Abstract
Purpose
The maturation of an arterio-venous fistula (AVF) requires remodeling of the arterial inflow and the venous outflow limbs to sustain flows sufficient to support hemodialysis. However, factors influencing remodeling of AVF are poorly understood. We hypothesized that AVF remodeling was an endothelium-dependent process.
Methods
This is a prospective cohort study of patients (n=25) undergoing autologous AVF formation. Brachial artery vasoreactivity studies were performed preoperatively to assess endothelium-dependent, flow-mediated vasodilation (FMD). High-resolution ultrasound was used to assess venous and arterial diameters intraoperatively, and at 3 months.
Results
The mean age was 64.5 ± 13.6 years. Twelve subjects (48%) had diabetes. The mean FMD for the entire cohort was (mean ± SEM) 5.82 ± 0.9%, (range) 0–17.3%. The vein increased in size 3.19 ± .28 to 6.11 ± .41 mm, 108.4 ± 17.9%, p=.0001, while the artery increased from 3.29 ± .14 to 4.48 ± .30 mm, 20.47 ± 10.8%, p=.013. There was a significant positive correlation between the degree of arterial and venous remodeling, r=.52, p=.023. Brachial artery FMD most strongly correlated with the magnitude of arterial remodeling, r=.47, p=.038. Patients with diabetes failed to undergo venous remodeling to the same extent as did those without diabetes, 59.2 ± 24.4% vs. 141.5 ± 25.4%, p=.04.
Conclusion
Impairment of endothelial function is associated with decreased arterial remodeling and final venous lumen diameter attained at three months. Further investigation is needed to determine whether modulation of endothelial function in this cohort can improve AVF maturation.
Keywords: Flow-mediated dilation, Arteriovenous fistula, Endothelial function
Introduction
Due to a lower failure rate and lower risk of infection, native arterio-venous fistula (AVF) is the preferred method of providing vascular access for long-term hemodialysis patients. However, 28–53% of newly created AVF never mature adequately to be used for hemodialysis. Traditional cardiovascular risk factors such as hypertension and hyperlipidemia poorly predict AVF non-maturation, prompting investigations into other risk factors such as hemodynamic profile or vessel morphology (1). Although strict definitions of maturation vary among vascular societies in Europe, Japan, and the United States, there is general agreement that a mature AVF can be routinely cannulated 3 times per week with 2 needles and deliver blood flow at 300 to 450 ml/min for 3–5 hours. Accordingly, the brachial artery supplying a maturing AVF must increase baseline blood flow by 10–20 fold, from about 50 ml/min to 500 ml/min or higher, to deliver the required blood volume for the AVF as well as distal tissues (2). This increase in flow is accompanied by geometric remodeling of the vasculature such that the diameter increases sufficiently to accommodate the increased flow (3).
It is well accepted that arterial dilation in response to a rapid increase in flow is mediated by endothelium-derived nitric oxide (NO) (4). The resultant diameter increase has been proven to be endothelium-dependent in both healthy subjects as well as those with cardiovascular risk factors including uremia (5–8). However, when the hemodynamic stimuli persist, such as in a newly created AVF, structural alterations within the vessel occur which normalize shear stress and wall tension. For example, previous investigations have demonstrated that removal of the radial artery as a conduit for coronary bypass grafting resulted in a sustained increase in blood flow in the ipsilateral ulnar artery. In response to this hemodynamic modification, the ulnar artery dilated by about 9% (9). Importantly, the increase in lumen diameter was associated with endothelium-dependent vasoreactivity, thereby suggesting that prolonged exposure to increased flow rates results in vasodilation that is, at least in part, endothelium-dependent. However, it is currently unknown to what extent maturation of the AVF relies on endothelium-dependent mechanisms.
Therefore, we suspect that functional characteristics of the vasculature are critical to normal AVF remodeling and maturation. Accordingly, we hypothesize that remodeling of the artery and vein in a newly created AVF is associated with endothelium-dependent mechanisms as quantified by brachial artery flow-mediated vasodilation (FMD).
