Abstract
Hypoxia (lack of oxygen) is a physiological stress often associated with solid tumors. Hypoxia correlates with poor prognosis since hypoxic regions within tumors are considered apoptosis-resistant. Autophagy (cellular “self digestion”) has been associated with hypoxia during cardiac ischemia and metabolic stress as a survival mechanism. However, although autophagy is best characterized as a survival response, it can also function as a mechanism of programmed cell death. Our results show that autophagic cell death is induced by hypoxia in cancer cells with intact apoptotic machinery. We have analyzed two glioma cell lines (U87, U373), two breast cancer cell lines (MDA-MB-231, ZR75) and one embryonic cell line (HEK293) for cell death response in hypoxia (<1% O2). Under normoxic conditions, all five cell lines undergo etoposide-induced apoptosis whereas hypoxia fails to induce these apoptotic responses. All five cell lines induce an autophagic response and undergo cell death in hypoxia. Hypoxia-induced cell death was reduced upon treatment with the autophagy inhibitor 3-methyladenine, but not with the caspase inhibitor z-VAD-fmk. By knocking down the autophagy proteins Beclin-1 or ATG5, hypoxia-induced cell death was also reduced. The pro-cell death Bcl-2 family member BNIP3 (Bcl-2/adenovirus E1B 19kDa-interacting protein 3) is upregulated during hypoxia and is known to induce autophagy and cell death. We found that BNIP3 over-expression induced autophagy, while expression of BNIP3 siRNA or a dominant-negative form of BNIP3 reduced hypoxia-induced autophagy. Taken together, these results suggest that prolonged hypoxia induces autophagic cell death in apoptosis-competent cells, through a mechanism involving BNIP3.
Keywords: autophagy, hypoxia, autophagic cell death, BNIP3, cancer
INTRODUCTION
Autophagy is a regulated lysosomal pathway used in the degradation and recycling of long-lived proteins and organelles. During autophagy, cytoplasmic constituents are sequestered into double-membraned autophagosomes which are delivered to lysosomes, where degradation occurs.1 Genetic screening in yeast have identified more than 30 ATG (autophagy-related) genes, many of which have mammalian homologs.2 Conserved from yeast to humans, autophagy functions in basic cellular homeostasis, and is known to be essential for survival during starvation.3 More recently it has been shown that under certain conditions, autophagy can also promote cell death. For example, chemical agents such as arsenic trioxide4 and over-expression of tumor suppressor proteins such as p19ARF5 have been shown to induce cell death through an autophagic mechanism independent of apoptosis. In other cases, autophagic cell death has been achieved in systems where apoptosis is blocked, either though the use of caspase inhibitors6, 7 or by elimination of the pro-apoptotic Bcl-2 family members Bax and Bak8.
Hypoxia is a physiological stress encountered during various pathologies including cancer, myocardial infarction, and stroke.9 Cells deprived of oxygen will initially employ adaptive and survival strategies, but if hypoxia is sustained, cell death will eventually occur. The precise mechanisms of hypoxia-induced cell death remain unclear as apoptosis, necrosis and autophagy have all been reported in response to hypoxic stress.10–12 Chronic hypoxia is typical of tumor development as rapid proliferation causes the tumor to outgrow its available oxygen supply. The extent of tumor hypoxia correlates with neoplastic aggression, resistance to therapy-induced apoptosis and decreased overall patient survival.13 Because hypoxic tumor cells are difficult to target effectively, it is important to understand the cell death mechanisms involved (and evaded) during sustained oxygen deprivation in order to develop better strategies to treat cancers.
Bcl-2/E1B 19kDa interacting protein (BNIP3) is a pro-cell death Bcl-2 family member that is upregulated under hypoxic conditions.14 In transformed and cancer cells, forced over-expression of BNIP3 induces ‘non-apoptotic’ cell death that is characterized by localization to the mitochondria, opening of the permeability transition pore, loss of membrane potential and reactive oxygen species production. However, in these cells, BNIP3-induced cell death is independent of caspase activation and cytochrome c release from the mitochondria.15 In other cell types, BNIP3 can induce cell death via Bax and Bak46 or through a necrotic mechanism45. BNIP3 has also been implicated in ceramide- and arsenic trioxide-induced autophagy4, 16 and in hypoxia-induced cell death14. It remains to be determined, however, whether hypoxia induces autophagy as a death mechanism, and whether BNIP3 plays a role in this process.
Herein, we demonstrate that autophagic cell death occurs in the absence of apoptosis in cancer cells under hypoxic conditions, and that this process involves BNIP3.
MATERIALS AND METHODS
Antibodies and chemical inhibitors
Cytochrome c mouse monoclonal (sc-13560), ATG5 goat polyclonal (sc-8667), and BECN1 goat polyclonal (sc-10086) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). LC3 rabbit polyclonal was purchased from Abgent (API1802a). HMGB1 rabbit polyclonal (ab18256) were purchased from Abcam (Cambridge, MA). Actin rabbit polyclonal(A02066) and goat anti-rabbit fluorescein isothiocyanate (FITC)-conjugated secondary antibody were purchased from Sigma-Aldrich (St. Louis, MO). Anti-mouse Rhodamine Red-X (R-6393) was purchased from Molecular Probes (Eugene, OR). BNIP3 rabbit polyclonal antibody (University of Manitoba) was described previously.17 Caspase inhibitor z-VAD-fmk was obtained from Calbiochem (San Diego, CA) and used at 100 μM. 3-methyladenine was obtained from Sigma (St. Louis, MO) and used at 2 mM or 5 mM, as indicated. Chloroquine was obtained from Sigma (St. Louis, MO) and used at 0.1 mM.
Cell culture
All cell lines (unless otherwise stated) were maintained in a humidified 5% CO2 environment, in DMEM supplemented with 100 units of penicillin per mL, 100 μg of streptomycin(Life Technologies) per mL and in 10% bovine calf serum (HEK293) or 5% fetal bovine serum (U87, U373, MDA-MB-231). Human glioblastoma cell lines U373 and U87 were additionally supplemented with 2 mM L-glutamine and 1 mM MEM sodium pyruvate. Stable GFP-LC3 clones were selected with G418 (3 mg/mL). For hypoxia treatment, cells were maintained under hypoxic conditions (<1% oxygen) at 37°C within a hypoxic chamber (Forma Scientific, Waltham, MA) filled with 5% CO2 balanced with N2. For starvation, cells were treated with unsupplemented EBSS (Hyclone).
BNIP3 transfection experiments
HEK293 cells were plated the day before transfection to achieve ~50% confluency. Cells were transiently transfected using Lipofectamine 2000 (Invitrogen) as per the manufacturer’s instructions. After transfection with 2 μg total DNA (PCDNA3, PCDNA3-BNIP3 or PCDNA3-BNIP3ΔTM with or without the GFP-LC3 expression construct), cells were incubated in normoxia or hypoxia for 24 hours with or without drug treatment (5 mM 3-MA or 100 μM z-VAD-fmk). After treatment, cells were cytospun and fixed as described previously.18 Immunofluorescence was performed as described below. Because fixed/immunostained cells cannot be assayed for membrane permeability as a measure of cell viability, we used an alternate method to assess cell death in these cells: after co-staining with DAPI, we examined the nuclear morphology of BNIP3-positive cells. Cells with atypical, “shriveled” nuclei were considered dead.
Immunofluorescence of BNIP3, cytochrome c and HMGB1
HEK 293 cells were untransfected (cytochrome c and HMGB1) or transiently transfected (BNIP3) and treated as described. After 24 or 48 hours of treatment, the cells were cytospun, fixed, and stained as described previously.18 Cells stained for HMGB1 were grown on sterile coverslips and therefore were not cytospun. Primary antibodies were mouse anti-cytochrome c (1:500 dilution) or rabbit anti-BNIP3 (1:1500 dilution) or rabbit anti-HMGB1 (1:500 dilution). Secondary antibodies were anti-mouse Rhodamine Red (1:500) or goat-anti-rabbit-FITC (1:500). Slides were additionally stained with DAPI + antifade reagent (Biorad) to counterstain for nuclei. Fluorescence was visualized and captured using a Zeiss Axiphot microscope with a cooled charge-coupled device camera. Cells were assessed for cytochrome c release, or nuclear condensation (as an indicator of cell viability), or GFP-LC3 puncta in BNIP3-positive cells, or for HMGB1 release. No fewer than 200 cells were counted per sample.
