Abstract
The foodborne gram-negative pathogen Salmonella must adapt to varied environmental conditions encountered within foods, the host gastrointestinal tract and the phagosomes of host macrophages. Adaptation is achieved through the coordinate regulation of gene expression in response to environmental signals such as temperature, pH, osmolarity, redox state, antimicrobial peptides, and nutrient deprivation. This review will examine mechanisms by which the integration of regulatory responses to a broad array of environmental signals can be achieved. First, in the most straightforward case, tandem promoters allow gene expression to respond to multiple signals. Second, versatile sensor proteins may respond to more than one environmental signal. Third, transcriptional silencing and counter-silencing as demonstrated by the H-NS paradigm provides a general mechanism for the convergence of multiple regulatory inputs. Fourth, signaling cascades allow gene activation by independent sensory elements. These mechanisms allow Salmonella to utilize common adaptive stress pathways in response to a diverse range of environmental conditions.
Coordinate and Integrated Regulation of Gene Expression
Salmonella enterica is a foodborne pathogen that poses a worldwide challenge to food safety. Despite intensive efforts, large outbreaks and millions of cases of salmonellosis continue to occur each year (Majowicz et al., 2010).
Salmonella must withstand a range of environmental conditions as the microbe travels between food sources, animal intestinal tracts and the intracellular environment of host phagocytes. To respond to these changing environments, Salmonella senses and responds to a variety of signals including temperature, pH, and osmolarity. Within a host, bacteria may also encounter antimicrobial peptides, nitrosative and oxidative stress, and nutrient deprivation. The ability to adapt rapidly to environmental change is essential for Salmonella survival and virulence, and both stress responses and virulence genes are expressed in response to environmental signals.
Environmental signals trigger changes in gene expression via a variety of mechanisms. Common mechanisms of transcriptional regulation in bacteria include two-component regulatory systems, alternative sigma factors, and transcriptional repressors. Coordinate regulation allows the simultaneous expression of multiple genes in response to a single environmental stimulus (Miller et al., 1989). Transcription and gene expression is dependent on RNA polymerase, which in bacteria is composed of five subunits: αI, αII, β, β’, and ω. In order to efficiently bind to promoters and initiate transcription, RNA polymerase interacts with a σ factor to form a holoenzyme (Borukhov and Nudler, 2003). The σ factor dissociates from RNA polymerase following the initiation of transcription. In Salmonella, σD (σ70) is responsible for the expression of housekeeping genes during normal growth. Five alternative sigma factors designated σE (σ24), σF (σ28), σH (σ32), σS (σ38), and σN (σ54) are activated during stress or changes in growth conditions (Gruber and Gross, 2003). For example, σS regulates a general stress response by activating up to 500 genes during starvation and in response to a host of other environmental changes (Hengge, 2009). Because alternative sigma factors may control the expression of large groups of genes, they provide an effective means for the cell to rapidly effect major changes in gene expression.
Two-component signal transduction systems (TCS), composed of sensors and transcriptional regulators, are used by bacteria to respond to changes in environment (Beier and Gross, 2006). The sensor is classically a membrane bound histidine protein kinase that undergoes autophosphorylation when activated by environmental signals (Stock et al., 2000). The phosphoryl groups are then transferred to the response regulator. Activation of the response regulator typically leads to a conformational change that allows the protein to bind DNA. TCS sense a variety of environmental signals such as temperature, pH, and osmolarity. Many TCS play a role in Salmonella virulence, including the PhoP/Q system (Groisman, 2001). Classical transcriptional activation involves direct contact between an activator and RNA polymerase, for example, at the carboxy-terminal domain of the α subunit or at region 4 of σD, in order to recruit the polymerase to a promoter or facilitate open complex formation (Rhodius and Busby, 1998). Cross-talk between a sensor-kinase and non-cognate response-regulator appears to be unusual, constrained by multiple insulating mechanisms, most notably at the level of molecular recognition (Laub and Goulian, 2007).
Another mechanism of transcriptional regulation involves a DNA-binding protein that represses transcription unless an inducer molecule is present (Lewis et al., 1996). Under non-inducing conditions, the repressor remains bound to the promoter region to prevent RNA polymerase from initiating transcription. Under activating conditions, an inducer causes a conformational change in the repressor so that it no longer binds the promoter region.
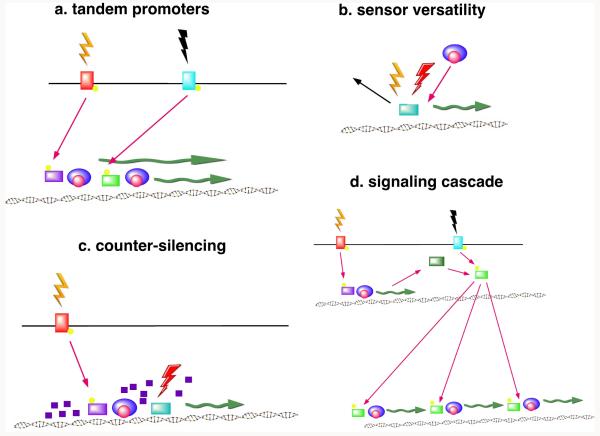
In addition to coordinately regulating the expression of multiple genes with individual regulators, Salmonella possesses mechanisms to integrate the control of specific genes or stress pathways to respond to multiple unrelated environmental signals. Through the integration of stress responses, Salmonella can express universal stress pathways in response to a diverse array of environmental signals, modulate gene responses by placing them under the influence of multiple regulatory pathways, and create regulatory check-points in which multiple conditions must be met in order for gene expression to occur. This brief review will consider four mechanisms by which such signal integration may be achieved: multiple promoters, sensor versatility, counter-silencing, and signaling cascades (Fig. 1).
Figure 1. Mechanisms of Signal Integration.
a. Tandem Promoters. Transcription of a gene driven by multiple tandem promoters controlled by different two-component regulatory systems. Two transcripts of different size are shown to emphasize their initiation at different promoters. b. Sensor Versatility. Sensing of different environmental signals by a single sensor protein. c. Counter-Silencing. Transcriptional silencing by an endogenous nucleoid-associated protein (small purple squares) is antagonized by a transcriptional activator (light purple) and a second DNA-binding protein (teal) in response to different environmental signals. d. Signaling Cascade. A transcriptional activator (light green) is maintained in an active state either by its cognate sensor-kinase (light blue) or by a protein (dark green) under the control of a separate two-component regulatory system.