Methods
Subjects
Twenty-five subjects undergoing autogenous AVF were enrolled in this study. Subjects were identified for participation in this study once a referral was made to a participating vascular surgeon for permanent vascular access. All subjects had an estimated glomerular filtration rate (eGFR) <30 mL/min/1.73m2 or were already on dialysis and had pre-operative venous mapping demonstrating adequate artery and vein for creation of an AVF. Subjects were excluded from participation if all or a portion of their fistula employed prosthetic material. Other exclusion criteria included the inability to withhold vasoactive medications or nicotine for 24 hr prior to vasoreacitvity studies. All participants provided written informed consent. The protocol was approved by the Partners Human Research Committee of the Brigham and Women’s Hospital, Boston, MA.
Pre-operative Vascular Reactivity Studies
Endothelium-dependent vasoreactivity of the brachial artery was studied in the post-absorptive state (fasting since the previous midnight) in a quiet, temperature-controlled room. All vasoactive medication, long acting nitrates, tobacco, and caffeine were held 24 hr prior to the study. High-resolution B-mode ultrasonography of the brachial artery and vein graft was performed using a GE Vivid 7 (GE Healthcare, Fairfield, CT) ultrasound machine and a 7.5 MHz linear array probe. The brachial artery ipsilateral to fistula placement was imaged longitudinally, just proximal to the antecubital fossa with the transducer position adjusted to obtain optimal B-mode images of the near and far walls of the intima. A 6.4 cm cuff was placed on the upper arm and inflated to supra-systolic pressures for 5 minutes to induce ischemia. Flow-induced endothelium-dependent vasodilation of the brachial artery was determined by acquiring images at 1 min after cuff deflation. The determination of endothelial function was performed in accordance with published guidelines (10). All post-processing imaging analysis was performed with the Brachial Analyzer, Medical Imaging Applications, Iowa City, Iowa. This software application uses edge-detection and wall-tracking software which is independent of investigator bias to capture 10 consecutive end-diastolic images.
Intra-operative and 3 month measurements of arterial and venous lumen diameters
After the satisfactory completion of the AV anastomosis and assurance of a palpable thrill within the fistula, intra-operative arterial and venous lumen measurements were undertaken. The arterial lumen dimensions were obtained 1 cm proximal to the anastomosis using a series of M-mode images with a cross section view of the artery. Five lumen diameter measurements were taken and averaged to determine the reported lumen dimension. Venous luminal dimensions under arterial pressures were obtained in a similar manner with M-mode ultrasound 1 cm distal to the anastomosis. Remodeling was calculated by subtracting the baseline lumen diameter from the 3-month lumen diameter and dividing by the baseline lumen diameter and multiplying by 100 to give a percent. Remodeling was reported separately for the artery and vein of the AVF.
Statistical analysis
Descriptive measures are reported as means ± standard deviation. Experimental measures are reported as means ± standard error of the mean (SEM). Experimental measures, including flow-mediated vasodilatation, for each subject group were compared using a paired Student’s t test. Linear regressions were performed to determine the correlation between continuous variables and Pearson’s correlation coefficient reported in variables with normal distributions. Statistical significance was accepted at the 95% confidence level (p < 0.05). All statistics were performed with STATA 10, College Station, TX.
Results
Demographics
Table I presents the baseline demographics for this cohort. Twenty-five subjects participated in this study. The mean age (mean ± S.D.) of the participants was 64.5 ± 13.3, of which 20 (80%) were men. Six subjects had already initiated hemodialysis at the time referral for the fistula and the remainder had stage IV chronic kidney disease with a mean eGFR of 17.2 ± 4.9 mL/min/1.73m2. The etiology of the renal impairment was variable but the majority was from diabetes or hypertension. Access type was determined by surgeon preference and suitability of the vein as determined on pre-operative vein mapping. Eight (32%) were radial-cephalic, 12 (48%) were brachial-cephalic, and four (16%) were brachial-basilic fistulas.
Table I.
