Abstract
Background
Therapy with HIV protease inhibitors (PI) has been associated with hyperglycemia, hyperlipidemia and changes in body composition. It is unclear whether these adverse effects are drug related, involve an interaction with the host response to HIV or reflect changes in body composition.
Methods
Indinavir 800 mg twice daily was given to 10 HIV-seronegative healthy men to distinguish direct metabolic effects of a PI from those related to HIV infection. Fasting glucose and insulin, lipid and lipoprotein profiles, oral glucose tolerance (OGTT), insulin sensitivity by hyperinsulinemic euglycemic clamp, and body composition were measured prior to and after 4 weeks of indinavir therapy.
Results
Fasting glucose (4.9 ± 0.1 versus 5.2 ± 0.2 mmol/l; P = 0.05) insulin concentrations (61.7 ± 12.2 versus 83.9 ± 12.2 pmol/l; P < 0.05), insulin : glucose ratio (12.6 ± 1.7 versus 15.9 ± 1.9 pmol/mmol; P < 0.05) and insulin resistance index by homeostasis model assessment (1.9 ± 0.3 versus 2.8 ± 0.5; P < 0.05) all increased significantly. During OGTT, 2 h glucose (5.1 ± 0.4 versus 6.5 ± 0.6 mmol/l; P < 0.05) and insulin levels (223.1 ± 48.8 versus 390.3 ± 108.8 pmol/l; P =0.05) also increased significantly. Insulin-mediated glucose disposal decreased significantly (10.4 ± 1.4 versus 8.6 ± 1.2 mg/kg · min per µU/ml insulin; 95% confidence interval 0.6–3.0; P < 0.01). There was no significant change in lipoprotein, triglycerides or free fatty acid levels. There was a small loss of total body fat (15.8 ± 1.4 versus 15.2 ± 1.4 kg; P = 0.01) by X-ray absorptiometry without significant changes in weight, waist : hip ratio, and visceral or subcutaneous adipose tissue by computed tomography.
Conclusions
In the absence of HIV infection, treatment with indinavir for 4 weeks causes insulin resistance independent of increases in visceral adipose tissue or lipid and lipoprotein levels.
Keywords: HIV protease inhibitors, indinavir, insulin resistance, body composition, cholesterol, triglycerides, diabetes, lipodystrophy, HIV, AIDS
Introduction
Therapy with HIV protease inhibitors (PI) has had a significant beneficial impact on HIV plasma viremia, immune function, opportunistic infections and mortality from AIDS [1,2]. However, treatment with a PI has been associated with abnormalities in carbohydrate and lipid metabolism, including insulin resistance, hyperglycemia [3–5] and hyperlipidemia [6–11]. Therapy with a PI is also reported to be accompanied by changes in body composition, which include visceral fat accumulation [12], symmetrical lipomatosis [13], dorsocervical fat deposition (buffalo hump) [14], and peripheral fat loss [15]. However, some of these changes in body composition and metabolism have also been reported in HIV-infected subjects not receiving PI therapy [16–18]. It is unclear whether adverse metabolic effects associated with PI are drug related, involve an interaction with the host response to HIV infection, or reflect changes in body composition.
In a recent prospective cohort study [10], treatment of HIV-infected subjects with a regimen that included a PI was found to induce hyperlipidemia and insulin resistance in the absence of changes in body composition. Studies on HIV-seronegative volunteers have shown that ritonavir caused significant hyperlipidemia-after only 2 weeks of treatment, but effects on glucose metabolism were not reported [19]. These results are compatible with direct drug effects of a PI inducing hyperlipidemia and insulin resistance.
Controversy still remains as to whether the observed metabolic effects are unique to individual drugs or common to all PI, as adverse metabolic effects may be more pronounced with some PI than others [11]. Additionally, effects may be more severe in patients with ethnic [20] and genetic risk factors [21] for dyslipidemia and the metabolic syndrome. Finally, HIV infection itself induces changes in lipid metabolism [22], and therapy with PI may exacerbate these effects.
To resolve these issues, we gave indinavir to HIV-negative healthy volunteers to determine the effects of this PI drug in the absence of HIV infection and immune dysregulation. We studied glucose and lipid metabolism before and after 4 weeks of treatment to optimize detection of direct metabolic effects and minimize changes in body composition.
Methods
The study protocol was approved by the Committee on Human Research of the University of California, San Francisco (UCSF) and informed consent was obtained from each subject. Healthy volunteer subjects were recruited from staff at UCSF and from the community. Subjects had no history of medical illnesses (including nephrolithiasis), showed no abnormalities on screening physical examination and had stable weight over the preceding 6 months. Family history alone was not used as a criterion for exclusion. All subjects had a negative HIV-1 antibody test prior to and at the end of the study.
