Abstract
Background
Longitudinal studies of head circumference growth in infants later diagnosed with autism are needed to understand the accelerated head growth in this disorder.
Methods
We analyzed longitudinal head circumference data from birth to 3 years in 28 children later diagnosed with autism spectrum disorder on the basis of individual growth curve analyses using hierarchical linear models.
Results
Head circumference Z scores relative to norms significantly increased in the autism sample from birth to 12 months, but this pattern did not persist beyond 12 months. Rather, the rate of change in head circumference from 12 to 36 months was not different from the normative sample.
Conclusions
These results suggest that a period of exceptionally rapid head growth occurs during the first year of life in autism; after 12 months of age, the rate of head circumference growth decelerates relative to the rate during the first year of life. Studies of behavioral development in infants later diagnosed with autism suggest that the period of acceleration of head growth precedes and overlaps with the onset of behavioral symptoms, and the period of deceleration coincides with a period of worsening of symptoms in the second year of life.
Keywords: Autism, head circumference, hierarchical linear models, infants, longitudinal
There is great interest in understanding the earliest manifestations of autism. To date, most accounts of early development in autism have relied on retrospective analysis of home videotapes of infants later diagnosed with autism. Recently, a few prospective accounts of the emergence of autism symptoms during infancy have appeared. One goal of such research is to identify young infants at risk for autism so that very early intervention might prevent, or at least ameliorate, the development of autism symptoms.
Studies of young infants at risk for autism, researchers have examined several autism symptoms known to be present by early preschool age in children with autism. Many of these symptoms pertain to behavioral skills present in typically developing infants during the first 2 years of life. A number of behavioral symptoms reliably distinguish preschool-age children with autism from those with developmental delay. These include impairments in social orienting, joint attention, imitation, responses to emotional displays of others, symbolic play, and language (e.g., Charman and Baron-Cohen 1997; Charman et al 1998; Dawson et al 1998a 1998b; Mundy et al 1986; Stone et al 1997, 1999). Relatively few controlled studies, however, have examined how children with autism below age 3 differ from children with related disabilities. Charman et al (1998) found that 20-month-olds with autism were more impaired in joint attention, responses to another's distress, pretend play, and imitation, compared with those with language delay. Other studies found that 24-month-olds with autism performed fewer joint attention gestures, including pointing and showing, and had more impaired language and imitation skills than typically developing and language-impaired children (Lord and Paul 1997; Stone et al 1997).
Retrospective studies of home videotapes have allowed investigators to observe the early social, language, motor, and play behaviors of infants who later receive a diagnosis of autism and to examine developmental differences between infants later diagnosed with autism and typically developing infants (Mars et al 1998; Osterling and Dawson 1994) and infants later diagnosed with mental retardation (Baranek 1999; Osterling, Dawson, Munson 2002). In one such study, Osterling and Dawson (1994) examined videotapes of first birthday parties and demonstrated that 1-year-olds later diagnosed with autism could be distinguished from 1-year-old typically developing infants. How often a child looked at the face of another person (“gaze”) correctly classified the greatest number of children (77%). When gaze was combined with the behaviors of showing, pointing, and orienting to name (i.e., social orienting), 91% of the infants with typical development and autism were correctly classified. These results were replicated by Mars et al (1998) who used blind scoring to evaluate home videotapes of first birthday parties of 25 infants later diagnosed with autism and 25 typically developing infants. Again, the variable “looks at faces” was found to be a powerful discriminator between the two groups, as well as joint attention (e.g., pointing) behaviors.
A subsequent home videotape study compared 1-year-olds later diagnosed with an autism spectrum disorder (ASD) with 1-year-olds later diagnosed with mental retardation and 1-year-olds with typical development. This study showed that 1-year-olds with ASD could be distinguished not only from typical 1-year-olds, but also from 1-year-olds with mental retardation (Osterling et al 2002). This is important because of the high comorbidity of autism and mental retardation. In this study, the infants with ASD were less likely to look at others and to orient to their names than infants with mental retardation. Joint attention behaviors, however, did not distinguish between ASD and developmental delay at 1 year of age, suggesting that other behaviors related to attending to people and other's speech might be important in distinguishing ASD from developmental delay at very young ages. In yet another home videotape study, a failure to orient to name was the best discriminator between 8- and 10-month-olds with ASD versus typical development (Werner et al 2000). In addition, some infants failed to display communicative babbling. Werner and Dawson (2005) used home videotapes of infants later diagnosed with autism to validate the phenomenon of autistic regression in the second year of life. One-year-old infants whose parents reported early onset of autism symptoms (i.e., symptoms evident in the first 12 months of life) displayed fewer joint attention and complex babbling and words at 12 months compared with infants with autistic regression.