Demographics of the study population.
| Age (mean ± S.D.) | 64.5 ± 13.3 |
|
| |
| Gender, men N (%) | 20 (80%) |
|
| |
| Access type* | |
| -RC (%) | 8 (25%) |
| -BC (%) | 12 (48%) |
| -BB (%) | 4 (16%) |
|
| |
| Median eGFR mL/min/1.73m2 | 17.2 ± 4.9 |
|
| |
| Median CRP mg/L (IQR)† | 5.2 (2.8–16.4) |
|
| |
| Diabetes | 12 (48%) |
|
| |
| Hypertension | 23 (92%) |
|
| |
| Coronary disease | 10 (40%) |
|
| |
| Tobacco use (current or former) | 19 (76%) |
|
| |
| Hypercholesterolemia | 16 (64%) |
RC radio-cephalic, BC brachial-cephalic, BB brachial-basilic,
IQR interquartile range.
Arterial and venous luminal remodeling
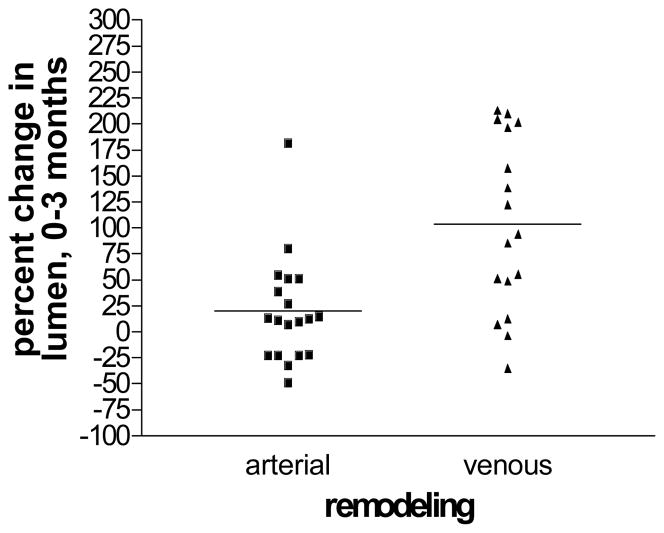
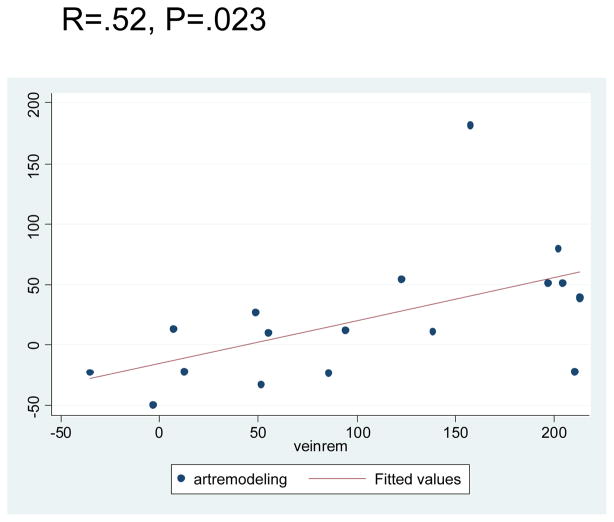
The baseline arterial lumen diameter for this cohort was (mean ± S.E.M.) 3.29 ± .14 mm (range 2.35–4.78 mm), while the baseline vein was 3.19 ± .28 mm (range 1.53–7.6). Arterial luminal dilation occurred from the time of implantation until 3 months, when the mean arterial lumen increased to 4.48 ± .30 mm (range 2.44–7.29mm) for a mean increase of 20.47% ± 10.81% (range −49%–182.13%) p=.013. The mean venous diameter achieved at 3 months was 6.11 ± .41 mm (range 2.89–10.56) for a 108.42% ± 17.9% (range −35.13% – 214.22%) percent increase, p=.001 (Fig. 1). There was a positive correlation between the magnitude of arterial luminal remodeling and venous remodeling, r=.52, p=.023 (Fig. 2).
Fig. 1.
A significant increase arterial and venous lumen diameter was observed over the first 3 months of AVF creation. Despite a mean increase in luminal enlargement, there is considerable heterogeneity of remodeling, [(3-month diameter-baseline diameter) ÷ baseline diameter] X 100, in the first 3 months.
Fig. 2.
There is a positive correlation between the magnitude of arterial and venous remodeling in the maturing AVF.
