Summary
Visceral obesity is associated with insulin resistance, but the association of other regional adipose depots with insulin resistance is not understood. In HIV infection, buffalo hump (upper trunk fat) is associated, but the association of upper trunk fat with insulin resistance has not been examined in controls. To determine the independent association of adipose depots other than visceral with insulin resistance, we performed a cross-sectional analysis of controls and HIV-infected subjects in the Fat Redistribution and Metabolic Change in HIV Infection (FRAM) study, who had measurements of glucose, insulin, and adipose tissue volumes by whole-body magnetic resonance imaging. We studied 926 HIV-positive persons from 16 academic medical center clinics and trials units with demographic characteristics representative of US patients with HIV infection and 258 FRAM controls from the population-based Coronary Artery Risk Development in Young Adults study. We measured visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT) volume in the legs, arms, lower trunk (back and abdomen), and upper trunk (back and chest) and assessed their association with the homeostasis model of assessment (HOMA) and HOMA >4 by stepwise multivariable analysis. The prevalence of HOMA >4 as a marker of insulin resistance was 28% among controls compared with 37% among HIV-infected subjects (P = 0.005). Among controls, those in the highest tertile of upper trunk SAT volume had an odds ratio (OR) of 9.0 (95% confidence interval [CI]: 2.4 to 34; P = 0.001) for having HOMA >4 compared with the lowest tertile, whereas in HIV-positive subjects, the OR was lower (OR = 2.09, 95% CI: 1.36 to 3.19; P = 0.001). Among controls, the highest tertile of VAT volume had an OR of 12.1 (95% CI: 3.2 to 46; P = 0.0002) of having HOMA >4 compared with the lowest tertile, whereas in HIV-positive subjects, the OR was 3.12 (95% CI: 2.0 to 4.8; P < 0.0001). After adjusting for VAT and upper trunk SAT, the association of other SAT depots with HOMA >4 did not reach statistical significance. Thus, VAT and upper trunk SAT are independently associated with insulin resistance in controls and in HIV-infected persons.
Keywords: buffalo hump, fat distribution, insulin resistance, lipodystrophy, visceral obesity
Insulin resistance has long been recognized as being associated with obesity,1 particularly with obesity in a central distribution at the waist rather than in a lower body distribution of hips and legs.2,3 As a consequence, the use of a waist-to-hip ratio (WHR) became a marker for what was referred to as central or upper body obesity.2,4,5 The recognition of the metabolic differences between visceral and subcutaneous fat provided a basis for the association of central or upper body obesity and insulin resistance.6,7 Measured visceral adipose tissue (VAT) was found to correlate with insulin resistance.8–14
Subsequently, it was found in most studies that waist circumference was a stronger marker of metabolic consequences than WHR.15–17 There is debate over the extent to which subcutaneous adipose tissue (SAT) in the abdomen or elsewhere is associated with insulin resistance after taking into account the association with VAT.11,14,18–20 Likewise, some but not all studies found that greater thigh fat or hip or thigh circumference was independently associated with less insulin resistance and improved glucose tolerance after adjustment for VAT.20–22
Most studies measure VAT and SAT by computed tomography (CT) scan, which involves radiation exposure; therefore only a limited number of abdominal slices are measured and thigh slices are obtained less frequently. As a consequence, the association of other adipose tissue depots with insulin resistance has not been evaluated. Magnetic resonance imaging (MRI) is an alternative method that can be used to measure whole-body regional adipose tissue volume without radiation exposure.23–25
In the setting of HIV infection, the introduction of combination antiretroviral (ARV) therapy was followed by the observations that changes in fat distribution were associated with metabolic abnormalities.26 An early example was the appearance of a “buffalo hump,” increased fat on the upper back often extending to the neck, which occurred in HIV infection in the absence of Cushing disease.27–29 Buffalo hump has been associated with insulin resistance in patients with HIV infection.30 In the absence of HIV infection, however, neck circumference has also been associated with insulin resistance.31,32 Neither identification of buffalo hump nor neck circumference is a direct measurement of fat; the relation of directly measured upper trunk (back and chest) SAT to insulin resistance has not been explored. Furthermore, because upper trunk fat is rarely measured by CT and VAT cannot be measured by dual-energy x-ray absorptiometry (DXA) or anthropometrics (circumferences or skin folds), the question of whether upper trunk fat is associated with insulin resistance independent of VAT has not been addressed.
Among the major objectives of the Fat Redistribution and Metabolic Change in HIV Infection (FRAM) study were to define the differences in fat distribution in subjects with HIV infection compared with controls by measuring multiple adipose tissue depot volumes using total-body MRI, to determine the association of fat in these depots with metabolic abnormalities, and to compare these associations in subjects with HIV infection with those in controls.33 In addition to measuring VAT, leg SAT, and arm SAT, we quantified lower trunk SAT (back and abdomen) and upper trunk SAT (back and chest) using total-body MRI, allowing us to separate a depot of “upper body” fat from central fat, which is composed of VAT and lower trunk SAT. We investigate here whether any of these adipose tissue depots were associated with insulin resistance independent of VAT in subjects with HIV infection and in controls.
METHODS
Subjects
HIV-infected and control subjects are from the FRAM study, whose entry criteria have been previously reported in detail elsewhere.33–35 In brief, HIV-infected subjects were recruited from randomized clinic or trial unit lists of 16 academic medical centers. After exclusion of those ineligible (age <18 years or unable to undergo imaging), 65% of those contacted were examined. Control subjects were recruited from 2 centers of the Coronary Artery Risk Development in Young Adults (CARDIA) study.36,37 CARDIA subjects were originally recruited as a population-based sample of healthy 18- to 30-yearold white and African-American men and women from 4 cities in 1985 to 1986 for a longitudinal study of cardiovascular risk factors. Of the CARDIA participants who agreed to the examination at year 15 who were approached to participate in the FRAM study, 83% were studied. Examinations were performed from June 2000 through September 2002.