Distribution of GFP-LC3
LC3 is recruited to the autophagosome membrane during autophagy. Cells were transiently transfected with the GFP-LC3 expression vector using either GenePORTER 2 (GTS) for U87, U373, and MDA-MB-231 cells or Lipofectamine 2000 (Invitrogen) for HEK293 cells. After overnight culture, cells were incubated in normoxia or hypoxia, fixed with 4% paraformaldehyde, and examined under an Olympus BX51 fluorescent microscope. GFP-LC3 cells present a diffuse distribution under control conditions, whereas a punctate pattern of GFP-LC3 expression is indicative of autophagy. GFP-LC3 stable clones (“U87-GFP-LC3 cells”) were generated in U87 cells after transfection and selection with G418. The GFP-LC3 expression vector was kindly provided by Drs. N. Mizushima and T. Yoshimori.
Western blot analysis
Cells were lysed for total protein as previously described.14 The lysates were separated by SDS-PAGE (with tricine for LC3 analysis) and transferred to nitrocellulose membranes. Membranes were blocked in 5% skim milk and western blotted with antibodies described above. Densitometry was performed using QuantityOne software (BioRad).
Electron microscopy
Cells were collected and fixed in 2% paraformaldehyde, 0.1% glutaraldehyde in 0.1 M sodium cacodylate for 2 h, postfixed with 1% OsO4 for 1.5 h, washed and finally stained for 1 h in 3% aqueous uranyl acetate. The samples were then washed again, dehydrated with graded alcohol, and embedded in Epon-Araldite resin (Canemco Inc.). Ultrathin sections were cut on a Reichert ultramicrotome, counterstained with 0.3% lead citrate and examined on a Philips EM420 electron microscope.
Detection and quantification of Acidic Vesicular Organelles (AVO)
Autophagy is characterized by the development of AVOs. To detect and quantify AVOs, cells were harvested and resuspended in PBS with acridine orange (1 μg/mL), incubated for 10 minutes at room temperature in the dark, followed by FACS analysis. This pH-sensitive dye is cell permeable, staining the nucleus and cytoplasm green and any acidic compartments red.
siRNA experiments
Non-targeting siRNA and siRNA targeted against beclin-1 or BNIP3 was obtained from Dharmacon (Lafayette, CO).5 siRNA targeted against ATG5 (5′-GCAACUCUGGAUGGGAUUG-3′) was obtained from Sigma-Proligo. Cells at 30% to 50% confluency were transfected with siRNA against beclin-1 or ATG5 using Oligofectamine (Invitrogen). For each transfection, 200 nmols of siRNA was added per 100 mm plate (final concentration 40 nM). Two days post-transfection, cells were seeded into 6-well plates. 24 hours later, cells were transferred to hypoxia or retained in normoxia (control). After a further 48 hours, cells were harvested and analyzed for autophagy or cell death. Protein expression was verified by western blot and densitometry as described previously.18
For BNIP3 silencing, cells were seeded in 12-well dishes (5×104 cells/well). Cells were transfected at the time of seeding using HiPerfect (QIAGEN), with a final siRNA concentration of 10 nM. Media was changed after 5 hours. 48 hours post-transfection, cells were transferred to hypoxia or retained in normoxia (control). Gene expression was verified by reverse-transcription PCR using the RNAEasy kit (QIAGEN) for RNA extraction and Superscript III Onestep kit (Invitrogen) for RT-PCR, according to the manufacturer’s instructions. The primers used were: BNIP3-FWD (5′-GCA TGA GTC TGG ACG GAG TA-3′) and BNIP3-REV (5′-GTT TCA GAA GCC CTG TTG GT-3′) with product size = 93 base pairs; GAPDH-FWD (5′-ACC CAC TCC TCC ACC TTT G-3′) and GAPDH-REV (5′-CTC TTG TGC TCT TGC TGG G-3′) with product size = 190 base pairs. The multiplex RT-PCR reaction consisted of 30 minutes at 55°C for cDNA synthesis, 2 minutes at 94°C for denaturation, and 28 cycles of [94°C (15 sec), 58°C (30 sec), 68°C (20 sec)] for amplification. PCR products were separated on a 2% agarose gel with ethidium bromide (0.1 μg/mL) and visualized with UV irradiation on a GelDoc2000/ChemiDoc System (Bio-Rad).
Membrane permeability assays for cell death
Cells were resuspended in 100 μL medium and 2 μL of acridine orange (100 μg/mL), and ethidium bromide (100 μg/mL) was added. Cells were viewed on an Olympus BX51 fluorescent microscope using the fluorescein filter set. The percentage of dead cells was calculated by counting the number of orange cells (permeablilized membrane allowing ethidium bromide to enter) in a population of diffused green cells as previously described.19, 20 At least 200 cells were counted for each assay. Alternatively, cells were harvested and suspended in PBS and stained with 0.04% trypan blue (Hartman-Leddon Co., Phila, PA) for 5–10 min at room temperature. Stained cells were analyzed by flow cytometry using the FACSscan cytometer and CellQuest software (Becton Dickinson, Mississauga, ON, Canada). Trypan Blue entering the cell (indicator of membrane permeabilization) was measured by the red filter (670 nm, FL3-H) on log scale as indicated by histogram.
MTT assay for cell viability
Cells were seeded in a 96-well dish (1000 cells/well) on day 1 and transferred to hypoxia (or retained in normoxia with fresh media) on day 3. Plates were transferred back to normoxia with fresh media after 24, 48 or 72 hours hypoxia (days 4, 5, or 6, respectively). The MTT colorimetric assay was performed on day 8: 150 μL of MTT (1 mg/mL) in RPMI media was added to each well, and plates were incubated for 1 hour at 37°C in normoxia. The plates were then spun at 1500 rpm for 10 min and 125 μL of DMSO was added to each well to dissolve the dark blue crystals and the absorbance was read at 540 nm on a microplate reader.
Nuclear condensation assay
To assess nuclear condensation (a hallmark of apoptosis), cells were stained with acridine orange and ethidium bromide as described above. Cells which showed intense staining of the DNA in the nucleus were defined as apoptotic versus the diffuse staining of the nucleus in healthy cells. At least 200 cells were counted per condition.
Caspase activity assay
Caspase activity was measured using the Caspase-3 Apoptosis Detection Kit (sc-4263) from Santa Cruz Biotechnology (Santa Cruz, CA) according to the manufacturer’s instructions. Briefly, 20 ug of total cell lysate was diluted in 200 uL reaction buffer with 10 mM DTT and 5 μL of DEVD-AFC substrate peptide. Reactions were performed in 96-well plates and incubated for 1 hour at 37°C. The level of free AFC was measured using a SpectraMax M5 plate reader from Molecular Devices (Sunnyvale, CA) with a 400 nm excitation filter and a 505 nm emission filter.
H2A.X phosphorylation assay
The histone H2A.X is phosphorylated at its carboxy-terminal in response to DNA double-strand breaks (a hallmark of apoptosis). H2A.X phosphorylation was measured using the H2A.X Phosphorylation Assay Kit (17–344) from Upstate Biotechnology Inc. (Lake Placid, NY) according to the manufacturer’s instructions. Briefly, cells were harvested and fixed for 20 minutes on ice in fixation solution at a cell density of 2 × 106 per mL. 50 μL of cells were then resuspended in permeabilization solution and incubated for 20 minutes on ice with 3.5 ul of anti-phospho-Histone H2A.X-FITC conjugate. After washing to remove excess antibody, cells were resuspended in PBS and analyzed by flow cytometry. Increased H2A.X phosphorylation is visualized as a shift in green fluorescence.
