributor Information
Laura N. Martin, Email: lnmartin@psychology.rutgers.edu.
Mauricio R. Delgado, Email: delgado@psychology.rutgers.edu.
References
- Abler B, Walter H, Erk S, Kammerer H, Spitzer M. Prediction error as a linear function of reward probability is coded in human nucleus accumbens. Neuroimage. 2006;31(2):790–795. doi: 10.1016/j.neuroimage.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13(7):266–271. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- Balleine BW, Delgado MR, Hikosaka O. The role of the dorsal striatum in reward and decision-making. Journal of Neuroscience. 2007;27(31):8161–8165. doi: 10.1523/JNEUROSCI.1554-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, O’Doherty JP. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology. 2010;35(1):48–69. doi: 10.1038/npp.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Ferri J, Duman EA. Genetics of emotion regulation. Neuroscience. 2009;164(1):43–54. doi: 10.1016/j.neuroscience.2009.06.049. [DOI] [PubMed] [Google Scholar]
- Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS Scales. Journal of Personality and Social Psychology. 1994;67(2):319–333. [Google Scholar]
- Christopoulos GI, Tobler PN, Bossaerts P, Dolan RJ, Schultz W. Neural correlates of value, risk, and risk aversion contributing to decision making under risk. Journal of Neuroscience. 2009;29(40):12574–12583. doi: 10.1523/JNEUROSCI.2614-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark L, Bechara A, Damasio H, Aitken MR, Sahakian BJ, Robbins TW. Differential effects of insular and ventromedial prefrontal cortex lesions on risky decision-making. Brain. 2008;131(Pt 5):1311–1322. doi: 10.1093/brain/awn066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR. Reward-related responses in the human striatum. Annals of the New York Academy of Sciences. 2007;1104:70–88. doi: 10.1196/annals.1390.002. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Gillis MM, Phelps EA. Regulating the expectation of reward via cognitive strategies. Nature Neuroscience. 2008;11(8):880–881. doi: 10.1038/nn.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR, Nearing KI, LeDoux JE, Phelps EA. Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron. 2008;59(5):829–838. doi: 10.1016/j.neuron.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drabant EM, McRae K, Manuck SB, Hariri AR, Gross JJ. Individual differences in typical reappraisal use predict amygdala and prefrontal responses. Biological Psychiatry. 2009;65(5):367–373. doi: 10.1016/j.biopsych.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eippert F, Veit R, Weiskopf N, Erb M, Birbaumer N, Anders S. Regulation of emotional responses elicited by threat-related stimuli. Human Brain Mapping. 2007;28(5):409–423. doi: 10.1002/hbm.20291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann JB, Tamir D. Individual differences in risk preference predict neural responses during financial decision-making. Brain Research. 2009;1290:28–51. doi: 10.1016/j.brainres.2009.06.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philosophical Transactions of the Royal Society of London: B Biological Sciences. 2008;363(1507):3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15(4):870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Green MJ, Malhi GS. Neural mechanisms of the cognitive control of emotion. Acta Neuropsychiatrica. 2006;18(3–4):144–153. doi: 10.1111/j.1601-5215.2006.00149.x. [DOI] [PubMed] [Google Scholar]
- Gross JJ, John OP. Individual differences in two emotion regulation processes: Implications for affect, relationships, and well-being. Journal of Personality and Social Psychology. 2003;85(2):348–362. doi: 10.1037/0022-3514.85.2.348. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35(1):4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324(5927):646–648. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Holmes A. Genetics of emotional regulation: the role of the serotonin transporter in neural function. Trends in Cognitive Sciences. 2006;10(4):182–191. doi: 10.1016/j.tics.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Holt CA, Laury SK. Risk aversion and incentive effects. American Economic Review. 2002;92(5):1644–1655. [Google Scholar]
