Abstract
We prepared 1-(4′-azido-2′-deoxy-2′-fluoro-β -D-arabinofuranosyl)cytosine (10) and its hydrochloride salt (11) as potential antiviral agents based on the favorable antiviral profiles of 4′-substituted nucleosides. Compounds 10 and 11 were synthesized from 1,3,5-O-tribenzoyl-2-deoxy-2-fluoro-D-arabinofuranoside in multiple steps, and their structures were unequivocally established by IR, 1H NMR, 13C NMR, and 19F NMR spectroscopy, HRMS, and X-ray crystallography. Compounds 10 and 11 exhibited potent anti-HIV-1 activity (EC50: 0.3 and 0.13 nM, respectively) without significant cytotoxicity in concentrations up to 100 μM. Compound 11 exhibited extremely potent anti-HIV activity against NL4-3 (wild-type), NL4-3 (K101E), and RTMDR viral strains, with EC50 values of 0.086, 0.15, and 0.11 nM, respectively. Due to the high potency of 11, it was also screened against an NIH Reagent Program NRTI-resistant virus panel containing eleven mutated viral strains and for cytotoxicity against six different human cell lines. The results of this screening indicated that 11 is a novel NRTI that could be developed as an anti-AIDS clinical trial candidate to overcome drug-resistance issues.
Keywords: 4′-Azido-2′-deoxy-2′-fluoro nucleosides, Anti-HIV activity, Nucleoside reverse transcriptase inhibitor (NRTI), Drug resistance
1. Introduction
Since the discovery of the human immunodeficiency virus (HIV) in 1983, AIDS has become the leading infectious cause of death worldwide. According to the World Health Organization (07 AIDS Epidemic Update), there were 33.2 million people living with AIDS worldwide in 2007, 2.1 million deaths from AIDS, and 2.5 million newly infected patients with AIDS. Over the last two decades, tremendous effort has been directed to the discovery and development of novel agents for the treatment of HIV infections and great successes have been achieved. So far, there are more than 20 approved anti-HIV drugs, belonging to seven classes [1].
Nucleoside reverse transcriptase inhibitors (NRTIs) are one of the most important classes of compounds active against HIV replication and have been used extensively to treat HIV infection [2]. However, the long term use of antiviral nucleoside analogs is inevitably associated with delayed toxicity and/or development of drug-resistant virus [3–6], which limits their effectiveness as an AIDS treatment. Therefore, there remains a need for the development of novel anti-HIV agents with better therapeutic indices, new mechanisms of action, and activity against HIV strains resistant to currently available drugs.
All clinical NRTIs belong to the family of 2′,3′-dideoxynucleoside (ddN) [7]. As the chain terminator of proviral DNA biosynthesis, the ddN structure has been assumed to be essential for an active nucleoside derivative. However, resistant HIV variants and other side effects have emerged. Resistance to these ddNs results from HIV mutants that have acquired the ability to discriminate between ddN and physiologic 2′-deoxynucleoside (dN), do not accept ddN into the active center of their reverse transcriptase (RT), and selectively cut off the incorporated ddN from their proviral DNA terminus. Therefore, nucleoside drugs that could prevent the emergence of drug-resistant HIV variants must have a 3′-OH as the chain terminator of proviral DNA biosynthesis.
