Abstract
Setting
A community-based voluntary counseling and testing (VCT) center in Moshi, Tanzania.
Objective
To compare rates of prior human immunodeficiency virus (HIV) testing among clients with and without previous tuberculosis (TB) treatment, and HIV seropositivity among those with and without current TB symptoms.
Design
Cross-sectional study of consecutive clients presenting for initial testing; sociodemographic and clinical data were collected via a structured questionnaire. HIV status was compared among clients with or without three or more TB-related symptoms: weight loss, fever, cough, hemoptysis or night sweats.
Results
Overall, 225 (3%) of 6583 VCT clients who responded to questions on previous TB treatment reported a history of TB, but only 34 (15%) reported previous HIV testing. This rate of HIV testing was not different from the rate among those clients without a history of TB (OR 0.77, P = 0.175). One hundred thirty-five (61%) clients with a history of TB were HIV-infected at VCT, compared with 17% of all clients. Of the total 6592 first-time testers who responded, 372 (6%) had at least three symptoms suggestive of TB at VCT. These symptoms were strongly associated with HIV seropositivity (OR 16.30, P < 0.001).
Conclusion
Missed opportunities for HIV diagnosis at the time of TB treatment appear frequent in this population, underscoring the need for integration of TB and HIV diagnostic services.
Keywords: tuberculosis, HIV, voluntary counseling and testing, Tanzania
The Global Burden of tuberculosis (TB) has increased dramatically in the past two decades, particularly in Africa. From 1990 to 2006, the estimated incidence of TB in the World Health Organization (WHO) Africa region grew from 829 000 to 2.8 million.1 In the East African country of Tanzania, TB prevalence has more than doubled since 1990. As in the rest of sub-Saharan Africa, this increase is coupled with the human immunodeficiency virus (HIV) epidemic in Tanzania, where HIV prevalence is estimated to be 6.2%.2 In one study of patients hospitalized from 1996 to 2001 in a large referral hospital in northern Tanzania, TB was the leading cause of hospitalization among HIV-infected patients.3
The HIV epidemic and the global burden of TB are closely intertwined. Approximately one third of HIV-infected persons worldwide are co-infected with Mycobacterium tuberculosis.4 HIV greatly increases the likelihood of both reactivating latent TB and progressing to active TB after primary infection. Globally, it is the most significant risk factor for developing active disease.5 TB is the most common cause of death in HIV-infected patients in the developing world, and it accelerates progression of the clinical course of HIV infection, leading to additional opportunistic infections and death.4,6,7
The onset of active TB is often the first manifestation of HIV infection. Recognizing this sentinel event has become critical, as access to antiretroviral therapy (ART) has increased in resource-limited settings. The need to better integrate HIV and TB services has been well documented.4,5,8–11 Understanding this need, Tanzania declared TB a national emergency in 2006, and has since developed national guidelines for collaborative TB and HIV activities, including a national policy of counseling and testing for HIV in all TB patients. WHO data show that rates of HIV testing among TB patients in sub-Saharan Africa, including Tanzania, have been quite low, but are improving.1,12 Outside of TB programs, clinicians and, anecdotally, individuals in the community are well aware of the interaction between TB and HIV. Although HIV counseling and testing is available at public and private hospitals and clinics, as well as at separate voluntary counseling and testing (VCT) centers, we found a paucity of data on HIV testing among TB patients in these other settings.
We conducted a cross-sectional study in collaboration with a community-based VCT organization and quantified the frequency with which clients with a history of active TB had previously been HIV tested. Our primary hypothesis was that clients with a history of active TB would have higher rates of prior HIV testing compared with VCT clients without a previous TB diagnosis. A secondary hypothesis was that VCT clients with current symptoms suggestive of TB would be more likely to be HIV-seropositive. We also explored other characteristics associated with previous HIV testing.