- Hsu M, Krajbich I, Zhao C, Camerer CF. Neural response to reward anticipation under risk is nonlinear in probabilities. Journal of Neuroscience. 2009;29(7):2231–2237. doi: 10.1523/JNEUROSCI.5296-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John OP, Gross JJ. Healthy and unhealthy emotion regulation: personality processes, individual differences, and life span development. Journal of Personality. 2004;72(6):1301–1333. doi: 10.1111/j.1467-6494.2004.00298.x. [DOI] [PubMed] [Google Scholar]
- Kalisch R, Wiech K, Herrmann K, Dolan RJ. Neural correlates of self-distraction from anxiety and a process model of cognitive emotion regulation. Journal of Cognitive Neuroscience. 2006;18(8):1266–1276. doi: 10.1162/jocn.2006.18.8.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Hamann S. Neural correlates of positive and negative emotion regulation. Journal of Cognitive Neuroscience. 2007;19(5):776–798. doi: 10.1162/jocn.2007.19.5.776. [DOI] [PubMed] [Google Scholar]
- Knutson B, Cooper JC. Functional magnetic resonance imaging of reward prediction. Current Opinion in Neurology. 2005;18(4):411–417. doi: 10.1097/01.wco.0000173463.24758.f6. [DOI] [PubMed] [Google Scholar]
- Kober H, Kross EF, Mischel W, Hart CL, Ochsner KN. Regulation of craving by cognitive strategies in cigarette smokers. Drug and Alcohol Dependence. 2009 doi: 10.1016/j.drugalcdep.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhnen CM, Knutson B. The neural basis of financial risk taking. Neuron. 2005;47(5):763–770. doi: 10.1016/j.neuron.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Kushner MG, Abrams K, Donahue C, Thuras P, Frost R, Kim SW. Urge to gamble in problem gamblers exposed to a casino environment. Journal of Gambling Studies. 2007;23(2):121–132. doi: 10.1007/s10899-006-9050-4. [DOI] [PubMed] [Google Scholar]
- Leland DS, Arce E, Feinstein JS, Paulus MP. Young adult stimulant users’ increased striatal activation during uncertainty is related to impulsivity. Neuroimage. 2006;33(2):725–731. doi: 10.1016/j.neuroimage.2006.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews SC, Simmons AN, Lane SD, Paulus MP. Selective activation of the nucleus accumbens during risk-taking decision making. Neuroreport. 2004;15(13):2123–2127. doi: 10.1097/00001756-200409150-00025. [DOI] [PubMed] [Google Scholar]
- McRae K, Hughes B, Chopra S, Gabrieli JD, Gross JJ, Ochsner KN. The neural bases of distraction and reappraisal. Journal of Cognitive Neuroscience. 2010;22(2):248–262. doi: 10.1162/jocn.2009.21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Basal-ganglia ‘projections’ to the prefrontal cortex of the primate. Cereb Cortex. 2002;12(9):926–935. doi: 10.1093/cercor/12.9.926. [DOI] [PubMed] [Google Scholar]
- Modinos G, Ormel J, Aleman A. Individual differences in dispositional mindfulness and brain activity involved in reappraisal of emotion. Social Cognitive and Affective Neuroscience. 2010;5(4):369–377. doi: 10.1093/scan/nsq006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson A, Killcross S. Amphetamine exposure enhances habit formation. Journal of Neuroscience. 2006;26(14):3805–3812. doi: 10.1523/JNEUROSCI.4305-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Doherty JP. Reward representations and reward-related learning in the human brain: Insights from neuroimaging. Current Opinion in Neurobiology. 2004;14(6):769–776. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JDE. Rethinking feelings: An fMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience. 2002;14(8):1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9(5):242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. Cognitive emotion regulation: Insights from social cognitive and affective neuroscience. Current Directions in Psychological Science. 2008;17(2):153–158. doi: 10.1111/j.1467-8721.2008.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, Gross JJ. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23(2):483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME. Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biological Psychiatry. 2005;57(3):210–219. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Preuschoff K, Quartz SR, Bossaerts P. Human insula activation reflects risk prediction errors as well as risk. Journal of Neuroscience. 2008;28(11):2745–2752. doi: 10.1523/JNEUROSCI.4286-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel A, Camerer C, Montague PR. A framework for studying the neurobiology of value-based decision making. Nature Reviews Neuroscience. 2008;9(7):545–556. doi: 10.1038/nrn2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray RD, Ochsner KN, Cooper JC, Robertson ER, Gabrieli JDE, Gross JJ. Individual differences in trait rumination and the neural systems supporting cognitive reappraisal. Cognitive, Affective, & Behavioral Neuroscience. 2005;5(2):156–168. doi: 10.3758/cabn.5.2.156. [DOI] [PubMed] [Google Scholar]
- Schonberg T, Daw ND, Joel D, O’Doherty JP. Reinforcement learning signals in the human striatum distinguish learners from nonlearners during reward-based decision making. Journal of Neuroscience. 2007;27(47):12860–12867. doi: 10.1523/JNEUROSCI.2496-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo MG, Barrett LF. Being Emotional during Decision Making-Good or Bad? An Empirical Investigation. Academy of Management Journal. 2007;50(4):923–940. doi: 10.5465/amj.2007.26279217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Lacadie C, Skudlarski P, Fulbright RK, Rounsaville BJ, Kosten TR, Wexler BE. Neural activity associated with stress-induced cocaine craving: a functional magnetic resonance imaging study. Psychopharmacology (Berl) 2005;183(2):171–180. doi: 10.1007/s00213-005-0147-8. [DOI] [PubMed] [Google Scholar]
- Sokol-Hessner P, Hsu M, Curley NG, Delgado MR, Camerer CF, Phelps EA. Thinking like a trader selectively reduces individuals’ loss aversion. Proceedings of the National Academy of Sciences, USA. 2009;106(13):5035–5040. doi: 10.1073/pnas.0806761106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudinger MR, Erk S, Abler B, Walter H. Cognitive reappraisal modulates expected value and prediction error encoding in the ventral striatum. Neuroimage. 2009;47(2):713–721. doi: 10.1016/j.neuroimage.2009.04.095. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain: 3-dimensional proportional system: an approach to cerebral imaging. New York: Thieme Medical Publishers, Inc; 1988. [Google Scholar]
- Tom SM, Fox CR, Trepel C, Poldrack RA. The neural basis of loss aversion in decision-making under risk. Science. 2007;315(5811):515–518. doi: 10.1126/science.1134239. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Telang F, Logan J, Jayne M, Ma Y, Pradhan K, Wong C, Swanson JM. Cognitive control of drug craving inhibits brain reward regions in cocaine abusers. Neuroimage. 2010;49(3):2536–2543. doi: 10.1016/j.neuroimage.2009.10.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Telang F, Jayne M, Ma Y, Pradhan K, Zhu W, Wong CT, Thanos PK, Geliebter A, Biegon A, Fowler JS. Proceedings of the National Academy of Sciences, USA. 4. Vol. 106. 2009. Evidence of gender differences in the ability to inhibit brain activation elicited by food stimulation; pp. 1249–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F. Neurobiology of craving, conditioned reward and relapse. Current Opinion in Pharmacology. 2005;5(1):9–19. doi: 10.1016/j.coph.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Yacubian J, Glascher J, Schroeder K, Sommer T, Braus DF, Buchel C. Dissociable systems for gain- and loss-related value predictions and errors of prediction in the human brain. J Neurosci. 2006;26(37):9530–9537. doi: 10.1523/JNEUROSCI.2915-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yacubian J, Sommer T, Schroeder K, Glascher J, Braus DF, Buchel C. Subregions of the ventral striatum show preferential coding of reward magnitude and probability. Neuroimage. 2007;38(3):557–563. doi: 10.1016/j.neuroimage.2007.08.007. [DOI] [PubMed] [Google Scholar]