Tandem Promoters
The simplest means of integrating different environmental signals is for gene expression to be regulated from multiple tandem promoters, with each promoter induced by a different environmental signal (Fig. 1a). One example of tandem promoters can be seen in the transcriptional regulation of the alternative sigma factor σH, encoded by the rpoH gene, which regulates genes involved in the heat shock stress response. The rpoH gene is transcribed from four promoters, three of which are recognized by σD during normal growth, and one that is transcribed by σE in response to extracytoplasmic stress conditions such as extreme heat (Erickson and Gross, 1989). Another example can be found within Salmonella Pathogenicity Island-1 (SPI-1). The Salmonella Pathogenicity Islands are AT-rich regions of the genome that encode many of the genes required for Salmonella virulence. SPI-1 encodes a type III secretory system that translocates effector proteins into host cells to stimulate cytoskeletal rearrangements, bacterial internalization and inflammatory cell death (Fink and Cookson, 2007; Lostroh and Lee, 2001; Patel and Galán, 2005). The hilE gene, which encodes a negative regulator of SPI-1 gene expression, possesses three promoters, one of which is controlled by the Mlc repressor (Lim et al., 2007), which responds to the availability of glucose, and another that is controlled by the FimZ activator (Baxter and Jones, 2005), which also regulates Salmonella attachment and adherence under static growth conditions. A third well-characterized example is the ugd gene, which is required for 4-aminoarabinose incorporation into lipopolysaccharide. The ugd gene has two promoters, one of which responds to the PhoP/Q and PmrA/B TCS and a second under control of the Rcs phosphorelay system (Mouslim and Groisman, 2003), which respond to distinct and independent environmental signals.
Sensor Versatility
Another mechanism by which signal integration can theoretically be achieved involves sensor proteins that are able to sense multiple environmental signals (Fig. 1b). If a single regulator can be activated by more than one signal, this can provide the basis for a common stress response triggered by diverse stimuli.
A classic example is provided by the SoxRS TCS, conserved between Salmonella and E. coli. SoxR is both a sensor and a transcription factor that senses and responds to two environmental conditions, oxidative stress and nitrosative stress. Both superoxide and nitric oxide (NO) are free radicals that can cause damage to the cell, and these reactive oxygen and nitrogen species share cellular targets such as iron-sulfur cluster-containing proteins and DNA. Superoxide is produced as a byproduct of respiration, whereas nitric oxide can be generated by metabolism or produced exogenously as a host defense mechanism. Each SoxR protein contains a single (2Fe-2S) cluster, which is necessary for sensing superoxide and nitric oxide. Superoxide oxidizes the (2Fe-2S) cluster of SoxR, activating SoxR as a transcription factor (Gaudu and Weiss, 1996). SoxR then induces the expression of SoxS, which is responsible for the activation of a diverse set of genes involved in antioxidant defense. Nitric oxide directly activates SoxR by forming a dinitrosyl-iron-dithiol complex with the (2Fe-2S) clusters (Nunoshiba et al., 1993; Ding and Demple, 2000).
However, SoxR is not the primary regulator of the cellular response to NO. NsrR, an FeS cluster-containing transcriptional repressor, controls the NO stress response. One of the most conserved genes in the NsrR regulon is hmp, which encodes a flavohemoprotein capable of detoxifying NO under both aerobic and anaerobic conditions (Bang et al., 2006; Bodenmiller and Spiro, 2006; Gardner et al., 1998; Hausladen et al., 1998; Tucker et al., 2010). Expression of hmp in the absence of NO can exacerbate oxidative stress by shuttling electrons to the flavin pool and promoting Fenton chemistry (Bang et al., 2006). Thus, hmp is regulated both by NO and iron availability. Nitrosylation of NsrR induces the expression of hmp as a protective response, but NsrR represses hmp expression if NO is absent and iron is available (Bang et al., 2006). Responsiveness to both signals allows cells to finely regulate levels of hmp expression, ameliorating nitrosative stress without aggravating oxidative stress.
The fumarate and nitrate reduction regulator (FNR) is a transcription factor that controls the expression of a large number of genes in response to the availability of oxygen. FNR contains a (4Fe-4S) cluster that is sensitive to the presence of oxygen. Under anaerobic conditions, FNR functions as a transcriptional activator through the acquisition of a (4Fe-4S)2+ cluster (Popescu et al., 1998). Upon exposure to oxygen, FNR is inactivated following the conversion of the (4Fe-4S)2+ cluster to a (2Fe-2S)2+ cluster, resulting in a decrease in DNA-binding ability (Khoroshilova et al., 1997). As with SoxR, NO can also nitrosylate the iron-sulfur cluster of FNR to reduce DNA-binding affinity of the protein and mimic the effects of oxygen (Cruz-Ramos et al., 2002).
The transcriptional regulator OxyR is a non-metal-containing protein in the LysR family that has been suggested to respond to multiple signals via a redox-sensitive thiol at Cys199. Oxidation of Cys199 to sulfenic acid by hydrogen peroxide promotes a reversible conformational change that allows OxyR to activate transcription of specific genes involved in resistance to oxidative stress (Kullik et al., 1995). An intramolecular disulfide bond involving Cys199 and Cys208 can stabilize OxyR in an activated form (Choi et al., 2001; Lee et al., 2004; Zheng et al., 1998). An alternative pathway of OxyR activation during nitrosative stress has been proposed, in which nitrosylation of Cys199 stimulates a similar conformational change (Hausladen et al., 1996; Kim et al., 2002). However, questions regarding the physiological significance of OxyR Cys199 modifications other than oxidation and disulfide bond formation remain (Helmann, 2002; Paget and Buttner, 2003).