Exclusion criteria included body mass index (BMI) > 27 kg/m2, fasting serum total cholesterol > 6.2 mmol/l, triglycerides > 3.84 mmol/l, fasting glucose > 7 mmol/l, serum aspartate or alanine aminotransferases > 50 U/l and creatinine > 1.4 mg/dl. Twelve subjects enrolled in the study. One subject failed to return after baseline studies; a second withdrew during the baseline study. The data from these two subjects were not used.
Study design
This was an open-label study of indinavir. Subjects were admitted to the General Clinical Research Center (GCRC) at San Francisco General Hospital (SFGH) for 5 days and placed on a constant calorie diet with fixed proportions of carbohydrate, fat and protein designed to maintain body weight and minimize dietary influences on metabolism [23]. After baseline studies, subjects were discharged, given indinavir (Crixivan, Merck & Co, Rahway, New Jersey, USA) 800 mg every 8 h (0700, 1500, and 2100 h) and seen weekly for safety and adherence monitoring (see below). They were instructed to resume their usual diet and physical activity. After 4 weeks on treatment, subjects were readmitted to the GCRC for a second 5-day period for repeat studies.
Measurements
Fasting lipids and free fatty acids were measured by enzymatic colorimetric methods (Sigma Diagnostics, St Louis, Missouri, and Wako Chemicals, Richmond, Virginia, USA, respectively). Lipoprotein subfractions and lipoprotein (a) were measured by microultracentrifugation (Atherotech, Birmingham, Alabama, USA). Whole blood and plasma glucose and lactate were measured using a glucose analyzer (YSI 2300 STAT-Plus Glucose & Lactate Analyzer, YSI Inc., Yellow Springs, Ohio, USA). Serum insulin levels were determined by Coat-A-Count radioimmunoassay (Diagnostic Products Corp, Los Angeles, California, USA) with interassay coefficient of variation of 7.3%, lower detection limit of 1.3 µU/ml and 40% cross-reactivity with pro-insulin. Indinavir levels were measured by liquid chromatography mass spectrometry (lower detection limit of 5 ng/ml) at the Drug Research Unit, SFGH.
Fat clearance
At 8:00 a.m., after a 10 h overnight fast, clearance of triglyceride-rich particles was determined by intra-venous fat tolerance test, as described previously [24]. Intralipid (Liposyn II 20%, Abbott Laboratories, Chicago Illinois, USA) was infused at 0.1 g/kg body weight over 2 min and blood samples were collected at 0, 5, 10, 15, 20, 30, 40 and 50 min for nephelometry.
Oral glucose tolerance test
At 8:00 a.m., after a 10 h overnight fast, subjects received 75 g glucose orally. Blood samples were collected at 0, 30, 60, 90, 120 and 180 min for the measurement of plasma glucose (NaF-containing tubes) and serum insulin. The areas under the curve (AUC) for glucose and insulin were calculated by the trapezoid method. Fasting plasma glucose (FPG) and serum insulin (FSI) levels were measured on 2 days and averaged data were used to calculate insulin resistance index (RI) by the homeostasis model assessment (HOMA) [25] (RI = FSI × FPG/22.5) and insulin : glucose (I:G) ratios.
Studies of body composition
BMI was calculated as weight in kilograms divided by height in meters squared (kg/m2). Total and regional body composition were measured by dual-energy X-ray absorptiometry (DEXA; Lunar model DPX, Madison, Wisconsin, USA, software version 3.65) [26]. Analysis of scans was performed as previously described [18] with coefficient of variation for repeated regional analysis of trunk, arm, and leg fat at 1.0, 2.7, and 1.4%, respectively. Central abdominal region (at L2–L4) was analyzed as described by Carey et al. [27]
Computed tomography was performed on a helical HiSpeed CTI Scanner (General Electric Medical Systems, Milwaukee, Wisconsin, USA). A single 7 mm slice obtained at the level of the L4–L5 intervertebral disc space was used for quantification of visceral and subcutaneous fat. All images were analyzed in a matrix of 512 × 512 pixels by one investigator, with a coefficient of variation < 1% on repeat analysis.
Waist (at the level of the umbilicus) and hip circumferences were measured in triplicate and waist : hip ratio calculated. Bioelectric impedance analysis was performed using a tetra polar BIA101 Quantum (RJL Systems Inc, Clinton Township, Michigan, USA). Body cell mass and total body water were calculated using software version 3.1b.
Hyperinsulinemic euglycemic clamp
The hyperinsuliemic euglycemic clamp was performed as described by DeFronzo et al. [28]. Polyethylene cannulae were placed into an antecubital vein for infusion and into a vein in the dorsum of the contralateral hand, which was kept in a heated box at 50–55°C for ‘arterialized’ blood sampling. Subjects fasted overnight prior to the procedure. Beginning at 9:00 a.m., insulin (Humulin R, Eli Lilly, Indianapolis, Indiana, USA) was administered as a primed continuous intravenous infusion, followed by a constant infusion at the rate of 40 mU/m2 = min for 180 min. Whole blood glucose concentration was measured every 5 min after the start of the insulin infusion. A variable infusion of 20% dextrose was used to maintain plasma glucose concentration at 4.4 mmol/l (80 mg/dl) with a coefficient of variation < 5% based on the negative feedback principle. Blood samples were collected for post-hoc determination of plasma glucose and serum insulin concentrations.