A few prospective studies of infants later diagnosed with autism have now been published. Dawson and colleagues (2000) published the first case study of a 1-year-old infant with autism. Because of early feeding difficulties, this infant was evaluated and closely monitored by a neurologist and occupational therapist from birth, allowing a fairly detailed accounting of his early development. Dawson and colleagues noted that, except for feeding difficulties associated with oral motor problems in the first few months of life, autism symptoms were not apparent until after 6 months, when the infant became less socially responsive. By 13 months, many symptoms were apparent, however, including language delay, social aloofness, and stereotyped motor behaviors. Klin and colleagues (2004) later reported a case study of an infant from 12 to 20 months of age, noting delays in language and joint attention at 12–14 months, and, by 15 months of age, impaired toy exploration and a restricted range of interests. Two larger studies of infants at risk for autism have been conducted. Zwaigenbaum et al (2005) studied 65 infants from aged 6 to 36 months who were at genetic risk for autism by virtue of having an older sibling with autism. By 12 months, siblings who were later diagnosed with autism showed poor eye contact and visual tracking, difficulty disengaging attention, and impairments in social orienting, imitation, and social smiling and interest. Other characteristics noted included passivity followed by distress reactions at 6 months, fixation on particular objects, decreased expression of positive affect, and delayed language onset. In the second study of 87 infants at genetic risk for autism, Landa and Garrett-Mayer (2006) reported no symptoms at 6 months of age, but by 14 months they observed delays in language and motor development. In summary, it appears that symptoms of autism are not readily apparent by 6 months of age; however, by 8–12 months, several behavioral symptoms associated with autism can be observed.
In contrast, few early biological markers of autism have yet been identified. One such marker is an atypical pattern of growth in head circumference characterized by small-to-normal head size at birth followed by an accelerated pattern of growth in head circumference that appears to begin at about 4 months (Courchesne and Pierce 2005; Gilberg and de Souza 2002; Redcay and Courchesne 2005). Courchesne and colleagues (2003) reported an increase in head circumference of 1.67 SD between birth and 6–14 months. In a meta-analysis using head circumference (converted to brain volume), brain volume measured from magnetic resonance imaging, and brain weight from autopsy studies, Redcay and Courchesne (2005) found that brain size changes from 13% smaller than control subjects at birth to 10% greater than control subjects at 1 year, and only 2% greater by adolescence. Sparks et al (2002) reported significantly larger total cerebral volume in 3- to 4-year-old children with autism compared with chronological- and mental-age-matched children with developmental delay and chronological-age-matched children with typical development. It is interesting to note that the timing of the onset of accelerated head growth between 4 and 12 months slightly precedes, then overlaps with the onset of behavioral symptoms.
The purpose of this report is twofold: first, we report longitudinal data on head circumference taken in the first 3 years of life from a group of children later diagnosed with ASD with the goal of examining the longitudinal patterns of head circumference growth during the first years of life in autism. Previous reports primarily have relied on cross-sectional data. We report evidence indicating that there exists a period of accelerated head growth during the first year, followed by a slowing in rate of head circumference growth at about 12 months in autism such that the rate of head circumference growth in the second year is not significantly different from the normal population rate of growth at that age. Second, we review the available studies examining early behavioral development in infants with autism and suggest that the period of accelerated head growth precedes and then overlaps with the onset of symptoms, whereas the period of deceleration coincides with the period of behavioral decline or worsening of symptoms in children with autism.
Methods and Materials
Participants
Participants were 28 male children with ASD (17 children with autistic disorder and 11 children with pervasive developmental disorder, not otherwise specified [PDD-NOS]) who were administered a diagnostic evaluation at age 3–4 years (M age = 42.7 months, SD = 4.1, range 37–52 months) as part of their participation in a National Institute of Child Health and Human Development–funded longitudinal study. The mean Mullen Composite Standard Score for the sample was 61.5 (SD = 17.8, range 49–106). Ethnicity was as follows: 23 whites, 1 Japanese, and 3 more than one race. Mother's education was as follows: 1 with some high school, 3 high school graduates, 6 with some college, 12 with college degrees, and 6 with graduate degrees. Participants were recruited from local parent advocacy groups, public schools, the Department of Developmental Disabilities, clinics, hospitals, and the University of Washington Infant and Child Subject Pool. Exclusionary criteria included presence of a known genetic etiology, significant sensory or motor impairment, premature birth or serious birth complications, head trauma, acute neurological disease, or a combination of these. This study was approved by the University of Washington institutional review board, and appropriate informed consent was obtained from all participants.