Study Population And Methods
Location and context
Participants were recruited through the VCT program at Kikundi cha Wanawake Kilimanjaro Kupambana na UKIMWI (KIWAKKUKI, Women Against AIDS in Kilimanjaro) in Moshi, Tanzania. KIWAKKUKI is an HIV/AIDS (acquired immune-deficiency syndrome) advocacy organization that provides education, VCT, home-based HIV/AIDS care services, and support to AIDS orphans in the Kilimanjaro region of Tanzania. The VCT program began in March 2003 and provides free VCT to adults and children.13 It is staffed by paid and volunteer counselors who are trained according to Tanzania Ministry of Health (MoH) guidelines.14 Data were collected between November 2003 and January 2008.
Procedures
All persons presenting for VCT received standard pretest counseling in accordance with Tanzania MoH guidelines. Those accepting VCT provided a 2 ml blood sample for HIV testing. Following pre-test counseling and blood sample collection, individuals aged ≥18 years were approached for study participation. Those providing informed consent were administered a structured questionnaire that collected information on sociodemographic, clinical and behavioral characteristics. Clinical information collected included symptoms in the preceding 2 or 3 months (such as weight loss, fever, cough, hemoptysis and night sweats), history of TB treatment, and month and year of TB treatment, if applicable. Clients were also asked if they had been tested previously for HIV, and the date, if applicable. Responses were recorded on paper questionnaires.
All blood samples were tested with both Capillus (Trinity Biotech PLC, Bray, Ireland) and Determine (Abbott Laboratories, Abbott Park, IL, USA) rapid HIV 1/2 antibody tests. Contradictory results were resolved with Vironostika HIV-1 microenzyme-linked immunosorbent assay (microELISA) assay (Organon Teknika, Charlotte, NC, USA) at the regional or zonal referral hospital. Furthermore, for quality assurance, one of every 20 samples was tested at the regional or zonal referral hospital with the Vironostika HIV-1 microELISA assay. All clients received results and appropriate post-test counseling in accordance with national guidelines, and clients testing positive were referred to KIWAKKUKI-based support services and the HIV Care and Treatment Centre at the regional or zonal referral hospital. Clients reporting three or more symptoms of active TB were referred to the District TB clinic. Data on client compliance with referrals, confirmed TB diagnoses and further treatment at HIV and TB centers were not collected.
Statistical analysis
We performed double data entry using Epi Info v3.3.2 (Centers for Disease Control and Prevention, Atlanta, GA, USA) for data collected from November 2003 to January 2006. In February 2006, we introduced the electronic Verity TeleForm v9.1 data entry system (CAYLX Software [Pacific] Pty Limited, Belrose, NSW, Australia) and merged the two databases. We developed and followed standard operating procedures and quality checks to ensure accurate data entry.
Clients testing for the first time at KIWAKKUKI who completed the study questionnaire were identified and selected for analysis (‘first-time’ testers). History of TB was determined by reported history or date of treatment. Clients who did not respond to these questions were excluded from subsequent analyses. To calculate time since TB treatment, a default date of July 1 was assigned to those clients who provided only the year of TB treatment. History of previous HIV test was also determined by reported history or date of test.
Analysis with Pearson χ2 was used to evaluate the relationship between a history of TB treatment and previous HIV testing, as well as the relationship between HIV seropositivity and reporting three or more symptoms of active TB at VCT Multivariate analysis was used to assess characteristics associated with previous HIV testing, including testing before and after 1 September 2004—the beginning of free ART in Tanzania. Also included was a ‘risk index’, for which one point was given for each of the following: sexual debut <16 years of age, >2 lifetime sexual partners, having concurrent partners in the past year, having a partner with other partners, and having ever exchanged gifts or money for sex. All data were analyzed using JMP 7.0 (SAS Institute, Cary, NC, USA).
Research ethics
This study was approved by the Institutional Review Board of Duke University Medical Center, the Research Ethics Committee of Kilimanjaro Christian Medical Centre, and the Medical Research Coordinating Committee of the Tanzania National Institute of Medical Research.
