Abstract
Identification of cancer stem cells is crucial for advancing cancer biology and therapy. Several markers including CD24, CD44, CD117, CD133, ABCG, ESA and ALDH are utilized to identify and investigate human epithelial cancer stem cells in the literature. We have now systemically analyzed and compared the expression of these markers in fresh ovarian epithelial carcinomas. Although the expression levels of these markers were unexpectedly variable and partially overlapping in fresh ovarian cancer cells from different donors, we reliably detected important levels of CD133 and ALDH in the majority of fresh ovarian cancer. Furthermore, most of these stem cell markers including CD133 and ALDH were gradually lost following in vitro passage of primary tumor cells. However, the expression of ALDH and CD133, but not CD24, CD44 and CD117, could be partially rescued by the in vitro serum free and sphere cultures, and the in vivo passage in the immune deficient xenografts. ALDH+ and CD133+ cells formed three dimensional spheres more efficiently than their negative counterparts. These sphere forming cells expressed high levels of stem cell core gene transcripts, and could be expanded and formed additional spheres in long-term culture. ALDH+, CD133+, and ALDH+CD133+ cells from fresh tumors developed larger tumors more rapidly than their negative counterparts. This property was preserved in the xenografted tumors. Altogether, the data suggest that ALDH+ and CD133+ cells are enriched with ovarian cancer initiating (stem) cells, and ALDH and CD133 may be widely utilized as reliable markers to investigate ovarian cancer stem cell biology.
Keywords: Stem cell, ovarian cancer, apoptosis resistance, ALDH, CD133, Tumorigenesis
Ovarian carcinoma is a deadly disease, characterized by late diagnosis, early metastasis, and resistance to therapy. Although the majority of patients initially respond to platinum based chemotherapy, most will subsequently succumb to chemoresistant, recurrent disease. Long term treatment success is limited by the development of chemoresistant disease. This may be partially due to the formed immune suppressive networks in the human ovarian cancer microenvironment. In the last several years our research team has focused on the human ovarian carcinoma microenvironment, and has demonstrated that immune cells in human ovarian carcinoma have been reprogrammed by active tumor-mediated processes to defeat tumor immunity, and in turn temper the clinical efficacy of chemotherapy1–4.
It is also possible that existing therapies target primarily the bulk of the ovarian carcinoma cell population, rather than cancer initiating cells (or stem cells, or stem-like cells)5–11. Although multiple markers including CD44, CD11712, CD13313, 14 and Hoechst positive ‘side population’15 are utilized to identify ovarian cancer stem cells, the concept of ovarian cancer stem cells remains controversial and the nature of these ovarian cancer stem cells has not been well defined in fresh ovarian cancer and primary tumor cells. ALDH has been used to investigate multiple human cancer stem cells16–22. Recent reports showed that ALDH+ ovarian cancer cells possess stem cell properties23, 24. However, it is not well understood if ovarian cancer stem cells universally express ALDH, CD133 and other markers, and if ALDH expression is phenotypically and functionally associated to many other reported markers for ovarian cancer stem cells. In the present study, we revisited this issue, and examined fresh ovarian cancer tissues, primary ovarian cancer cells and xenografted ovarian cancer cells in our laboratory, and focused our studies on identifying and comparing potential ovarian cancer stem cells, and the regulatory effects of in vitro and in vivo environments on the property of ovarian cancer stem cells.
Materials & Methods
Human subjects
We studied previously-untreated patients with epithelial ovarian carcinomas (n = 25). Patients gave written, informed consent. The study was approved by the University of Michigan.
Cells and tissues
Cells and tissues were obtained from ascites and tumors as described25–27. Fresh tumors were processed into single cell suspension as described and immediately used for enrichment or flow analysis25–27. Potential cancer stem cells were enriched by depleting CD45-PE positive immune cells including macrophages, myeloid dendritic cells, plasmacytoid dendritic cells, B and T cell subsets (PE-selection kit, StemCell Technology, Vancouver, Canada) and sorted with FACSaria (Becton Dickinson, San Jose, CA) as we described25–27. Dead cells were excluded. Cell purity was > 98% as confirmed by flow cytometry (LSR II, BD). Primary ovarian cancer cells were established from fresh ascites or/and tumor tissues. Tumor cells were initially enriched with double Ficoll separation with 100% Ficoll-Metrizoate (1.077 g/ml) on the bottom, followed by a layer of 75 % Ficoll-Metrizoate on the top (1.057 g/ml). Tumor cells were enriched on the top layer. Other cells were on the middle layer, and debris containing erythrocytes and polynuclear cells were on the lower layer. The enriched tumor cells were further sorted with high speed sorter (FACSAria, BD). The cells were either cultured under conventional condition (10% FCS, RPMI medium, all from GIBCO, Invitrogen) as a monolayer or serum-free (X-vivo20, Lonza) and sphere culture conditions (nonadherent, X-vivo20, Lonza).
Flow cytometry analysis (FACS)
Cells were stained with specific antibodies against human CD3, CD4, CD8, CD11b, CD11c, CD14, CD19, CD24, CD44, CD117, CD133, Annexin V (BD Biosciences) and ESA (StemCell technologies Inc). Samples were acquired on a LSR II and data were analyzed with DIVA software (BD). The ALDEFLUOR (ALDH) kit (StemCell technologies Inc) was used to identify and sort ALDH+ cells with high ALDH enzymatic activity by FACSAria as described16, 17. Briefly, single cells were suspended in ALDH assay buffer containing ALDH substrate-BAAA and incubated at 37°C for 40 minutes. In each experiment, the specific ALDH inhibitor diethylaminobenzaldehyde (DEAB) was used as negative control at 50 mmol/L. Other cells including CD133+ and ABCG2+ were sorted based on the surface antigen expression.
Immunofluorescence analysis
Immunofluorescence analysis was performed as described27, 28. Tissues were stained with monoclonal mouse anti-human-CD133 (1/100 dilution, Miltenyi) followed by Alexa Fluor 568-conjugated goat anti-mouse IgG (2 µg/ml, Molecular Probes), and with FITC conjugated mouse anti-human ESA (1/100 dilution, StemCell Technologies Inc). Positive cells were quantified by ImagePro Plus software and expressed as the mean of the percent positive cells ± standard deviation in 10 high powered fields using confocal microscopy.
Sphere formation
The sphere assay was performed as described16, 17. Briefly, tumor cells or electronically sorted tumor cell subsets were plated in ultra-low attachment plates (Corning, MA) in serum-free EBM-2 or X-VIVO medium (Lonza) supplemented with 5 µg/mL insulin (Sigma), 20 ng/mL human recombinant epidermal growth factor (EGF; Invitrogen), at a density of 1,000–10,000 viable cells/well. Spheres (> 50 µm) were counted for 1–6 weeks.