Diagnosis was based on the Autism Diagnostic Interview—Revised (ADI-R; Lord et al 1994), the Autism Diagnostic Observation Schedule—Generic (ADOS-G; Lord et al 2000), and clinical judgment of diagnosis based on presence or absence of autism symptoms as defined in the fourth edition of the DSM–IV (American Psychiatric Association 1994). Diagnosis of autism was defined as meeting criteria for Autistic Disorder on the ADOS-G and ADI-R and meeting DSM-IV criteria for autistic disorder based on clinical judgment. In addition, if a child received a diagnosis of autistic disorder on the ADOS-G and based on DSM-IV clinical diagnosis, and came within 2 points of meeting criteria on the ADI-R, the child was also considered to have autistic disorder. Diagnosis of pervasive developmental disorder (PDD-NOS or PDD) was defined as meeting criteria for PDD on the ADOS-G, meeting criteria for autistic disorder on the ADI-R or missing criteria on the ADI-R by 2 or fewer points, and meeting DSM-IV criteria for PDD based on clinical judgment.
Head Circumference Data
Head circumference data were derived from medical records of occipital–frontal circumference (OFC) measures between birth and 3 years. These are considered a good measure of brain volume in children aged less than 7 years (Bartholomeusz et al 2002). Only participants for whom at least three valid head circumference measurements were obtained from birth through 36 months were included in the analysis. The mean number of OFC measurements per participant was 7.0. The number of participants with OFC measurements available at each of the following age ranges was similar: birth–5 months, 26 subjects; 6–11 months, 25 subjects; 12–17 months, 24 subjects, and 18–24 months, 20 subjects.
Height and Weight Data
The body length and weight of each individual (taken at the same time the head circumference measures were recorded) were collected to determine whether significant differences from averages of age-matched healthy individuals from the National Center for Health Statistics/Center of Disease Control Normative (CDC) values existed. Analyses of the head circumference growth were performed after partitioning out the influence of individual height measurements, after converting to Z scores based on the normative values of typically developing age- and gender-matched children.
Results
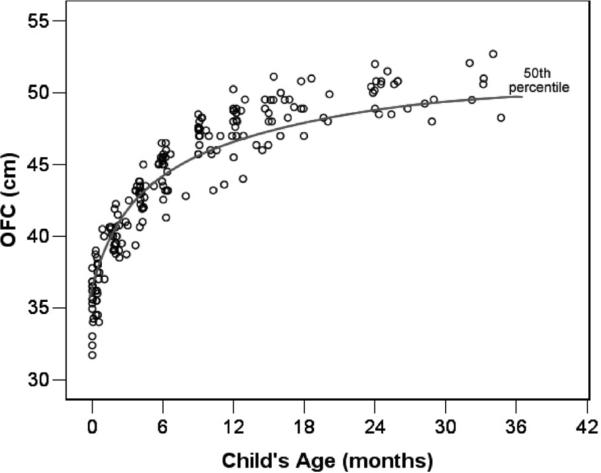
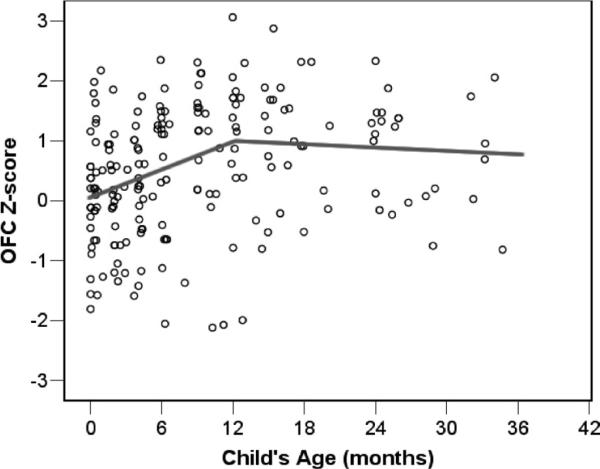
OFC scores obtained from medical records are shown in Figure 1. To compare the growth rates for children measured at varying points in time from birth to 3 years, the metric data for head circumference, height, and weight were transformed to Z scores using the Centers for Disease Control (2002) Growth Charts normative data for gender- and age-matched children, developed by the National Center for Health Statistics. If an individual child's rate of change in OFC Z scores is positive (Z scores are increasing over time), this indicates growth in head circumference that exceeds the typical rate of growth as represented in the CDC norms tables. Similarly, a negative rate of change in OFC Z scores reflects head growth that is slower than that seen in the normative sample. The OFC Z scores are shown in Figure 2.