RESULTS
Hypoxia induces autophagy in cancer cells
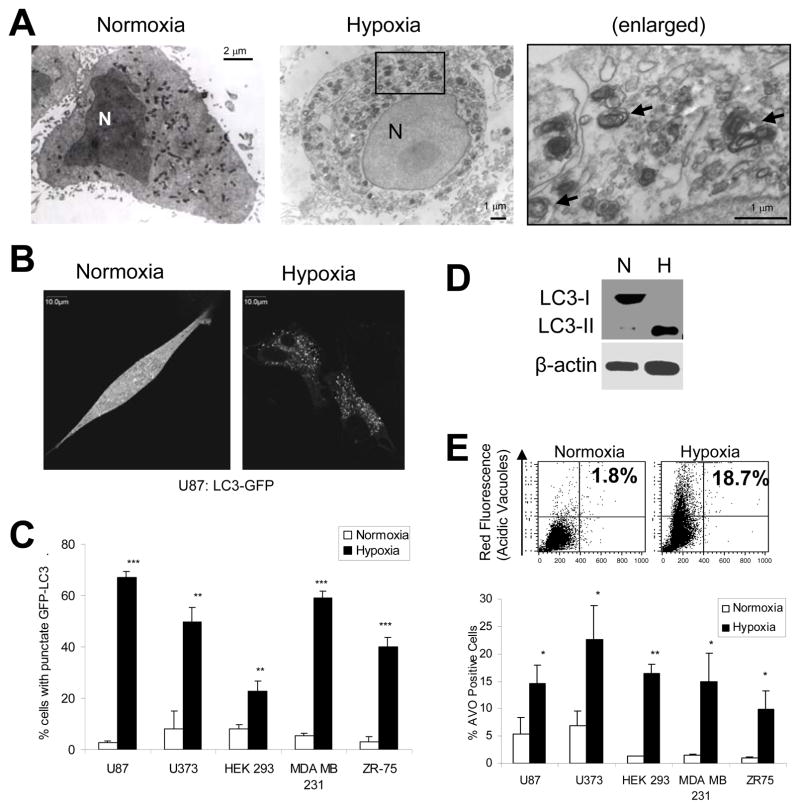
Autophagy is best characterized in response to nutrient deprivation, an energy-limiting cellular stress.3 Hypoxia is another energy-limiting stress, known to decrease ATP levels via inhibition of the oxidative phosphorylation pathway.21 Previously, “metabolic stress” (hypoxia combined with nutrient deprivation) has been shown to induce autophagy.10 To determine whether hypoxia alone can induce autophagy, we exposed five human cell lines (two glioma: U87, U373; two breast cancer: ZR75, MDA-MB-231, and one embryonic: HEK293) to prolonged hypoxia (< 1% O2, 24 to 72 hours). We found that, in all five cell lines, autophagy was induced by hypoxia. Using electron microscopy, we identified double-membraned autophagosomes (arrows) in U87 cells under hypoxic conditions that are absent under normal conditions (Fig. 1A). The autophagosomes observed by electron microscopy frequently contained mitochondria. Similar results were obtained using HEK293 cells (data not shown).
Figure 1.
Hypoxia induces autophagy in cancer cells. (A) Electron microscopy for detection of autophagosomes. U87 cells were incubated in normoxia or hypoxia for 24 hours followed by ultrastructural analysis by electron microscopy. Arrows indicate double-membraned autophagic vacuoles, in some cases containing mitochondria. (B) LC3-GFP distribution. U87-GFP-LC3 cells were incubated in normoxia or hypoxia for 24 hours. GFP-LC3 displayed diffuse intracellular localization under normoxic conditions, while membrane translocation (punctate localization) indicative of autophagy was observed in hypoxic cells. Images A and B were obtained using identical microscope settings, exposure times, and manipulations for brightness and contrast. (C) Quantification of GFP-LC3 translocation in transiently-transfected normoxic and hypoxic cells. 200 cells per sample were counted for punctate versus diffuse staining (representative of three independent experiments). (D) LC3 processing. U87 cells were incubated in normoxia (N) or 24 hours hypoxia (H). Total cell lysates were prepared and analyzed by Western Blot as described. LC3-II, which is produced during autophagy, migrates faster on SDS-PAGE. (E) Production of acidic vesicular organelles (AVOs). (Top) Cells incubated in normoxia or hypoxia for 48 hours were stained with acridine orange (1 μg/mL) and analyzed by flow cytometry to measure acid vacuole (AVO) production. Cytoplasm and nuclei fluoresce green while AVO/autophagosomes fluoresce red. (Bottom) Quantification of AVO production in normoxic and hypoxic cells (representative of three independent experiments). Error bars indicate standard deviation and statistical analysis was by unpaired student’s t-test: *p<.05, **p<.01, ***p<.001.
We also transfected cells with the GFP-tagged autophagy protein LC3. LC3 (mammalian ATG8), is recruited to the autophagosome membrane during autophagy.22 Under normoxia, the GFP-LC3 was diffusely fluorescent whereas under hypoxia GFP-LC3 became punctate, indicating formation of autophagosomes (Fig. 1B). The level of GFP-LC3 punctate staining in the five cell lines ranged from 23% to 67% after 48 hours of hypoxia, compared to 3% to 8% in normoxia (Fig. 1C). In order to be recruited to autophagosome membranes, cytosolic LC3 (LC3-I) is conjugated to phosphatidyl-ethanolamine. This processed form (LC3-II) migrates faster than LC3-I on SDS-PAGE, and is detected more readily by Western blot. We observed a drastic increase in LC3 processing in U87 cells under hypoxia (Fig. 1D). To verify that hypoxia-induced accumulation of LC3-II is due to autophagic flux, and not blockage of lysosomal degradation, we repeated this experiment in the presence or absence of the lysosomal protease inhibitor, chloroquine. Hypoxia-induced accumulation of LC3-II was amplified in the presence of chloroquine (Fig. S1). Thus, LC3-II is degraded in the lysosome during hypoxia, an indication of autophagic flux.
Finally, we assessed the production of acidic vesicular organelles (AVOs). AVOs include lysosomes as well as autophagolysosomes, which are characteristic of autophagy and can be detected and quantified by acridine orange staining and flow cytometry.23 In U87 cells, AVO production was increased from 1.8% in normoxia to 18.7% in hypoxia (Fig. 1E, top). Similar increases were observed in the other four cell lines, corresponding with increased GFP-LC3 punctate staining and LC3 processing (Fig. 1E, bottom).
Prolonged hypoxia induces autophagic cell death without apoptosis
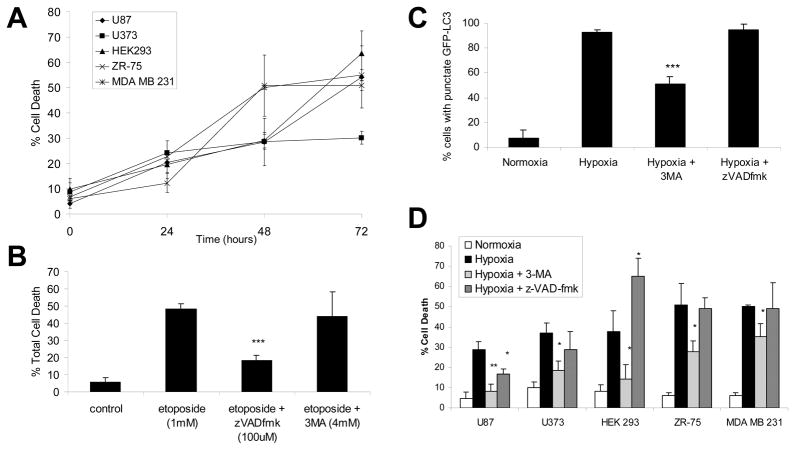
To determine whether the autophagy observed in hypoxic cells is involved in hypoxia-induced cell death, cells were placed under hypoxia for a 72-hour time course and the amount of total cell death was determined by membrane permeability assay (Fig. 2A) and MTT assay (Fig. S2). In all five cell lines, hypoxia induced significant cell death by 48 hours. The breast cancer cell lines showed the most sensitivity to hypoxia with 50% cell death at 48 hours compared to 30% cell death for the other cell lines. We then employed specific inhibitors for both apoptosis (caspase inhibitor z-VAD-fmk) and autophagy (3-methyladenine, 3-MA, which blocks autophagosome formation). We confirmed the specificity of these inhibitors by measuring cell death, caspase activity and GFP-LC3 puncta: only z-VAD-fmk blocked etoposide-induced caspase activation (data not shown) and cell death (Fig. 2B), while only 3-MA blocked the formation of GFP-LC3 puncta during starvation (Fig. S3) and hypoxia (Fig. 2C and Fig. S3).