Quantitative real-time PCR
Total RNA was isolated with Qiagen Reagent (Qiagen). The RNA was reverse transcribed into cDNA using oligo-dT primers and SuperScript II Reverse Transcriptase (Invitrogen), according to manufacturer's instructions. The primer sequence combinations spanned contact sequences of subsequent exons. For amplification, the SyberGreen qPCR mix was used (Invitrogen). Each reaction was run in triplicate on the Mastercycler machine (Eppendorf) and was normalized to housekeeping gene GAPDH transcripts.
In vivo tumor formation
Ovarian tumor cell subsets (102−5 ×106) in 100 µl of buffered saline were subcutaneously injected into dorsal tissues of female NOD/Shi-scid/IL-2Rγnull (NSG) mice (6–8 weeks old, Jackson Lab, Bar Harbor, Maine), similar to our studies in NOD.SCID mice28, 29. Tumor size was measured twice weekly using calipers fitted with a Vernier scale. Tumor volume was calculated based on three perpendicular measurements28, 29.
Statistical analysis
Differences in cell surface molecule expression were determined by X2 test, and in other variables by Mann-Whitney test, with P < 0.05 being considered significant.
Results
Expression of multiple potential cancer stem cell markers in fresh ovarian tumors
CD24, CD44, CD117, CD133, ABCG2 and ESA are used to define cancer stem cells in multiple human epithelial cancers30–32 including ovarian cancer12–14. Recently, ALDH was also applied for identifying cancer stem cells7–9, 19, 20, 30–33. We initially examined the expression of these markers in fresh ovarian tumor cells. To this end, ovarian tumors were excised, and single cell suspensions were made. Suspended tumor cells were stained with lineage markers (anti-CD45, anti-CD34, and anti-ESA), stem cell markers and 7-AAD. All analyses were gated on viable cells. Immune cells (CD45+), endothelial cells and hematopoeic progenitor cells (CD34+) and other non-epithelial cells (ESA−) were gated out for stem cell marker analysis (Fig. 1a). Multiple color flow cytometry analysis revealed a significant lin−CD45−CD34−ESA+ cell population in fresh ovarian cancer tissues (Fig. 1a). This population was also observed in the fresh ascites fluid in patients with ovarian cancer (not shown). We reasoned that the potential ovarian cancer stem cells could be included in lin−CD45−CD34−ESA+ cell population.
Figure 1. Cancer stem cell markers in fresh ovarian cancer.
Fresh ovarian tumors were separated into single cell suspensions. Cells were stained for lineage specific and cancer stem cell markers and apoptotic cells. (a) Phenotype of fresh ovarian cancer cells. Multiple color FACS analysis was performed on the cells by gating on viable lin−CD45−CD34− cells. Epithelial ovarian cancer cells were defined as viable lin−CD45−CD34−ESA+ cells. The characteristics of lin−CD45−CD34−ESA+ cells in Forward scatter (FSC)/Side scatter (SSC) are shown. One of 25 representative patients is shown. (b, c) Cancer stem cell markers in fresh ovarian cancer cells. Results are expressed as the percentage of specific population in lin−CD45−CD34−ESA+ cells (b, c). Original dot plots showed high (upper panel) and low (lower panel) expression of given cancer stem cell marker in fresh ovarian cancer cells (c). (d, e) the expression of CD133 and ESA in fresh ovarian cancer tissues. High levels of CD133 (d), and low levels of CD133 (e).
Lin−CD45−CD34−ESA+ cell population was further analyzed for potential cancer stem cell markers. We observed that there were 7 to 100% CD24+ cells, 0.5 to 85% CD44+ cells, 0 to 10% CD117+ cells, 0.7 to 6.2% CD133+ cells, 1.8 to 14% ABCG2+ cells, and 0.3 to 7.1% ALDH+ cells in epithelial cells isolated from fresh ovarian tumors (Fig. 1b, c). Immune fluorescence staining confirmed that CD133+ cells were ESA+ in fresh ovarian cancer tissues (Fig. 1d, e). As the expression levels of cancer stem cell markers were considerably variable from patient to patient, we showed the levels of cancer stem cell marker expression in individual patients (Fig. 1b), as well as the high levels of expression (Fig. 1c upper panel, and Fig. 1d) and low levels of expression (Fig. 1c lower panel, and Fig. 1e) of each marker from two representative ovarian cancers. The data indicate that fresh ovarian cancer cells express variable levels of multiple potential cancer stem cell markers.
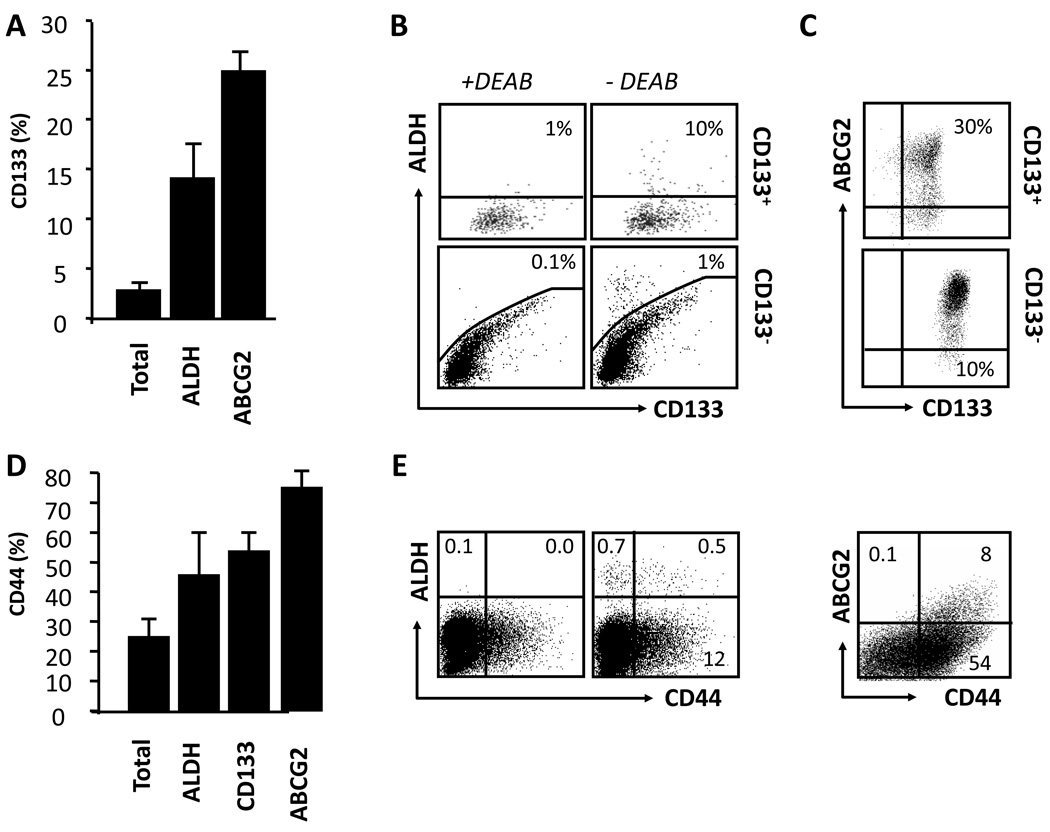
Relationships between multiple cancer stem cell markers in fresh ovarian tumors
We next examined the relationships between cancer stem cell markers in fresh ovarian tumors. High levels of CD24 expression and limited CD117 expression were detected in the majority of fresh ovarian cancer cells (Fig. 1b). We focused on the expression of CD133, ALDH, ABCG2 and CD44, but not CD24 and CD117. On average, CD133 expression overlapped significantly with expression of ALDH and ABCG2. Only 3% of all epithelial tumor cells were CD133+, whereas 14% of ALDH+ and 25% of ABCG2+ cells also expressed CD133 (Fig. 2a, b). There were 10 fold more ALDH+ cells in CD133+ than CD133− cells (Fig. 2b). Similarly, the fraction of ABCG2+ cells was higher in CD133+ than CD133− cells (Fig. 2c). We next analyzed CD44+ cells. Approximately 40–80% ALDH+, ABCG2+ and CD133+ cells also expressed CD44 (Fig. 2d, e). Thus, the expression of these markers is highly variable and overlapping from patient to patient.