Figure 1.
Occipital–frontal (OFC) measurements (N = 195) for 28 children with autism spectrum disorder from birth to 36 months of age.
Figure 2.
Occipital–frontal (OFC) Z score measurements (N = 195) with mean estimated growth trajectory for 28 children with autism spectrum disorder (hierarchical linear model two-piece linear model centered at 12 months).
To address the question of whether rate of growth in head circumference changes over the first 3 years of life in children with autism, individual growth curve analyses using hierarchical linear models (HLM; Bryk and Raudenbush 1992) were conducted. The advantage of these methods is that individual growth is explicitly modeled for each child, and thus the question of whether the head circumference growth rate changes during this period can be directly tested. In this study, individual growth in head circumference was modeled with a two-piece linear model in which all OFC Z scores were modeled using three parameters: one that represents the linear rate or change (slope) across the entire period, a second that measures the change in slope at a predefined inflection point, and a third that represents the estimated level of the child's head circumference at the inflection point. In the first analysis, the inflection point was set at 12 months of age allowing this model to estimate change rates for OFC measurements before 12 months and those that occur at or after 12 months.
As seen in Table 1, the estimated OFC Z score at 12 months is significantly greater than zero (coefficient [coeff.] = .919, p < .001). This reflects the fact that children in this sample had head circumference measurements at 12 months that were nearly 1 SD larger than the national CDC norms (OFC Z score = .919, equivalent to the 82nd percentile). In comparison, at birth the sample as a whole had an estimated OFC Z score of .007, which does not significantly differ from the national average of zero. When the OFC Z scores for children taken at birth to 2 weeks of age is examined separately, the sample average is nearly identical to that estimated from the HLM growth curve results (mean OFC Z score = .003, SE = .209, n = 21). As seen in Table 1, the rate of change in OFC Z scores significantly increases across the first year of life at a rate of .076 Z scores per month. In comparison, the rate of change (slope) in OFC Z scores from 12 to 36 months decreases (coeff. = −.083, p = .001). The value of −.083 reflects a decrement in the rate of change. During this period, OFC Z scores are decreasing at an average rate of .007 Z score units per month (.076−.083 = −.007). Coding the two-piece model in a manner to test explicitly whether the slope between 12 and 36 significantly differs from zero, results showed that it does not [coeff. = −.007, SE = .008, t(27) = −.84, p = .409]. In summary, the OFC Z scores for the sample significantly increased from birth to 12 months, but this pattern did not persist beyond 12 months. Rather, the rate of change in head circumference from 12 to 36 months was not different from the normative sample.
Table 1.
Hierarchical Linear Model Growth Trajectory Model of OFC Z Scores with Inflection at 12 Months
| Growth Parameter | Coefficient | SE | t(df) | p |
|---|---|---|---|---|
| OFC Z Score (12 mo) | .919 | .225 | 4.08 (27) | <.001 |
| Slope (Birth–12 mo) | .076 | .015 | 5.07 (27) | <.001 |
| Slope (12–36 mo) | −.007 | .008 | −.84 (27) | .409 |
| Change in Slope (12 moa) | −.083 | .021 | −4.03 (27) | .001 |
mo, months; OFC, occipital–frontal circumference.
The change in slope parameter was coded using an increment–decrement model (Raudenbush & Bryk, 2002, p. 178).
Next, length/height was added as a time-varying covariate to the model. As shown in Table 2, results indicated that even after correcting for measurements of the child's stature, head circumference measurements were increased at 12 months. Furthermore, increases at a rate of .09 Z scores per month from birth to age 12 months remain, and the rate of change drops to near zero after 12 months. Length/height is significantly related to head circumference; children who are longer/taller tend to have larger heads. The greater growth rate of head circumference in the first year, however, and its return to normal rates thereafter is not accounted for by an overall growth in stature.
Table 2.
Hierarchical Linear Model Growth Trajectory Model of OFC Z Scores Controlling for Length/Height
| Growth Parameter | Coefficient | SE | t(df) | p |
|---|---|---|---|---|
| OFC Z Score (12 mo) | .813 | .208 | 3.91 (27) | .001 |
| Length/Height | ||||
| Z Score Covariate | .365 | .061 | 5.97 (27) | <.001 |
| Slope (Birth to 12 mo) | .090 | .015 | 6.01 (27) | <.001 |
| Change in Slope (12 moa) | −.096 | .022 | −4.30 (27) | <.001 |
mo, month; OFC, occipital–frontal circumference.