Flow-mediated, endothelium-dependent vasodilation and correlation with remodeling
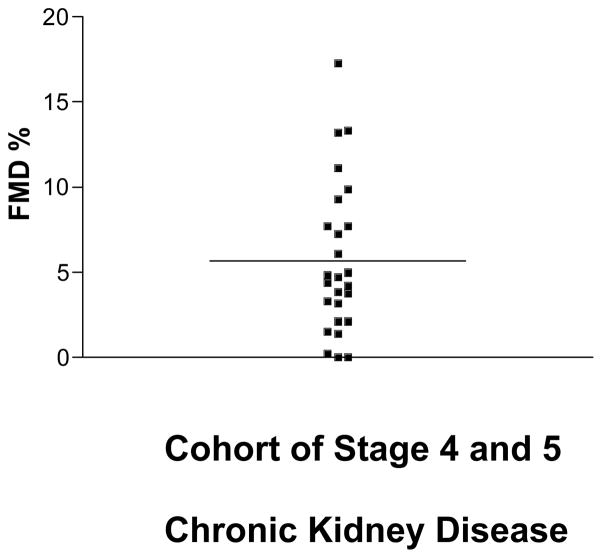
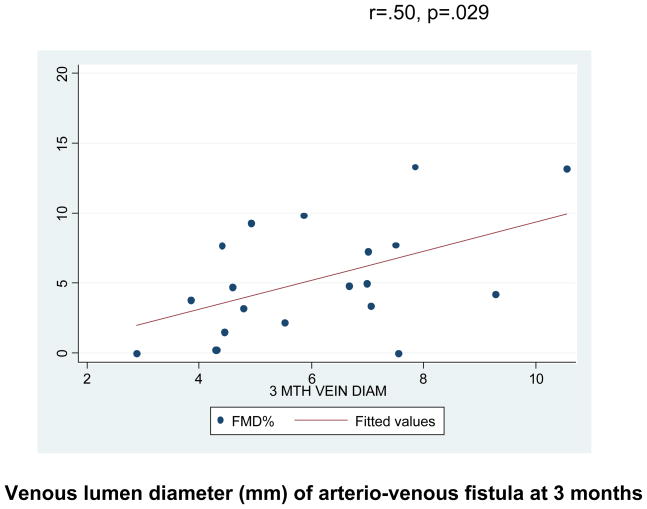
The FMD for the entire cohort was 5.82% ± .9% indicating severely impaired endothelial function (normal value for our lab is 11–13%, data not shown) (6) (Fig. 3). However, the range of FMD was from (0–17.25%) indicating considerable variability in endothelial function in these patients (Fig. 3). There was a significant positive correlation of ipsilateral brachial artery endothelial function and subsequent percent arterial luminal remodeling, r=.47, p=.038, as well as final venous diameter at 3 months, r=.50, p=.029 (Fig. 4). The presence of diabetes was associated with a significant impairment in the extent to which the venous side of the AVF remodeled, 59.2 ± 24.4% vs. 141.5 ± 25.4%, p=.04.
Fig. 3.
Endothelium-dependent, flow mediated, brachial artery dilation was 5.82% ± .9%, indicating severe impairment of endothelial function in this population.
Fig. 4.
There is a significant positive correlation between brachial artery flow mediated dilation and final venous luminal dimension.
Discussion
This study has several salient findings. First, there is rapid (≤ 3 months) and significant arterial and venous outward luminal remodeling following the creation of an AVF as it adapts to the increase in blood flow. However, despite the mean increase in diameter, there is a considerable variability of fistula remodeling indicating heterogeneity within the population. Further, the magnitude of both arterial and venous remodeling, appear to be correlated with one another indicating the importance of arterial as well as venous remodeling in maturing fistula. Second, endothelial function is severely impaired in this cohort of patients with stage IV or V chronic kidney disease (CKD) compared to historically normal patients examined in our laboratory. Once again, there exists a considerable variability (0–17.25%) in endothelial-dependent vasodilation even amongst these patients with severe uremia and often multiple cardiovascular risk factors. Most notably, we have shown for the first time that the magnitude of endothelial function is associated with the percent arterial luminal remodeling and the final attained venous diameter indicating that these dynamic processes, associated with chronic exposure to a high flow milieu, are in part, endothelial dependent.