As in previous FRAM reports, subjects with recent opportunistic infections (OIs) were excluded. For this study, subjects with diabetes, defined as having a fasting blood glucose level ≥126 mg/dL (7.0 mmol/L) or being on a hypoglycemic medication, were also excluded, because fasting insulin measurements are less accurate predictors of insulin resistance in such subjects. Ninety-two percent of FRAM participants had MRI performed. We report here on 258 controls and 926 HIV-positive subjects who had measurements of adipose tissue depots by MRI, glucose, and insulin.
Measurements
Body composition was measured by MRI, with subjects in the supine position and arms extended over head, and analyzed as described in detail elsewhere.24,33–35 In brief, using the intervertebral space between the fourth and fifth lumbar vertebrae as the origin, transverse images (10-mm slice thickness) were obtained every 40 mm from hand to foot. Using image analysis software (Tomovision, Montreal, Quebec, Canada), tissue areas (cm2) were calculated by summing specific tissue pixels and then multiplying by individual pixel surface area. Volume per slice (cm3) of each tissue was calculated by multiplying area by thickness. Volume of each tissue for the space between 2 consecutive slices was calculated by means of a mathematic algorithm.25
Glucose and insulin were measured in a central laboratory (Linco Research, St. Louis, MO).
Physical activity, alcohol intake, smoking, illicit drug use, education, and food intake were assessed and graded by standard instruments, as previously described in detail.37–40
Statistics
We first compared characteristics of HIV-infected and control subjects. For numeric values, data are presented as median values and 95% confidence intervals (CIs), with distribution-free confidence intervals constructed for the median41 and P values calculated using the Mann-Whitney U test. The Fisher exact test was used for categoric values.
To assess the independent associations of body fat depots and other factors with insulin resistance (using the homeostasis model of assessment [HOMA] or HOMA >442), we performed multivariable regression analysis in separate models for controls and HIV-positive subjects. In this first analysis, factors related to HIV infection were initially excluded. The primary predictors were trichotomized amounts of adipose tissue volume from anatomic sites measured by MRI (upper trunk, lower trunk, arm, and leg) and total SAT, VAT, and total fat.34 Trichotomized versions of the anatomic site measurements were created using tertile cutoffs from the control group (men and women were assessed separately) to facilitate comparison of similar quantities of adipose tissue. We evaluated body mass index (BMI) in the model as a possible alternative to adipose tissue depots for predicting insulin resistance, but it was weaker. Demographic predictors unrelated to HIV infection, such as gender, age, and ethnicity, were also included. The effect of age was modeled linearly but with potentially different slopes in the ranges of 18 to 40, 40 to 50, and over 50 years old. Other predictors included as candidates in the model were level of physical activity, current smoking status, current illicit drug use (marijuana, crack, cocaine, or combination use of crack and cocaine), food consumption, alcohol drinks used in the past year, and albumin.37–40
Multivariable logistic and linear regression models were built using stepwise regression, with P = 0.05 for entry and retention, testing for interactions at each step; gender, age, and ethnicity were forced to be included in every model. A fat depot was included in the model if testing showed significance at the 0.05 level for each group analyzed. We tested for colinearity for depots in the model and found that it was not substantial between VAT and upper trunk SAT; therefore, both depots were kept in the final model. We performed stepwise regression by evaluating possible models on an individual basis rather than with an automated stepwise procedure so as to avoid exclusion of observations that had missing data only on unselected candidate variables. To ensure the validity of pooling men and women in this analysis, interactions between gender and other factors in the model were tested and none had P < 0.05. Odds ratios (ORs) for the control and HIV groups were compared using a test of difference in estimates.43 Because of its skewed distribution, HOMA was log-transformed in all linear regression analyses; results were back-transformed to produce estimated percentage differences in HOMA attributable to each factor.
In a further stepwise multivariable analysis, we tested whether the addition of factors related to HIV infection affected the association of adipose tissue volumes with HOMA >4, using the complete HIV-infected cohort. HIV-related factors screened in the model were CD4 cell count, HIV RNA level, history of AIDS by OI, and current ARV therapy in models similar to those previously presented, with current CD4 cell counts and HIV RNA levels forced to be included in the model.34,35
Another objective was to compare insulin resistance among HIV-infected and control subjects after adjusting for the common predictors measured in both groups. We used a stepwise multivariable analysis similar to the first analysis that was performed but with HIV infected versus control added as a factor. For this analysis, age was restricted to 33 to 45 years old and only data from whites and African Americans were used to match the demographics of the controls.
RESULTS
Subject Demographics
More than a quarter of HIV-infected subjects were women (Table 1). Half were white, and more than a third were African American. The control group was divided approximately equally by gender and ethnicity. HIV-infected subjects were slightly older, weighed less, had lower BMI, and had lower SAT in all depots but had similar VAT to controls.
TABLE 1.
Demographics of Subjects
| Control | HIV Positive | P | |
|---|---|---|---|
| n | 258 | 926 | |
| Age (y) | |||
| Mean ± SD | 40.2 ± 3.5 | 42.7 ± 8.6 | <0.0001 |
| Range | 33.0 to 45.0 | 19.0 to 75.0 | |
| Gender | |||
| Female | 47% | 27% | <0.0001 |
| Male | 53% | 73% | |
| Race | |||
| White | 54% | 51% | 0.35* |
| African American | 46% | 37% | |
| Hispanic | 0% | 10% | |
| Asian | 0% | 1% | |
| Other | 0% | 2% | |
| HIV risk factors | |||
| MSM | n/a | 49% | |
| Heterosexual | 22% | ||
| IDU | 20% | ||
| Other | 10% | ||
| Duration HIV (y) | |||
| Mean ± SD | n/a | 8.5 ± 4.1 | |
| Range | 1.1 to 21.1 | ||
| HIV RNA (1000 copies/mL) | |||
| Median | n/a | 0.4 | |
| Range | 0.4 to 751.0 | ||
| CD4 (cells/µL) | |||
| Mean ± SD | n/a | 392 ± 245 | |
| Range | 2 to 1499 | ||
| Current NRTI use | n/a | 84% | |
| Current PI use | n/a | 55% | |
| Current NNRTI use | n/a | 39% | |
| Current HAART use | n/a | 77% | |
| Current use of any ARV | n/a | 85% | |
| Height (cm) | |||
| Mean ± SD | 172.6 ± 9.6 | 171.9 ± 9.3 | 0.48 |
| Weight (kg) | |||
| Mean ± SD | 81.7 ± 16.6 | 74.2 ± 13.9 | <0.0001 |
| BMI (kg/m2) | |||
| Mean ± SD | 27.4 ± 5.2 | 25.1 ± 4.4 | <0.0001 |
| Fat depots (mean ± SD) | |||
| VAT (L) | 1.75 ± 1.44 | 1.85 ± 1.54 | 0.52 |
| Leg (L) | 7.20 ± 3.82 | 4.45 ± 3.32 | <0.0001 |
| Lower trunk (L) | 8.01 ± 4.34 | 5.17 ± 3.89 | <0.0001 |
| Upper trunk (L) | 4.07 ± 2.24 | 3.53 ± 2.40 | <0.0001 |
| Arm (L) | 1.59 ± 0.79 | 1.28 ± 0.66 | <0.0001 |
P from Mann-Whitney test or Fisher exact test.