An interesting example of a versatile sensor not involving a metal center has been recently proposed. The Salmonella mgtA gene encodes an ATP-dependent magnesium transporter. Expression of mgtA is responsive to low magnesium, which is sensed both at the level of the PhoP/Q TCS and a riboswitch contained within the 5′ leader of the mgtA mRNA (Choi et al., 2009; Cromie and Groisman, 2010). A proline-rich open reading frame also located within the mgtA leader mRNA, designated mgtL, has been shown to confer responsiveness to proline availability and osmolarity (Park et al., 2010). While this observation is intriguing, it has not yet been shown whether mgtA plays an essential role during proline deprivation or hyperosmolar stress. Although sensor versatility is a theoretically attractive mechanism of signal integration, the small number of examples in fact suggests that true versatility is difficult to achieve. Even for the known examples, it is controversial whether response to multiple signals represents actual signal integration or merely adventitious triggering of a stress response by promiscuous or incidental sensing.
Xenogeneic Silencing and Counter-Silencing
H-NS is a nucleoid-associated protein that globally silences genes acquired via horizontal gene transfer (Lucchini et al., 2006; Navarre et al., 2006). Foreign genes acquired through gene transfer typically have a lower GC content than the resident genome. In a process designated “xenogeneic silencing,” H-NS specifically binds and represses the transcription of AT-rich DNA sequences, thereby limiting the expression of foreign genes and facilitating their later incorporation into regulatory networks (Navarre et al., 2007). Following acquisition, evolutionary modification of H-NS-bound sequences can produce mechanisms of regulated counter-silencing that permit the expression of H-NS-silenced genes under specific conditions. Several mechanisms of counter-silencing have been described (Navarre et al., 2007; Stoebel et al., 2008). First, antagonists can bind to H-NS, disrupting the H-NS multimeric complexes. Second, H-NS can be out-competed by DNA-binding proteins with higher binding affinity. Third, genes may be expressed via alternative sigma factors that have higher affinity to AT-rich DNA. Finally, geometry of the promoter region may be changed by environmental factors resulting in disruption of H-NS binding. It is becoming clear that counter-silencing is responsible for a substantial proportion of bacterial gene regulation in response to environmental conditions, particularly with regard to virulence genes. The varied mechanisms by which H-NS-mediated silencing can be countered can facilitate signal integration.
For example, counter-silencing has been shown to play a role in the regulation of Salmonella biofilms. Salmonella forms biofilms both within and outside the host in response to a variety of environmental signals such as variations in nutrient availability, temperature, pH, and osmolarity. Biofilm formation contributes to host colonization and protects cells outside the host from desiccation, disinfectants, and antibiotics (Steenackers et. al., 2011). Curli fimbriae promote cell-surface and cell-cell interactions during biofilm formation. The csgBAC genes can be transcribed by σ70 in the absence of H-NS, but require the alternative sigma factor σS to overcome silencing by H-NS under normal conditions (Olsen et. al., 1993). Under low nutrient and low temperature conditions, the regulator protein Crl recruits σS to the csgBAC promoter, enabling expression of Curli and biofilm formation (Bougdour et. al., 2004). The switch between planktonic to multicellular states is regulated by the transcriptional regulator CsgD, whose expression provides another example of signal integration. Several regulatory proteins, including OmpR, IHF, H-NS, and MlrA, bind directly to a large intergenic region upstream of the csgD promoter to modulate gene expression in response to changing environmental stimuli (Gerstel and Romling, 2003).
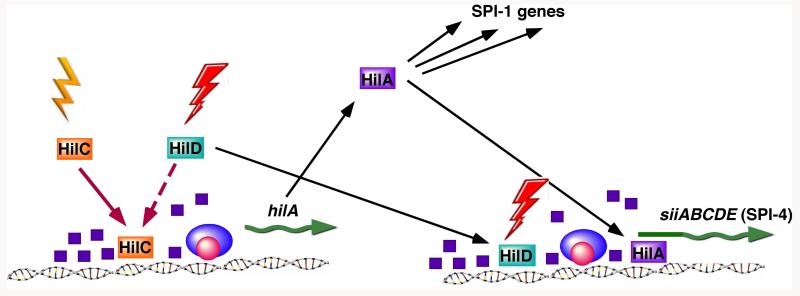
Along with other horizontally acquired sequences, the aforementioned Salmonella pathogenicity islands (SPIs) are silenced by H-NS until activated by environmental signals encountered in the intestinal tract or within host cell phagosomes. Expression of SPI-1 genes is regulated by multiple transcription factors in addition to HilE, including HilA (Lostroh and Lee, 2001). The expression of hilA, also located in SPI-1, is controlled by a number of regulatory factors in response to changing environmental conditions. The hilA gene is silenced by H-NS under low-osmolarity conditions, until H-NS-mediated repression is relieved by HilC and HilD, two other transcription factors encoded within SPI-1 (Schechter et al., 2003). HilC and HilD respond to different environmental conditions, but either alone is capable of activating hilA expression (Lucas and Lee, 2001). Thus, counter-silencing allows SPI-1 expression to occur under either of two environmental conditions (Fig. 2).
Figure 2. Counter-silencing allows signal integration and checkpoint regulation of Salmonella Pathogenicity Island genes.
HilA, a transcription factor involved in the regulation of SPI-1 genes, is silenced by H-NS (purple squares) under repressing conditions. Either HilC or HilD is capable of relieving HNS-mediated repression, allowing hilA to be expressed under either of two environmental conditions. HilA is also required for the expression of SPI-4 genes siiABCDE. However, SPI-4 genes are activated only when both HilA and HilD are present, ensuring that SPI-4 genes are expressed only when specific environmental conditions are met.
The SPI-4 pathogenicity island encodes six genes that, like SPI-1 genes, are involved in the intestinal phase of Salmonella infection (Kiss et al., 2007). SPI-4 is co-regulated with SPI-1 (Gerlach et al., 2007); the expression of SPI-4 genes requires two SPI-1-encoded transcription factors, HilA and HilD (Main-Hester, 2008; Main-Hester et al., 2008). As with SPI-1, SPI-4 genes are normally repressed by H-NS until de-repressed by HilA under inducing conditions encountered in the intestines. However, both HilA and HilD are required for SPI-4 expression. This can be viewed as a “checkpoint” mechanism, since two different environmental conditions must be met for gene expression to take place (Fig. 2). Hybrid arrangements in which classical transcriptional activation and counter-silencing are combined also occur (Fig. 1c), as in the co-regulation of SPI-2 pathogenicity island genes by OmpR and SsrB (Walthers et al., 2007), or the co-regulation of the OmpS1 porin by OmpR and Leu regulation of OmpS1. (De la Cruz et al., 2007). The participation of transcriptional activators as both classical activators and counter-silencers, and the ability to achieve both signal integration and checkpoint regulation, illustrates the versatility of the counter-silencing paradigm in achieving diverse regulatory goals.