Endothelial function in uremic patients
Previous investigations have demonstrated impaired endothelial function in patients with renal impairment (8, 11–14). For example, Annuk et al (8), used methacholine, a receptor-dependent, endothelium-dependent, mechanism to increase forearm blood flow. These investigators noted that compared to healthy controls, endothelial function was blunted in subjects with even moderate chronic renal failure. These findings have led the authors and others to speculate that endothelial dysfunction accounts for the increased mortality in seen renal failure patients even after accounting for traditional cardiovascular risk factors. Others have shown that the arteries of CKD patients are capable of dilating to the same extent as control patients when given exogenous NO donors such as sodium nitroprusside. This observation suggests that there is no deficit in smooth muscle cell relaxation but rather the deficiency lies in the bioavailability of NO in these patients (11, 14). Indeed, these findings are consistent with other investigators who note total body NO is low in patients with chronic renal impairment (15, 16). One notable difference from these previous investigations is that we used the more physiological relevant flow stimulus to detect endothelial function as opposed to a pharmacologic stimulus (methylcholine).
AVF hemodynamics and remodeling
Other investigators have characterized brachial artery diameter changes of the maturing AVF. Dammers et al noted that the brachial artery luminal diameter increased 37% from baseline to 3 months (17). Remarkably, 16% of lumen gain occurred by the first post-operative day. Others have noted increase in vein dimensions over the first 3 months of the maturing fistula (18). However, until now systematic studies of geometric remodeling of both arterial and venous limbs of the AVF have been lacking.
While this study is the first investigation to date to demonstrate that endothelial function is associated with arterial and venous remodeling, this finding is concordant with basic hemodynamic principles. As more blood is delivered through the venous outflow limb of the AVF, wall shear stress, proportional to the quotient of blood flow velocity and lumen diameter, will rise. Theoretically, this should lead to venous dilation to normalize the shear stress. Wedgwood et al demonstrated a high degree of correlation between the flow rate through an AVF and the venous diameter indicating that flow is a primary determinant of final venous diameter (19). It is likely that the magnitude of remodeling depends on the hemodynamic stimuli, endothelium-derived mediators, and the baseline stiffness of the conduit; all of which can be impacted by traditional cardiovascular risk factors.
Limitations
While we have established a relationship between endothelial function and AVF remodeling, we are unable to comment on whether endothelial function is associated with clinical maturation of the fistula. Several patients have not yet initiated HD, three have died; and therefore, there is limited power to make the observation. Pre-existing vein disease, previous phlebotomy, and arterial calcification also significantly impact clinical maturation and were not considered in the present study. Nevertheless, the focus of the study was on the physiological relationship between remodeling and endothelial function and not powered for clinical outcomes. Finally, multivariable modeling techniques are needed incorporating, structural, functional, demographic, as well as biochemical factors that may contribute to the maturing fistula.
Conclusion
We have shown for the first time that AVF remodeling is, in part, endothelium-dependent. As such, efforts to improve endothelial function in these patients may have clinical merit. However, further investigation is required to evaluate how this mechanism impacts clinically relevant endpoints such as fistula maturation and patency. Nevertheless, these data do suggest that brachial artery flow-mediated vasodilation (FMD) may play a role in identifying patients with a high probability of successful AVF maturation and function.
Acknowledgments
Financial support: Supported by funding from the National Heart, Lung, and Blood Institute K23 HL 92163
Footnotes
Competition of Interest: None
Meeting presentation: Presented at the Association of VA Surgeons Annual Meeting April 20, 2009.