Figure 2. Relationships between multiple cancer stem cell markers in fresh ovarian cancer.
Fresh ovarian tumors were separated into single cell suspensions. Cells were stained for stem cell markers (CD133, ALDH, ABCG2 and CD44) and linkage markers (CD45, CD34 and ESA). Tumor cells were determined as described in figure 1a. (a–c) The phenotypic characteristics of CD133+ tumor cell populations. Results are shown as the percentage of CD133+ cells in different tumor populations. Original dot plots showed the relationship between ALDH, ABCG2and CD133 (b, c). As a control for ALDH activity, the DEAB inhibitor has been used (see Materials and methods) (b). (d, e) The phenotypic characteristic of CD44+ tumor cells. Results are shown as the percentage of CD44+ cells in different tumor populations. Original dot plots showed the relationship between ALDH and ABCG2. n = 12. DEAB, specific ALDH inhibitor diethylaminobenzaldehyde.
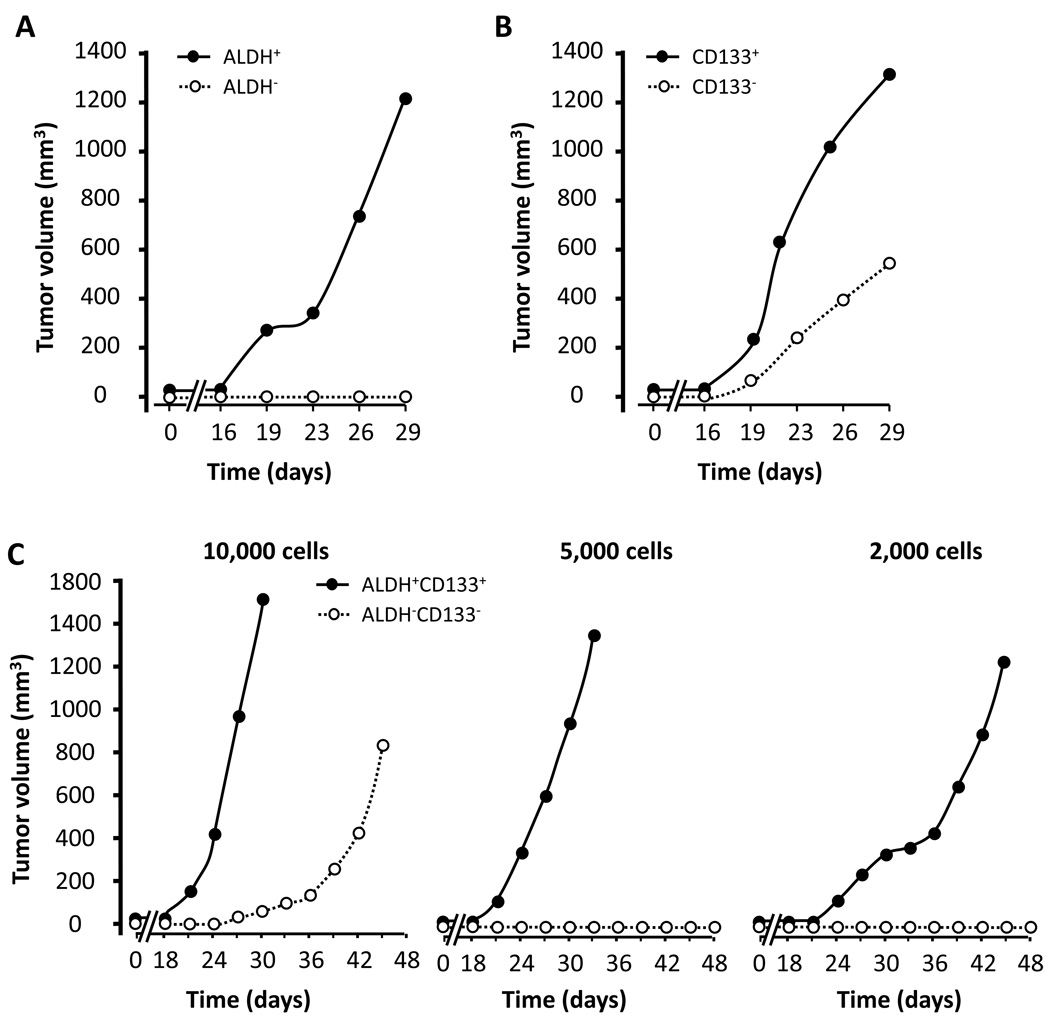
In vivo tumorigenesis of ALDH+ and CD133+ cells in fresh ovarian tumors
ALDH, CD133 and other potential stem cell markers have not been directly compared as stem cell markers in fresh ovarian cancer from the same patients. To determine and compare the in vivo tumorigenesis of cancer stem cells, we electronically sorted ALDH+, CD133+ and ABCG2+ cells from fresh ovarian tumors. Variable numbers of sorted cells were immediately implanted into NOD/Shi-scid/IL-2Rγnull (NSG) mice by subcutaneously injection, and tumor formation was followed for up to 48 days. The capacity of in vivo tumor formation with the sorted cells was different from patient to patient (Table 1). In 6 individual patients, we found that the rate of tumor formation was reliably higher in mice received different numbers of ALDH+ cells (Fig. 3a), CD133+ cells (Fig. 3b) and ALDH+CD133+ cells (Fig. 3c) (Table 1), than that of their negative counterparts. Furthermore, although ALDH−, CD133− and ALDH−CD133− cells could be tumorigenic (Table 1, Fig. 3a–c), the tumor volumes were smaller than their positive counterparts (Fig. 3b, c). When we isolated and analyzed tumor cells from the first xenografted tumors, similar percentages of ALDH+ and CD133+ cells were generated from the NSG mice inoculated with ALDH+CD133+, ALDH+CD133− and ALDH−CD133+ cells populations from the same donor (Table 2). When ALDH+, CD133+, ALDH+CD133+ or ALDH−CD133− populations were further isolated from the newly formed xenografted tumors, and inoculated into NSG mice, similar tumor forming capacity was observed (Fig. 3c, not shown). Interestingly, Hematoxylin & Eosin staining revealed that tumors forming from ALDH+, CD133+, ALDH+CD133+ or ALDH−CD133− populations were histologically similar (Supplementary Fig. 1). ABCG2+ and ABCG2− cells were tumorigenic but formed comparable tumors (not shown). The data indicate that ALDH+ and CD133+ cells are able to generate heterogeneous tumor populations in vivo, and further support that fresh tumor ALDH+ and CD133+ cells are enriched with cancer stem cells.