Results
Characteristics of VCT clients
Between November 2003 and January 2008, 6632 first-time testers were identified. Of these, 6583 (99%) responded to questions regarding previous TB treatment (Table 1). Sociodemographic characteristics did not differ significantly between all first-time testers and those who responded to TB questions only (all P > 0.96). Among the 6583 clients, the median age was 30 years (range, 18-87). Female clients numbered 3639 (55%), and 3040 (47%) participants lived in the more urban town of Moshi (population 143 799), while the rest lived in more rural towns and villages in the region (total population 1 381 149).15
Table 1. Characteristics of clients presenting for VCT at KIWAKKUKI, Moshi, Tanzania, November 2003–January 2008.
| All first-time testers* (N = 6583) n (%) |
First-time testers with TB history (n = 225) n (%) |
P value† | |
|---|---|---|---|
| Sex | |||
| Male | 2927 (45) | 109 (48) | 0.251 |
| Female | 3639 (55) | 116 (52) | |
| Age, years | |||
| <25 | 2054 (31) | 27 (12) | <0.001‡ |
| 25–34 | 2354 (36) | 85 (38) | 0.556 |
| 35–44 | 1216 (19) | 67 (30) | <0.001‡ |
| >44 | 939 (14) | 46 (20) | 0.010‡ |
| Residence | |||
| Town | 3040 (47) | 95 (43) | 0.182 |
| Village | 3409 (53) | 128 (57) | |
| Marital status | |||
| Single | 3192 (49) | 71 (32) | <0.001‡ |
| Cohabiting | 562 (9) | 22 (10) | 0.520 |
| Married | 1570 (24) | 68 (30) | 0.029‡ |
| Divorced/separated | 589 (9) | 23 (10) | 0.517 |
| Widowed | 627 (10) | 40 (18) | <0.001‡ |
| Education | |||
| None/primary (1–7 years) | 4395 (67) | 175 (78) | <0.001‡ |
| Occupation | |||
| Business | 1841 (28) | 71 (32) | 0.183 |
| Farming | 1390 (21) | 69 (32) | <0.001‡ |
| Salaried worker | 918 (14) | 19 (9) | 0.022‡ |
| Skilled worker | 623 (10) | 18 (9) | 0.502 |
| Unskilled worker | 472 (7) | 15 (7) | 0.820 |
| Student | 553 (8) | 4 (2) | <0.001‡ |
| Weekly expenses§ | |||
| 0–3000 Tsh | 1123 (18) | 43 (20) | 0.523 |
| 3000–7000 Tsh | 1292 (21) | 40 (18) | 0.390 |
| 7000–10 000 Tsh | 1319 (21) | 48 (22) | 0.766 |
| >10 000 Tsh | 2494 (40) | 87 (40) | 0.968 |
Excludes first-time testers who did not respond to questions about TB history.
P for χ2
P significant, i.e., <0.05.
Tanzanian minimum wage = 65 000 Tsh/month.
VCT = voluntary counseling and testing; KIWAKKUKI = Kikundi cha Wanawake Kilimanjaro Kupambana na UKIMWI (Women Against AIDS in Kilimanjaro); TB = tuberculosis; Tsh = Tanzanian shillings.
Of these 6583 clients, 225 (3%) reported a history of TB. Compared with other first-time testers, clients with a TB history did not differ significantly by sex, residence or weekly expenses. They were, however, more likely to be older, married or widowed, farmers or unemployed, and less likely to be single, salaried workers or students, or to have more than a primary education (Table 1). Of 194 clients reporting a TB treatment date, 59 (30%) had been treated in the previous year (±6 months), and the median time since treatment was 35 months; 125 (64%) participants were treated in the previous 5 years (±6 months).