References
- 1.Kharboutly Z, Fenech M, Treutenaere JM, Claude I, Legallais C. Investigations into the relationship between hemodynamics and vascular alterations in an established arteriovenous fistula. Med Eng Phys. 2007;29(9):999–1007. doi: 10.1016/j.medengphy.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 2.Dixon BS. Why don't fistulas mature? Kidney Int. 2006;70(8):1413–22. doi: 10.1038/sj.ki.5001747. [DOI] [PubMed] [Google Scholar]
- 3.Zarins CK, Zatina MA, Giddens DP, Ku DN, Glagov S. Shear stress regulation of artery lumen diameter in experimental atherogenesis. J Vasc Surg. 1987;5(3):413–20. [PubMed] [Google Scholar]
- 4.Joannides R, Haefeli WE, Linder L, et al. Nitric oxide is responsible for flow-dependent dilatation of human peripheral conduit arteries in vivo. Circulation. 1995;91(5):1314–9. doi: 10.1161/01.cir.91.5.1314. [DOI] [PubMed] [Google Scholar]
- 5.Lieberman EH, Gerhard MD, Uehata A, et al. Flow-induced vasodilation of the human brachial artery is impaired in patients <40 years of age with coronary artery disease. Am J Cardiol. 1996;78(11):1210–4. doi: 10.1016/s0002-9149(96)00597-8. [DOI] [PubMed] [Google Scholar]
- 6.Beckman JA, Liao JK, Hurley S, et al. Atorvastatin restores endothelial function in normocholesterolemic smokers independent of changes in low-density lipoprotein. Circ Res. 2004;95(2):217–23. doi: 10.1161/01.RES.0000134628.96682.9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson TJ, Uehata A, Gerhard MD, et al. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol. 1995;26(5):1235–41. doi: 10.1016/0735-1097(95)00327-4. [DOI] [PubMed] [Google Scholar]
- 8.Annuk M, Lind L, Linde T, Fellstrom B. Impaired endothelium-dependent vasodilatation in renal failure in humans. Nephrol Dial Transplant. 2001;16(2):302–6. doi: 10.1093/ndt/16.2.302. [DOI] [PubMed] [Google Scholar]
- 9.Vita JA, Holbrook M, Palmisano J, et al. Flow-induced arterial remodeling relates to endothelial function in the human forearm. Circulation. 2008;117(24):3126–33. doi: 10.1161/CIRCULATIONAHA.108.778472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39(2):257–65. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 11.Thambyrajah J, Landray MJ, McGlynn FJ, Jones HJ, Wheeler DC, Townend JN. Abnormalities of endothelial function in patients with predialysis renal failure. Heart. 2000;83(2):205–9. doi: 10.1136/heart.83.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Guldener C, Lambert J, Janssen MJ, Donker AJ, Stehouwer CD. Endothelium-dependent vasodilatation and distensibility of large arteries in chronic haemodialysis patients. Nephrol Dial Transplant. 1997;12 (Suppl 2):14–8. [PubMed] [Google Scholar]
- 13.Joannides R, Bakkali EH, Le Roy F, et al. Altered flow-dependent vasodilatation of conduit arteries in maintenance haemodialysis. Nephrol Dial Transplant. 1997;12(12):2623–8. doi: 10.1093/ndt/12.12.2623. [DOI] [PubMed] [Google Scholar]
- 14.Morris ST, McMurray JJ, Rodger RS, Jardine AG. Impaired endothelium-dependent vasodilatation in uraemia. Nephrol Dial Transplant. 2000;15(8):1194–200. doi: 10.1093/ndt/15.8.1194. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt RJ, Baylis C. Total nitric oxide production is low in patients with chronic renal disease. Kidney Int. 2000;58(3):1261–6. doi: 10.1046/j.1523-1755.2000.00281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wever R, Boer P, Hijmering M, et al. Nitric oxide production is reduced in patients with chronic renal failure. Arterioscler Thromb Vasc Biol. 1999;19(5):1168–72. doi: 10.1161/01.atv.19.5.1168. [DOI] [PubMed] [Google Scholar]
- 17.Dammers R, Tordoir JH, Kooman JP, et al. The effect of flow changes on the arterial system proximal to an arteriovenous fistula for hemodialysis. Ultrasound Med Biol. 2005;31(10):1327–33. doi: 10.1016/j.ultrasmedbio.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 18.Corpataux JM, Haesler E, Silacci P, Ris HB, Hayoz D. Low-pressure environment and remodelling of the forearm vein in Brescia-Cimino haemodialysis access. Nephrol Dial Transplant. 2002;17(6):1057–62. doi: 10.1093/ndt/17.6.1057. [DOI] [PubMed] [Google Scholar]
- 19.Wedgwood KR, Wiggins PA, Guillou PJ. A prospective study of end-to-side vs. side-to-side arteriovenous fistulas for haemodialysis. Br J Surg. 1984;71(8):640–2. doi: 10.1002/bjs.1800710831. [DOI] [PubMed] [Google Scholar]