Adherence
Subjects were instructed to take indinavir 800 mg three times daily with two glasses of water. Adherence was monitored at each weekly visit by four methods: (i) self-report and direct questioning; (ii) manual pill counts; (iii) electronic pill counts using a Medication Event Management System (MEMS, Aprex Corp. Union City, California, USA) for quantification of adherence rate and dosing intervals; and (iv) measurement of plasma indinavir trough levels.
Statistical analyses
Data were analyzed using Sigma Stat v. 2.03 (SPSS, Inc. San Rafael, California, USA). Paired t-tests were used for normally distributed data. Non-parametric data were analyzed using Mann–Whitney or Wilcoxon Rank Sum test. Data are presented as mean ± SEM. P values are two-tailed.
Results
Ten subjects completed the study. Subjects ranged in age from 30 to 65 years (mean 42.1 ± 3.9); four were non-white (two Hispanic, two African-American). Average weekly indinavir trough levels were 0.726 ± 0.205 µmol/l (range, 0.161–1.239) (446 ± 126 ng/ml) (range, 99–761 ng/ml). Average therapeutic coverage as measured by MEMS was 95 ± 2% (range, 86–100). The most common reported adverse effects were dry skin and dry mouth (in seven) followed by nausea and muscle aches (in three). Three subjects developed asymptomatic elevations in total bilirubin (peak levels 23, 17, and 16 mg/l) and one had aspartate aminotransferase elevation to twice the upper limit of normal value. No subject withdrew from the study because of adverse events related to indinavir.
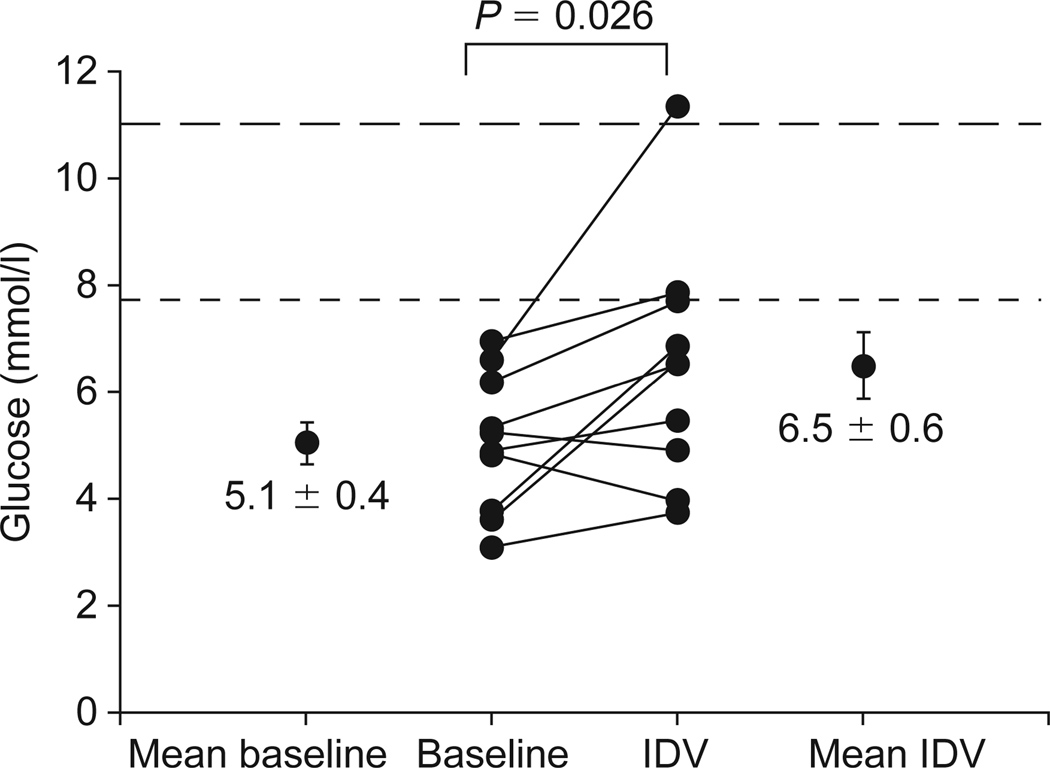
Fasting glucose, insulin, I : G ratio and insulin resistance index by HOMA all increased significantly with indinavir (Table 1). Fasting lactate levels did not change. Seven subjects had an increase in HOMA index while three had no change (Fig. 1). During OGTT, glucose and insulin levels at 2 h also increased (Table 1). Of note, based on glucose levels at 2 h on OGTT, one subject developed diabetes (2 h glucose 11.3 mmol/l), one became glucose intolerant (2 h glucose 7.9 mmol/l), and one nearly developed impaired glucose tolerance (2 h glucose 7.7 mmol/l) (Fig. 2). A trend towards an increase in AUC for glucose was observed (Fig. 3a). Insulin levels at 2 h were significantly increased (Table 1 and Fig. 3b).