Excluding diabetes mellitus (glucose ≥126 or hypoglycemic) and OI with nonmissing HOMA and nonmissing MRI.
Race comparison is proportion of whites versus African Americans.
HAART indicates highly active antiretroviral therapy; IDU, injection drug user; MSM, men who have sex with men; n/a, not applicable; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
Glucose, Insulin, and Homeostatic Model of Assessment Levels
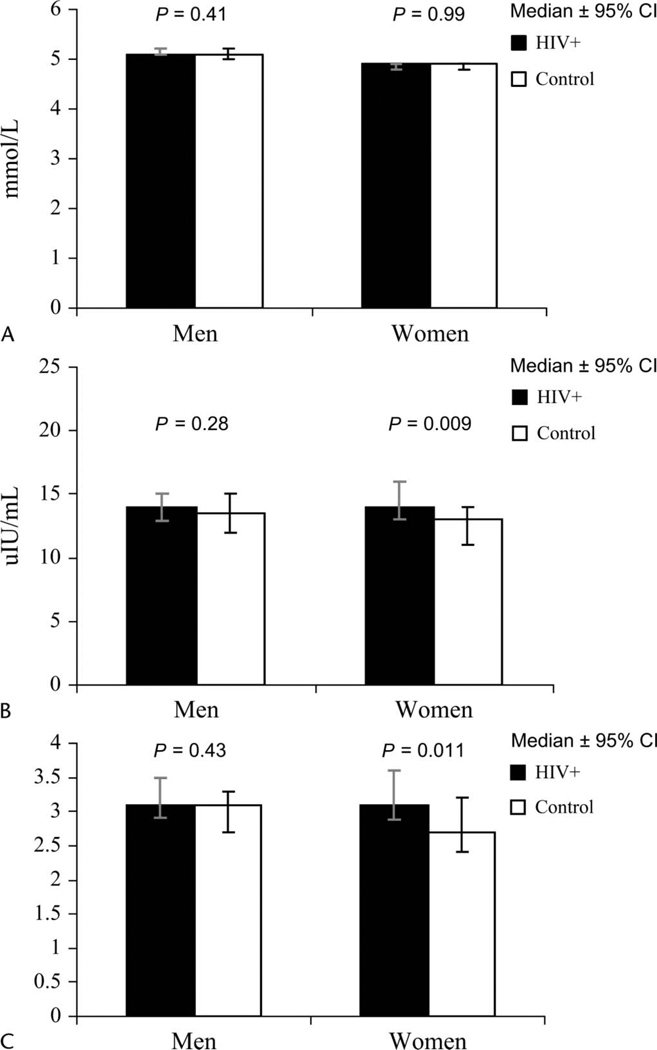
For men and women, median glucose levels were similar in control and HIV-infected subjects (Fig. 1). Control and HIV-infected men also had similar insulin and HOMA levels. HIV-infected women had slightly higher insulin and HOMA levels than control women, however (see Fig. 1). The prevalence of HOMA >4 as a marker of insulin resistance42 was 37% among HIV-infected subjects compared with 28% among controls (P = 0.005).
FIGURE 1.
Glucose, insulin, and HOMA stratified by gender. A, Glucose levels in mmol/L. B, Insulin levels in µU/mL. C, HOMA calculated by the equation: Insulin × Glucose/22.5. Men are shown on the left, and women are shown on the right. Closed bars indicate HIV-positive subjects; open bars, controls. Results are median ± 95% CI.
Adipose Tissue Depots as Predictors of Homeostatic Model of Assessment >4
After adjusting for demographic and other factors that might affect insulin resistance, the association of adipose tissue volume with HOMA >4 was assessed in a stepwise multivariable logistic regression analysis. This analysis revealed that higher levels of upper trunk SAT and of VAT were independently associated with greater odds of insulin resistance, as assessed by HOMA >4 (Table 2) in control and HIV-infected subjects. Colinearity was not substantial; therefore, upper trunk SAT and VAT were kept in the final model. Other factors associated with insulin resistance in HIV-infected subjects included increasing age, whereas women, alcohol users, and crack/cocaine users had lower odds of insulin resistance. In control subjects, African Americans had higher odds of insulin resistance, whereas more physical activity and inadequate food intake were associated with lower odds of insulin resistance.
TABLE 2.
Multivariable Logistic Regression Analysis of Adipose Tissue Associations With HOMA Level >4 in HIV-Positive Subjects and Controls*
| Control | HIV-Positive | |||||||
|---|---|---|---|---|---|---|---|---|
| HOMA >4† | OR | 95% CI | P | HOMA >4† | OR | 95% CI | P | |
| Upper trunk SAT | ||||||||
| Third vs. first tertile | 61% | 9.0 | (2.4, 33.9) | 0.001 | 57% | 2.1 | (1.36, 3.2) | 0.001 |
| Second vs. first tertile | 20% | 2.4 | (0.63, 9.1) | 0.20 | 36% | 1.21 | (0.82, 1.78) | 0.33 |
| First tertile (reference) | 5% | 27% | ||||||
| VAT | ||||||||
| Third vs. first tertile | 63% | 12.1 | (3.2, 45.6) | 0.0002 | 55% | 3.1 | (2.0, 4.8) | <0.0001 |
| Second vs. first tertile | 18% | 1.94 | (0.54, 6.9) | 0.31 | 38% | 1.90 | (1.26, 2.8) | 0.002 |
| First tertile (reference) | 6% | 22% | ||||||
Excludes those currently on hypoglycemics or glucose ≥ 126.