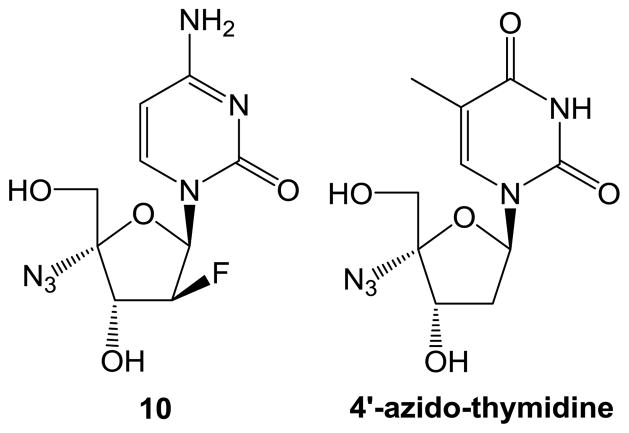
A 4′-C-substituted-2′-deoxynucleoside (4′sdN) satisfies these conditions, because 4′sdN has all of the functional groups of dN, making it difficult for HIV to discriminate between a 4′sdN and dN. At the same time, the 3′-OH group of 4′sdN would not be useful for elongation of proviral DNA biosynthesis. Thus, 4′sdN could be the chain-terminator of proviral DNA biosynthesis and active against HIV as well as HIV strains resistant to current ddN drugs. In addition, modification of the carbohydrate moiety of nucleosides with electron-withdrawing groups can greatly affect the nucleoside’s electronic properties and conformational shape [8–14], which often leads to dramatically improved activities. Therefore, we incorporated a fluorine atom, which has the strongest electron-withdrawing property of the halogens atoms, into our newly designed 4′sdN. Moreover, azido, cyano, and ethynyl groups, which have characteristic electronic and structural features due to their sp hybridization, are present in many biologically active compounds [15–17]. It has been proposed that the furanose ring of 4′-C-azidothymidine exhibits an unnatural 3′-C-endo conformation, and the compound was active against multidrug-resistant strains of HIV (EC50: 0.34 μM) [18]. Therefore, we were interested in designing novel 4′sdN compounds with such substitutions at the 4′-position. Herein, we report the synthesis and evaluation of 1-(4′-azido-2′-deoxy-2′-fluoro-β -D-arabinofuranosyl)cytosine (10) (Fig. 1) as a novel potent NRTI that couldovercome drug -resistanceproblems [ 19–21].
Fig. 1.
Structures of 1-(4′-Azido-2′-deoxy-2′-fluoro-β -D-arabinofuranosyl)cytosine (10) and Its Design Lead 4′-Azido-thymidine.
2. Chemistry
The synthesis of 10 is illustrated in Scheme 1. According to the reported method [8, 13, 19–25], treatment of commercially available 1,3,5-O-tribenzoyl-2-deoxy-2-fluoro-D-arabinofuranoside (1) with HBr-HOAc (45%) in dichloromethane (DCM) gave exclusively the α-bromide 2, which was coupled with silylated uracil in chloroform to provide the β -nucleoside analog 3 in a good yield after recrystallization. Deprotection of 3 with methanolic ammonia afforded nucleoside 4 in excellent yield. The 4′-azido substitution was introduced using the following procedure modified from the previously reported method [26]. Treatment of 4 with I2/Ph3P in tetrahydrofuran (THF) followed by elimination in the presence of sodium methoxide (NaOMe) gave 4′-methylene-nucleoside 6. Treatment of 6 with ICl/NaN3 in THF afforded 4′-azido-nucleoside 7. Benzoylation of 7 followed by treatment with m-chloroperbenzoic acid (m-CPBA) in the presence of m-chlorobenzoic acid (m-CBA) yielded a protected 4′-azido-uridine analog 9. Treatment of 9 with triazole/POCl3 followed by methanolic ammonia gave our target 10. Compound 10 was dissolved in methanol and treated with a solution of hydrogen chloride in ethyl acetate. The product separated as a solid and was collected by filtration to afford the hydochloride salt (11) of 10. The solid-state structure of 11 was determined by single-crystal X-ray crystallography (Fig. 2). Crystallographic data (excluding structure factors) for the structures in this paper have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication (nos. CCDC 740473). Copies of the data can be obtained, free of charge, on application to CCDC, 12 Union Road, Cambridge CB2 1EZ, UK, (fax: +44-(0)1223-336033 or e-mail: deposit@ccdc.cam.ac.uk).
Scheme 1.
Reagents and conditions: a) HBr/HOAc/DCM; b) silylated uracil(5-fluorouracil)/CHCl3; c) NH3/MeOH; d) Ph3P/I2/DMF; e) NaOMe/THF; f) ICl/NaN3/THF; g) BzCl/Pyr.DCM; h) mCPBA/mCBA/DCM; i) 1. triazole/POCl3/pyridine/DCM, 2. NH4OH, 3. NH3/MeOH; j) HCl, MeOH, EtOAc.
Fig. 2.

X-ray Crystallographic Structure of Hydrochloride Salt 11.