Table 1.
Glucose metabolism at baseline and during indinavir therapy.
| Baseline | Indinavir for 4 weeks | P value | |
|---|---|---|---|
| FPG (mmol/l)a | 4.9 ± 0.1 | 5.2 ± 0.2 | 0.05 |
| FSI (pmol/l)a | 61.7 ± 12.2 | 83.9 ± 12.2 | 0.04 |
| FSI/FPG (pmol/mmol)a | 12.5 ± 1.2 | 15.9 ± 1.9 | 0.04 |
| HOMA IRa | 1.9 ± 0.3 | 2.8 ± 0.5 | 0.03 |
| Lactate (mmol/l)a | 0.99 ± 0.08 | 1.08 ± 0.08 | 0.23 |
| Glucose 2 h (OGTT) (mmol/l) | 5.1 ± 0.4 | 6.5 ± 0.6 | 0.03 |
| Insulin 2 h (OGTT) (pmol/l) | 223.1 ± 48.8 | 390.3 ± 108.8 | 0.03 |
| AUC glucose (OGTT) (mmol · h/l) | 17.4 ± 0.9 | 18.9 ± 2.4 | 0.08 |
| AUC insulin (OGTT) (pmol · h/l) | 768 ± 86 | 926 ± 165 | 0.23 |
Data are mean ± SEM. All P values are by paired-test (n = 10).
FPG, fasting plasma glucose; FSI, fasting serum insulin; OGTT, oral glucose tolerance test; HOMA IR, homeostasis model assessment of insulin resistance index; AUC, area under curve (3 h).
To convert lactate from mmol/L to mg/dL, multiply by 9.0.
To convert glucose from mmol/L to mg/dL, multiply by 18.1.
To convert insulin from pmol/L to µU/mL, multiply by 0.14.
Average of 2 days.
Fig 1.
Homeostasis model assessment [24] (HOMA) insulin resistance index at baseline and after 4 weeks therapy with indinavir (IDV) (see text for calculation). Data are mean ± SEM and represent the average of 2 days.
Fig. 2.
Plasma glucose levels 2 h following 75 g oral glucose at baseline and after 4 weeks taking indinavir (IDV). One subject developed diabetes (2 h glucose 11.3 mmol/l), another became glucose intolerant (2 h glucose 7.9 mmol/L), and one nearly developed impaired glucose tolerance (2 h glucose 7.7 mmol/l). (To convert glucose from mmol/l to mg/dl, multiply by 18.1.)
Fig. 3.
Glucose (a) and insulin (b) levels during oral glucose tolerance test at baseline (·) and after 4 weeks taking indinavir (IDV; ·). Area under the curve (AUC) for glucose showed a trend towards an increase (P = 0.08). The second-phase insulin secretion at 2 h was increased with IDV but the 3 h AUC did not reach statistical significance (P = 0.25). (To convert insulin from pmol/l to µU/ml, multiply by 0.14.)
During hyperinsulinemic euglycemic clamp, a steady-state insulin level of ≃500 pmol/l was achieved after 30 min (Fig. 4a). Steady-state glucose levels of ≃4.5 mmol/l were reached at 60 min and were maintained for 180 min (Fig. 4a). After 4 weeks of indinavir treatment, insulin-mediated glucose disposal rate per unit of insulin from 60 to 180 min declined in nine out ten subjects by an average of 1.4 mg/kg · min per µU/ml (95% confidence interval, 0.6–3.0; P < 0.01) (Fig. 4b).
Fig. 4.
(a) Summary of the steady-state plasma insulin and glucose concentrations and glucose infusion rate during hyperinsulinemic euglycemic clamp: insulin (◆) and glucose (●) levels during baseline study; insulin (◇) and glucose (○) levels during study on indinavir. (b) Insulin-mediated glucose disposal rate (mg/kg per min) per unit of plasma insulin (µU/ml) (M/I) expressed as mean ± SEM during steady state (60–180 min) at baseline and after 4 weeks taking indinavir (IDV).
Total, direct cholesterol levels in low density lipoproteins (LDL), high density lipoproteins (HDL) and intermediate density lipoproteins did not change (Table 2). The mean difference in plasma triglyceride levels was not significant (P = 0.22), nor was there a significant change in plasma free fatty acids, lipoprotein (a) or subclasses of LDL and HDL. The density pattern of LDL particles increased in two subjects [type A (light) to type B (dense) and type AB (intermediate) to type B] and decreased in another (type AB to type A). The clearance time of triglycerides as measured by an intravenous fat tolerance test increased slightly (Table 2). After 4 weeks of indinavir treatment, there was no significant change in weight, waist : hip ratio, percentage body fat, body cell mass, total body water by bioelectric impedance analysis, or in visceral fat or subcutaneous fat by computed tomography. There was a small loss of total fat and trunk fat, but no change in L2–L4 fat. Total and regional lean body mass remained unchanged (Table 3).