Table 1.
The rate of tumor formation from different tumor populations in NSG mice
| Number of inoculated cells: |
2,000 cells | 5,000 cells | 10,000 cells | |||
|---|---|---|---|---|---|---|
| Rate of tumor formation | % | N | % | N | % | N |
| CD133+ | 33% | 1/3 | 60% | 3/5 | 100% | 2/2 |
| CD133− | 20% | 1/5 | 20% | 1/5 | 50% | 2/4 |
| ALDH+ | 80% | 4/5 | 83% | 5/6 | 100% | 2/2 |
| ALDH− | 20% | 1/5 | 33% | 2/6 | 75% | 3/4 |
| CD133+ALDH+ | 100% | 2/2 | 100% | 2/2 | 100% | 2/2 |
| CD133−ALDH− | 0% | 0/2 | 0% | 0/2 | 50% | 1/2 |
Note: CD133+, ALDH+, CD133+ALDH+ and their negative counterparts were sorted from fresh human ovarian cancer, and injected into NSG mice as described in Fig. 1a, b. The rate of tumor formation (%) was shown in mice that received different numbers of tumor cell subpopulations. The observation time is up to 48 days.
Figure 3. In vivo tumorigenicity of ALDH+, CD133+ and ALDH+CD133+ cells.
(a, b), In vivo tumor formation. 2000 ALDH+ and ALDH− cells (a), 2000 CD133+ and CD133− cells (b), and 2000–10,000 ALDH+CD133+ cells, and ALDH−CD133− cells were electronically sorted from fresh ovarian tumors and injected into NSG mice (n = 5). Tumor volumes were measured. Cells in a and b were from one donor. Cells in c were from a different donor. One of 3 independent experiments is shown.
Table 2.
The percentage of different tumor populations from tumor xenograft in NSG mice
| Inoculated cell type |
Xenograft tumor cell type | |||
|---|---|---|---|---|
| Patient 1 | Patient 2 | |||
| CD133+ | ALDH+ | CD133+ | ALDH+ | |
| ALDH+CD133+ | 28% | 6% | 11% | 11% |
| ALDH+CD133− | 26% | 3% | 15% | 13% |
| ALDH−CD133+ | 25% | 3% | 14% | 9% |
Note: CD133+, ALDH+, and CD133+ALDH+ cells were isolated and sorted from tumors formed in the NSG mice, and were injected into new NSG mice as described in Fig. 1a, b. The percentage of CD133+ and ALDH+ cells was analyzed in the total tumor population from NSG mice from two patients. The observation time is up to 48 days.
ALDH+ and CD133+ cells form spheres
One of the key features of cancer stem cells is their capacity of self-renewal. To further functionally define the stem cell property, we performed the sphere assay with sorted fresh ovarian cancer cells as described16, 17. To minimize in vitro manipulation, we initially cultured the bulk cells. We observed that culturing the bulk of fresh ovarian cancer cells resulted in sphere formation. However, deletion of ALDH+ cells or CD133+ cells dramatically reduced the quantity and size of spheres formed. Simultaneous deletion of ALDH+ and CD133+ cells resulted in drastic reduction of sphere formation (Fig. 4a, b). As a confirmatory experiment, we showed that sorted ALDH+, CD133+, and ALDH+CD133+ cells were more efficient in sphere formation than their negative counterparts (Fig. 4c). We next examined if the sphere cells could be expanded and kept sphere forming capacity. The sphere cells were harvested and subject to further sphere culture. As expected, the sphere cells were able to form and expand spheres in culture for more than 2 months (Fig. 4d). Furthermore, although it was mechanistically unknown, we noticed that the morphological appearance of the spheres was different from one donor to another (Fig. 4d). We quantified and compared selected stem cell core genes in sphere cells and conventional cultured primary parental tumor cells. Real-time PCR revealed that the levels of SOX2, OCT3/4 and NANOG were higher in sphere cells than parental cells (Fig. 4e). ALDH+ and CD133+ cells were enriched in the sphere cells (Fig. 4b, c). Altogether, these data suggest that ALDH+ and CD133+ cells are capable of self-renewal in vitro (Fig. 4) and in vivo (Fig. 3), and that tumor initiating cells or cancer stem cells are enriched with ALDH+ and/or CD133+ cells.
Figure 4. Sphere formation of ALDH+, CD133+ and ALDH+CD133+ cells.
(a, b) Sphere forming ovarian cancer cells were enriched in bulk ALDH+ and CD133+ cells. The sphere forming assay was performed with bulk ovarian cancer cells, and cells depleted for CD133 and/or ALDH. Numbers of spheres were expressed as Mean ± SEM, n = 4, derived from 3 different patients. (c) The sphere forming ovarian cancer cells were enriched in sorted primary ALDH+, CD133+ and ALDH+CD133+ cells. The sphere forming assay was performed with sorted ALDH+, CD133+, ALDH+CD133+ and total cells. Numbers of spheres were expressed as Mean ± SEM, n = 4, derived from 3 different patients. (d) Different morphological appearance of spheres and sphere expansion from different patients. Different sphere appearances were observed from different patients (type A, upper panel, and type B, lower panel). Primary sphere cells formed additional spheres in the long-term culture. Results were shown from two patients. (e) High levels of stem cell core gene transcripts in sphere cells. Real-time PCR was conducted with parental cells and sphere forming cells for stem cell core genes. Results are expressed as the mean values relative to GAPDH ± SD. Three experiments with triplicates, P < 0.01.