3. Results and discussion
The anti-HIV activity of 10 and 11 was first evaluated in vitro according to standard procedures using efavirenz (EFV) and zidovudine (AZT) as controls (Table 1) [27–31]. Compounds 10 and 11 exhibited extremely potent antiviral activity with EC50 values of 0.3 and 0.13 nM, respectively, compared with EFV (EC50: 1.2 nM) and AZT (EC50: 47 nM). The increased activity of 11 compared with 10 likely reflects its increased solubility of 11 in water [32]. Both compounds did not show significant cytotoxicity in concentrations up to 100 μM against the MT-2 cell line using the MTT assay. In the further evaluation of 11, we discovered that it retained its sub-nanomolar activity against drug-resistant HIV strains NL4-3 (K101E) and RTMDR (Table 2). K101E tends to decrease viral susceptibility to all nucleoside RT inhibitors, while RTMDR is a multiple RT inhibitor-resistant strain, which is insensitive to AZT, ddI, nevirapine, and other NNRTIs. In our screening, 11 exhibited extremely potent anti-HIV activity against NL4-3 (wild-type), NL4-3 (K101E), and RTMDR, with EC50 values of 0.086, 0.15, and 0.11 nM, respectively. These findings suggested that 11 has a great potential to be developed as a novel NRTI.
Table 1.
Anti-HIV activity of 10 and 11 against wild-type HIV-1 virus.
| Compound | EC50 (nM) (wild-type) a |
|---|---|
| 10 | 0.3 |
| 11 | 0.13 |
| EFV | 1.2 |
| AZT | 47 |
EC50 (nM) is the concentration that inhibits HIV by 50%. Results were averaged from at least two separate experiments.
Table 2.
Anti-HIV activity of 11 against NL4-3 (wild-type), NL4-3 (K101E), and RTMDR.
| Compound 11 | EC50 (nM) a |
|---|---|
| NL4-3 | 0.086 |
| NL4-3.K101E | 0.15 |
| HIV-1RTMDR | 0.11 |
EC50 (nM) is the concentration that inhibits HIV by 50%. Results were averaged from at least two separate experiments.
Therefore, in the following study, we further evaluated the anti-HIV potential of 11 using a panel of eleven NRTI resistant viral strains from the NIH Reagent Program. As shown in Table 3, 11 retained its nanomolar activity against resistant strains 7324-1, 7324-4, 7303-3, 35764-2, and 56252-1 with EC50 values of 0.595, 0.735, 0.56, 0.42, 0.525 nM, respectively, which were similar to that of 11 against the wild type NL4-3 (EC50: 0.318 nM). However, 11 partially or completely lost its nanomolar inhibitory activity against 10076-4, 7295-1, 4755-5, 6463-13, 1617-1, and 29129-2. After carefully analyzing the mutations of these resistant viral strains, we discovered that one single mutation M184V may lead to the substantially reduced antiviral activity of 11. This M184V mutation in RT was discovered previously to be 3TC treatment-associated, and it was also found that the M184V mutation increases the HIV sensitivity to AZT in AZT-resistant viruses, suggesting that compound 11 may be used in combination with AZT to reduce the influence of resistance issues.
Table 3.
Anti-HIV activity of compound 11 against NIH reagent program NRTI-resistant virus panel.
| NRTI Resistant Virus Panel | EC50 (nM) a |
|---|---|
| 7324-1 (M41L, D67N, K70R, T215F, K219E, T69N) | 0.595 |
| 7324-4 (M41L, K70R, T215F, K219E) | 0.735 |
| 10076-4 (M41L, T215Y, M184V) | >40,000 b |
| 7295-1 (D67N, K70R, T215F, K219Q, M184V, T69N) | >40,000 |
| 4755-5 (M41L, D67N, L210W, T215Y, M184V, T69D, E44D, V118I) | >40,000 |
| 6463-13 (M41L, D67N, L210W, T215Y, M184V, V118I) | >40,000 |
| 7303-3 (M41L, D67N, L210W, T215Y, T69D, E44D, V118I) | 0.56 |
| 1617-1 (K70G, M184V, T69K, V75I, F77L, F116Y, Q151M) | 32.2 |
| 35764-2 (V75I, F77L, F116Y, Q151M) | 0.42 |
| 29129-2 (M41L, D67N, L210W, T215Y, M184V) | >40,000 |
| 56252-1 (K70R, V75I, F77L, F116Y, Q151M, K65R) | 0.525 |
| NL4-3 (wild-type) | 0.318 |
EC50 (nM) is the concentration that inhibits HIV by 50%. Results were averaged from at least two separate experiments.