Table 2.
Lipoproteins and lipids at baseline and during indinavir therapy.
| Baseline | Indinavir for 4 weeks | P value | |
|---|---|---|---|
| Total cholesterol (mmol/l) | 4.7 ± 0.2 | 5.1 ± 0.2 | 0.16 |
| LDL cholesterol (mmol/l) | 3.2 ± 0.2 | 3.3 ± 0.2 | 0.48 |
| LDL-A (mmol/l) | 1.6 ± 0.2 | 1.5 ± 0.2 | 0.40 |
| LDL-B (mmol/l) | 1.0 ± 0.3 | 1.3 ± 0.3 | 0.31 |
| HDL cholesterol (mmol/l) | 1.1 ± 0.1 | 1.0 ± 0.1 | 0.70 |
| HDL2 (mmol/l) | 0.26 ± 0.05 | 0.23 ± 0.03 | 0.32 |
| HDL3 (mmol/l) | 0.80 ± 0.03 | 0.80 ± 0.05 | 0.87 |
| VLDL cholesterol (mmol/l) | 0.49 ± 0.08 | 0.52 ± 0.08 | 0.56 |
| IDL cholesterol (mmol/l) | 0.36 ± 0.05 | 0.36 ± 0.03 | 0.49 |
| Lipoprotein (a) (mmol/l) | 0.14 ± 0.03 | 0.14 ± 0.02 | 0.90 |
| Triglycerides (mmol/l) | 1.4 ± 0.2 | 1.7 ± 0.4 | 0.22 |
| Free fatty acids (mmol/l) | 0.30 ± 0.02 | 0.22 ± 0.04 | 0.31 |
| Half-life fat clearance (min) | 19.3 ± 2.3 | 21.9 ± 3.1 | 0.03 |
Data are mean ± SEM.
All P values are by paired-t test (n = 10).
HDL, high density lipoprotein; LDL, low density lipoprotein; VLDL, very low density lipoprotein; IDL, intermediate density lipoprotein.
To convert cholesterol from mmol/L to mg/dL, multiply by 38.7.
To convert triglycerides from mmol/L to mg/dL, multiply by 88.6.
To convert free fatty acids from mmol/L to mg/dL, multiply by 26.5.
Table 3.
Body composition at baseline and on indinavir.
| Baseline | Indinavir for 4 weeks | P value | |
|---|---|---|---|
| Weight (kg) | 70.8 ± 2.6 | 70.7 ± 2.5 | 0.85 |
| Body mass index (kg/m2) | 23.4 ± 0.5 | 23.4 ± 0.4 | 0.62 |
| Waist : hip ratio | 0.87 ± 0.02 | 0.88 ± 0.02 | 0.15 |
| Fat | |||
| Visceral (mm2) | 8896 ± 1865 | 9449 ± 2178 | 0.28 |
| Subcutaneous (mm2) | 14762 ± 1577 | 14629 ± 1651 | 0.72 |
| Visceral : subcutaneous | 0.58 ± 0.08 | 0.55 ± 0.10 | 0.27 |
| Visceral : total | 0.34 ± 0.03 | 0.35 ± 0.04 | 0.34 |
| Total adipose tissues (kg) | 15.8 ± 1.9 | 15.2 ± 1.9 | 0.01 |
| Appendicular fat (kg) | 6.7 ± 0.7 | 6.5 ± 0.6 | 0.08 |
| Trunk fat (kg) | 8.3 ± 1.2 | 7.9 ± 1.2 | 0.03 |
| L2–L4 fat (kg) | 1.2 ± 0.2 | 1.2 ± 0.2 | 0.42 |
| Total lean tissue (kg) | 52.0 ± 1.6 | 52.4 ± 1.7 | 0.22 |
| Appendicular lean (kg) | 24.6 ± 1.0 | 24.6 ± 1.2 | 0.88 |
| Trunk lean (kg) | 23.9 ± 0.7 | 24.3 ± 0.5 | 0.64 |
| L2–L4 lean (kg) | 3.2 ± 0.1 | 3.4 ± 0.2 | 0.35 |
| Body cell mass (kg) | 28.7 ± 0.8 | 28.1 ± 1.0 | 0.26 |
| Total body water (l) | 41.3 ± 1.3 | 41.1 ± 1.4 | 0.25 |
Data are mean ± SEM. All P values are by paired t-test (n = 10).
Discussion
The metabolic effects of indinavir in HIV-seronegative subjects was studied to distinguish direct drug effects from those caused by the host response to HIV that occurs in the context of immune reconstitution. Following 4 weeks of treatment, there was a small decrease in total fat by DEXA but no increase in abdominal visceral fat and subcutaneous fat or the ratio of visceral to total adipose tissue by computed tomographic scanning. Consequently the effects on intermediary metabolism cannot be attributed to an increase in visceral fat, although they could be part of a more complex syndrome that subsequently would lead to changes in body composition.