Signaling Cascades
Signaling cascades, as seen in the Salmonella flagellar regulon, the SPI-1 and SPI-2 pathogenicity islands and many other examples, involve the sequential activation of multiple genes, which may amplify a response and allow input from multiple signaling pathways and environmental signals (Fig. 1d). One of the best-studied examples of a Salmonella signaling cascade involves the PhoP/Q and PmrA/B TCS. PhoP/Q activation can be stimulated by acid pH and antimicrobial peptides or low magnesium concentrations (Bader et al., 2005; Garcia Vescovi et al., 1996; Prost et al., 2007), while PmrA/B activation responds both to acid stress and elevated iron(III) concentrations (Perez and Groisman, 2007; Wosten et al., 2000). PhoP activates the expression of the pmrD gene, encoding a TCS connector protein that binds and stabilizes phosphorylated PmrA (Kato and Groisman, 2004; Kox et al., 2000; Mitrophanov, 2008). Thus, the PmrA/B regulon is pH-responsive and can further be stimulated by iron in a PhoP/Q-independent manner, or by cationic peptides or low magnesium in a PhoP/Q-dependent manner (Gunn and Miller, 1996; Soncini and Groisman, 1996). In an analogous fashion, the osmoregulated auxiliary regulatory protein TviA encoded by Salmonella Pathogenicity Island 7 modulates expression of the RcsB regulon in Salmonella Typhi and thereby integrates changes in osmolarity with cell envelope stress in the regulation of Salmonella motility, invasion and capsular synthesis (Winter et al., 2009).
Although sigma factors directly control the expression of discrete subsets of genes, the σS–dependent general stress response can be triggered by multiple environmental signals and pathways, including those regulated by the alternative sigma factors σE and σH. σS is required for bacterial survival during stationary phase and for general stress responses to signals such as changes in osmolarity, temperature and pH (Hengge-Aronis, 2002). σE belongs to the family of of extracytoplasmic function (ECF) sigma factors, which play essential roles in membrane and periplasmic homeostasis, and are activated by the presence of unfolded membrane or periplasmic proteins (Alba and Gross, 2004; Erickson and Gross, 1989; Rouviere et al., 1995). σE has also been found to have a role in stationary phase survival and resistance to oxidative stress (Testerman et al., 2002). In addition, σS and σE are both required for virulence in Salmonella (Fang et al., 1992; Humphreys et al., 1999; Testerman et al., 2002). σH is responsible for regulating genes required for resistance to heat shock, and in addition plays a role in stationary phase survival (Jenkins et al., 1991). Recent observations have shown that regulatory interactions link σE, and σH and σS in a signaling cascade (Bang et al., 2005). σE is able to enhance levels of σS during stationary phase via increased expression of rpoH and hfq. Hfq, a σH-dependent protein, promotes σS translation by binding the small RNAs DsrA and RprA and stabilizing their interaction with rpoS mRNA to promote translation (McCullen et al., 2010; Sledjeski et al., 1996; Soper et al., 2010). Activation of either σE or σH can thereby increase expression of σS in an Hfq-dependent fashion (Bang et al., 2005). Thus, a sigma factor cascade integrates extracytoplasmic (σE) and cytoplasmic (σH) stress responses with the σS–dependent general stress response (Fang, 2005). In fact, regulation of the σS general stress response at transcriptional, translational and post-translational levels allows modulation by many environmental conditions including growth rate, cell density, temperature, osmolarity, pH, and nutrient availability (Klauck et al., 2007).
Proteolytic Signaling Cascade
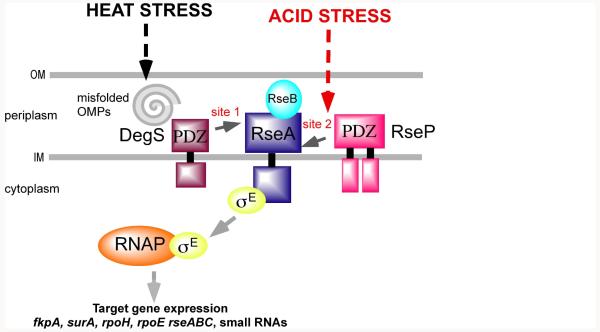
The mechanism of σE activation in response to misfolded outer membrane proteins (OMPs) has been well characterized (Ades, 2008; Alba and Gross, 2004). Under non-stress conditions, σE is sequestered by the anti-sigma factor RseA at the inner membrane (Fig. 3). During heat stress, RseA is sequentially cleaved by the DegS and RseP proteases. Initial cleavage of the RseA periplasmic domain by DegS is followed by cleavage of the RseA transmembrane domain by RseP, to release σE and a residual RseA cytoplasmic domain that is degraded by ClpXP. The presence of misfolded OMPs leads to DegS activation via interaction with the protease PDZ domain (Walsh et al., 2003). RseP is unable to proteolyze RseA until DegS has first acted upon the periplasmic domain of RseA.
Figure 3. Activation of σE by a Proteolytic Cascade.
Canonical activation of the alternative sigma factor σE is initiated by the presence of misfolded outer membrane proteins that relieve inhibition of the periplasmic DegS protease (see text). Site 1 proteolysis of the periplasmic domain of the RseA anti-sigma by DegS allows subsequent intramembrane proteolysis of RseA at site 2 by the RseP protease, resulting in release of σE and the expression of σE–dependent genes. A second route of activation that also involves RseA proteolysis by RseP is stimulated by acid pH stress in a DegS-independent manner (modified from Müller et al, 2009).
However, studies in Salmonella have revealed a second pathway of RseA proteolysis and σE activation. Although σE is essential in E. coli, this sigma factor is non-essential in Salmonella. A null mutation in degS is less attenuating for Salmonella virulence compared to a null mutation in rpoE encoding σE (Rowley et al., 2005). This is because σE can be activated during acid stress by a DegS-independent mechanism that does not require the presence of misfolded OMPs (Müller et al., 2009). The acid pH-triggered DegS-independent activation of σE is essential for Salmonella survival within the acidified phagosomes of host macrophages (Müller et al., 2009). Acid pH activation of σE still requires RseA cleavage by the RseP protease, and it has been suggested that acid stress may alleviate inhibition of RseA proteolysis by RseP without requiring initial RseA processing by DegS. The DegS-RseP proteolytic cascade thus allows the integration of different environmental signals (misfolded OMPs, acid pH) to activate a common stress response controlled by σE.