Rates of previous HIV testing
Contrary to our primary hypothesis, participants with a history of TB were no more likely than those without a history of TB to report previous HIV testing (Table 2). There was also no discernible improvement over time, despite the publication of the WHO interim guidelines for collaborative TB-HIV activities in 2004.8 The rate of previous HIV testing among clients treated for TB before 2004 was 19%, compared with 9% among those treated in 2004 or later, demonstrating no trend toward improvement (P = 0.067). Strikingly, 61% of clients with a history of TB were HIV-positive at this VCT encounter compared with 17% of those without a TB history (P < 0.001). Of the 135 clients with a TB history who were HIV-positive, only 18 (13%) reported previous HIV testing.
Table 2. Rates of previous HIV testing and HIV seropositivity among first-time testers presenting for VCT at KIWAKKUKI, Moshi, Tanzania, November 2003–January 2008.
| Clients without TB history* (n = 6312) n (%) |
Clients with TB history* (n = 223) n (%) |
OR | 95%CI | P value | |
|---|---|---|---|---|---|
| Reported previous HIV test | 1190 (19) | 34 (15) | 0.77 | 0.53–1.12 | 0.175 |
| HIV-positive at current VCT | 1074 (17) | 135 (61) | 7.48 | 5.67–9.86 | <0.001 |
Excludes participants who did not respond to questions about previous HIV testing.
HIV = human immunodeficiency virus; VCT = voluntary counseling and testing; KIWAKKUKI = Kikundi cha Wana wake Kilimanjaro Kupambana na UKIMWI (Women Against AIDS in Kilimanjaro); TB = tuberculosis; OR = odds ratio; CI = confidence interval.
Characteristics associated with previous HIV testing
Although a history of TB was not associated with previous HIV testing, several characteristics were (Table 3). These were older age, residence in town, more than primary education, married, weekly expenses >7000 Tanzanian shillings, risk index >2 and testing prior to the introduction of free ART. Among clients with a history of TB, the only factor significantly associated with previous HIV testing was a risk index >2.
Table 3. Characteristics associated with having had a previous HIV test among clients presenting for VCT at KIWAKKUKI, Moshi, Tanzania, November 2003–January 2008*.
| All first-time clients† (n = 5967) |
First-time clients with TB history (n = 211) |
|||
|---|---|---|---|---|
| OR | P value | OR | P value | |
| Female | 0.88 | 0.082 | 0.84 | 0.686 |
| Age >30 years | 0.84‡ | 0.028 | 1.40 | 0.527 |
| Lives in town | 1.26‡ | 0.001 | 0.82 | 0.639 |
| Married | 1.54§ | <0.001 | 1.64 | 0.253 |
| Primary education or less | 0.70§ | <0.001 | 0.42 | 0.064 |
| Weekly expenses >7000 Tsh | 1.61§ | <0.001 | 1.32 | 0.521 |
| Risk index >2 | 1.42§ | <0.001 | 3.08‡ | 0.017 |
| Tested prior to free ART | 2.54§ | <0.001 | 2.12 | 0.062 |
| History of TB treatment | 0.80 | 0.249 | — | — |
Results from multivariate logistic regression for factors associated with history of previous HIV test.
Includes only clients who responded to questions about all characteristics.
P < 0.05.
P < 0.001.
HIV = human immunodeficiency virus; VCT = voluntary counseling and testing; KIWAKKUKI = Kikundi cha Wanawake Kilimanjaro Kupambana na UKIMWI (Women Against AIDS in Kilimanjaro); OR = odds ratio; Tsh = Tanzanian shillings; ART = antiretroviral therapy; TB = tuberculosis.
Symptoms of active TB among VCT clients
Of all first-time testers, 6592 answered questions about symptoms in the past 2 or 3 months; 371 (6%) reported at least three symptoms of active TB during this period. This was strongly associated with HIV seropositivity (74% vs. 15%, among those reporting 0–2 symptoms, P < 0.001, odds ratio [OR] 16.30, 95% confidence interval [CI] 12.78–20.79; Figure).
Figure.