Figure 2.
Hypoxia-induced cell death is blocked by the autophagic inhibitor 3-MA, but not by the caspase inhibitor z-VAD-fmk. (A) Total cell death in hypoxia. Cells were cultured in hypoxia over a 72-hour time course. Total cell death was determined by acridine orange membrane permeability assay as described in Materials and Methods. Compared to normoxic cells (time zero), cell death in hypoxic cells (48 and 72 hours) was significantly increased in all five cell types (p<.05, 1-tailed t-test). (B) Effect of autophagy and apoptosis inhibitors on etoposide-induced cell death. Cells were treated with 1mM etoposide for 48 hours in the presence or absence of specific inhibitors for autophagy (PI3-Kinase inhibitor 3-MA, 4 mM) or apoptosis (caspase inhibitor z-VAD-fmk, 100 μM). Total cell death was determined as in (A). (C) Effect of autophagy and apoptosis inhibitors on hypoxia-induced autophagy. U87-GFP-LC3 cells were incubated for 24 hours in normoxia or hypoxia, in the presence or absence if 3-MA or z-VAD-fmk. 200 cells per sample were counted for punctate versus diffuse staining. (D) Effect of autophagy and apoptosis inhibitors on hypoxia-induced cell death. Cells were incubated in hypoxia for 48 hours in the presence or absence of 3-MA or z-VAD-fmk. Total cell death was determined as in (B). All results represent three independent experiments and error bars indicate standard deviation. Statistical analysis was by unpaired t-test: *p<.05, **p<.01, ***p<.001.
In ZR75 cells, 48 hours of hypoxia induced 51% cell death, which was unchanged (49%) upon z-VAD-fmk treatment but reduced to 28% upon 3-MA treatment (Fig. 2D). The suppression of hypoxia-induced cell death by 3-MA was only partial, possibly because 3-MA only partially blocked hypoxia-induced autophagy (Fig. 2C). Alternatively, the remaining cell death after 3-MA treatment could be necrotic, as we have evidence that necrosis also occurs in hypoxia: the nuclear protein HMGB1, which is specifically released only during necrosis, exhibited nuclear staining in normoxic cells but was released in a proportion of hypoxic cells (Fig. S4). Similar results in the other cell lines indicate that while z-VAD-fmk treatment had little effect on hypoxia-induced cell death, 3-MA significantly protected cells against hypoxia-induced cell death (Fig. 2D), suggesting that cell death in hypoxia is due to autophagy, and not apoptosis.
Surprisingly, in the case of HEK293 cells, hypoxia-induced cell death was enhanced by caspase inhibition: cell death was 38% in untreated cells, reduced to 14% by 3-MA, and increased to 65% by z-VAD-fmk (Fig. 2D). Although unexpected, this response is in agreement with previous reports that caspase inhibitors can promote alternative death pathways including both necrosis and autophagic cell death.47
To further confirm that autophagy is a mechanism for hypoxia-induced cell death, we used siRNA to knock-down the autophagy proteins Beclin-1 and ATG5.24, 25 Beclin-1 (mammalian ATG6) is a regulator of the Class III PI3K (phosphoinositide 3-kinase) complex involved in autophagosome formation25 while ATG5 is part of a ubiquitin-like pathway required for the formation of autophagic vesicles26. As expected, siRNA against beclin-1 or Atg5 strongly reduced the expression of the corresponding gene products (Fig. 3A). In addition, siRNA knockdown of beclin-1 and Atg5 significantly reduced hypoxia-induced autophagy as determined by AVO production in HEK293 cells (Fig. 3B). As shown in Fig. 1, AVO production in all cell lines tested correlated with induction of autophagy as demonstrated by other detection methods (GFP-LC3 localization, LC3 processing and EM detection of autophagosomes). More importantly, we found that knock-down of beclin-1 or Atg5 significantly reduced hypoxia-induced cell death as determined by membrane permeability assay, described in Materials and Methods. In control cells we observed 60% cell death, whereas beclin-1 or Atg5 knock-down reduced cell death to 28% and 18%, respectively (Fig. 3C). Non-targeting siRNA failed to prevent hypoxia-induced cell death (Fig. 3C). These results indicate that hypoxia-induced cell death is dependent on the autophagy proteins Beclin-1 and ATG5. Collectively, our results show that hypoxia-induced cell death involves autophagy, but not apoptosis.
Figure 3.
Hypoxia-induced cell death is blocked by knock-down of Beclin-1 and ATG5. HEK293 cells were untransfected (control) or transiently transfected with siRNA against beclin-1 or Atg5, or a non-targetting control siRNA. (A) Western blot analysis with β-actin as a loading control at 72 hours post-transfection. Numerical values indicate protein quantification by densitometry, normalized to β-actin. (B) 72 hours post-transfection, cells were incubated in normoxia or hypoxia for a further 48 hours. Autophagy was assessed by detection of AVOs as described. (C) (Top) Total cell death was determined by trypan blue membrane permeability assay using flow cytometry as described in Materials and Methods. Red fluorescence yields two observable peaks, representing viable cells (first peak – weak fluorescence) and dead cells (second peak, unable to exclude the dye and therefore strongly fluorescent). (Bottom) Quantification of trypan blue assay using CellQuest software, representing four independent experiments. Error bars indicate standard deviation; statistical analysis was by unpaired student’s t-test: *p<.05, **p<.01, ***p<.001.
Hypoxia fails to induce apoptosis in apoptosis-competent cells
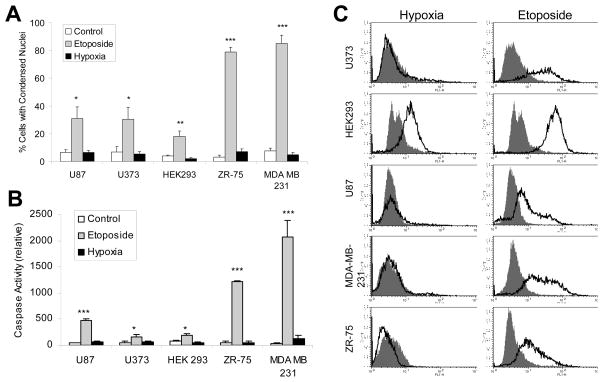
It has been reported that oxygen deprivation can induce apoptosis11, and that autophagy may promote or occur simultaneously with apoptosis 27. Therefore, we investigated the apoptotic response to hypoxia in the five cell lines mentioned above. To first confirm that these cells are competent for apoptosis we used the anti-cancer drug etoposide, a DNA damaging agent and known apoptotic stimulus. In all five cell lines, nuclear condensation (a hallmark of apoptosis) was detected in cells treated with etoposide but failed to be detected under hypoxic conditions at 48 hours (Fig. 4A), even though cell death was detected (Fig. 2A). Depending on the cell line, nuclear condensation occurred in 30% to 85% of etoposide-treated cells compared to just 2% to 7% of hypoxia-treated cells, which did not differ significantly from untreated cells (3% to 8%). We also determined the level of caspase activity using a fluorescently labeled peptide substrate for caspase 3 (DEVD-AFC). Caspase activation was detected following etoposide treatment in all five cell lines (ranging from 160 to 2071 relative units). However, hypoxia failed to significantly activate caspases after 48 hours in all five cell lines tested (40 to 130 relative units, compared to between 25 and 83 for controls) (Fig. 4B). U87, U373 and HEK293 cells had reduced (but significant) caspase activation upon etoposide treatment compared to ZR-75 and MDA MB 231 cells, correlating with the level of nuclear condensation detected.