Model also controls for ethnicity, age, gender, alcohol (HIV only), current crack or cocaine use (HIV only), physical activity (control only), and adequacy of food (controls only).
Prevalence (%) of HOMA >4.
There was not complete concordance in the tertiles of VAT and upper trunk SAT. Among controls, 31% of those in the highest tertile of VAT were not in the highest tertile of upper trunk SAT and 32% of those in the highest tertile of upper trunk SAT were not in the highest tertile of VAT. Among HIV-infected subjects, there was even less concordance. Nearly half (46%) of those in the highest tertile of VAT were not in the highest tertile of upper trunk SAT, and 28% of those in the highest tertile of upper trunk SAT were not in the highest tertile of VAT.
Subjects in the upper tertile of upper trunk SAT had a high prevalence of HOMA >4 (61% of controls and 57% of HIV-infected subjects; P = 0.52). HIV-infected subjects in the lowest tertile of upper trunk SAT had a 27% prevalence of HOMA >4, whereas controls had a prevalence of 5% (P < 0.0001). Controls in the highest tertile of upper trunk SAT had a 9-fold odds (OR = 9.0, 95% CI: 2.4 to 34; P = 0.001) of having HOMA >4 compared with controls in the lowest tertile (see Table 2). Among HIV-infected subjects, those in the highest tertile of upper trunk SAT for controls had more than 2-fold odds (OR = 2.1, 95% CI: 1.36 to 3.26; P = 0.001) of having HOMA >4 compared with those in the lowest tertile. The OR for the association of upper trunk SAT with HOMA >4 in controls was significantly higher than the OR for the association of upper trunk SAT with HOMA >4 in HIV-infected subjects (P = 0.040). When compared with controls, the lower OR in the HIV-infected group for HOMA >4 in the higher tertile versus the lowest tertile of upper trunk SAT is a result of the higher prevalence of HOMA >4 of HIV-infected subjects in the lowest tertile of upper trunk SAT.
Subjects in the upper tertile of VAT had a high prevalence of HOMA >4 (63% of controls and 55% of HIV-infected subjects; P = 0.26). HIV-infected subjects in the lowest tertile of VAT had a 22% prevalence of HOMA >4, whereas controls had a prevalence of 6% (P = 0.0003). Controls in the highest tertile of VAT also had greater odds of HOMA >4 (OR = 12.1, 95% CI: 3.2 to 46; P = 0.0002) compared with controls in the lowest tertile in this multivariable analysis. In the multivariable analysis of HIV-infected subjects, we also found greater odds of HOMA >4 for those in the highest VAT tertile (OR = 3.1, 95% CI: 2.0 to 4.8; P < 0.0001) compared with those in the lowest tertile. The OR for the association of VAT with HOMA >4 in controls showed a similar trend to be higher than the OR for HIV-infected subjects (P = 0.058). Likewise, compared with controls, the lower OR in the HIV-infected group for HOMA >4 in the higher tertile versus the lowest tertile of VAT is attributable to the higher prevalence of HOMA >4 of HIV-infected subjects in the lowest tertile of VAT.
Other fat depots (lower trunk, leg, and arm SAT) did not enter into the model after adjusting for upper trunk SAT and VAT. When leg SAT was forced into the model, it was associated with less insulin resistance, but the association did not reach significance (OR for HOMA >4 of being in highest tertile vs. lowest tertile: controls: OR = 0.60, 95% CI: 0.19 to 1.96, P = 0.40; HIV-positive subjects: OR = 0.75, 95% CI: 0.45 to 1.25, P = 0.27) and had little effect on the association of upper trunk SAT and VAT with HOMA >4.
To evaluate the effect of HIV infection on insulin resistance, we combined the HIV and control cohorts. For this analysis, age was restricted to 33 to 45 years old and only data from whites and African Americans were used, thereby matching the demographics of the controls. After multivariable adjustment for adipose tissue depots, demographics, and lifestyle factors, HIV infection was associated with greater insulin resistance (OR = 1.62 for having HOMA >4; 95% CI: 1.10 to 2.4; P = 0.015).
Within the full cohort of HIV-infected subjects, we then controlled for HIV-related factors, including ARV therapy, in addition to the factors in the models included in Table 2 but found no change in association of adipose tissue volumes with HOMA >4. After multivariable adjustment, HIV-infected subjects in the highest tertile of upper trunk SAT still had a 2-fold odds of having HOMA >4 (OR = 2.1, 95% CI 1.35 to 3.2; P = 0.001) compared with those in lowest tertile. Likewise, HIV-infected subjects in the highest tertile of VAT still had more than a 3-fold greater odds of having HOMA >4 (OR = 3.04, 95% CI: 1.96 to 4.7; P < 0.001) compared with those in the lowest tertile.
In adjusted models, current CD4 cell count showed little association, but increasing HIV viral load was associated with lower odds of insulin resistance (OR = 0.82 per 10-fold increase in viral load, 95% CI: 0.68 to 0.99; P = 0.040). Diagnosis of AIDS was associated with slightly elevated odds of insulin resistance but did not reach statistical significance (OR = 1.28, 95% CI: 0.88 to 1.87; P = 0.19). In unadjusted models, nucleoside reverse transcriptase inhibitor (NRTI) use was associated with higher HOMA levels (median: 3.5 vs. 2.9 for users vs. nonusers; P = 0.013) and slightly higher prevalence of HOMA >4 (38% vs. 30%; P = 0.062). After adjustment for adipose tissue, demographics, and lifestyle factors, however, associations of ARV therapies with insulin resistance were weak and no individual ARV or ARV class reached statistical significance.