Indinavir caused insulin resistance in these HIV-negative subjects. Insulin resistance could be demonstrated using fasting samples (HOMA index and insulin : glucose ratio), OGTT, and, more significantly, hyperinsulinemic euglycemic clamp. Based on 2 h glucose during OGTT, diabetes mellitus developed in one subject, impaired glucose tolerance in another and near impaired glucose tolerance in a third. These changes occurred despite only a small increase in fasting glucose and insulin levels. The increase of 5% in fasting glucose after 4 weeks of treatment was less than the average increase of 11% after 3 months on a PI drug seen in HIV-positive subjects in a prospective study [10], and the 15% reported in a cross-sectional study with average duration on PI of 18 months [5]. One previous study reported a delay in the peak of insulin levels in PI-treated compared with PI-naive HIV-infected patients [5]. However, there was a typical early peak of insulin levels in our HIV-negative patients treated with indinavir.
Genetic factors may play a role in susceptibility to the effects of indinavir. Three subjects had first-degree relatives with diabetes. These included the subject who developed diabetes and the subject who developed near impaired glucose tolerance by OGTT criteria. The third subject with a family history of diabetes had the greatest decline in glucose disposal rate (M value) during hyperinsulinemic euglycemic clamp on indinavir.
Peripheral insulin resistance has been reported as a complication of therapy with PI in HIV-infected patients, but the mechanism is not completely understood [5,10,15,19]. In this study there was a 20% decrease in sensitivity of the peripheral tissues to insulin action (i.e. insulin-mediated glucose disposal) during a hyperinsulinemic euglycemic clamp. Insulin-mediated glucose disposal occurs primarily in muscle and adipose tissues [29]. Insulin signaling induces translocation of the glucose transporter vesicles (predominantly GLUT4) to the plasma membrane [30]. In vitro study of the effect of PI on glucose uptake in cultured adipocytes has shown that indinavir at near-peak therapeutic concentration of 10 µmol/l (7120 ng/ml) caused inhibition of the insulin-stimulated glucose uptake [31]. This 26% decrease is similar to our finding of 20% decline in insulin-mediated glucose disposal, although findings in cultured cells cannot be directly extrapolated to results in vivo. Moreover, our data do not exclude another independent effect of indinavir on the liver that causes insulin resistance.
Our study demonstrates that treatment with indinavir for 4 weeks has little direct effect on lipid and lipoprotein levels in HIV-seronegative subjects. Total cholesterol values were within 8% of baseline and lipoprotein (a) remained unchanged. Triglycerides were 26% higher; however, this increase was not statistically significant as the increase in the mean level after indinavir treatment was driven primarily by a single subject. Lipoprotein subfraction analysis revealed no significant alteration in HDL subclasses. It is, therefore, unlikely that a defect in lipid metabolism is the cause of indinavir-induced insulin resistance. However, the finding of a small change in plasma triglyceride levels and an increased rate of clearance of triglycerides suggest that some changes occur that may increase in magnitude with longer exposure to PI or as a consequence of subsequent changes in body composition.
In the prospective cohort study of HIV-infected subjects cited above [10], those who initiated therapy with a PI had an average increase of 22% in total cholesterol, 20% in LDL cholesterol and 47% in triglycerides, consistent with other prospective studies of similar duration [9] in HIV-infected subjects. Also in contrast to our data, Purnell et al. found that HIV-seronegative subjects treated with ritonavir for only 2 weeks had an average increase of 24% in total cholesterol, 40% in lipoprotein (a), and 245% in triglycerides [32]. Though apparently contradictory, these data are supported by other observations indicating that ritonavir has a more potent effect on plasma lipids than other PI [8,11,21]. For example, ritonavir increased triglycerides, while nelfinavir and indinavir did not; ritonavir and nelfinavir caused statistically greater increases in LDL than did indinavir (150%, 144% and 126%, respectively) [11]. These data, therefore, support an early and more potent effect of ritonavir on lipids than with indinavir or nelfinavir. The differing effects of individual PI on lipids support the concept of drug-specific rather than class-specific effects.
Our study has a number of potential limitations. We have studied only one PI and therefore our data may not be applicable to all drugs in this class. We studied men only; metabolic effects might be different in pre- and postmenopausal women. While it was advantageous to study metabolism after 4 weeks of treatment with indinavir, we cannot determine if longer duration of treatment would lead to significant changes in body composition. Furthermore, we cannot determine whether changes in body composition, if they occurred, would compound the metabolic effects we have reported in this study. Data presented in this paper cannot be used to determine whether indinavir causes insulin resistance in the liver. Finally, combination drug regimens (e.g. indinavir in combination with ritonavir or stavudine) may have additional unique metabolic effects.