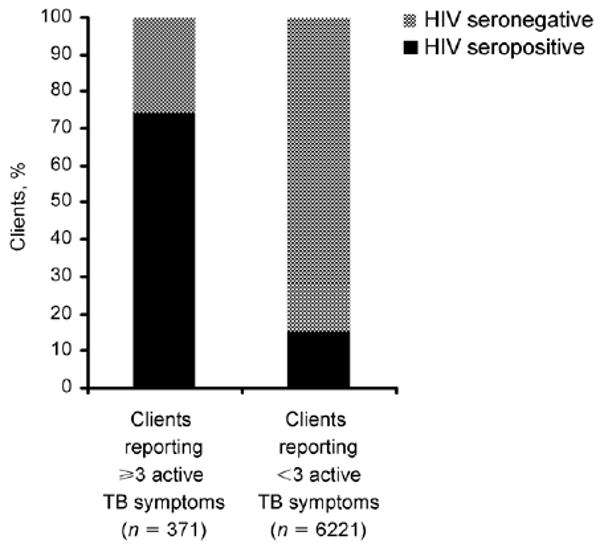
HIV serostatus among clients presenting for VCT stratified by number of active TB symptoms, Moshi, Tanzania, November 2003–January 2008. HIV = human immunodeficiency virus; TB = tuberculosis; VCT = voluntary counseling and testing.
Discussion
Sixty per cent of our clients with a history of TB were found to be HIV-positive at their VCT encounter. As the majority of clients with a history of TB reported treatment in the previous 5 years, it is highly likely that these TB cases were related to early, undiagnosed, underlying HIV infection. Because our study was not population- or TB clinic-based, we cannot determine how many individuals with TB were tested for HIV at the time of their TB diagnosis. What we can say is that, among individuals with previous TB who found their way to KIWAKUKKI, previous HIV testing was no more common than testing in the adult Tanzanian population at large.16 This finding is contrary to our original hypothesis and confirms the need to strengthen capacity within TB programs to reduce missed opportunities for diagnosis. Our study was not designed to measure the consequences of missed HIV diagnoses over the ensuing time period, but future population-based studies should address these, including estimates of ongoing disease spread and attributable morbidity and mortality among infected adults and children born to infected women.
The reason for low rates of previous HIV testing despite months of treatment for TB is not known. WHO data are similar; in 2006, only 11% of reported TB cases in Tanzania were HIV-tested, although 50% of those tested were HIV-infected.1 These rates may be improving; in 2007, collaborative TB-HIV activities were expanded to the entire country, followed by a ‘massive’ increase in the 2008 TB control budget and funding.1 Prior to these increased activities, VCT may not have been readily accessible at TB programs or not offered free of charge, although data for this are not available. Patients may have been referred for testing but did not comply due to the stigma associated with both TB and HIV. Rates of VCT acceptance among TB patients have been high in most other settings: 92% in Côte d'Ivoire, 91% in Malawi and Guyana, 84% in Ukraine, and 83% in London, UK, although rates were 59% in a second study in Malawi and only 35% in southern Ethiopia.17–23 This suggests that, were VCT offered, the majority of patients would have accepted it. Rates of refusal in Tanzania, however, may be higher. VCT was free of charge in Côte d'Ivoire, Malawi, Guyana, and London (not clarified in Ukraine and Ethiopia), but has not always been free in Tanzania. Also, VCT was available at all study sites; Tanzanian data regarding this are unavailable. In addition to access and fees, TB patients may decline HIV testing for other reasons. In London, the most common reason for refusal was perceived inability to cope with a second diagnosis.21 This was also identified as a potential barrier to VCT uptake in South Africa, along with fear of AIDS-related stigma, lack of partner's consent, asymptomatic or incurable disease, and uncertainty about ART eligibility while still receiving TB treatment.24
This study also investigated the need for TB referral among VCT clients. Previous studies in this cohort demonstrated a strong association between HIV seropositivity and both previous TB treatment and symptoms consistent with active TB.13 The present study confirmed these associations in a much larger population. These findings are not surprising, and they underscore the need for integration of HIV and TB services. They also support the utility of VCT in TB case finding, which has been promoted by the WHO ProTEST initiative25 and shown to be successful and cost-effective in other settings.26–28
Our study has several limitations, most notably referral bias. Individuals previously tested in the TB program and found to have HIV would not need to come to a VCT center. However, this does not negate the fact that 3% of our VCT population had a history of active TB, resulting in an extraordinarily high TB ‘case rate’ of 3000/100 000 population, and 6% had symptoms highly suggestive of TB. Furthermore, both groups had astonishingly high rates of HIV co-infection—respectively 61% and 74%. At least at this time, VCT centers may thus be serving an important stop-gap function pending adequate funding for TB-HIV program integration.