Conclusions
The integration of transcriptional responses to different signals allows bacteria to respond to diverse environmental conditions through the expression of common stress responses. Recent observations are revealing new insights into the mechanisms of signal integration that include tandem promoters, sensor versatility, counter-silencing and signaling cascades. The versatile pathogen Salmonella enterica utilizes each of these strategies as it adapts to the varied conditions encountered in food vehicles, the host intestine and the intracellular environment.
Acknowledgments
The authors are grateful for support from research grants from the National Institutes of Health (AI39557, AI44486, AI77629) and thank Linda Kenney and Stephen Libby for critical comments and discussions. S.S. received support from an NIH Training Grant (AI55396).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ades SE. Regulation by destruction: design of the sigmaE envelope stress response. Current Opinion in Microbiology. 2008;11:535–540. doi: 10.1016/j.mib.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Alba BM, Gross CA. Regulation of the Escherichia coli sigma-dependent envelope stress response. Molecular Microbiology. 2004;52:613–619. doi: 10.1111/j.1365-2958.2003.03982.x. [DOI] [PubMed] [Google Scholar]
- 3.Bader MW, Sanowar S, Daley ME, Schneider AR, Cho U, Xu W, Klevit RE, Le Moual H, Miller S. l. Recognition of antimicrobial peptides by a bacterial sensor kinase. Cell. 2005;122:461–472. doi: 10.1016/j.cell.2005.05.030. [DOI] [PubMed] [Google Scholar]
- 4.Bang IS, Frye JE, McClelland M, Velayudhan J, Fang FC. Alternative sigma factor interactions in Salmonella: sigma(E) and sigma(H) promote antioxidant defences by enhancing sigma(S) levels. Molecular Microbiology. 2005;56:811–823. doi: 10.1111/j.1365-2958.2005.04580.x. [DOI] [PubMed] [Google Scholar]
- 5.Bang IS, Liu L, Vazquez-Torres A, Crouch ML, Stamler JS, Fang FC. Maintenance of nitric oxide and redox homeostasis by the Salmonella flavohemoglobin Hmp. Journal of Biological Chemistry. 2006;281:28039–28047. doi: 10.1074/jbc.M605174200. [DOI] [PubMed] [Google Scholar]
- 6.Baxter MA, Jones BD. The fimYZ genes regulate Salmonella enterica Serovar Typhimurium invasion in addition to type 1 fimbrial expression and bacterial motility. Infection and Immunity. 2005;73:1377–1385. doi: 10.1128/IAI.73.3.1377-1385.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beier D, Gross R. Regulation of bacterial virulence by two-component systems. Current Opinion in Microbiology. 2006;9:143–152. doi: 10.1016/j.mib.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Bodenmiller DM, Spiro S. The yjeB (nsrR) gene of Escherichia coli encodes a nitric oxide-sensitive transcriptional regulator. Journal of Bacteriology. 2006;188:874–881. doi: 10.1128/JB.188.3.874-881.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borukhov S, Nudler E. RNA polymerase holoenzyme: structure, function and biological implications. Current Opinion in Microbiology. 2003;6:93–100. doi: 10.1016/s1369-5274(03)00036-5. [DOI] [PubMed] [Google Scholar]
- 10.Bougdour A, Lelong C, Geiselmann J. Temperature-induced protein in Escherichia coli that binds directly to the stationary phase sigma subunit of RNA polymerase. Journal of Biological Chemistry. 2004;279:19540–19550. doi: 10.1074/jbc.M314145200. [DOI] [PubMed] [Google Scholar]
- 11.Choi H, Kim S, Mukhopadhyay P, Cho S, Woo J, Storz G, Ryu SE. Structural basis of the redox switch in the OxyR transcription factor. Cell. 2001;105:103–113. doi: 10.1016/s0092-8674(01)00300-2. [DOI] [PubMed] [Google Scholar]
- 12.Choi E, Groisman EA, Shin D. Activated by different signals, the PhoP/PhoQ two-component system differentially regulates metal uptake. Journal of Bacteriology. 2009;191:7174–7181. doi: 10.1128/JB.00958-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cromie MJ, Groisman EA. Promoter and riboswitch control of the Mg2+ transporter MgtA from Salmonella enterica. Journal of Bacteriology. 2010;192:604–607. doi: 10.1128/JB.01239-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cruz-Ramos H, Crack J, Wu G, Hughes MN, Scott C, Thomson AJ, Green J, Poole RK. NO sensing by FNR: regulation of the Escherichia coli NO-detoxifying flavohaemoglobin, Hmp. EMBO Journal. 2002;21:3235–3244. doi: 10.1093/emboj/cdf339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De La Cruz MA, Fernández-Mora M, Guadarrama C, Flores-Valdez MA, Bustamante VH, Vázquez A, Calva E. LeuO antagonizes H-NS and StpA-dependent repression in Salmonella enterica ompS1. Molecular Microbiology. 2007;66:727–743. doi: 10.1111/j.1365-2958.2007.05958.x. [DOI] [PubMed] [Google Scholar]
- 16.Ding H, Demple B. Direct nitric oxide signal transduction via nitrosylation of iron-sulfur centers in the SoxR transcription activator. Proceedings of the National Academy of Sciences U.S.A. 2000;97:5146–5150. doi: 10.1073/pnas.97.10.5146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erickson JA, Gross CA. Identification of the sigma E subunit of Escherichia coli RNA polymerase: a second alternate sigma factor involved in high-temperature gene expression. Genes and Development. 1989;3:1462–1471. doi: 10.1101/gad.3.9.1462. [DOI] [PubMed] [Google Scholar]
- 18.Fang FC. Sigma cascades in prokaryotic regulatory networks. Proceedings of the National Academy of Sciences U.S.A. 2005;102:4933–4934. doi: 10.1073/pnas.0501417102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fang FC, Libby SJ, Buchmeier NA, Loewen PC, Switala J, Harwood J, Guiney DG. The alternative sigma factor KatF (RpoS) regulates Salmonella virulence. Proceedings of the National Academy of Sciences U.S.A. 1992;89:11978–11982. doi: 10.1073/pnas.89.24.11978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fink SL, Cookson BT. Pyroptosis and host cell death responses during Salmonella infection. Cellular Microbiology. 2007;9:2562–2570. doi: 10.1111/j.1462-5822.2007.01036.x. [DOI] [PubMed] [Google Scholar]