We also assessed the association of regional body fat depots with absolute HOMA levels in a stepwise multivariable linear regression analysis in the full control and HIV-infected cohorts. For controls and HIV infected subjects, being in the highest tertiles of VAT and upper trunk SAT was associated with higher levels of HOMA (percent effects of being in the upper and middle tertiles are given in Table 3).
TABLE 3.
Multivariable Linear Regression Analysis of Adipose Tissue Associations With HOMA† in HIV-Positive Subjects and Controls*
| Control | HIV-Positive | |||||
|---|---|---|---|---|---|---|
| % Est. | 95% CI | P | % Est. | 95% CI | P | |
| Upper trunk SAT | ||||||
| Third vs. first tertile | 35 | (6, 74) | 0.017 | 39 | (25, 55) | <0.0001 |
| Second vs. first tertile | 5 | (−14, 27) | 0.65 | 13 | (2, 24) | 0.014 |
| VAT | ||||||
| Third vs. first tertile | 46 | (23, 75) | <0.0001 | 27 | (14, 42) | <0.0001 |
| Second vs. first tertile | 13 | (−2, 31) | 0.10 | 15 | (4, 27) | 0.006 |
Excludes those currently on hypoglycemics or glucose ≥126.
Model also controls for ethnicity, age, gender, alcohol (HIV only), current crack or cocaine use (HIV only), and adequacy of food (controls only).
HOMA was log-transformed; results were back-transformed to produce estimated percentage differences in HOMA attributable to each factor
Est. indicates estimated.
Upper Trunk Subcutaneous Adipose Tissue, Visceral Adipose Tissue, and Homeostatic Model of Assessment Levels
The associations between tertiles of upper trunk SAT or VAT and HOMA levels are presented in Figure. 2. For controls and all HIV-infected subjects, the median HOMA levels for the highest and middle tertiles of VAT and upper trunk SAT were higher than the median HOMA levels in the lowest tertile. For control and HIV-infected subjects, the median HOMA levels were >4.0 for the highest tertiles of upper trunk SAT (controls = 4.4, 95% CI: 4.0 to 5.4; HIV-infected subjects = 4.6, 95% CI: 4.0 to 5.0) and of VAT (controls = 4.8, 95% CI: 4.2 to 5.3; HIV-infected subjects = 4.3, 95% CI: 4.0 to 4.7).
FIGURE 2.
HOMA and tertile of upper trunk SAT and VAT. HOMA values are plotted versus tertiles of the 2 selected fat depots. Tertiles are based on the values for controls. Tertiles are presented as lowest on the left to highest on the right. A, Controls. B, HIV-positive subjects. Hatched bars indicate tertiles of upper trunk SAT; open bars, tertiles of VAT. Results are median ± 95% CI.
DISCUSSION
Using direct measurements of adipose tissue volumes by MRI, we have shown that more VAT and upper trunk fat (SAT) were independently associated with insulin resistance in control and HIV-infected subjects, whether assessed by HOMA or HOMA >4. Increased VAT was previously well known to be associated with insulin resistance.8–10,12–14 The association with measured upper trunk fat reported here is novel, however; to our knowledge, this is the first time that upper trunk SAT was directly measured and its association with insulin resistance assessed by multivariable analysis in a carefully characterized population. Only a limited number of studies (reviewed by Garg44) have examined the association of insulin resistance with adipose tissue measured by CT or MRI across the entire body (as opposed to the combination of VAT and abdomen/lower trunk SAT). Most of those studies assessed the effect of VAT and total SAT. Our data support the observation that truncal skin folds, which include the upper and lower trunk, correlate better with insulin resistance than abdomen/lower trunk SAT or total SAT, however.19,45
The association of insulin resistance with buffalo hump (an increase in upper trunk fat on the back) has previously been described in patients with HIV infection.30 We report here that greater upper trunk fat is also strongly associated with insulin resistance among control subjects, however, whether assessed by continuous HOMA or dichotomized as HOMA >4. These associations of HOMA with direct measurements of upper trunk SAT may also provide the basis for the previously reported association of neck size with insulin resistance in subjects who are not HIV infected.31,32
Our results are expressed based on the tertiles of adipose tissue volume found in controls as a norm to allow comparison of similar amounts of adipose tissue. We have previously shown that subcutaneous lipoatrophy is less prominent in the upper trunk than in the legs in the HIV-associated syndrome of lipodystrophy.34,35 In HIV-positive subjects and controls, upper trunk fat is associated with more insulin resistance. Thus, for a given amount of subcutaneous fat, HIV-infected subjects have more fat in the depot associated with insulin resistance (upper trunk fat) compared with controls and less fat in a depot that may be associated with insulin sensitivity (leg fat) compared with controls. Even after adjusting for adipose tissue volume and other factors associated with insulin resistance, however, HIV infection was still associated with higher odds of having HOMA >4 (OR = 1.60), suggesting that HIV-related factors other than fat distribution contribute to the insulin resistance of HIV infection. Lower viral loads were associated with insulin resistance. Use of NRTIs was associated with increased insulin resistance in unadjusted models but showed little association after adjustment. Adjusting for these HIV-related factors did not substantially affect the associations of upper trunk SAT and VAT with insulin resistance.
Given the association of VAT with insulin resistance, waist circumference is likely to remain a useful marker for insulin resistance, at least in those without HIV infection, and should continue to be utilized to diagnose the metabolic syndrome,46–48 although the use of the metabolic syndrome has recently been called into question.49 Finding a simple surrogate for upper trunk fat may be more difficult, because chest circumference is confounded by lung volume and skin folds are not in common use. Until further studies are done on standardized neck measurements, clinicians may have to rely on pattern recognition for diagnosing excess upper trunk fat. This need is reinforced by the observation that a substantial number of participants who were in the highest tertile of VAT were not in the highest tertile of upper trunk fat (33% of controls and 49% of HIV-infected subjects).