The highest concentration tested was 40,000 nM.
The cytotoxicity profiles of 11 were further evaluated using six human cell lines different from the previously used MT-2 cell line (Table 4). A Promega CellTiter-Glo Luminescent Cell Viability Assay was used to determine the number of viable cells based on quantitation of the presence of ATP, which signals the presence of metabolically active cells. Compound 11 showed little cytotoxicity against TZM-BL, HEPG-2, and 293T cells with IC50 values over 140 μM, resulting in therapeutic index (TI) values of >280,000. Comparatively, 11 showed some cytotoxicity against MT4, ACH-2, and PBMC cells with IC50 values of 0.12, 0.11, and 0.15 μM, and corresponding TI values of 1418, 1255, and 1709. Overall, 11 showed less toxicity to adherent cells compared to suspension cells. Nevertheless, the TI values were still greater than at least one thousand.
Table 4.
Cytotoxicity screening of compound 11 against six human cell lines.
| Cells | IC50 (μM) a |
|---|---|
| TZM-BL (derived from Hela Cells) | >140 b |
| HEPG-2 | >140 |
| 293T | >140 |
| MT4 | 0.12 |
| ACH-2 | 0.11 |
| PBMC (PHA activated) | 0.15 |
IC50 (μM) is the concentration that inhibits cell growth by 50%. Results were averaged from at least three separate experiments.
The highest concentration tested was 140 μM.
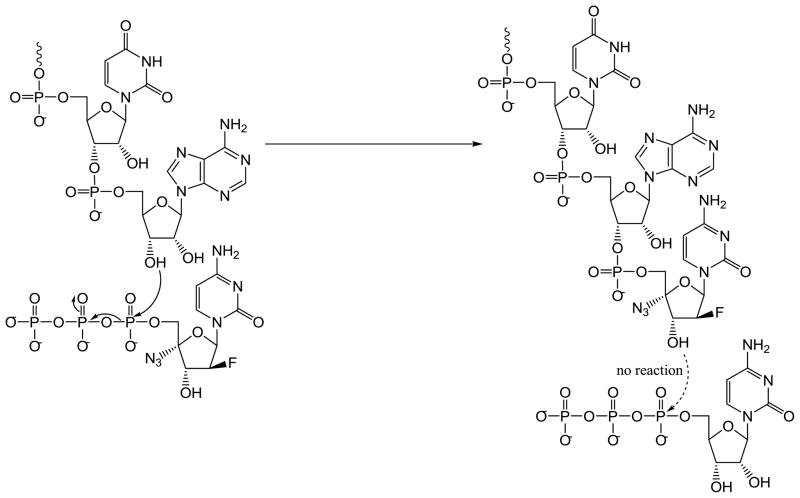
Regarding mechanism of action, compound 10 is likely to have a strong binding in the active site of HIV RT due to the introduction of fluorine at the 2′-position. Although compound 10 possesses a 3’-hydroxy group, it might act like a DNA chain terminator similar to 4’-azido thymidine (Fig. 3). Based on the drug profile shown in Table 3 against resistant virus strains, D67 and M184 are likely the two critical amino acid residues in the NRTI binding site of HIV-1 RT that interact intimately with the compound. NRTI resistant viruses with D67N or M184V mutation are markedly resistant to compound 10. Thus, the compound is likely to bind between the palm and the finger subdomains of HIV-1 RT and make critical interactions with residues including D67 and M184.
Fig 3.
Mechanism of Phosphorylated Compound 10.
4. Conclusion
In summary, novel 4′sdN compounds 10 and 11 were designed, synthesized, and evaluated for anti-HIV activity. The hydrochloride salt 11 retained its sub-nanomolar activity against NRTI-resistant (K101E) and multi-drug-resistant HIV strains (RTMDR). In further studies, 11 also showed nanomolar activity against resistant viral strains, except for the M184V single mutation. Compound 11 showed little cytotoxicity against adherent cells and marginal cytotoxicity against suspension cells with TI values over one thousand. Overall, compound 11 merits further development as an anti-AIDS clinical trial candidate.