Cancer stem cell markers in fresh cancer cells, primary cancer cells and xenograft tumors
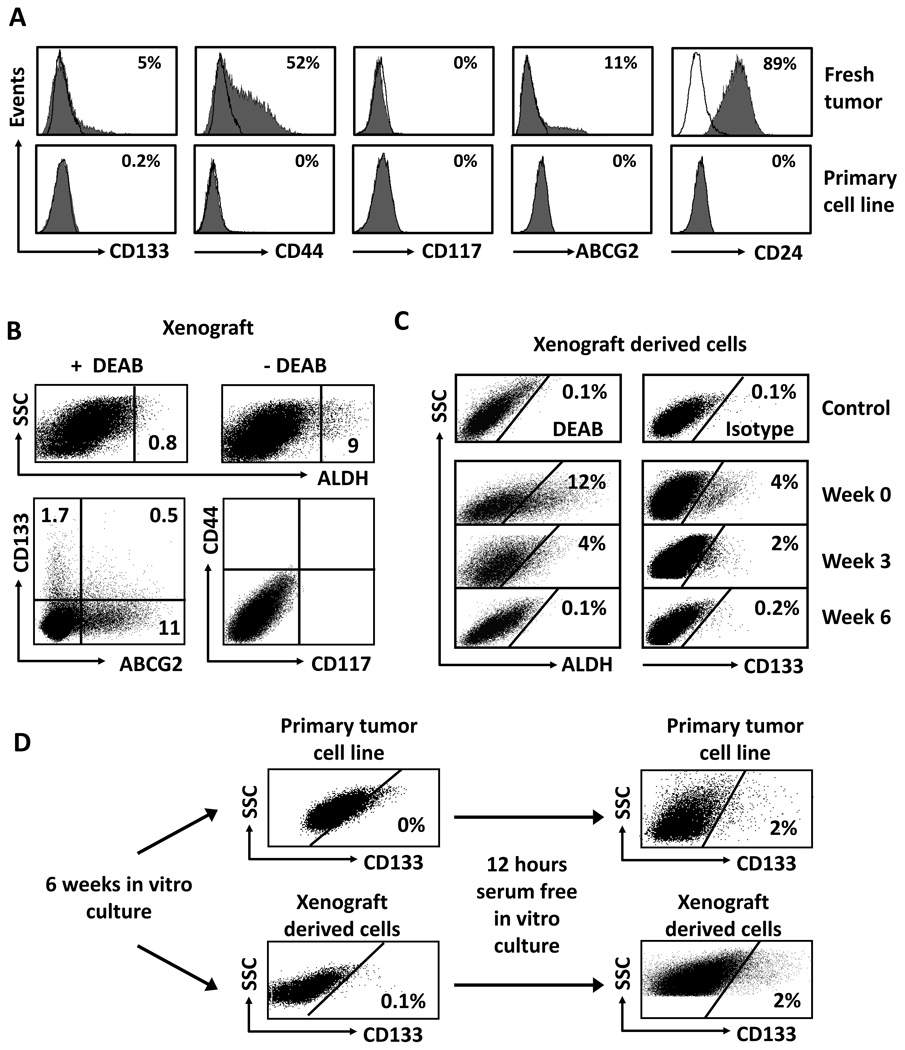
Majority of previous investigators have used established or/and commercialized ovarian cancer cell lines to conduct cancer stem cell research. Our research team has established multiple primary cancer cell lines from ovarian cancer patients. We examined and compared cancer stem cell markers in fresh tumor cells, primary ovarian cancer cell lines, and tumor cells cultured in vitro in different conditions, and tumor cells isolated from xenograft tumors. We observed that fresh OC18 cells contained a significant CD133+ population (Fig. 5a). However, following the conventional culture with 10% fetal calf serum (FCS), the proportion of cells expressing CD133 gradually decreased from 4–5% (Fig. 5a, upper panel) of the total population to 0–0.2% (Fig. 5a, lower panel) after 6 weeks in culture. Similar loss was observed for ALDH expression (not shown). The loss of CD133 expression was universally observed in all primary ovarian cancer cell lines we established. Furthermore, after more than 6–8 weeks in conventional culture, the expression of CD24, CD44, CD117 and ABCG2 was either largely reduced or lost completely as compared to fresh ovarian cancer cells (Fig. 5a).
Figure 5. Cancer stem cell markers in fresh, primary and xenografted ovarian tumor cells.
(a) Cancer stem cell markers in fresh and primary ovarian cancer cell lines. Fresh tumor cells were directly isolated fresh ovarian cancer ascites or tumor tissues. The cells were cultured for 3–6 weeks in conventional culture medium (10% Fcs). The expression of cancer stem cells was determined by FACS. Results were expressed as the percentage of certain stem cell marker positive cells. One of 6 experiments is shown. (b) Cancer stem cell markers in xenograft-derived ovarian cancer cell lines. 5 × 106 cells from the culture of primary ovarian cancer cell lines were injected into NSG mouse to form tumor. Xenograft-derived tumor cells were stained for stem cell markers. Results were expressed as the percent of positive cells in total tumor cells. Human tumor cells in the xenografts were determined by gating on H-2Kb-7-AAD− cells. (c) Cancer stem cell markers in the cultured xenograft-derived ovarian cancer cell lines in conventional culture. Human tumor cells in the xenografts were obtained from xenografts as described (b). The cells were cultured with 10% Fcs from 0–6 weeks. The cultured Xenograft-derived tumor cells were stained for stem cell markers. Results were expressed as the percent of positive cells in total tumor cells. (d). Cancer stem cell markers in the cultured primary and xenograft-derived ovarian cancer cell lines in serum free condition. Primary tumor cells were cultured for 6 weeks in conventional condition (upper panel), and subsequently subject to serum-free culture for 12 hours (upper panel). The cells were stained for stem cell markers. Results were expressed as the percent of positive cells in total tumor cells. Similar experiments were realized with xenograft-derived tumor cells. One of 5 is shown (b–d).