A limitation of this study is that the data are cross-sectional. Longitudinal studies are needed to understand the consequences of increased upper trunk SAT more fully. Another limitation is that for studies of this size, HOMA must be used rather than more rigorous measurements, such as the clamp.50
In summary, upper trunk (chest and back) SAT is strongly and independently associated with insulin resistance in analyses that simultaneously adjusted for VAT rather than lower trunk SAT. The association of upper trunk fat with insulin resistance was not unique to HIV infection, because it was even stronger in controls. This new understanding of the metabolic correlates of upper body fat may help clinicians to profile better those at risk for insulin resistance and metabolic complications.
Acknowledgments
Supported by National Institutes of Health (NIH) grants R01-DK57508, HL74814, and HL 53359 and by NIH General Clinical Research Center (GCRC) grants M01-RR00036, -RR00051, -RR00052, -RR00054, -RR00083, -RR0636, and -RR00865.
The funding agency had no role in the collection or analysis of the data.
APPENDIX
Writing Team: Carl Grunfeld, David Rimland, Cynthia Gibert, William Powderly, Stephen Sidney, Michael Shlipak, Peter Bacchetti, Rebecca Scherzer, Steven Haffner, and Steven Heymsfield
Role of Authors: Drs. Grunfeld, Scherzer, and Bacchetti had full access to the data in the study and take responsibility for the integrity of the data and the accuracy of the analysis.
Dr. Grunfeld contributed to the conception and design, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, statistical analysis, obtaining of funding, and administrative support and supervision.
Dr. Rimland contributed to the acquisition of data, analysis and interpretation of data, and critical revision of the manuscript for important intellectual content.
Dr. Gibert contributed to the acquisition of data, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, and administrative support.
Dr. Powderly contributed to the conception and design, acquisition of data, and critical revision of the manuscript for important intellectual content.
Dr. Sidney contributed to acquisition of data, critical revision of the manuscript for important intellectual content, and supervision.
Dr. Shlipak contributed to analysis and interpretation of data, drafting of the manuscript, and statistical analysis.
Dr. Bacchetti contributed to the conception and design, analysis and interpretation of data, critical revision of the manuscript for important intellectual content, and statistical analysis.
Dr. Scherzer contributed to the analysis and interpretation of data, critical revision of the manuscript for important intellectual content, and statistical analysis.
Dr. Haffner contributed to the conception and design, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, and statistical analysis.
Dr. Heymsfield contributed to the conception and design, analysis and interpretation of data, drafting of the manuscript, statistical analysis, and obtaining of funding.
Sites and Investigators: University Hospitals of Cleveland, Cleveland, OH (Barbara Gripshover, MD); Tufts University, Boston, MA (Abby Shevitz, MD, and Christine Wanke, MD); Stanford University, Stanford, CA (Andrew Zolopa, MD, and Lisa Gooze, MD); University of Alabama at Birmingham, Birmingham, AL (Michael Saag, MD, and Barbara Smith, PhD); John Hopkins University, Baltimore, MD (Joseph Cofrancesco and Adrian Dobs); University of Colorado Heath Sciences Center, Denver, CO (Constance Benson, MD, and Lisa Kosmiski, MD); University of North Carolina at Chapel Hill, Chapel Hill, NC (Charles van der Horst, MD); University of California at San Diego, San Diego, CA (W. Christopher Mathews, MD, and Daniel Lee, MD); Washington University, St. Louis, MO (William Powderly, MD, and Kevin Yarasheski, PhD); Veterans Affairs Medical Center, Atlanta, GA (David Rimland, MD); University of California at Los Angeles, Los Angeles, CA (Judith Currier, MD, and Matthew Leibowitz, MD); Veterans Affairs Medical Center, New York, NY (Michael Simberkoff, MD, and Juan Bandres, MD); Veterans Affairs Medical Center, Washington, DC (Cynthia Gibert, MD, and Fred Gordin, MD); St. Luke’s–Roosevelt Hospital Center, New York, NY (Donald Kotler, MD, and Ellen Engelson, PhD); University of California at San Francisco, San Francisco, CA (Morris Schambelan, MD, and Kathleen Mulligan, PhD); Indiana University, Bloomington, IN (Michael Dube, MD); Kaiser Permanente, Oakland, CA (Stephen Sidney, MD); University of Alabama at Birmingham, Birmingham, AL (Cora E. Lewis, MD)
Data Coordinating Center: University of Alabama at Birmingham, Birmingham, AL (O. Dale Williams, PhD, Heather McCreath, PhD, Charles Katholi, PhD, George Howard, PhD, Tekeda Ferguson, and Anthony Goudie)
Image Reading Center: St. Luke’s–Roosevelt Hospital Center, New York, NY (Steven Heymsfield, MD, Jack Wang, MS, and Mark Punyanitya)
Office of the Principal Investigator: University of California, San Francisco, Veterans Affairs Medical Center, San Francisco, CA, and the Northern California Institute for Research and Development, San Francisco, CA (Carl Grunfeld, MD, PhD, Phyllis Tien, MD, Peter Bacchetti, PhD, Dennis Osmond, PhD, Andrew Avins, MD, Michael Shlipak, MD, Rebecca Scherzer, PhD, Mae Pang, RN, MSN, Heather Southwell, MS, RD, and Yong Kyoo Chang, MS)
REFERENCES