5. Experimental
All chemical reagents were commercially available and column chromatography was performed on silica gel 200–300 mesh (Yantai Silica Gel Co. LTD). Analytical TLC was performed on silica gel GF254. Melting points were recorded with an XT-4 melting point apparatus and are uncorrected. IR spectra were obtained on a NICOLET 6700 spectrometer. 1H, 19F, and 13C NMR spectra were obtained with a Bruker AV-300 spectrometer. Chemical shifts are reported as δ (ppm) downfield with respect to an internal standard of tetramethylsilane (TMS). High-resolution mass spectra (HRMS-ESI) were obtained on a MicroTM Q-TOF Mass Spectrometer. Elemental analyses were performed with a Carlo-Erba 1106 C, H, and N analyzer. LC-MS spectra were measured on an Agilent MSD-1100 ESI-MS/MS system. X-ray crystallography was obtained on Rigaku Saturn 724 CCD diffractometer.
5.1. Synthesis of 1-(2-deoxy-2-fluoro-3,5-di-O-benzoyl-β-D-arabino-furanosyl)uracil (3)
To a solution of 1,3,5-O-tribenzoyl-2-deoxy-2-fluoro-D-arabinofuranoside (1) (20.0 g, 43.1 mmol) in anhydrous DCM (400 mL) was added HBr-HOAc (45% v/v, 50 mL, 271 mmol) dropwise under nitrogen at 0 C and the resulting solution was stirred at room temperature for 20 h. The solution was washed with saturated NaHCO3 (3×100 mL), dried over Na2SO4, filtered, and concentrated to give compound 2 as syrup, which was used in the next step without further purification.
A mixture of uracil (12.6 g, 113.0 mmol) and (NH4)2SO4 (1.57 g) in hexamethyldisilazane (HMDS) (1.5 L) was refluxed under nitrogen for 20 h, and the homogeneous solution obtained was evaporated to dryness under vacuum to give the silylated uracil. To the silylated uracil residue was added a solution of compound 2 in CHCl3 (800 mL) and the mixture was refluxed under nitrogen for 24 h. The reaction was quenched by addition of ice-water and filtered. The aqueous layer was extracted with DCM (3×200 mL) and the combined organic solution was washed with brine, dried over Na2SO4, filtered, and concentrated to give a white solid, which was recrystallized with 5% EtOH-DCM to give compound 3 (16.7 g, 84.2%). 1H NMR (300 MHz, CDCl3) δ 8.35 (1H, brs, NH), 7.43-8.11 (11H, m, Ar-H, H-6), 6.33 (1H, dd, J = 21.59, 2.93 Hz, H-1′), 5.69 (1H, d, J = 8.05 Hz, H-5), 5.63 (1H, dd, J = 17.20, 2.93 Hz, H-3′), 5.34 (1H, dd, J = 50.13, 2.93 Hz, H-2′), 4.78 (2H, d, J = 4.39 Hz, H-5′), 4.52 (1H, m, H-4′); ESI- MS: m/z 455 [M+H]+; Anal. calcd (C23H19FN2O7): C, 60.79; H, 4.21; N, 6.16; found: C, 60.75; H, 4.24; N, 6.11.
5.2. Synthesis of 1-(2-deoxy-2-fluoro-β-D-arabinofuranosyl)uracil (4)
A solution of compound 3 (7.79 g, 17.1 mmol) in saturated NH3-MeOH (200 mL) was stirred at room temperature for 24 h and evaporated to dryness under reduced pressure. DCM (25 mL) was added to the residue and the mixture was stirred at room temperature for 1 h. The white solid was collected by filtration to give compound 4 (3.93 g, 93.0%). 1H NMR (300 MHz, DMSO-d6) δ 11.35 (1H, brs, NH), 7.71 (1H, dd, J = 8.05, 1.46 Hz, H-6), 6.10 (1H, dd, J = 16.10, 4.39 Hz, H-1′), 5.87 (1H, d, J = 5.12 Hz, 3′-OH), 5.64 (1H, d, J = 8.05 Hz, H-5), 5.08 (1H, t, J = 5.85 Hz, 5′-OH), 5.03 (1H, ddd, J = 52.69, 4.03, 2.93 Hz, H-2′), 4.21 (1H, dm, J = 19.76 Hz, H-3′), 3.79 (1H, m, H-4′), 3.60 (2H, m, H-5′); ESI-MS: m/z 269 [M+Na]+; Anal. calcd (C9H11FN2O5): C, 43.91; H, 4.50; N, 11.38;found : C, 43.90; H, 4.29; N, 11.33.