We next investigated whether the lost stem cell markers could be recovered in the in vivo xenograft passages or in vitro serum-free culture. We inoculated the in vitro cultured primary tumor cells (Fig. 5a) into NSG mice. These cells formed tumors in vivo. The tumor cells were subsequently isolated from the NSG mice for multiple stem cell marker analysis (Fig. 5b–d). We found that xenograft-derived tumors expressed appreciable levels of ALDH (Fig. 5b, upper panel), CD133, and ABCG2 (Fig. 5b, lower panel), but negligible levels of CD44 and CD117 (Fig. 5b, lower panel). When the xenograft-derived tumor cells were further cultured under conventional condition with 10% FCS, the expression of ALDH (Fig. 5c, left panel), CD133 (Fig. 5c, right panel) and ABCG2 (not shown) was gradually disappeared. When the primary tumor cells (Fig. 5d, upper panel) and xenograft-derived tumor cells (Fig. 5d, lower panel) were in vitro cultured in serum free conditions, the expression of CD133 was partially and rapidly recovered (Fig. 5d). Similar results were obtained for ALDH expression. However, serum-free and sphere culture did not rescue (induce) the expression of CD24, CD44, ABCG2 and CD117. Altogether, the data indicate that serum free conditions and in vivo tumor transplantation are able to rescue (or induce) the expression of CD133 and ALDH, but not CD44 and CD117.
Discussion
In this study we have examined and compared the expression of multiple cancer stem cell markers in fresh ovarian cancer and established primary ovarian cancer cell lines, and investigated the stem cell properties of potential ovarian cancer stem cells in vitro and in vivo.
We have shown that although the levels of ALDH and CD133 expression are variable, expression is detectable in the majority of fresh ovarian tumors. Consistent with the cancer stem cell concept, ALDH+ and CD133+ cells are able to efficiently form spheres and heterogeneous tumors in vivo with limited numbers of cells. ALDH is thought to be a marker for defining stem cells in multiple human epithelial cancers including breast cancer16, 21, colon cancer19, 20, hepatocellular carcinoma18, head and neck squamous cell carcinoma22 and ovarian cancer23, 24. Based on these reports and current criteria including in vivo tumor formation with limited cells, and sphere formation, we suggest that ALDH+ and CD133+ cells may be enriched with cancer stem cells in the majority of human ovarian cancer.
Although ALDH and CD133 can be used to identify ovarian cancer stem cells in fresh ovarian tumors, the expression of CD133, ALDH and other markers is gradually reduced following prolonged in vitro cell passages. In support of our observation, it has been demonstrated that tumor cells grown under standard serum-containing cell culture conditions result in the loss of tumor stem cells34. The loss of stem cell markers in vitro culture system suggests that the stem cell phenotype or/and properties may possibly need to be supported in vivo in the tumor microenvironment, and that the in vitro conventional culture conditions may not be appropriate for maintaining the cancer stem cell phenotype. In support of this possibility, the expression of cancer stem cell markers CD133 and ALDH is partially recovered in the in vivo formed xenograft tumors and in the in vitro serum free culture. Our results also indicate that the loss of stem cell markers may be reversible. However, it is unknown whether the recovered CD133+ and ALDH+ cells are from original CD133dim and ALDHdim cells (which may not be detectable by current flow cyometry technique), or CD133− and ALDH− cells. It has been suggested that the capacities for self-renewal and tumor initiation may not be restricted to a uniform population of stem-like cells, but can be shared by a lineage of self-renewing cell types35. Further genetic and functional studies are needed to dissect if CD133 and ALDH are functionally and genetically relevant for controlling cancer stem cell properties, and if fresh and induced (or rescued) CD133 and ALDH expressing cells are genetically and functionally distinct36. Interestingly, once the cells are exposed to conventional culture conditions, the expression of CD133 and ALDH rescued (or induced) by the serum-free culture conditions or the in vivo tumor passages gets lost again. Nonetheless, given that the expression of CD133 and ALDH appears within 12 hour in the in vitro serum-free culture, and the appearance of their expression is dependent on the environmental conditions, we speculate that genetic mutations may not be the major cause of driving CD133 and ALDH induction in our experimental conditions. This does not contradict with the notion that the combination of multiple genetic changes or/instability is of fundamental importance in tumorigenesis. Furthermore, fresh and induced CD133 and ALDH expressing cells express high levels of stem cell core genes, efficiently form spheres and in vivo tumors. Altogether, the data support the conclusion that CD133 and ALDH expressing cells are enriched with cancer stem cells, and these cells are important tools for studying ovarian cancer stem cell biology.
In addition to ALDH and CD133, other markers may be used in ovarian cancer stem cell research. ESA is expressed in fresh epithelial ovarian tumor cells. The expression of CD24 and CD44 is highly expressed in many fresh ovarian tumor cells we examined. ABCG2+ and ABCG2− ovarian cancer cells are equally tumorigenic. It has been reported that CD44+ and CD117+ can be used to identify ovarian cancer stem cells12, 37. Our data show that the expression of CD117 is not detectable in more than 50% of fresh ovarian tumors, and in 100% primary ovarian cancer cells established in 10% FCS conventional culture. Furthermore, the loss of CD44 and CD117 expression can’t be rescued by in vivo xenograft tumor passage and in vitro sphere culture. Based on these results, our data suggest that CD133 and ALDH can more accurately identify ovarian cancer stem cells and can be broadly used for ovarian cancer stem cell research in the majority of ovarian cancer. However, given the high heterogeneity of ovarian cancer types, it is important to note that CD44, CD117 and other markers could be used to investigate cancer stem cells in certain ovarian cancer types. Additionally, we have shown that CD133− and ALDH− cells could be tumorigenic in vivo. It also indicates that CD133 and ALDH are not exclusive markers for ovarian cancer stem cells.
The majority of published reports on human ovarian cancer stem cells utilized commercially available established ovarian cancer cell lines, or the unsorted tumors, or “the cells” isolated from the in vitro formed spheres or the mouse xenografts12–15, 37. Macrophages, fibroblasts and many other cells express some stem cell markers including CD44 and CD24. As these cells are substantial populations in the tumor mass and may promote tumorigenesis, it is important to absolutely avoid their contamination in the ovarian cancer stem cell compartments. To this end, based on our multiple color flow cytometry analysis, we have excluded all the possible non-epithelial cell fractions including the immune cells (e.g. macrophages, T cells, and B cells), fibroblasts, vascular endothelial cells and CD34+ progenitors and hematopoietic cells in our studies. We systemically compared multiple stem cell markers, and directly sorted these cells from fresh human ovarian tumors, and investigated their stemness properties, including self-renewal and in vivo tumorigenesis. Given that long-term culture may alter cancer stem cell properties, we have minimized the potential impact of long-term in vitro culture, and experimental manipulation on the properties of ovarian cancer stem cells. One of the key issues in cancer stem cell studies is the regulation of cancer stem cell self-renewal and expansion. These properties are not autonomous to stem cells, and recent evidence points to a level of external control from the microenvironment that defines the stem cell niche38, 39. This may explain why tumor stem cell markers are lost in the in vitro culture and are partially rescued in the in vivo model. Recent studies demonstrate that IL-640, 41 and IL-842 promote cancer stem cell-mediated tumorigenesis in vivo. We suggest that cancer stem cells may renew and expand in the tumor environment in vivo. The next step is to further define the tumor environmental factors and molecular signaling pathway crucial for regulating ovarian cancer stem cell properties.