- 21.García Véscovi E, Soncini F, Groisman EA. Mg2+ as an extracellular signal: environmental regulation of Salmonella virulence. Cell. 1996;84:165–174. doi: 10.1016/s0092-8674(00)81003-x. [DOI] [PubMed] [Google Scholar]
- 22.Gardner PR, Gardner AM, Martin LA, Salzman AL. Nitric oxide dioxygenase: an enzymic function for flavohemoglobin. Proceedings of the National Academy of Sciences U.S.A. 1998;95:10378–10383. doi: 10.1073/pnas.95.18.10378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gaudu P, Weiss B. SoxR, a [2Fe-2S] transcription factor, is active only in its oxidized form. Proceedings of the National Academy of Sciences U.S.A. 1996;93:10094–10098. doi: 10.1073/pnas.93.19.10094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gerlach RG, Jäckel D, Geymeier N, Hensel M. Salmonella pathogenicity island 4-mediated adhesion is coregulated with invasion genes in Salmonella enterica. Infection and Immunity. 2007;75:4697–4709. doi: 10.1128/IAI.00228-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gerstel U, Park C, Römling U. Complex regulation of csgD promoter activity by global regulatory proteins. Molecular Microbiology. 2003;49:639–654. doi: 10.1046/j.1365-2958.2003.03594.x. [DOI] [PubMed] [Google Scholar]
- 26.Groisman EA. The pleiotropic two-component regulatory system PhoP-PhoQ. Journal of Bacteriology. 2001;183:1835–1842. doi: 10.1128/JB.183.6.1835-1842.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gruber TM, Gross CA. Multiple sigma subunits and the partitioning of bacterial transcription space. Annual Review of Microbiology. 2003;57:441–466. doi: 10.1146/annurev.micro.57.030502.090913. [DOI] [PubMed] [Google Scholar]
- 28.Gunn JS, Miller SI. PhoP-PhoQ activates transcription of pmrAB, encoding a two-component regulatory system involved in Salmonella typhimurium antimicrobial peptide resistance. Journal of Bacteriology. 1996;178:6857–6864. doi: 10.1128/jb.178.23.6857-6864.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hausladen A, Gow AJ, Stamler JS. Nitrosative stress: metabolic pathway involving the flavohemoglobin. Proceedings of the National Academy of Sciences U.S.A. 1998;95:14100–14105. doi: 10.1073/pnas.95.24.14100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hausladen A, Privalle CT, Keng T, DeAngelo J, Stamler JS. Nitrosative stress: activation of the transcription factor OxyR. Cell. 1996;86:719–729. doi: 10.1016/s0092-8674(00)80147-6. [DOI] [PubMed] [Google Scholar]
- 31.Helmann JD. OxyR: a molecular code for redox sensing? Science’s STKE. 2002;2002:pe46. doi: 10.1126/stke.2002.157.pe46. [DOI] [PubMed] [Google Scholar]
- 32.Hengge-Aronis R. Signal transduction and regulatory mechanisms involved in control of the sigma(S) (RpoS) subunit of RNA polymerase. Microbiology and Molecular Biology Reviews. 2002;66:373–395. doi: 10.1128/MMBR.66.3.373-395.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hengge R. Proteolysis of sigmaS (RpoS) and the general stress response in Escherichia coli. Research in Microbiology. 2009;160:667–676. doi: 10.1016/j.resmic.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 34.Humphreys S, Stevenson A, Bacon A, Weinhardt AB, Roberts M. The alternative sigma factor, sigmaE, is critically important for the virulence of Salmonella typhimurium. Infection and Immunity. 1999;67:1560–1568. doi: 10.1128/iai.67.4.1560-1568.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jenkins DE, Auger EA, Matin A. Role of RpoH, a heat shock regulator protein, in Escherichia coli carbon starvation protein synthesis and survival. Journal of Bacteriology. 1991;173:1992–1996. doi: 10.1128/jb.173.6.1992-1996.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kato A, Groisman EA. Connecting two-component regulatory systems by a protein that protects a response regulator from dephosphorylation by its cognate sensor. Genes and Development. 2004;18:2302–2313. doi: 10.1101/gad.1230804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khoroshilova N, Popescu C, Münck E, Beinert H, Kiley PJ. Iron-sulfur cluster disassembly in the FNR protein of Escherichia coli by O2: [4Fe-4S] to [2Fe-2S] conversion with loss of biological activity. Proceedings of the National Academy of Sciences U.S.A. 1997;94:6087–6092. doi: 10.1073/pnas.94.12.6087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim SO, Merchant K, Nudelman R, Beyer WF, Jr., Keng T, DeAngelo J, Hausladen A, Stamler JS. OxyR: a molecular code for redox-related signaling. Cell. 2002;109:383–396. doi: 10.1016/s0092-8674(02)00723-7. [DOI] [PubMed] [Google Scholar]
- 39.Kiss T, Morgan E, Nagy G. Contribution of SPI-4 genes to the virulence of Salmonella enterica. FEMS Microbiology Letters. 2007;275:153–159. doi: 10.1111/j.1574-6968.2007.00871.x. [DOI] [PubMed] [Google Scholar]
- 40.Klauck E, Typas A, Hengge R. The sigmaS subunit of RNA polymerase as a signal integrator and network master regulator in the general stress response in Escherichia coli. Science in Progress. 2007;90:103–127. doi: 10.3184/003685007X215922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kox LF, Wösten MM, Groisman EA. A small protein that mediates the activation of a two-component system by another two-component system. EMBO Journal. 2000;19:1861–1872. doi: 10.1093/emboj/19.8.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kullik I, Toledano MB, Tartaglia LA, Storz G. Mutational analysis of the redox-sensitive transcriptional regulator OxyR: regions important for oxidation and transcription activation. Journal of Bacteriology. 1995;177:1275–1284. doi: 10.1128/jb.177.5.1275-1284.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Laub MT, Goulian M. Specificity in two-component signal transduction pathways. Annual Review of Genetics. 2007;41:121–145. doi: 10.1146/annurev.genet.41.042007.170548. [DOI] [PubMed] [Google Scholar]