In summary, our study suggests that the earliest direct effect of treatment with indinavir on metabolism is peripheral insulin resistance, which can be detected within 4 weeks in the absence of any increases in visceral fat tissue. In that period, indinavir has little effect on lipid and lipoprotein levels in healthy HIV-negative subjects. The mechanism by which indinavir induces insulin resistance is unknown. Future studies are needed to assess whether these effects are common to other PI and how they might be influenced by genetic predisposition and interact with the changes in body composition.
Acknowledgments
The authors thank Miyin Pang, Barbara Chang, Joy Hirai, Natalie Patterson, Carlynn Yee-Hicaiji and the GCRC nursing staff for technical assistance and Dr Francesca Aweeka for measuring indinavir levels.
Sponsorship: grants from Merck Research Laboratories (Merck & Co. Inc., Rahway, New Jersey, USA), the AIDS Clinical Research Center of University of California, San Francisco, and the Universitywide AIDS Research Program F99SF-044 (M.N) and by grants from the National Institutes of Health (NIH) DK45833 (M.S) and DK52615 (K.M). The studies were conducted in the General Clinical Research Center (GCRC) at San Francisco General Hospital supported by a grant (RR-00083) from the National Center for Research Resources (NCRR) NIH. J.L. is a Clinical Associate Physician supported by the NCRR. C.G. is supported by the University of California AIDS Clinical Research Center and the Research Service of the Department of Veterans Affairs.
References
- 1.Autran B, Carcelain G, Li TS, et al. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science. 1997;277:112–116. doi: 10.1126/science.277.5322.112. [DOI] [PubMed] [Google Scholar]
- 2.Cameron DW, Heath-Chiozzi M, Danner S, et al. Randomised placebo-controlled trial of ritonavir in advanced HIV-1 disease. The Advanced HIV Disease Ritonavir Study Group. Lancet. 1997;351:543–549. doi: 10.1016/s0140-6736(97)04161-5. [DOI] [PubMed] [Google Scholar]
- 3.Dube MP, Johnson DL, Currier JS, Leedom JM. Protease inhibitor-associated hyperglycaemia. Lancet. 1997;350:713–714. doi: 10.1016/S0140-6736(05)63513-1. [DOI] [PubMed] [Google Scholar]
- 4.Walli R, Herfort O, Michl GM, et al. Treatment with protease inhibitors associated with peripheral insulin resistance and impaired oral glucose tolerance in HIV-1-infected patients. AIDS. 1998;12:F167–F173. doi: 10.1097/00002030-199815000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Behrens G, Dejam A, Schmidt H, et al. Impaired glucose tolerance, beta cell function and lipid metabolism in HIV patients under treatment with protease inhibitors. AIDS. 1999;13:F63–F70. doi: 10.1097/00002030-199907090-00001. [DOI] [PubMed] [Google Scholar]
- 6.Sullivan AK, Nelson MR. Marked hyperlipidaemia on ritonavir therapy. AIDS. 1997;11:938–939. [PubMed] [Google Scholar]
- 7.Segerer S, Bogner JR, Walli R, Loch O, Goebel FD. Hyperlipidemia under treatment with proteinase inhibitors. Infection. 1999;27:77–81. doi: 10.1007/BF02560501. [DOI] [PubMed] [Google Scholar]
- 8.Sullivan AK, Feher MD, Nelson MR, Gazzard BG. Marked hypertriglyceridaemia associated with ritonavir therapy. AIDS. 1998;12:1393–1394. doi: 10.1097/00002030-199811000-00024. [DOI] [PubMed] [Google Scholar]
- 9.Berthold HK, Parhofer kg, Ritter MM, et al. Influence of protease inhibitor therapy on lipoprotein metabolism. J Intern Med. 1999;246:567–575. doi: 10.1046/j.1365-2796.1999.00615.x. [DOI] [PubMed] [Google Scholar]
- 10.Mulligan K, Grunfeld C, Tai VW, et al. Hyperlipidemia and insulin resistance are induced by protease inhibitors independent of changes in body composition in patients with HIV infection. J Acquir Immune Defic Syndr. 2000;23:35–43. doi: 10.1097/00126334-200001010-00005. [DOI] [PubMed] [Google Scholar]
- 11.Periard D, Telenti A, Sudre P, et al. Atherogenic dyslipidemia in HIV-infected individuals treated with protease inhibitors. The Swiss HIV Cohort Study. Circulation. 1999;100:700–705. doi: 10.1161/01.cir.100.7.700. [DOI] [PubMed] [Google Scholar]
- 12.Miller KD, Jones E, Yanovski JA, Shankar R, Feuerstein I, Falloon J. Visceral abdominal-fat accumulation associated with use of indinavir. Lancet. 1998;351:871–875. doi: 10.1016/S0140-6736(97)11518-5. [DOI] [PubMed] [Google Scholar]