Figure 4.
Hypoxia fails to induce apoptosis in apoptosis-competent cells. (A) Cells were untreated or treated with etoposide (100 μM) or incubated in hypoxia for 48 hours. Nuclear condensation was assessed by acridine orange and ethidium bromide staining as described in Materials and Methods. Results represent three independent experiments. (B) Cells treated as in (A) were assayed for caspase activity by a fluorometric caspase 3 substrate cleavage assay as described in Materials and Methods. Results represent three independent experiments. (C) Whole cell lysates from cells treated as in (A) were incubated with a FITC-tagged anti-phospho-H2A.X antibody, followed by flow cytometry. Solid curve represents untreated cells and line curve represents hypoxia or etoposide treated cells. The figure is representative of three independent experiments. Error bars indicate standard deviation; statistical significance was determined by unpaired t-test (*p<.05, **p<.01, ***p<.001).
Another marker for apoptosis is DNA double strand breaks, where phosphorylation of histone H2A.X occurs.28 Using antibodies specific for phoshporylated H2A.X, we determined the level of H2A.X phosphorylation by flow cytometry. We found that etoposide treatment induced H2A.X phosphorylation whereas hypoxia did not (Fig. 4C).
HEK293 cells did show some H2A.X phosphorylation under hypoxia compared with other cell lines, but to a lesser extent as compared to etoposide treatment. To further confirm that HEK293 cells fail to undergo apoptosis under hypoxia, we immunostained HEK293 cells with antibodies against cytochrome c. We found that mitochondrial cytochrome c failed to be released under hypoxia (as denoted by punctate staining) whereas etoposide effectively induced mitochondrial cytochrome c release (as denoted by diffuse and reduced staining) (Fig. S5A). To further confirm these findings in U87 cells we co-stained with the mitochondrial dye, mitotracker. We found that cytochrome c co-localized with mitochondria in control and hypoxia-treated cells, whereas a proportion of etoposide-treated cells showed release of cytocrhome c (Fig. S5B). In addition, phase contrast images of HEK293 cells under normoxia, hypoxia and etoposide treatment showed that only etoposide induced the membrane-blebbing and rounding features characteristic of apoptosis. Under hypoxia the cells appeared larger without membrane blebbing (Fig. S5C). The mechanism for the preference between autophagy and apoptosis under hypoxia in specific cells is unknown. Nevertheless, these results suggest that prolonged hypoxia could induce autophagic cell death in the absence of apoptosis in apoptosis-competent cells.
BNIP3 plays a role in hypoxia-induced autophagic cell death
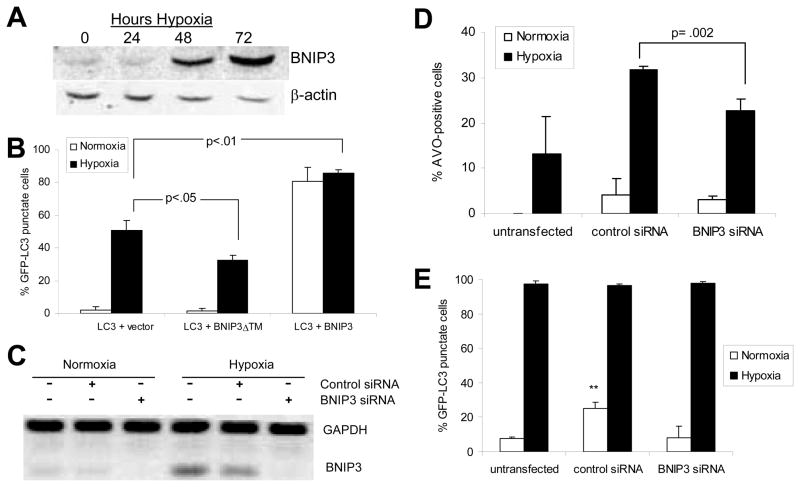
BNIP3, a pro-death member of the Bcl-2 family, is upregulated in hypoxia by the transcription factor HIF1-α.14 Indeed, we found that BNIP3 protein levels were elevated in HEK293 cells (Fig. 6A) and U87 cells (not shown) at 48 and 72 hours hypoxia, time points that correspond with increased cell death (Fig. 2A). Forced over expression of BNIP3 is known to induce a ‘non-apoptotic’ form of cell death which is independent of caspases and cytochrome c release, and which involves extensive cytoplasmic vacuolization.15 Additionally, autophagic cell death induced by the pharmaceutical agent arsenic trioxide involves upregulation of BNIP3.4 We therefore investigated whether BNIP3 is also involved hypoxia-induced autophagic cell death.
Figure 6.
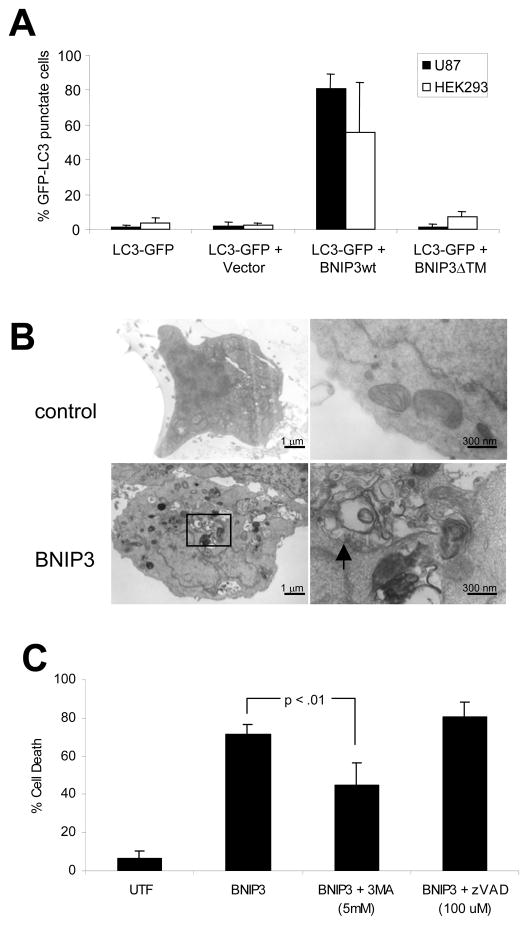
BNIP3 plays a role in hypoxia-induced autophagic cell death. (A) Western blot showing upregulation of BNIP3 in hypoxia in HEK293 cells. (B) HEK293 cells transfected as in Fig. 6A were incubated in normoxia or hypoxia for 24-hours post-transfection. GFP-LC3 distribution (in co-transfected cells only) was assessed as in Fig. 1B. (C) U87 cells were untransfected, or transfected with control siRNA or BNIP3 siRNA and silencing of gene expression was verified by RT-PCR analysis as described in Materials and Methods. (D) Two days after siRNA silencing, U87 cells were retained in normoxia or exposed to 24 hours hypoxia, and AVO production was measured as in Fig. 1E. (E) Two days after siRNA silencing, U87-GFP-LC3 cells were retained in normoxia or exposed to 24 hours hypoxia, and GFP-LC3 distribution was assessed and quantified as in Fig. 1C. Error bars indicate standard deviation of three independent experiments. Statistical significance was determined by an unpaired t-test: **p<.01.