The change in slope parameter was coded using an increment–decrement model (Raudenbush & Bryk, 2002, p. 178).
We next conducted a similar set of analyses, using 6 months as the inflection point to assess whether the return to normative rates of growth had occurred by this point in time. As shown in Table 3, the rate of change in OFC Z scores during the period from 6 to 36 months was significantly greater than zero [coeff. = .012, SE = .006, t(27) = 2.14, p = .041]. Thus, including the 6- to 12-month observations in the second piece of the model served to increase the estimated growth rate. This suggests that the increased head circumference growth rate does indeed persist into the second half of the first year of life.
Table 3.
Hierarchical Linear Model Growth Trajectory Model of OFC Z Scores with Inflection at 6 Months
| Growth Parameter | Coefficient | SE | t(df) | p |
|---|---|---|---|---|
| OFC Z score (6 mo) | .641 | .198 | 3.23 (27) | .004 |
| Slope (Birth to 6 mo) | .119 | .030 | 3.99 (27) | .001 |
| Slope (6–36 mo) | .012 | .006 | 2.14 (27) | .041 |
| Change in Slope (6 moa) | −.107 | .033 | −3.28 (27) | .003 |
mo, months; OFC, occipital–frontal circumference.
The change in slope parameter was coded using an increment–decrement model (Raudenbush & Bryk, 2002, p. 178).
Discussion
In this study, we examined head circumference growth longitudinally in 28 children with autism spectrum disorder from birth through 36 months of age. Whereas children with ASD, on average, did not have significantly larger head circumference at birth, by 1 year of age head circumference was nearly 1 SD larger than the national CDC norms. This unusual and rapid increase in head growth from birth to 12 months was reflected in a significant difference in slope in OFC Z scores during this period. In comparison, although children's head circumference was larger than normal by 12 months, the rate of growth in head circumference after 12 months was not significantly different from the normative sample. Including length/height as a covariate did not alter these findings. In other words, these longitudinal data suggest that rate of head circumference growth decelerates in infants with autism after 12 months of age relative to the rate from birth to 12 months and that the early period of exceptionally rapid head growth is restricted to the first year of life.
As discussed in the introduction, several studies now suggest that behavioral symptoms of autism first become readily apparent at about 8–12 months of age (Dawson et al 2000; Klin et al 2004; Zwaigenbaum et al 2005). Thus, the period of accelerated head growth appears to precede and then overlap with the onset of autism behavioral symptoms. Interestingly, and perhaps coincidentally, the period after 12 months, during which deceleration of rate of head growth was detected, appears to be associated with a slowing in acquisition or actual loss of skills in infants with autism. The first evidence supporting this idea came from a case study reported by Dawson et al (2000) of an infant who met criteria for autism at 13 months. It was noted in that report that “standardized testing suggested a slight cognitive decline (relative to chronological age) between 1 and 2 years. Whereas at 1 year, [the infant with autism] achieved a developmental index of 82 (twelfth percentile rank), at 2 years of age, he achieved a composite score of 63, which placed him at the first percentile in terms of overall cognitive ability” (Dawson et al 2000, p. 308). The second piece of evidence came from findings derived from a subsample of the children who were part of the head circumference study reported here (Werner and Dawson 2005). In this study, home videotapes of children's first- and second-year birthday parties were collected on young children with ASD with and without a reported history of regression as well as typically developing children. Observations of infants' communicative, social, affective, and repetitive behaviors and their toy play were coded from videotapes of the first and second birthday parties. Infants with ASD whose parents reported a history of regression were indistinguishable from typical infants at 12 months; they showed normal levels of joint attention and communication skills at this age. In contrast, infants with ASD whose parents reported early onset of symptoms displayed fewer joint attention and communicative behaviors at 12 months. By 24 months, both groups of toddlers with ASD displayed fewer instances of word use, vocalizations, declarative pointing, social gaze, and orienting to name, compared with typically developing 24-month-olds. Interestingly, however, both early onset and regressed infants with ASD showed a significant decrease in their use of social gaze between 12 and 24 months, and the decrease was of approximately the same magnitude for each of the groups. Social gaze worsened between ages 1 and 2 years for all the infants with ASD, not only those with regression. The typical children increased their use of complex babble and words dramatically between 12 and 24 months, whereas both the early-onset and regressed infants with ASD showed decline in use of vocalizations and complex babbling.