We conclude that ALDH and CD133 are useful and reliable markers for investigating human ovarian cancer stem cells in the majority of ovarian cancer patients. Our data indicate that the expression levels of multiple stem cell markers gradually diminish following prolonged culture in vitro. Fresh tumor cells are needed to investigate cancer stem cell biology.
Supplementary Material
CD133+, ALDH+, CD133+ALDH+ and their negative counterparts were injected into NSG mice as described in Fig. 1a, b. Tumors formed from these cells were subjected to HE staining. Similar morphology was observed in the tumors formed from different tumor cell populations. One of 6 different patients is shown.
Acknowledgements
We thank to Deborah Postiff in the tissue procurement core for her technical assistance.
Grant sponsor: This research is supported (in part) by the National Institutes of Health grants (5P30 CA46592; CA123088; CA099985; CA156685), the Ovarian Cancer Research Fund, and Marsha Rivkin Center for Ovarian Cancer Research.
Abbreviations
- ALDH
Aldehyde Dehydrogenase
- DEAB
Diethylaminobenzaldehyde
- NSG
nitric oxide dismutate-severe combined immunodeficiency IL-2 receptor γ null mice
Footnotes
Funding Disclosure
We have no financial conflict of interest.
References
- 1.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 2.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 3.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8:467–477. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 4.Zou W, Restifo NP. T(H)17 cells in tumour immunity and immunotherapy. Nat Rev Immunol. 2010;10:248–256. doi: 10.1038/nri2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 6.Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–284. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- 7.Wicha MS. Cancer stem cells and metastasis: lethal seeds. Clin Cancer Res. 2006;12:5606–5607. doi: 10.1158/1078-0432.CCR-06-1537. [DOI] [PubMed] [Google Scholar]
- 8.Wicha MS. Identification of murine mammary stem cells: implications for studies of mammary development and carcinogenesis. Breast Cancer Res. 2006;8:109. doi: 10.1186/bcr1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wicha MS, Liu S, Dontu G. Cancer stem cells: an old idea--a paradigm shift. Cancer Res. 2006;66:1883–1890. doi: 10.1158/0008-5472.CAN-05-3153. discussion 95-6. [DOI] [PubMed] [Google Scholar]
- 10.Huntly BJ, Gilliland DG. Leukaemia stem cells and the evolution of cancer-stem-cell research. Nat Rev Cancer. 2005;5:311–321. doi: 10.1038/nrc1592. [DOI] [PubMed] [Google Scholar]
- 11.Bjerkvig R, Tysnes BB, Aboody KS, Najbauer J, Terzis AJ. Opinion: the origin of the cancer stem cell: current controversies and new insights. Nat Rev Cancer. 2005;5:899–904. doi: 10.1038/nrc1740. [DOI] [PubMed] [Google Scholar]
- 12.Zhang S, Balch C, Chan MW, Lai HC, Matei D, Schilder JM, Yan PS, Huang TH, Nephew KP. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 2008;68:4311–4320. doi: 10.1158/0008-5472.CAN-08-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baba T, Convery PA, Matsumura N, Whitaker RS, Kondoh E, Perry T, Huang Z, Bentley RC, Mori S, Fujii S, Marks JR, Berchuck A, et al. Epigenetic regulation of CD133 and tumorigenicity of CD133+ ovarian cancer cells. Oncogene. 2009;28:209–218. doi: 10.1038/onc.2008.374. [DOI] [PubMed] [Google Scholar]
- 14.Curley MD, Therrien VA, Cummings CL, Sergent PA, Koulouris CR, Friel AM, Roberts DJ, Seiden MV, Scadden DT, Rueda BR, Foster R. CD133 expression defines a tumor initiating cell population in primary human ovarian cancer. Stem Cells. 2009;27:2875–2883. doi: 10.1002/stem.236. [DOI] [PubMed] [Google Scholar]
- 15.Szotek PP, Pieretti-Vanmarcke R, Masiakos PT, Dinulescu DM, Connolly D, Foster R, Dombkowski D, Preffer F, Maclaughlin DT, Donahoe PK. Ovarian cancer side population defines cells with stem cell-like characteristics and Mullerian Inhibiting Substance responsiveness. Proc Natl Acad Sci U S A. 2006;103:11154–11159. doi: 10.1073/pnas.0603672103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG, Liu S, Schott A, Hayes D, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu S, Ginestier C, Charafe-Jauffret E, Foco H, Kleer CG, Merajver SD, Dontu G, Wicha MS. BRCA1 regulates human mammary stem/progenitor cell fate. Proc Natl Acad Sci U S A. 2008;105:1680–1685. doi: 10.1073/pnas.0711613105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma S, Chan KW, Lee TK, Tang KH, Wo JY, Zheng BJ, Guan XY. Aldehyde dehydrogenase discriminates the CD133 liver cancer stem cell populations. Mol Cancer Res. 2008;6:1146–1153. doi: 10.1158/1541-7786.MCR-08-0035. [DOI] [PubMed] [Google Scholar]
- 19.Carpentino JE, Hynes MJ, Appelman HD, Zheng T, Steindler DA, Scott EW, Huang EH. Aldehyde dehydrogenase-expressing colon stem cells contribute to tumorigenesis in the transition from colitis to cancer. Cancer Res. 2009;69:8208–8215. doi: 10.1158/0008-5472.CAN-09-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang EH, Hynes MJ, Zhang T, Ginestier C, Dontu G, Appelman H, Fields JZ, Wicha MS, Boman BM. Aldehyde dehydrogenase 1 is a marker for normal and malignant human colonic stem cells (SC) and tracks SC overpopulation during colon tumorigenesis. Cancer Res. 2009;69:3382–3389. doi: 10.1158/0008-5472.CAN-08-4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Charafe-Jauffret E, Ginestier C, Iovino F, Tarpin C, Diebel M, Esterni B, Houvenaeghel G, Extra JM, Bertucci F, Jacquemier J, Xerri L, Dontu G, et al. Aldehyde dehydrogenase 1-positive cancer stem cells mediate metastasis and poor clinical outcome in inflammatory breast cancer. Clin Cancer Res. 2010;16:45–55. doi: 10.1158/1078-0432.CCR-09-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clay MR, Tabor M, Owen JH, Carey TE, Bradford CR, Wolf GT, Wicha MS, Prince ME. Single-marker