Another limitation was reliance on reported history of both TB disease and HIV testing, which may be inaccurate. Finally, although our study is prospectively collecting data about individuals coming for VCT, we were not able to investigate the availability of HIV testing at the time of TB diagnosis or the reasons for accepting or declining testing at that time. Furthermore, integration of HIV testing into TB programs in Tanzania may be improving. Future studies are important to more accurately determine rates of HIV testing among TB patients in the Kilimanjaro region of Tanzania. Likewise, it would be beneficial to examine the success of referring clients with symptoms of active TB to chest clinics and their ultimate diagnosis and treatment.
The integration of TB and HIV diagnosis is an essential component of care and prevention for both TB and HIV/AIDS. Efforts are already underway throughout Tanzania and sub-Saharan Africa to facilitate integration. Initial studies indicate that this can be successful, particularly provider-initiated HIV testing and counseling for TB patients.29–32 Barriers to VCT access and uptake among TB patients should continue to be investigated and addressed, and integration of TB and HIV activities should continue to be a top priority for both local and national organizations.
Acknowledgments
The authors are very grateful to the study participants and the staff and volunteers of the KIWAKKUKI AIDS Information Centre, especially the VCT counselors. This study was funded in part by Roche Laboratories. Additional support was obtained from the AIDS Clinical Trials Group (U01 AI-39156) and the Mid-career Investigator Award (K24 AI-0744-01) from the National Institute of Allergy and Infectious Diseases (JAB), NIAID K24 AI-001833-01 (CDH), the US Department of State Fulbright Program (NMT), National Institute for Health (NIH) awards International Studies on AIDS Associated Co-infections (U01 AI062563-04; CDH, JAC, JFS, JAB and NMT), a Fogarty International Center AIDS International Training and Research Program (D43 PA-03-018; JAC, JFS, JAB, and NMT and EMN), and the Duke Clinical Trials Unit and Clinical Research Sites (U01 AI069484-01; JAC, JAB, JFS and NMT). JAB is also supported by the Duke University Center for AIDS Research, an NIH-funded program (P30 AI 64518). CDH, JAB, JAC and NMT all receive support from the National Institute of Allergy and Infectious Diseases-supported International Studies of AIDS Associated Co-infections (ISAAC) project. ACT was supported by the Hubert-Yeargan Center for Global Health at Duke University Medical Center, and by her research mentor, CDH.
This article was presented in part at the 44th Annual Meeting of the Infectious Diseases Society of America, Toronto, Ontario, Canada, 12–15 October 2006 (Abstract 897).