In Table 4, we review results of studies that have noted a worsening in symptoms or developmental decline (or both) in the second year of life. One of these studies (Dawson et al 2000) reported on an infant's development from birth. In this case study, oral motor impairments were apparent in the first few months, and mild impairments in social responsiveness were evident by 9 months. By 13 months, the infant met criteria for autism. These impairments and symptoms became more obvious and were combined with a decline in cognitive skills, however, between ages 13 and 24 months. Similarly, in a case study by Klin and colleagues (2004), the infant's parents and pediatrician reported fairly normal development until shortly after age 12 months, at which time the infant stopped using words and became less socially engaged. Between 15 and 34 months, this infant demonstrated a continued decline in skills, most notably in exploration of novel toys, attention, response to change, and range of interests. Werner and Dawson (2005) reported a decline in complex babble, pointing, social gaze, and orienting to name as evidenced from home videotapes. Landa and Garrett-Mayer (2006) reported a slowing and loss of fine and gross motor skills, as well as receptive and expressive language skills, between 12 and 24 months.
Table 4.
Studies Documenting Loss or Decline in Skills in Infants with Autism During Second Year of Life
| Author | Year | Ages Studied | Skill Domain | Measures | Method | Age at Which Loss/Decline Noted | Skill Area in Which Loss/Decline Was Observed |
|---|---|---|---|---|---|---|---|
| Dawson et al | 2000 | 1 mo | Oral–motor | OT evaluation | Record review | ||
| 2.5 mo | Sensorimotor | Pediatric neurology evaluation | Record review | ||||
| Self-regulation | |||||||
| 4 mo | Motor | OT evaluation | Record review | ||||
| Self-regulation | |||||||
| 9 mo | Oral–motor | Pediatric neurology evaluation | Record review | ||||
| Sensorimotor | |||||||
| Self-regulation | |||||||
| Social responsive | |||||||
| Social responsiveness | |||||||
| 11–13 mo | Cognitive | Bayley Scales | Direct observation | ||||
| Motor | OT evaluation | Record review | |||||
| 13–15 mo | Diagnostic | ADI-R | Direct observation | ||||
| Cognitive | Bayley Scales | Parent report | |||||
| Speech and language | Preschool Language Scale | ||||||
| Visual–spatial | MacArthur CDI | ||||||
| Social–communicative | Speech perception | ||||||
| Motor imitation | Free play | ||||||
| Object permanence | Social orienting | ||||||
| Neuropsychological | Joint attention | ||||||
| Imitation battery | |||||||
| Visual paired comparison | |||||||
| Delayed response task | |||||||
| 24 mo | Diagnostic | ADOS | Direct observation | 12–24 mo | Social responsiveness | ||
| Cognitive | Mullen Scales of Early Learning | Cognitive ability | |||||
| Klin et al | 2004 | 12 mo | Language | Parent report | Parent report | ||
| Social interaction | |||||||
| 15 mo | Diagnostic | ADOS/ADI | Direct observation | ||||
| Cognitive | Mullen Scales of Early Learning | ||||||
| Speech and language | |||||||
| Adaptive behavior | MacArthur CDI | ||||||
| CSBS-DP | |||||||
| Vineland Adaptive Behavior Scales | |||||||
| 17,20 mo | Head circumference | Record review | |||||
| Weight, length | |||||||
| 23 mo | Diagnostic | ADOS | Direct observation | ||||
| Cognitive | Mullen Scales of Early Learning | Parent report | |||||
| Adaptive behavior | |||||||
| Vineland Adaptive Behavior Scales | |||||||
| 34 mo | Diagnostic | ADOS | Direct observation | 12–34 mo | Words | ||
| Cognitive | Mullen Scales of Early Learning | Parent report | Social (showing, requesting) | ||||
| Adaptive behavior | |||||||
| Vineland Adaptive Behavior Scales | Exploration of novel toys | ||||||
| Attention | |||||||
| Response to change | |||||||
| Range of interests | |||||||
| Werner and Dawson | 2005 | 12, 24 mo | Simple/complex babble | Behavioral coding | Videotape analysis | 12–24 mos | Complex babble/words |
| Words and phrases | |||||||
| Joint attention | Declarative pointing | ||||||
| Gaze (to people/objects) | Social gaze | ||||||
| Orienting to name | Orienting to name | ||||||
| Repetitive behavior | |||||||
| Affect | |||||||
| Toy play | |||||||
| Landa and Garrett-Mayer | 2006 | 6, 14 mo | Cognitive | Mullen Scales of Early Learning | Direct observation | ||
| 24mos | Diagnostic | ADOS | Direct observation | 14–24 mo | Both slowing and loss of skills in fine/gross motor, receptive, and expressive language domains | ||