identification of head and neck squamous cell carcinoma cancer stem cells with aldehyde dehydrogenase. Head Neck. 2010 doi: 10.1002/hed.21315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Landen CN, Goodman BW, Katre AA, Steg AD, Nick AM, Stone R, Miller L, Vivas-Mejia PE, Jennings NB, Gershenson DM, Bast RC, Jr, Coleman RL, et al. Targeting Aldehyde Dehydrogenase Cancer Stem Cells in Ovarian Cancer. Mol Cancer Ther. 2010 doi: 10.1158/1535-7163.MCT-10-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deng S, Yang X, Lassus H, Liang S, Kaur S, Ye Q, Li C, Wang LP, Roby KF, Orsulic S, Connolly DC, Zhang Y, et al. Distinct expression levels and patterns of stem cell marker, aldehyde dehydrogenase isoform 1 (ALDH1), in human epithelial cancers. Plos One. 2010;5:e10277. doi: 10.1371/journal.pone.0010277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kryczek I, Wei S, Zou L, Altuwaijri S, Szeliga W, Kolls J, Chang A, Zou W. Cutting Edge: Th17 and Regulatory T Cell Dynamics and the Regulation by IL-2 in the Tumor Microenvironment. J Immunol. 2007;178:6730–6733. doi: 10.4049/jimmunol.178.11.6730. [DOI] [PubMed] [Google Scholar]
- 26.Kryczek I, Wei S, Vatan L, Escara-Wilke J, Szeliga W, Keller ET, Zou W. Cutting Edge: Opposite Effects of IL-1 and IL-2 on the Regulation of IL-17+ T Cell Pool IL-1 Subverts IL-2-Mediated Suppression. J Immunol. 2007;179:1423–1426. doi: 10.4049/jimmunol.179.3.1423. [DOI] [PubMed] [Google Scholar]
- 27.Kryczek I, Zou L, Rodriguez P, Zhu G, Wei S, Mottram P, Brumlik M, Cheng P, Curiel T, Myers L, Lackner A, Alvarez X, et al. B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J Exp Med. 2006;203:871–881. doi: 10.1084/jem.20050930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 29.Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, Krzysiek R, Knutson KL, Daniel B, Zimmermann MC, David O, Burow M, et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003;9:562–567. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 30.Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 31.Dalerba P, Dylla SJ, Park IK, Liu R, Wang X, Cho RW, Hoey T, Gurney A, Huang EH, Simeone DM, Shelton AA, Parmiani G, et al. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci U S A. 2007;104:10158–10163. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang EH, Heidt DG, Li CW, Simeone DM. Cancer stem cells: a new paradigm for understanding tumor progression and therapeutic resistance. Surgery. 2007;141:415–419. doi: 10.1016/j.surg.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 33.Dontu G, Al-Hajj M, Abdallah WM, Clarke MF, Wicha MS. Stem cells in normal breast development and breast cancer. Cell Prolif. 2003;36 Suppl 1:59–72. doi: 10.1046/j.1365-2184.36.s.1.6.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee J, Kotliarova S, Kotliarov Y, Li A, Su Q, Donin NM, Pastorino S, Purow BW, Christopher N, Zhang W, Park JK, Fine HA. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9:391–403. doi: 10.1016/j.ccr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 35.Chen R, Nishimura MC, Bumbaca SM, Kharbanda S, Forrest WF, Kasman IM, Greve JM, Soriano RH, Gilmour LL, Rivers CS, Modrusan Z, Nacu S, et al. A hierarchy of self-renewing tumor-initiating cell types in glioblastoma. Cancer Cell. 2010;17:362–375. doi: 10.1016/j.ccr.2009.12.049. [DOI] [PubMed] [Google Scholar]
- 36.Curtis SJ, Sinkevicius KW, Li D, Lau AN, Roach RR, Zamponi R, Woolfenden AE, Kirsch DG, Wong KK, Kim CF. Primary tumor genotype is an important determinant in identification of lung cancer propagating cells. Cell Stem Cell. 2010;7:127–133. doi: 10.1016/j.stem.2010.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alvero AB, Chen R, Fu HH, Montagna M, Schwartz PE, Rutherford T, Silasi DA, Steffensen KD, Waldstrom M, Visintin I, Mor G. Molecular phenotyping of human ovarian cancer stem cells unravels the mechanisms for repair and chemoresistance. Cell Cycle. 2009;8:158–166. doi: 10.4161/cc.8.1.7533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bendall SC, Stewart MH, Menendez P, George D, Vijayaragavan K, Werbowetski-Ogilvie T, Ramos-Mejia V, Rouleau A, Yang J, Bosse M, Lajoie G, Bhatia M. IGF and FGF cooperatively establish the regulatory stem cell niche of pluripotent human cells in vitro. Nature. 2007;448:1015–1021. doi: 10.1038/nature06027. [DOI] [PubMed] [Google Scholar]
- 39.Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441:1075–1079. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]
- 40.Sansone P, Storci G, Tavolari S, Guarnieri T, Giovannini C, Taffurelli M, Ceccarelli C, Santini D, Paterini P, Marcu KB, Chieco P, Bonafe M. IL-6 triggers malignant features in mammospheres from human ductal breast carcinoma and normal mammary gland. J Clin Invest. 2007;117:3988–4002. doi: 10.1172/JCI32533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gao SP, Mark KG, Leslie K, Pao W, Motoi N, Gerald WL, Travis WD, Bornmann W, Veach D, Clarkson B, Bromberg JF. Mutations in the EGFR kinase domain mediate STAT3 activation via IL-6 production in human lung adenocarcinomas. J Clin Invest. 2007;117:3846–3856. doi: 10.1172/JCI31871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ginestier C, Liu S, Diebel ME, Korkaya H, Luo M, Brown M, Wicinski J, Cabaud O, Charafe-Jauffret E, Birnbaum D, Guan JL, Dontu G, et al. CXCR1 blockade selectively targets human breast cancer stem cells in vitro and in xenografts. J Clin Invest. 2010;120:485–497. doi: 10.1172/JCI39397. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CD133+, ALDH+, CD133+ALDH+ and their negative counterparts were injected into NSG mice as described in Fig. 1a, b. Tumors formed from these cells were subjected to HE staining. Similar morphology was observed in the tumors formed from different tumor cell populations. One of 6 different patients is shown.