- 13.Hengel RL, Watts NB, Lennox JL. Benign symmetric lipomatosis associated with protease inhibitors. Lancet. 1997;350:1596. doi: 10.1016/s0140-6736(05)64011-1. [DOI] [PubMed] [Google Scholar]
- 14.Lo JC, Mulligan K, Tai VW, Algren H, Schambelan M. ‘Buffalo hump’ in men with HIV-1 infection. Lancet. 1998;351:867–870. doi: 10.1016/S0140-6736(97)11443-X. [DOI] [PubMed] [Google Scholar]
- 15.Carr A, Samaras K, Burton S, et al. A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. AIDS. 1998;12:F51–F58. doi: 10.1097/00002030-199807000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Gervasoni C, Ridolfo AL, Trifiro G, et al. Redistribution of body fat in HIV-infected women undergoing combined antiretroviral therapy. AIDS. 1999;13:465–471. doi: 10.1097/00002030-199903110-00004. [DOI] [PubMed] [Google Scholar]
- 17.Saint-Marc T, Partisani M, Poizot-Martin I, et al. A syndrome of peripheral fat wasting (lipodystrophy) in patients receiving long-term nucleoside analogue therapy. AIDS. 1999;13:1659–1667. doi: 10.1097/00002030-199909100-00009. [DOI] [PubMed] [Google Scholar]
- 18.Lo JC, Mulligan K, Tai VW, Algren H, Schambelan M. Body shape changes in HIV-infected patients. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;19:307–308. doi: 10.1097/00042560-199811010-00015. [DOI] [PubMed] [Google Scholar]
- 19.Walli K, Michl G, Segerer S. Dyslipidemia and insulin resistance in HIV-infected patients treated with reverse transcriptase inhibitors alone and in combination with protease inhibitors. Sixth Conference on Retroviruses and Opportunistic Infections; Chicago. 1999. [abstract 645] [Google Scholar]
- 20.Dever LL, Oruwari PA, Figueroa WE, O’Donovan CA, Eng RH. Hyperglycemia associated with protease inhibitors in an urban HIV-infected minority patient population. Ann Pharmacother. 2000;34:580–584. doi: 10.1345/aph.19231. [DOI] [PubMed] [Google Scholar]
- 21.Lister RK, Youle M, Nair DR, Winder AF, Rustin MH. Latent dysbetalipoproteinaemia precipitated by HIV-protease inhibitors. Lancet. 1999;353:1678. doi: 10.1016/s0140-6736(99)01449-x. [DOI] [PubMed] [Google Scholar]
- 22.Grunfeld C, Pang M, Doerrler W, Shigenaga JK, Jensen P, Feingold KR. Lipids, lipoproteins, triglyceride clearance, and cytokines in human immunodeficiency virus infection and the acquired immunodeficiency syndrome. J Clin Endocrinol Metab. 1992;74:1045–1052. doi: 10.1210/jcem.74.5.1373735. [DOI] [PubMed] [Google Scholar]
- 23.Schwarz JM, Neese RA, Turner S, Dare D, Hellerstein MK. Short-term alterations in carbohydrate energy intake in humans. Striking effects on hepatic glucose production, de novo lipogenesis, lipolysis, and whole-body fuel selection. J Clin Invest. 1995;96:2735–2743. doi: 10.1172/JCI118342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carlson LA, Reossner S. A methodological study of an intravenous fat tolerance test with Intralipid R emulsion. Scand J Clin Lab Invest. 1972;29:271–280. doi: 10.3109/00365517209080242. [DOI] [PubMed] [Google Scholar]
- 25.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 26.Mazess RB, Barden HS, Bisek JP, Hanson J. Dual-energy X-ray absorptiometry for total-body and regional bone-mineral and soft-tissue composition. Am J Clin Nutr. 1990;51:1106–1112. doi: 10.1093/ajcn/51.6.1106. [DOI] [PubMed] [Google Scholar]
- 27.Carey DG, Nguyen TV, Campbell LV, Chisholm DJ, Kelly P. Genetic influences on central abdominal fat: a twin study. Int J Obes Relat Metab Disord. 1996;20:722–726. [PubMed] [Google Scholar]
- 28.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 29.Czech MP, Corvera S. Signaling mechanisms that regulate glucose transport. J Biol Chem. 1999;274:1865–1868. doi: 10.1074/jbc.274.4.1865. [DOI] [PubMed] [Google Scholar]
- 30.Pessin JE, Thurmond DC, Elmendorf JS, Coker KJ, Okada S. Molecular basis of insulin-stimulated GLUT4 vesicle trafficking. Location! Location! Location! J Biol Chem. 1999;274:2593–2596. doi: 10.1074/jbc.274.5.2593. [DOI] [PubMed] [Google Scholar]
- 31.Murata H, Hruz PW, Mueckler M. The mechanism of insulin resistance caused by HIV protease inhibitor therapy. J Biol Chem. 2000;275:20251–20254. doi: 10.1074/jbc.C000228200. [DOI] [PubMed] [Google Scholar]
- 32.Purnell JQ, Zambon A, Knopp RH, et al. Effect of ritonavir on lipids and postheparin lipase activities in normal subjects. AIDS. 2000;14:51–57. doi: 10.1097/00002030-200001070-00006. [DOI] [PubMed] [Google Scholar]