- 44.Lee C, Lee SM, Mukhopadhyay P, Kim SJ, Lee SC, Ahn WS, Yu MH, Storz G, Ryu SE. Redox regulation of OxyR requires specific disulfide bond formation involving a rapid kinetic reaction path. Nature Structural and Molecular Biology. 2004;11:1179–1185. doi: 10.1038/nsmb856. [DOI] [PubMed] [Google Scholar]
- 45.Lewis M, Chang G, Horton NC, Kercher MA, Pace HC, Schumacher MA, Brennan RG, Lu P. Crystal structure of the lactose operon repressor and its complexes with DNA and inducer. Science. 1996;271:1247–1254. doi: 10.1126/science.271.5253.1247. [DOI] [PubMed] [Google Scholar]
- 46.Lim S, Yun J, Yoon H, Park C, Kim B, Jeon B, Kim D, Ryu S. Mlc regulation of Salmonella pathogenicity island I gene expression via hilE repression. Nucleic Acids Research. 2007;35:1822–1832. doi: 10.1093/nar/gkm060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lostroh CP, Lee CA. The Salmonella pathogenicity island-1 type III secretion system. Microbes and Infection. 2001;3:1281–1291. doi: 10.1016/s1286-4579(01)01488-5. [DOI] [PubMed] [Google Scholar]
- 48.Lucas RL, Lee CA. Roles of hilC and hilD in regulation of hilA expression in Salmonella enterica serovar Typhimurium. Journal of Bacteriology. 2001;183:2733–2745. doi: 10.1128/JB.183.9.2733-2745.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lucchini S, Rowley G, Goldberg MD, Hurd D, Harrison M, Hinton JC. H-NS mediates the silencing of laterally acquired genes in bacteria. PLoS Pathogens. 2006;2:e81. doi: 10.1371/journal.ppat.0020081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Main-Hester KL, Colpitts KM, Thomas GA, Fang FC, Libby SJ. Coordinate regulation of Salmonella pathogenicity island 1 (SPI1) and SPI4 in Salmonella enterica serovar Typhimurium. Infection and Immunity. 2008;76:1024–1035. doi: 10.1128/IAI.01224-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Main-Hester KL. Ph.D. thesis. University of Washington; Seattle, WA: 1998. Counter-silencing of laterally acquired genes, in Salmonella pathogenicity island 4, by three DNA binding proteins, HilA, HilD, and SlyA. [Google Scholar]
- 52.Majowicz SE, Musto J, Scallan E, Angulo FJ, Kirk M, O’Brien SJ, Jones TF, Fazil A, Hoekstra RM, International Collaboration on Enteric Disease ‘Burden of Illness’ Studies The global burden of nontyphoidal Salmonella gastroenteritis. Clinical Infectious Diseases. 2010;50:882–889. doi: 10.1086/650733. [DOI] [PubMed] [Google Scholar]
- 53.McCullen C, Benhammou J, Majdalani N, Gottesman S. Mechanism of Positive Regulation by DsrA and RprA sRNAs: Pairing increases Translation and Protects rpoS mRNA From Degradation. Journal of Bacteriology. 2010;192:5559–5571. doi: 10.1128/JB.00464-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miller JF, Mekalanos JJ, Falkow S. Coordinate regulation and sensory transduction in the control of bacterial virulence. Science. 1989;243:916–922. doi: 10.1126/science.2537530. [DOI] [PubMed] [Google Scholar]
- 55.Mitrophanov AY, Groisman EA. Signal integration in bacterial two-component regulatory systems. Genes and Development. 2008;22:2601–2611. doi: 10.1101/gad.1700308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mouslim C, Latifi T, Groisman EA. Signal-dependent requirement for the co-activator protein RcsA in transcription of the RcsB-regulated ugd gene. Journal of Biological Chemistry. 2003;278:50588–50595. doi: 10.1074/jbc.M309433200. [DOI] [PubMed] [Google Scholar]
- 57.Müller C, Bang IS, Velayudhan J, Karlinsey J, Papenfort K, Vogel J, Fang FC. Acid stress activation of the sigma(E) stress response in Salmonella enterica serovar Typhimurium. Molecular Microbiology. 2009;71:1228–1238. doi: 10.1111/j.1365-2958.2009.06597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Navarre WW, McClelland M, Libby SJ, Fang FC. Silencing of xenogeneic DNA by H-NS-facilitation of lateral gene transfer in bacteria by a defense system that recognizes foreign DNA. Genes and Development. 2007;21:1456–1471. doi: 10.1101/gad.1543107. [DOI] [PubMed] [Google Scholar]
- 59.Navarre WW, Porwollik S, Wang Y, McClelland M, Rosen H, Libby SJ, Fang FC. Selective silencing of foreign DNA with low GC content by the H-NS protein in Salmonella. Science. 2006;313:236–238. doi: 10.1126/science.1128794. [DOI] [PubMed] [Google Scholar]
- 60.Nunoshiba T, deRojas-Walker T, Wishnok JS, Tannenbaum SR, Demple B. Activation by nitric oxide of an oxidative-stress response that defends Escherichia coli against activated macrophages. Proceedings of the National Academy of Sciences U.S.A. 1993;90:9993–9997. doi: 10.1073/pnas.90.21.9993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Olsén A, Arnqvist A, Hammar M, Sukulpolvi S, Normark S. The RpoS sigma factor relieves H-NS-mediated transcriptional repression of csgA, the subunit gene of fibronectin-binding curli in Escherichia coli. Molecular Microbiology. 1993;7:523–536. doi: 10.1111/j.1365-2958.1993.tb01143.x. [DOI] [PubMed] [Google Scholar]
- 62.Paget MS, Buttner MJ. Thiol-based regulatory switches. Annual Review of Genetics. 2003;37:91–121. doi: 10.1146/annurev.genet.37.110801.142538. [DOI] [PubMed] [Google Scholar]