- 1.Rabinowitz D, Zierler KL. Forearm metabolism in obesity and its response to intra-arterial insulin. Characterization of insulin resistance and evidence for adaptive hyperinsulinism. J Clin Invest. 1962;41:2173–2181. doi: 10.1172/JCI104676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evans DJ, Hoffmann RG, Kalkhoff RK, et al. Relationship of body fat topography to insulin sensitivity and metabolic profiles in premenopausal women. Metabolism. 1984;33:68–75. doi: 10.1016/0026-0495(84)90164-1. [DOI] [PubMed] [Google Scholar]
- 3.Despres JP. Health consequences of visceral obesity. Ann Med. 2001;33:534–541. doi: 10.3109/07853890108995963. [DOI] [PubMed] [Google Scholar]
- 4.Evans DJ, Murray R, Kissebah AH. Relationship between skeletal muscle insulin resistance, insulin-mediated glucose disposal, and insulin binding. Effects of obesity and body fat topography. J Clin Invest. 1984;74:1515–1525. doi: 10.1172/JCI111565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haffner SM, Fong D, Hazuda HP, et al. Hyperinsulinemia, upper body adiposity, and cardiovascular risk factors in non-diabetics. Metabolism. 1988;37:338–345. doi: 10.1016/0026-0495(88)90133-3. [DOI] [PubMed] [Google Scholar]
- 6.Bjorntorp P. Metabolic implications of body fat distribution. Diabetes Care. 1991;14:1132–1143. doi: 10.2337/diacare.14.12.1132. [DOI] [PubMed] [Google Scholar]
- 7.Kissebah AH. Insulin resistance in visceral obesity. Int J Obes. 1991;15 Suppl 2:109–115. [PubMed] [Google Scholar]
- 8.Seidell JC, Bjorntorp P, Sjostrom L, et al. Visceral fat accumulation in men is positively associated with insulin, glucose, and C-peptide levels, but negatively with testosterone levels. Metabolism. 1990;39:897–901. doi: 10.1016/0026-0495(90)90297-p. [DOI] [PubMed] [Google Scholar]
- 9.Pouliot MC, Despres JP, Nadeau A, et al. Visceral obesity in men. Associations with glucose tolerance, plasma insulin, and lipoprotein levels. Diabetes. 1992;41:826–834. doi: 10.2337/diab.41.7.826. [DOI] [PubMed] [Google Scholar]
- 10.Marin P, Andersson B, Ottosson M, et al. The morphology and metabolism of intraabdominal adipose tissue in men. Metabolism. 1992;41:1242–1248. doi: 10.1016/0026-0495(92)90016-4. [DOI] [PubMed] [Google Scholar]
- 11.Ross R, Rissanen J, Hudson R. Sensitivity associated with the identification of visceral adipose tissue levels using waist circumference in men and women: effects of weight loss. Int J Obes Relat Metab Disord. 1996;20:533–538. [PubMed] [Google Scholar]
- 12.Sidney S, Lewis CE, Hill JO, et al. Association of total and central adiposity measures with fasting insulin in a biracial population of young adults with normal glucose tolerance: the CARDIA study. Obes Res. 1999;7:265–272. doi: 10.1002/j.1550-8528.1999.tb00405.x. [DOI] [PubMed] [Google Scholar]
- 13.Phillips GB, Jing T, Heymsfield SB. Relationships in men of sex hormones, insulin, adiposity, and risk factors for myocardial infarction. Metabolism. 2003;52:784–790. doi: 10.1016/s0026-0495(03)00072-6. [DOI] [PubMed] [Google Scholar]
- 14.Rattarasarn C, Leelawattana R, Soonthornpun S, et al. Regional abdominal fat distribution in lean and obese Thai type 2 diabetic women: relationships with insulin sensitivity and cardiovascular risk factors. Metabolism. 2003;52:1444–1447. doi: 10.1016/s0026-0495(03)00257-9. [DOI] [PubMed] [Google Scholar]
- 15.Pouliot MC, Despres JP, Lemieux S, et al. Waist circumference and abdominal sagittal diameter: best simple anthropometric indexes of abdominal visceral adipose tissue accumulation and related cardiovascular risk in men and women. Am J Cardiol. 1994;73:460–468. doi: 10.1016/0002-9149(94)90676-9. [DOI] [PubMed] [Google Scholar]
- 16.Kunesova M, Hainer V, Hergetova H, et al. Simple anthropometric measurements—relation to body fat mass, visceral adipose tissue and risk factors of atherogenesis. Sb Lek. 1995;96:257–267. [PubMed] [Google Scholar]
- 17.Macor C, Ruggeri A, Mazzonetto P, et al. Visceral adipose tissue impairs insulin secretion and insulin sensitivity but not energy expenditure in obesity. Metabolism. 1997;46:123–129. doi: 10.1016/s0026-0495(97)90288-2. [DOI] [PubMed] [Google Scholar]
- 18.Lovejoy JC, de la Bretonne JA, Klemperer M, et al. Abdominal fat distribution and metabolic risk factors: effects of race. Metabolism. 1996;45:1119–1124. doi: 10.1016/s0026-0495(96)90011-6. [DOI] [PubMed] [Google Scholar]
- 19.Abate N, Garg A, Peshock RM, et al. Relationships of generalized and regional adiposity to insulin sensitivity in men. J Clin Invest. 1995;96:88–98. doi: 10.1172/JCI118083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goodpaster BH, Thaete FL, Simoneau JA, et al. Subcutaneous abdominal fat and thigh muscle composition predict insulin sensitivity independently of visceral fat. Diabetes. 1997;46:1579–1585. doi: 10.2337/diacare.46.10.1579. [DOI] [PubMed] [Google Scholar]
- 21.Seidell JC, Perusse L, Despres JP, et al. Waist and hip circumferences have independent and opposite effects on cardiovascular disease risk factors: the Quebec Family Study. Am J Clin Nutr. 2001;74:315–321. doi: 10.1093/ajcn/74.3.315. [DOI] [PubMed] [Google Scholar]
- 22.Snijder MB, Visser M, Dekker JM, et al. Low subcutaneous thigh fat is a risk factor for unfavourable glucose and lipid levels, independently of high abdominal fat. The Health ABC Study. Diabetologia. 2005;48:301–308. doi: 10.1007/s00125-004-1637-7. [DOI] [PubMed] [Google Scholar]
- 23.Ross R. Magnetic resonance imaging provides new insights into the characterization of adipose and lean tissue distribution. Can J Physiol Pharmacol. 1996;74:778–785. [PubMed] [Google Scholar]