First, we examined the effect of forced BNIP3 over-expression in HEK293 and U87 cells. We found that BNIP3 induced an autophagic response in both cell types: GFP-LC3 punctate staining increased from 2% or 3% in cells transfected with empty vector, to 81% or 56% in cells transfected with BNIP3 (Fig. 5A). There was no increase in GFP-LC3 punctate staining in cells transfected with the dominant-negative BNIP3ΔTM, indicating that the TM domain (which targets BNIP3 to mitochondria) is critical for BNIP3’s ability to induce autophagy. By electron microscopy, we observed the formation of double-membraned vacuoles upon BNIP3 expression in HEK293 cells (Fig. 5B, arrows).
Figure 5.
BNIP3 induces autophagy and autophagic cell death. (A) HEK293 and U87 cells were transiently transfected with the autophagy marker GFP-LC3, alone or with PCDNA3 (empty vector) or PCDNA3-BNIP3 or PCDNA3-BNIP3ΔTM. Immunofluorescence for BNIP3 was performed as described in Materials and Methods. GFP-LC3 distribution (in co-transfected cells only) was assessed and quantified as in Fig. 1C. Error bars indicate standard deviation of three independent experiments. (B) HEK293 cells were untransfected (control) or transfected with BNIP3. Cells were analyzed by electron microscopy for detection of autophagosomes. (C) HEK293 cells were untransfected (control) or transfected with BNIP3. Cells were untreated or treated with 3-MA (5 mM) or z-VAD-fmk (100 μM) for 24 hours post-transfection. Immunofluorescence for BNIP3 was performed and cell death in BNIP3-positive cells was determined by assessing nuclear morphology as described. Error bars indicate standard deviation of three independent experiments. Statistical significance was determined by a two-tailed t-test.
As expected, BNIP3 over-expression also induced cell death. We found that BNIP3-induced cell death was reduced by the autophagy inhibitor 3-MA, but not by the caspase inhibitor z-VAD-fmk: cell death was reduced from 71% to 45% upon treatment with 3-MA, while there was no significant difference in cell death after z-VAD-fmk treatment (Fig. 5C). Our results suggest that in these cells, BNIP3-induced cell death is autophagic and not apoptotic.
Next, we investigated the role of BNIP3 in hypoxia-induced autophagy and cell death. We have previously shown that hypoxia-induced cell death is partially blocked by expression of the dominant-negative BNIP3ΔTM mutant or by knock-down of the BNIP3 gene.14 Here we show that hypoxia-induced autophagy is also reduced by BNIP3ΔTM or by BNIP3 knock-down, while it is enhanced by over-expression of wild type BNIP3: GFP-LC3 punctate staining at 24 hours hypoxia was 53% for empty vector cells, reduced to 34% in BNIP3ΔTM-expressing cells, and increased to 86% for BNIP3-expressing cells (Fig. 6B). Additionally, BNIP3 knock-down by siRNA (Fig. 6C) reduced hypoxia-induced AVO production from 32% to 23%, compared to cells transfected with control siRNA (Fig. 6D). Notably, BNIP3 knock-down did not affect the formation of hypoxia-induced GFP-LC3 puncta in stable GFP-LC3 clones (Fig. 6E). This could be because siRNA transfection itself induces autophagy (Fig. 6C and 6D: control siRNA cells induce autophagy in normoxia), possibly to an extent that masks the effect of BNIP3 knockdown in this particular assay. However it is more likely that since BNIP3 expression occurs relatively late in hypoxia (24 hours, Fig. 6A) compared to autophagosome formation (as early as 2 hours, data not shown), BNIP3 knock-down in hypoxia could selectively affect AVO production (“late autophagy”) but not the formation GFP-LC3 puncta (“early autophagy”). This would indicate that BNIP3 is involved in autophagy at the stage of autophagosome-lysosome fusion as opposed to autophagosome formation. In either case, our results indicate that hypoxia-induced BNIP3 plays an important role in hypoxia-induced autophagic cell death, and that the TM domain is critical for this function.
DISCUSSION
Although autophagy has been characterized in many contexts, it has not yet been extensively studied in hypoxia-induced cell death of cancer cells. Oxygen deprivation is known to induce autophagy during cardiac ischemia, but it has generally been regarded as a protective response in this context.12 By contrast, in mice lacking the RB tumor suppressor gene, hypoxia in the developing fetal liver appears to promote autophagic cell death.45 Previously, autophagic cell death has often been observed alongside apoptosis.29, 30 Apoptosis-independent autophagic cell death has generally been described in systems using caspase inhibitors or genetic manipulation to directly block apoptosis.6–8, 10 We have shown that hypoxia induces autophagic cell death without inducing apoptosis in apoptosis-competent cancer cells. Furthermore, we have determined that BNIP3 is involved in this process.
It is well established that autophagy occurs at low basal levels to maintain cellular homeostasis. For example, in animal models where the autophagy proteins ATG7 or ATG5 are eliminated from neurons, neurodegenerative disorders are found, suggesting a role for autophagy in maintaining normal neuronal function.24, 31
It is also accepted that autophagy can be transiently induced by stress, such as nutrient deprivation, as a survival response.32 This concept has been illustrated many times at the cellular level, and more recently in an animal model of ATG5-deficient mice. These mice, although nearly normal at birth, could not survive the early neonatal starvation period since they failed to induce autophagy.33 In another study, ischemia-induced apoptosis was increased upon blockage of autophagy.12 Thus, autophagy contributes to cell survival functions in various physiological contexts.
The role of autophagy as a form of programmed cell death (PCD type II) has also been demonstrated, but remains controversial. For example, treatment of glioma cells with arsenic trioxide induced autophagy without any apoptotic morphology, and blocking autophagy resulted in decreased cell death.34 In another study, over-expression of a short isoform of tumor suppressor p19ARF (smARF) also resulted in formation of autophagosomes and cell death independent of caspase activation, due to localization of smARF to the mitochondria and reduced mitochondrial membrane potential. Knock-down of beclin-1 and ATG5 reduced smARF-mediated cell death, indicating PCD II.5 A third report showed that upon nutrient withdrawal or metabolic stress, cells lacking Bax and Bak induced autophagy instead of undergoing apoptosis. Blockage of autophagy resulted in reduced cell death.8 Our results provide additional evidence that autophagy can function as a distinct mechanism for programmed cell death: herein, we provide a clear demonstration of prolonged autophagy contributing to hypoxia-induced cell death without induction of apoptosis, in cancer cells that are fully competent to induce apoptosis by a DNA damaging agent.
The apparently contradictory functions for autophagy could be explained by a “dual role” for autophagy in cell survival and cell death: early induction of autophagy may contribute to a protective response, whereas prolonged autophagy could lead to cell death. Indeed, we have detected autophagy in viable cells following acute hypoxia without induction of BNIP3, likely as an initial survival strategy (data not shown). There is evidence for such a pro-survival role of autophagy in hypoxia: when autophagy is blocked by over-expression of AKT or knock-down of Beclin-1, cells show increased sensitivity to “metabolic stress” (hypoxia combined with nutrient deprivation).10 In contrast, we have shown that prolonged autophagy under hypoxic conditions without nutrient deprivation contributes to cell death.
There is also increasing evidence that autophagy functions as an anti-cancer mechanism. In cancers, blockade of the apoptotic pathway is a requirement for tumor progression.35 Recent evidence suggests that autophagic cell death is suppressed in cancers, similar to apoptosis.36 The autophagy gene beclin-1 is a proposed tumor suppressor as haploinsufficiency promotes tumorigeneisis in mice, and expression is reduced in many human tumors.25 Additionally, pro-survival Bcl-2 and Bcl-XL (which are overexpressed in many cancers) can bind to Beclin-1, preventing autophagic cell death.37, 38 Another anti-autophagy strategy employed by many cancers is hyperactivation of the PI3K/AKT pathway, which leads to activation of mammalian target of rapamycin (mTOR) and subsequent inhibition of autophagy.39 Finally, it is known that growth factor withdrawal induces autophagy36, and since growth factors and their receptors are often constitutively activated in cancers20, this could represent another mechanism for evading autophagic cell death. In agreement with this hypothesis, and the evidence presented here for hypoxia-induced autophagic cell death, we have previously demonstrated that epidermal growth factor protects cancer cells from hypoxia-induced cell death.14 In addition, others have shown that cancer cells resist hypoxia-induced cell death through hypoxia-mediated up-regulation of growth factors and angiogenesis factors.40 Thus blockage of autophagy might contribute to tumour progression.