- 63.Patel JC, Galán JE. Manipulation of the host actin cytoskeleton by Salmonella-- all in the name of entry. Current Opinion in Microbiology. 2005;8:10–15. doi: 10.1016/j.mib.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 64.Park SY, Cromie MJ, Lee EJ, Groisman EA. A bacterial mRNA leader that employs different mechanisms to sense disparate intracellular signals. Cell. 2010;142:737–748. doi: 10.1016/j.cell.2010.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Perez JC, Groisman EA. Acid pH activation of the PmrA/PmrB two-component regulatory system of Salmonella enterica. Molecular Microbiology. 2007;63:283–293. doi: 10.1111/j.1365-2958.2006.05512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Popescu CV, Bates DM, Beinert H, Münck E, Kiley PJ. Mossbauer spectroscopy as a tool for the study of activation/inactivation of the transcription regulator FNR in whole cells of Escherichia coli. Proceedings of the National Academy of Sciences U.S.A. 1998;95:13431–13435. doi: 10.1073/pnas.95.23.13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Prost LR, Daley ME, Le Sage V, Bader MW, Le Moual H, Klevit RE, Miller SI. Activation of the bacterial sensor kinase PhoQ by acidic pH. Molecular Cell. 2007;26:165–174. doi: 10.1016/j.molcel.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 68.Rhodius VA, Busby SJ. Positive activation of gene expression. Current Opinion in Microbiology. 1998;1:152–159. doi: 10.1016/s1369-5274(98)80005-2. [DOI] [PubMed] [Google Scholar]
- 69.Rouvière PE, De Las Peñas A, Mecsas J, Lu CZ, Rudd KE, Gross CA. rpoE, the gene encoding the second heat-shock sigma factor, sigma E, in Escherichia coli. EMBO Journal. 1995;14:1032–1042. doi: 10.1002/j.1460-2075.1995.tb07084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rowley G, Stevenson A, Kormanec J, Roberts M. Effect of inactivation of degS on Salmonella enterica serovar Typhimurium in vitro and in vivo. Infection and Immunity. 2005;73:459–463. doi: 10.1128/IAI.73.1.459-463.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schechter LM, Jain S, Akbar S, Lee CA. The small nucleoid-binding proteins H-NS, HU, and Fis affect hilA expression in Salmonella enterica serovar Typhimurium. Infection and Immunity. 2003;71:5432–5435. doi: 10.1128/IAI.71.9.5432-5435.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sledjeski DD, Gupta A, Gottesman S. The small RNA, DsrA, is essential for the low temperature expression of RpoS during exponential growth in Escherichia coli. EMBO Journal. 1996;15:3993–4000. [PMC free article] [PubMed] [Google Scholar]
- 73.Soncini FC, Groisman EA. Two-component regulatory systems can interact to process multiple environmental signals. Journal of Bacteriology. 1996;178:6796–6801. doi: 10.1128/jb.178.23.6796-6801.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Soper T, Mandin P, Majdalani N, Gottesman S, Woodson SA. Positive regulation by small RNAs and the role of Hfq. Proceedings of the National Academy of Sciences U.S.A. 2010;107:9602–9607. doi: 10.1073/pnas.1004435107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Steenackers H, Hermans K, Vanderleyden J, De Keersmaecker CJ. Salmonella biofilms: an overview on occurrence, structure, regulation and eradication. Food Research International. 2011 January 18; 2011. epub ahead of print, doi: 10.1016/j.foodres.2011.1.038. [Google Scholar]
- 76.Stock AM, Robinson V, Goudreau P. Two-component signal transduction. Annual Review of Biochemistry. 2000;69:183–215. doi: 10.1146/annurev.biochem.69.1.183. [DOI] [PubMed] [Google Scholar]
- 77.Stoebel DM, Free A, Dorman CJ. Anti-silencing: overcoming H-NS-mediated repression of transcription in Gram-negative enteric bacteria. Microbiology. 2008;154:2533–2545. doi: 10.1099/mic.0.2008/020693-0. [DOI] [PubMed] [Google Scholar]
- 78.Testerman TL, Vazquez-Torres A, Xu Y, Jones-Carson J, Libby SJ, Fang FC. The alternative sigma factor sigmaE controls antioxidant defences required for Salmonella virulence and stationary-phase survival. Molecular Microbiology. 2002;43:771–782. doi: 10.1046/j.1365-2958.2002.02787.x. [DOI] [PubMed] [Google Scholar]
- 79.Tucker NP, Le Brun NE, Dixon R, Hutchings MI. There’s NO stopping NsrR, a global regulator of the bacterial NO stress response. Trends in Microbiology. 2010;18:149–156. doi: 10.1016/j.tim.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 80.Walsh NP, Alba BM, Bose B, Gross CA, Sauer RT. OMP peptide signals initiate the envelope-stress response by activating DegS protease via relief of inhibition mediated by its PDZ domain. Cell. 2003;113:61–71. doi: 10.1016/s0092-8674(03)00203-4. [DOI] [PubMed] [Google Scholar]
- 81.Walthers D, Carroll RK, Navarre WW, Libby SJ, Fang FC, Kenney LJ. The response regulator SsrB activates expression of diverse Salmonella pathogenicity island 2 promoters and counters silencing by the nucleoid-associated protein H-NS. Molecular Microbiology. 2007;65:477–493. doi: 10.1111/j.1365-2958.2007.05800.x. [DOI] [PubMed] [Google Scholar]
- 82.Winter SE, Winter MG, Thiennimitr P, Gerriets VA, Nuccio S-P, Rüssmann H, Bäumler AJ. The TviA auxiliary protein renders the Salmonella enterica serotype Typhi RcsB regulon responsive to changes in osmolarity. Molecular Microbiology. 2009;74:175–193. doi: 10.1111/j.1365-2958.2009.06859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wösten MM, Kox LF, Chamnongpol S, Soncini FC, Groisman EA. A signal transduction system that responds to extracellular iron. Cell. 2000;103:113–125. doi: 10.1016/s0092-8674(00)00092-1. [DOI] [PubMed] [Google Scholar]
- 84.Zheng M, Aslund F, Storz G. Activation of the OxyR transcription factor by reversible disulfide bond formation. Science. 1998;279:1718–1721. doi: 10.1126/science.279.5357.1718. [DOI] [PubMed] [Google Scholar]