| Cognitive | Mullen Scales of Early Learning | Parent report | |||||
| Language | |||||||
| Social communication | Preschool Language School | ||||||
| Joint attention | |||||||
| Adaptive behavior | CSBS | ||||||
| Vineland Adaptive Behavior Scale | |||||||
| Vineland SEEC |
In summary, in this report we offer evidence that, whereas, the first year of life in autism is associated with rapid and atypical increases in head growth, this pattern does not persist beyond the first year of life. Rather, rate of head growth decelerates in the second year of life relative to the rate during the first year, such that it is no longer significantly different from the rate of normative growth. The second year of life in autism also appears to be characterized by a slowing in acquisition of behavioral skills, and in some domains, a loss of skills. We hypothesize that this pattern is characteristic of a large proportion, if not the majority, of children with autism and is only accentuated in those with clear autistic regression. In other words, even those children who are clearly symptomatic at 12 months appear to show a regression in the second year of life. This hypothesis will need to be tested in prospective studies that assess both behavioral development and head growth during early life. If this hypothesis is supported by future studies, we propose that the second year of life represents a period of particular vulnerability in autism and that early rapid head growth precedes this period of behavioral decline and vulnerability. As early behavioral and biological markers of risk for autism are identified, it will be possible to detect autism and provide intervention before this period of decline. This offers the possibility of altering the early course of autism with the hope of preventing or at least ameliorating the loss of skills during toddlerhood. (Gillberg and de Souza, 2002, Lainhart et al 1997).
Acknowledgments
This research was funded by a grant from the National Institute of Child Health and Human Development (NICHD; Grant No. U19HD34565), which is part of the NICHD Collaborative Program of Excellence in Autism, and a center grant from the National Institute of Mental Health (NIH; Grant No. U54MH066399), which is part of the NIH STAART Centers Program. The authors thank the children and parents who participated in this study.
References
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th ed. Author; Washington, DC: 1994. [Google Scholar]
- Baranek G. Autism during infancy: A retrospective video analysis of sensory-motor and social behaviors at 9–12 months of age. J Autism Dev Disord. 1999;29:213–224. doi: 10.1023/a:1023080005650. [DOI] [PubMed] [Google Scholar]
- Bartholomeusz HH, Courchesne E, Karns C. Relationship between head circumference and brain volume in healthy normal toddlers, children, and adults. Neuropediatrics. 2002;22:239–241. doi: 10.1055/s-2002-36735. [DOI] [PubMed] [Google Scholar]
- Bryk AS, Raudenbush SW. Hierarchical Linear Models: Applications and Data Analysis Methods. Sage; Newbury Park, CA: 1992. [Google Scholar]
- Centers for Disease Control 2002 CDC Growth charts for the United States: Methods and Development. (Series 11). [Accessed November 15 2004];Vital Health Statistics. 2002 Number 246, May 2002. NCHS/CDC. Available at: http://www.cdc.gov/growthcharts. [PubMed]
- Charman T, Baron-Cohen S. Brief report: Prompted pretend play in autism. J Autism Dev Disord. 1997;27:325–332. doi: 10.1023/a:1025806616149. [DOI] [PubMed] [Google Scholar]
- Charman T, Swettenham J, Baron-Cohen S, Cox A, Baird G, Drew A. An experimental investigation of social-cognitive abilities in infants with autism: Clinical implications. Infant Ment Health J. 1998;19:260–275. [Google Scholar]
- Courchesne E, Carper R, Akshoomoff N. Evidence of brain overgrowth in the first year of life in autism. JAMA. 2003;290:337–344. doi: 10.1001/jama.290.3.337. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Pierce K. Brain overgrowth in autism during a critical time in development: Implications for frontal pyramidal neuron and interneuron development and connectivity. Int J Dev Neurosci. 2005;23:153–170. doi: 10.1016/j.ijdevneu.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Dawson G, Meltzoff A, Osterling J, Rinaldi J. Neuropsychological correlates of early autistic symptoms. Child Dev. 1998a;69:1247–1482. [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Meltzoff AN, Osterling J, Rinaldi J, Brown E. Children with autism fail to orient to naturally occurring social stimuli. J Autism Dev Disord. 1998b;28:479–485. doi: 10.1023/a:1026043926488. [DOI] [PubMed] [Google Scholar]