Hypoxia is a poor prognostic indicator for solid tumours due to increased resistance to radiation, restricted access to drug delivery through lack of blood vessels, and adaptation to the microenvironment through genomic instability.40 This study provides evidence for autophagy as a mechanism for hypoxia-induced cell death in cancer cells that is distinct and independent from apoptosis. This indicates that inducing prolonged autophagy in hypoxic cells could be a strategy for cancer treatment. Indeed, prolonged induction of autophagy in cancer cells that are resistant to apoptosis has already been suggested as a possible target for cancer therapy.36, 40, 41
The pro-death Bcl-2 family member BNIP3 is upregulated under hypoxic conditions, including poorly oxygenated regions of solid tumors.42 Hypoxic cancer cells have therefore developed mechanisms to evade BNIP3-induced cell death, including methylation and silencing of the BNIP3 gene43 and sequesteration of BNIP3 protein in the nucleus18. Recently it has been shown that BNIP3 may have opposing functions at different stages of tumor progression. BNIP3 expression was associated with good survival outcome in invasive breast cancer, but with poor outcome in pre-invasive disease.44 This “dual role” for BNIP3 could be explained by its role in autophagy. Our data confirms that BNIP3 expression is a strong inducer of autophagy and demonstrates that BNIP3 is important for hypoxia-induced autophagic cell death. Induction of autophagy through BNIP3 in pre-invasive cancers may provide tumour cells with extra nutrients and promote further tumor progression. However at later stages, BNIP3 expression could promote prolonged autophagy in hypoxic cancer cells possibly through increased autophagosome- lysosome fusions, leading to autophagic cell death and better patient outcome. In a recent study, RB−/− MEF cells had increased HIF-1-mediated BNIP3 expression, contributing to increased autophagy and cell death.45 Moreover, in glioma cells BNIP3 has been identified as having a pivotal role in both ceramide- and arsenic trioxide-induced autophagic cell death.4, 16 Thus, BNIP3 and the autophagy pathway may be important targets for cancer therapy.
In summary, our work shows that hypoxia can induce autophagic cell death in apoptosis-competent cancer cells, though a pathway involving BNIP3. Additional studies will be required to further elucidate BNIP3’s regulation of autophagy and apoptosis in hypoxic cancer cells, and to characterize the transition from beneficial to suicidal autophagic activity in cells under hypoxic stress.
Supplementary Material
Acknowledgments
Ms. Azad has as studentship from the National Sciences and Engineering Research Council of Canada. Dr. Spencer Gibson has a new investigator award from the Canadian Institutes for Health Research. Dr. Chen has a fellowship from CancerCare Manitoba Foundation. This research is supported from a grant by the Canadian Institutes for Health Research.
Abbreviations and acronyms
- BNIP3
Bcl-2/adenovirus E1B 19kDa-interacting protein 3
- ATG
autophagy-related gene
- 3-MA
3-methyladenine
- PCD
programmed cell death
- LC3
microtubule-associated light chain 3 (mammalian ATG8)
- AVO
acidic vacuole
- TM
transmembrane
- siRNA
small interfering RNA
Footnotes
I have no conflict of interest or financial disclosure statements to declare.
References
- 1.Levine B, Klionsky DJ. Development by self-digestion: Molecular mechanisms and biological functions of autophagy. Dev Cell. 2004;6:463–477. doi: 10.1016/s1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- 2.Suzuki K, Ohsumi Y. Molecular machinery of autophagosome formation in yeast, saccharomyces cerevisiae. FEBS Lett. 2007;581:2156–2161. doi: 10.1016/j.febslet.2007.01.096. [DOI] [PubMed] [Google Scholar]
- 3.Mizushima N, Yamamoto A, Matsui M, Yoshimori T, Ohsumi Y. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol Biol Cell. 2004;15:1101–1111. doi: 10.1091/mbc.E03-09-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanzawa T, Zhang L, Xiao L, Germano IM, Kondo Y, Kondo S. Arsenic trioxide induces autophagic cell death in malignant glioma cells by upregulation of mitochondrial cell death protein BNIP3. Oncogene. 2005;24:980–991. doi: 10.1038/sj.onc.1208095. [DOI] [PubMed] [Google Scholar]
- 5.Reef S, Zalckvar E, Shifman O, Bialik S, Sabanay H, Oren M, Kimchi A. A short mitochondrial form of p19ARF induces autophagy and caspase-independent cell death. Mol Cell. 2006;22:463–475. doi: 10.1016/j.molcel.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 6.Xu Y, Kim SO, Li Y, Han J. Autophagy contributes to caspase-independent macrophage cell death. J Biol Chem. 2006 doi: 10.1074/jbc.M513377200. [DOI] [PubMed] [Google Scholar]
- 7.Yu L, Alva A, Su H, Dutt P, Freundt E, Welsh S, Baehrecke EH, Lenardo MJ. Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase-8. Science. 2004;304:1500–1502. doi: 10.1126/science.1096645. [DOI] [PubMed] [Google Scholar]
- 8.Shimizu S, Kanaseki T, Mizushima N, Mizuta T, Arakawa-Kobayashi S, Thompson CB, Tsujimoto Y. Role of bcl-2 family proteins in a non-apoptotic programmed cell death dependent on autophagy genes. Nat Cell Biol. 2004;6:1221–1228. doi: 10.1038/ncb1192. [DOI] [PubMed] [Google Scholar]
- 9.Pouyssegur J, Dayan F, Mazure NM. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature. 2006;441:437–443. doi: 10.1038/nature04871. [DOI] [PubMed] [Google Scholar]
- 10.Degenhardt K, Mathew R, Beaudoin B, Bray K, Anderson D, Chen G, Mukherjee C, Shi Y, Gelinas C, Fan Y, Nelson DA, Jin S, White E. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell. 2006;10:51–64. doi: 10.1016/j.ccr.2006.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brunelle JK, Chandel NS. Oxygen deprivation induced cell death: An update. Apoptosis. 2002;7:475–482. doi: 10.1023/a:1020668923852. [DOI] [PubMed] [Google Scholar]
- 12.Hamacher-Brady A, Brady NR, Gottlieb RA. Enhancing macroautophagy protects against ischemia/reperfusion injury in cardiac myocytes. J Biol Chem. 2006;281:29776–29787. doi: 10.1074/jbc.M603783200. [DOI] [PubMed] [Google Scholar]
- 13.Vaupel P. The role of hypoxia-induced factors in tumor progression. Oncologist. 2004;9 (Suppl 5):10–17. doi: 10.1634/theoncologist.9-90005-10. [DOI] [PubMed] [Google Scholar]
- 14.Kothari S, Cizeau J, McMillan-Ward E, Israels SJ, Bailes M, Ens K, Kirshenbaum LA, Gibson SB. BNIP3 plays a role in hypoxic cell death in human epithelial cells that is inhibited by growth factors EGF and IGF. Oncogene. 2003;22:4734–4744. doi: 10.1038/sj.onc.1206666. [DOI] [PubMed] [Google Scholar]
- 15.Vande Velde C, Cizeau J, Dubik D, Alimonti J, Brown T, Israels S, Hakem R, Greenberg AH. BNIP3 and genetic control of necrosis-like cell death through the mitochondrial permeability transition pore. Mol Cell Biol. 2000;20:5454–5468. doi: 10.1128/mcb.20.15.5454-5468.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daido S, Kanzawa T, Yamamoto A, Takeuchi H, Kondo Y, Kondo S. Pivotal role of the cell death factor BNIP3 in ceramide-induced autophagic cell death in malignant glioma cells. Cancer Res. 2004;64:4286–4293. doi: 10.1158/0008-5472.CAN-03-3084. [DOI] [PubMed] [Google Scholar]