- 24.Gallagher D, Belmonte D, Deurenberg P, et al. Organ-tissue mass measurement allows modeling of REE and metabolically active tissue mass. Am J Physiol. 1998;27(5):E249–E258. doi: 10.1152/ajpendo.1998.275.2.E249. [DOI] [PubMed] [Google Scholar]
- 25.Shen W, Wang Z, Tang H, et al. Volume estimates by imaging methods: model comparisons with visible woman as the reference. Obes Res. 2003;11:217–225. doi: 10.1038/oby.2003.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Safrin S, Grunfeld C. Fat distribution and metabolic changes in patients with HIV infection. AIDS. 1999;13:2493–2505. doi: 10.1097/00002030-199912240-00002. [DOI] [PubMed] [Google Scholar]
- 27.Hengel RL, Watts NB, Lennox JL. Benign symmetric lipomatosis associated with protease inhibitors [letter] Lancet. 1997;350:1596. doi: 10.1016/s0140-6736(05)64011-1. [DOI] [PubMed] [Google Scholar]
- 28.Lo JC, Mulligan K, Tai VW, et al. “Buffalo hump” in men with HIV-1 infection. Lancet. 1998;351:867–870. doi: 10.1016/S0140-6736(97)11443-X. [DOI] [PubMed] [Google Scholar]
- 29.Miller KK, Daly PA, Sentochnik D, et al. Pseudo-Cushing’s syndrome in human immunodeficiency virus-infected patients. Clin Infect Dis. 1998;27:68–72. doi: 10.1086/514638. [DOI] [PubMed] [Google Scholar]
- 30.Mallon PW, Wand H, Law M, et al. Buffalo hump seen in HIV-associated lipodystrophy is associated with hyperinsulinemia but not dyslipidemia. J Acquir Immune Defic Syndr. 2005;38:156–162. doi: 10.1097/01.qai.0000147527.64863.1a. [DOI] [PubMed] [Google Scholar]
- 31.Dixon JB, O’Brien PE. Neck circumference a good predictor of raised insulin and free androgen index in obese premenopausal women: changes with weight loss. Clin Endocrinol (Oxf) 2002;57:769–778. doi: 10.1046/j.1365-2265.2002.01665.x. [DOI] [PubMed] [Google Scholar]
- 32.Laakso M, Matilainen V, Keinanen-Kiukaanniemi S. Association of neck circumference with insulin resistance-related factors. Int J Obes Relat Metab Disord. 2002;26:873–875. doi: 10.1038/sj.ijo.0802002. [DOI] [PubMed] [Google Scholar]
- 33.Tien PC, Benson C, Zolopa AR, et al. The study of Fat Redistribution and Metabolic Change in HIV Infection (FRAM): methods, design, and sample characteristics. Am J Epidemiol. 2006;163:860–869. doi: 10.1093/aje/kwj111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.The Study of Fat Redistribution and Metabolic Change in HIV Infection (FRAM). Fat distribution in men with HIV infection. J Acquir Immune Defic Syndr. 2005;40:121–131. doi: 10.1097/01.qai.0000182230.47819.aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.The Study of Fat Redistribution and Metabolic Change in HIV Infection (FRAM). Fat distribution in women with HIV infection. J Acquir Immune Defic Syndr. 2006;42:562–571. doi: 10.1097/01.qai.0000229996.75116.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hughes GH, Cutter G, Donahue R, et al. Recruitment in the Coronary Artery Disease Risk Development in Young Adults (CARDIA) study. Control Clin Trials. 1987;8 Suppl:68S–73S. doi: 10.1016/0197-2456(87)90008-0. [DOI] [PubMed] [Google Scholar]
- 37.Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41:1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 38.Sidney S, Jacobs DR, Jr, Haskell WL, et al. Comparison of two methods of assessing physical activity in the Coronary Artery Risk Development in Young Adults (CARDIA) study. Am J Epidemiol. 1991;133:1231–1245. doi: 10.1093/oxfordjournals.aje.a115835. [DOI] [PubMed] [Google Scholar]
- 39.Hoegerman GS, Lewis CE, Flack J, et al. Lack of association of recreational cocaine and alcohol use with left ventricular mass in young adults. The Coronary Artery Risk Development in Young Adults (CARDIA) study. J Am Coll Cardiol. 1995;25:895–900. doi: 10.1016/0735-1097(94)00469-7. [DOI] [PubMed] [Google Scholar]
- 40.Hill JO, Sidney S, Lewis CE, et al. Racial differences in amounts of visceral adipose tissue in young adults: the CARDIA (Coronary Artery Risk Development in Young Adults) study. Am J Clin Nutr. 1999;69:381–387. doi: 10.1093/ajcn/69.3.381. [DOI] [PubMed] [Google Scholar]
- 41.Hahn GJ, Meeker WQ. Statistical Intervals: A Guide for Practitioners. New York: John Wiley & Sons; 1991. [Google Scholar]
- 42.Reinehr T, Andler W. Changes in the atherogenic risk factor profile according to degree of weight loss. Arch Dis Child. 2004;89:419–422. doi: 10.1136/adc.2003.028803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ. 2003;326:219. doi: 10.1136/bmj.326.7382.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garg A. Regional adiposity and insulin resistance. J Clin Endocrinol Metab. 2004;89:4206–4210. doi: 10.1210/jc.2004-0631. [DOI] [PubMed] [Google Scholar]
- 45.Abate N, Garg A, Peshock RM, et al. Relationship of generalized and regional adiposity to insulin sensitivity in men with NIDDM. Diabetes. 1996;45:1684–1693. doi: 10.2337/diab.45.12.1684. [DOI] [PubMed] [Google Scholar]
- 46.Stern MP, Haffner SM. Body fat distribution and hyperinsulinemia as risk factors for diabetes and cardiovascular disease. Arteriosclerosis. 1986;6:123–130. doi: 10.1161/01.atv.6.2.123. [DOI] [PubMed] [Google Scholar]
- 47.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 48.Grundy SM, Brewer HB, Jr, Cleeman JI, et al. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 49.Kahn R, Buse J, Ferrannini E, et al. The metabolic syndrome: time for a critical appraisal: joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2005;28:2289–2304. doi: 10.2337/diacare.28.9.2289. [DOI] [PubMed] [Google Scholar]
- 50.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487–1495. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]