References
- 1.World Health Organization. WHO report 2008 WHO/HTM/TB/2008.393. Geneva, Switzerland: WHO; 2008. Global tuberculosis control: surveillance, planning, financing. [Google Scholar]
- 2.Joint United Nations Programme on HIV/AIDS. 2008 Report on the global AIDS epidemic: United Republic of Tanzania epidemiological fact sheet. Geneva, Switzerland: UNAIDS; 2008. [Google Scholar]
- 3.Ole-Nguyaine S, Crump JA, Kibiki GS, et al. HIV-associated morbidity, mortality and diagnostic testing opportunities among inpatients at a referral hospital in northern Tanzania. Ann Trop Med Parasitol. 2004;98:171–179. doi: 10.1179/000349804225003163. [DOI] [PubMed] [Google Scholar]
- 4.Corbett EL, Watt CJ, Walker N, et al. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch Intern Med. 2003;163:1009–1021. doi: 10.1001/archinte.163.9.1009. [DOI] [PubMed] [Google Scholar]
- 5.Sharma SK, Mohan A, Kadhiravan T. HIV-TB co-infection: epidemiology, diagnosis and management. Indian J Med Res. 2005;121:550–567. [PubMed] [Google Scholar]
- 6.Whalen C, Horsburgh CR, Hom D, Lahart C, Simberkoff M, Ellner J. Accelerated course of human immunodeficiency virus infection after tuberculosis. Am J Respir Crit Care Med. 1995;151:129–135. doi: 10.1164/ajrccm.151.1.7812542. [DOI] [PubMed] [Google Scholar]
- 7.Toossi Z. Virological and immunological impact of tuberculosis on human immunodeficiency virus type 1 disease. J Infect Dis. 2003;188:1146–1155. doi: 10.1086/378676. [DOI] [PubMed] [Google Scholar]
- 8.Stop TB Department and Department of HIV/AIDS, World Health Organization. WHO/HTM/TB/2004.330; WHO/HTM/HIV/2004.1. Geneva, Switzerland: WHO; 2004. Interim policy on collaborative TB/HIV activities. [Google Scholar]
- 9.Reid A, Scano F, Getahun H, et al. Towards universal access to HIV prevention, treatment, care, and support: the role of tuberculosis/HIV collaboration. Lancet Infect Dis. 2006;6:483–495. doi: 10.1016/S1473-3099(06)70549-7. [DOI] [PubMed] [Google Scholar]
- 10.Hopewell PC, Pai M, Maher D, Uplekar M, Raviglione MC. International standards for tuberculosis care. Lancet Infect Dis. 2006;6:710–725. doi: 10.1016/S1473-3099(06)70628-4. [DOI] [PubMed] [Google Scholar]
- 11.Gunneberg C, Reid A, Williams BG, Floyd K, Nunn P. Global monitoring of collaborative TB-HIV activities. Int J Tuberc Lung Dis. 2008;12(Suppl 1):S2–S7. [PubMed] [Google Scholar]
- 12.World Health Organization. WHO report 2007 WHO/HTM/TB/2008.376. Geneva, Switzerland: World Health Organization; 2007. Global tuberculosis control: surveillance, planning, financing. [Google Scholar]
- 13.Chu HY, Crump JA, Ostermann J, et al. Sociodemographic and clinical characteristics of clients presenting for HIV voluntary counselling and testing in Moshi, Tanzania. Int J STD AIDS. 2005;16:691–696. doi: 10.1258/095646205774357307. [DOI] [PubMed] [Google Scholar]
- 14.Tanzania Ministry of Health. National guidelines for HIV voluntary counselling and testing, 2005. Dar Es Salaam, Tanzania: National AIDS Control Program, Tanzania MoH; 2005. [Google Scholar]
- 15.United Republic of Tanzania. 2002 Population and housing census. Dar es Salaam, Tanzania: National Bureau of Statistics Tanzania; 2002. [Google Scholar]
- 16.Tanzania Commission for AIDS (TACAIDS) National Bureau of Statistics (NBS), ORC Macro. Tanzania HIV/AIDS indicator survey 2003-04. Calverton, MD, USA: TACAIDS, NBS and ORC Macro; 2005. [Google Scholar]