- 17.Chen G, Cizeau J, Vande Velde C, Park JH, Bozek G, Bolton J, Shi L, Dubik D, Greenberg A. Nix and Nip3 form a subfamily of pro-apoptotic mitochondrial proteins. J Biol Chem. 1999;274:7–10. doi: 10.1074/jbc.274.1.7. [DOI] [PubMed] [Google Scholar]
- 18.Burton TR, Henson ES, Baijal P, Eisenstat DD, Gibson SB. The pro-cell death bcl-2 family member, BNIP3, is localized to the nucleus of human glial cells: Implications for glioblastoma multiforme tumor cell survival under hypoxia. Int J Cancer. 2006;118:1660–1669. doi: 10.1002/ijc.21547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCall K, Peterson JS. Detection of apoptosis in drosophila. Methods Mol Biol. 2004;282:191–205. doi: 10.1385/1-59259-812-9:191. [DOI] [PubMed] [Google Scholar]
- 20.Kabore AF, Johnston JB, Gibson SB. Changes in the apoptotic and survival signaling in cancer cells and their potential therapeutic implications. Curr Cancer Drug Targets. 2004;4:147–163. doi: 10.2174/1568009043481551. [DOI] [PubMed] [Google Scholar]
- 21.Kristian T. Metabolic stages, mitochondria and calcium in hypoxic/ischemic brain damage. Cell Calcium. 2004;36:221–233. doi: 10.1016/j.ceca.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 22.Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paglin S, Hollister T, Delohery T, Hackett N, McMahill M, Sphicas E, Domingo D, Yahalom J. A novel response of cancer cells to radiation involves autophagy and formation of acidic vesicles. Cancer Res. 2001;61:439–444. [PubMed] [Google Scholar]
- 24.Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I, Okano H, Mizushima N. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- 25.Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, Levine B. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999;402:672–676. doi: 10.1038/45257. [DOI] [PubMed] [Google Scholar]
- 26.Mizushima N, Yamamoto A, Hatano M, Kobayashi Y, Kabeya Y, Suzuki K, Tokuhisa T, Ohsumi Y, Yoshimori T. Dissection of autophagosome formation using Apg5-deficient mouse embryonic stem cells. J Cell Biol. 2001;152:657–668. doi: 10.1083/jcb.152.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gozuacik D, Kimchi A. Autophagy as a cell death and tumor suppressor mechanism. Oncogene. 2004;23:2891–2906. doi: 10.1038/sj.onc.1207521. [DOI] [PubMed] [Google Scholar]
- 28.Fillingham J, Keogh MC, Krogan NJ. GammaH2AX and its role in DNA double-strand break repair. Biochem Cell Biol. 2006;84:568–577. doi: 10.1139/o06-072. [DOI] [PubMed] [Google Scholar]
- 29.Lee CY, Clough EA, Yellon P, Teslovich TM, Stephan DA, Baehrecke EH. Genome-wide analyses of steroid- and radiation-triggered programmed cell death in drosophila. Curr Biol. 2003;13:350–357. doi: 10.1016/s0960-9822(03)00085-x. [DOI] [PubMed] [Google Scholar]
- 30.Gorski SM, Chittaranjan S, Pleasance ED, Freeman JD, Anderson CL, Varhol RJ, Coughlin SM, Zuyderduyn SD, Jones SJ, Marra MA. A SAGE approach to discovery of genes involved in autophagic cell death. Curr Biol. 2003;13:358–363. doi: 10.1016/s0960-9822(03)00082-4. [DOI] [PubMed] [Google Scholar]
- 31.Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, Ueno T, Koike M, Uchiyama Y, Kominami E, Tanaka K. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441:880–884. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- 32.Levine B. Eating oneself and uninvited guests: Autophagy-related pathways in cellular defense. Cell. 2005;120:159–162. doi: 10.1016/j.cell.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 33.Kuma A, Hatano M, Matsui M, Yamamoto A, Nakaya H, Yoshimori T, Ohsumi Y, Tokuhisa T, Mizushima N. The role of autophagy during the early neonatal starvation period. Nature. 2004;432:1032–1036. doi: 10.1038/nature03029. [DOI] [PubMed] [Google Scholar]
- 34.Kanzawa T, Kondo Y, Ito H, Kondo S, Germano I. Induction of autophagic cell death in malignant glioma cells by arsenic trioxide. Cancer Res. 2003;63:2103–2108. [PubMed] [Google Scholar]
- 35.Russo A, Terrasi M, Agnese V, Santini D, Bazan V. Apoptosis: A relevant tool for anticancer therapy. Ann Oncol. 2006;17(Suppl 7):vii115–vii123. doi: 10.1093/annonc/mdl963. [DOI] [PubMed] [Google Scholar]
- 36.Hait WN, Jin S, Yang JM. A matter of life or death (or both): Understanding autophagy in cancer. Clin Cancer Res. 2006;12:1961–1965. doi: 10.1158/1078-0432.CCR-06-0011. [DOI] [PubMed] [Google Scholar]
- 37.Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, Packer M, Schneider MD, Levine B. Bcl-2 antiapoptotic proteins inhibit beclin 1-dependent autophagy. Cell. 2005;122:927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 38.Maiuri MC, Le Toumelin G, Criollo A, Rain JC, Gautier F, Juin P, Tasdemir E, Pierron G, Troulinaki K, Tavernarakis N, Hickman JA, Geneste O, Kroemer G. Functional and physical interaction between bcl-X(L) and a BH3-like domain in beclin-1. EMBO J. 2007 doi: 10.1038/sj.emboj.7601689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lefranc F, Kiss R. Autophagy, the trojan horse to combat glioblastomas. Neurosurg Focus. 2006;20:E7. doi: 10.3171/foc.2006.20.4.4. [DOI] [PubMed] [Google Scholar]
- 40.Bacon AL, Harris AL. Hypoxia-inducible factors and hypoxic cell death in tumour physiology. Ann Med. 2004;36:530–539. doi: 10.1080/07853890410018231. [DOI] [PubMed] [Google Scholar]
- 41.Kondo Y, Kondo S. Autophagy and cancer therapy. Autophagy. 2006;2:85–90. doi: 10.4161/auto.2.2.2463. [DOI] [PubMed] [Google Scholar]
- 42.Lee H, Paik SG. Regulation of BNIP3 in normal and cancer cells. Mol Cells. 2006;21:1–6. [PubMed] [Google Scholar]
- 43.Murai M, Toyota M, Suzuki H, Satoh A, Sasaki Y, Akino K, Ueno M, Takahashi F, Kusano M, Mita H, Yanagihara K, Endo T, Hinoda Y, Tokino T, Imai K. Aberrant methylation and silencing of the BNIP3 gene in colorectal and gastric cancer. Clin Cancer Res. 2005;11:1021–1027. [PubMed] [Google Scholar]
- 44.Tan EY, Campo L, Han C, Turley H, Pezzella F, Gatter KC, Harris AL, Fox SB. BNIP3 as a progression marker in primary human breast cancer; opposing functions in in situ versus invasive cancer. Clin Cancer Res. 2007;13:467–474. doi: 10.1158/1078-0432.CCR-06-1466. [DOI] [PubMed] [Google Scholar]
- 45.Tracy K, Dibling BC, Spike BT, Knabb JR, Schumacker P, Macleod KF. BNIP3 is an RB/E2F target gene required for hypoxia-induced autophagy. Mol Cell Biol. 2007 Jun 18; doi: 10.1128/MCB.02246-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kubli DA, Ycaza JE, Gustafsson AB. Bnip3 mediates mitochondrial dysfunction and cell death through Bax and Bak. Biochem J. 2007;405:407–15. doi: 10.1042/BJ20070319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vandenabeele P, Vanden Berghe T, Festjens N. Caspase inhibitors promote alternative cell death pathways. Sci STKE. 2006;358:pe44. doi: 10.1126/stke.3582006pe44. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.