- Dawson G, Osterling J, Meltzoff AN, Kuhl P. Case study of the development of an infant with autism from birth to two years of age. J Applied Dev Psychol. 2000;21:299–313. doi: 10.1016/S0193-3973(99)00042-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillberg C, de Souza L. Head circumference in autism, Asperger syndrome and ADHD: A comparative study. Dev Med Child Neurol. 2002;44:296–300. doi: 10.1017/s0012162201002110. [DOI] [PubMed] [Google Scholar]
- Klin A, Chawarska K, Paul R, Rubin E, Morgan T, Wiesner L, et al. Autism in a 15-month-old child. Am J Psychiatry. 2004;161:1981–1988. doi: 10.1176/appi.ajp.161.11.1981. [DOI] [PubMed] [Google Scholar]
- Lainhart JE, Piven J, Wzorek M, Landa R, Santangelo SL, Coon H, et al. Macrocephaly in children and adults with autism. J Am Acad Child Adolesc Psychiatry. 1997;36:282–290. doi: 10.1097/00004583-199702000-00019. [DOI] [PubMed] [Google Scholar]
- Landa R, Garrett-Mayer E. Development in infants with autism spectrum disorders: A prospective study. J Child Psychol Psychiatry Allied Discipline. 2006;47:629–638. doi: 10.1111/j.1469-7610.2006.01531.x. [DOI] [PubMed] [Google Scholar]
- Lord C, Paul R. Language and communication in autism. In: Cohen DJ, Volkmar FR, editors. Handbook of Autism and Pervasive Developmental Disorders. 2nd ed. Wiley; New York: 1997. pp. 195–225. [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, Pickles A, et al. The Autism Diagnostic Observation Schedule—Generic: A standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30:205–223. [PubMed] [Google Scholar]
- Lord C, Rutter ML, LeCouteur A. Autism Diagnostic Interview—Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Mars AE, Mauk JE, Dowrick PW. Symptoms of pervasive developmental disorders as observed in prediagnostic home videos of infants and toddlers. J Pediatr. 1998;132:1–5. doi: 10.1016/s0022-3476(98)70027-7. [DOI] [PubMed] [Google Scholar]
- Mundy P, Sigman M, Ungerer J, Sherman T. Defining the social deficits of autism: The contribution of non-verbal communication measures. J Child Psychol Psychiatry. 1986;27:657–669. doi: 10.1111/j.1469-7610.1986.tb00190.x. [DOI] [PubMed] [Google Scholar]
- Osterling J, Dawson G. Early recognition of children with autism: A study of first birthday home videotapes. J Autism Dev Disord. 1994;24:247–257. doi: 10.1007/BF02172225. [DOI] [PubMed] [Google Scholar]
- Osterling J, Dawson G, Munson J. Early recognition of one year old infants with autism spectrum disorder versus mental retardation: A study of first birthday party home videotapes. Dev Psychopathol. 2002;14:239–252. doi: 10.1017/s0954579402002031. [DOI] [PubMed] [Google Scholar]
- Redcay E, Courchesne E. When is the brain enlarged in autism? A meta-analysis of all brain size reports. Biol Psychiatry. 2005;58:1–9. doi: 10.1016/j.biopsych.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Sparks BF, Friedman SD, Shaw DW, Aylward EH, Echelard D, Artru AA, et al. Brain structural abnormalities in young children with autism spectrum disorder. Neurology. 2002;59:184–192. doi: 10.1212/wnl.59.2.184. [DOI] [PubMed] [Google Scholar]
- Stone WL, Lee EB, Ashford L, Brissie J, Hepburn SL, Coonrod E, et al. Can autism be diagnosed accurately in children under 3 years? J Child Psychol Psychiatry. 1999;40:219–226. [PubMed] [Google Scholar]
- Stone WL, Ousley OY, Littleford CD. Motor imitation in young children with autism: What's the object? J Abnorm Child Psychol. 1997;25:475–485. doi: 10.1023/a:1022685731726. [DOI] [PubMed] [Google Scholar]
- Werner E, Dawson G. Validation of the phenomenon of autistic regression using home videotapes. Arch Gen Psychiatry. 2005;62:889–895. doi: 10.1001/archpsyc.62.8.889. [DOI] [PubMed] [Google Scholar]
- Werner E, Dawson G, Osterling J, Dinno J. Recognition of autism before 1 year of age: A retrospective study based on home videotapes. J Autism Dev Disord. 2000;30:157–162. doi: 10.1023/a:1005463707029. [DOI] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bryson S, Rogers T, Roberts W, Brian J, Szatmari P. Behavioral manifestations of autism in the first year of life. Int J Dev Neurosci. 2005;23:143–152. doi: 10.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]