- 17.Abouya L, Coulibaly IM, Wiktor SZ, et al. The Cote d'Ivoire national HIV counseling and testing program for tuberculosis patients: implementation and analysis of epidemiologic data. AIDS. 1998;12:505–512. doi: 10.1097/00002030-199805000-00012. [DOI] [PubMed] [Google Scholar]
- 18.Zachariah R, Spielmann MP, Harries AD, Salaniponi FL. Voluntary counselling, HIV testing and sexual behaviour among patients with tuberculosis in a rural district of Malawi. Int J Tuberc Lung Dis. 2003;7:65–71. [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention. HIV counseling, testing and care of tuberculosis patients at chest clinics—Guyana, 2005–2006. MMWR. 2006;55:849–851. [PubMed] [Google Scholar]
- 20.van der Werf MJ, Yegorova OB, Chechulin Y, Hasker E, Veen J, Turchenko LV. HIV testing practices of TB patients after introduction of a new testing policy in Kiev City, Ukraine. Int J Tuberc Lung Dis. 2005;9:733–739. [PubMed] [Google Scholar]
- 21.Dart S, Alder D, Mamdani M, et al. HIV testing in TB clinics: a problem in practice? Thorax. 2006;61:271–272. doi: 10.1136/thx.2005.048066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chimzizi RB, Harries AD, Manda E, Khonyongwa A, Salaniponi FM. Counselling, HIV testing and adjunctive cotrimoxazole for TB patients in Malawi: from research to routine implementation. Int J Tuberc Lung Dis. 2004;8:938–944. [PubMed] [Google Scholar]
- 23.Jerene D, Endale A, Lindtjorn B. Acceptability of HIV counselling and testing among tuberculosis patients in south Ethiopia. BMC Int Health Hum Rights. 2007;7:4. doi: 10.1186/1472-698X-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daftary A, Padayatchi N, Padilla M. HIV testing and disclosure: a qualitative analysis of TB patients in South Africa. AIDS Care. 2007;19:572–577. doi: 10.1080/09540120701203931. [DOI] [PubMed] [Google Scholar]
- 25.Godfrey-Faussett P, Maher D, Mukadi YD, Nunn P, Perriens J, Raviglione M. How human immunodeficiency virus voluntary testing can contribute to tuberculosis control. Bull World Health Organ. 2002;80:939–945. [PMC free article] [PubMed] [Google Scholar]
- 26.Godfrey-Faussett P, Ayles H. Can we control tuberculosis in high HIV prevalence settings? Tuberculosis (Edinb) 2003;83:68–76. doi: 10.1016/s1472-9792(02)00083-5. [DOI] [PubMed] [Google Scholar]
- 27.Mugisha B, Bock N, Mermin J, et al. Tuberculosis case finding and preventive therapy in an HIV voluntary counseling and testing center in Uganda. Int J Tuberc Lung Dis. 2006;10:761–767. [PubMed] [Google Scholar]
- 28.Hausler HP, Sinanovic E, Kumaranayake L, et al. Costs of measures to control tuberculosis/HIV in public primary care facilities in Cape Town, South Africa. Bull World Health Organ. 2006;84:528–536. doi: 10.2471/blt.04.018606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gasana M, Vandebriel G, Kabanda G, et al. Integrating tuberculosis and HIV care in rural Rwanda. Int J Tuberc Lung Dis. 2008;12(Suppl 1):S39–S43. [PMC free article] [PubMed] [Google Scholar]
- 30.Harris JB, Hatwiinda SM, Randels KM, et al. Early lessons from the integration of tuberculosis and HIV services in primary care centers in Lusaka, Zambia. Int J Tuberc Lung Dis. 2008;12:773–779. [PubMed] [Google Scholar]
- 31.Odhiambo J, Kizito W, Njoroge A, et al. Provider-initiated HIV testing and counselling for TB patients and suspects in Nairobi, Kenya. Int J Tuberc Lung Dis. 2008;12(Suppl 1):S63–S68. [PubMed] [Google Scholar]
- 32.Van Rie A, Sabue M, Jarrett N, et al. Counseling and testing TB patients for HIV: evaluation of three implementation models in Kinshasa, Congo. Int J Tuberc Lung Dis. 2008;12(Suppl 1):S73–S78. [PubMed] [Google Scholar]