5.3. Synthesis of 1-(2,5-dideoxy-2-fluoro-5-iodo-β-D-arabino-furanosyl)uracil (5)
To a solution of compound 4 (3.73 g, 15.2 mmol), imidazole (2.06 g, 30.3 mmol), and triphenylphosphine (5.96 g, 22.7 mmol) in THF (100 mL) was added a solution of iodine (5.77 g, 22.7 mmol) in THF (50 mL) dropwise at 0 °C. The reaction mixture was stirred at room temperature overnight and quenched with a saturated solution of Na2SO3, extracted with EtOAc (2×100 mL). The combined organic solution was washed with water, brine, dried over Na2SO4, filtered, and concentrated to yield a thick oil, which was purified by silica gel column chromatography (1–5% MeOH in DCM) to afford compound 5 (4.51 g, 83.5%). 1H NMR (300 MHz, DMSO–d6) δ 11.50 (1H, br s, NH), 7.58 (1H, dd, J = 8.14, 1.95 Hz, H-6), 6.17 (1H, dd, J = 18.40, 3.99 Hz, H-1′), 6.15 (1H, br, 3’-OH), 5.67 (1H, d, J = 8.14Hz, H-5), 5.08 (1H, ddd, J = 52.41, 3.84, 2.55 Hz, H-2′), 4.14 (1H, ddd, J = 19.68, 4.21, 2.65 Hz, H-3′), 3.87 (1H, dd, J = 11.02, 5.19 Hz, H-4′), 3.43-3.57 (m, 2H); 13C NMR (75 MHz, DMSO-d6) δ 6.2, 76.7, 82.0, 83.0, 95.3, 101.4, 141.2, 150.0, 162.8; ESI-MS: m/z 379 [M+23]+; Anal. calcd (C9H10FIN2O4): C, 30.36; H, 2.83; N, 7.87; found: C, 30.30; H, 2.88; N, 7.84.
5.4. Synthesis of 1-(2,5-dideoxy-2-fluoro-β-D-arabino-4-eno-furanosyl)uracil (6)
A solution of compound 5 (4.2 g, 11.8 mmol) in dry MeOH (40 mL) containing MeONa (25%wt in MeOH, 10.2 mL, 47.3 mmol) was heated to 65 °C for 3 h and an additional aliquot of MeONa (25%wt in MeOH, 3.4 mL) was added. After 30 min, the reaction mixture was allowed to stay at room temperature overnight and brine was added. The mixture was adjusted to pH 3 by addition of 1N HCl and the mixture was extracted with EtOAc (2×300 mL). The combined organic solution was dried over Na2SO4, filtered, and concentrated. The residue was purified by silica gel column chromatography (2–5% MeOH in DCM) to afford compound 6 (1.94 g, 72.1%). 1H NMR (300 MHz, CDCl3) δ 9.05(1H, br s, NH), 7.32 (1H, dd, J = 8.16, 2.31 Hz, H-6), 6.62 (1H, dd, J = 20.97, 2.79 Hz, H-1′), 5.76 (1H, d, J = 8.22Hz, H-5), 5.09 (1H, dd, J = 51.44, 2.95 Hz, H-2′), 4.76 (1H, d, J = 2.74 Hz, =CH2 ), 4.73 (1H, d, J = 11.46 Hz, H-3′), 4.52 (1H, d, J =2.74Hz, =CH2); ESI-MS: m/z 227 [M-H]+; Anal. calcd (C9H9FN2O4): C, 47.37; H, 3.98; N, 12.28;found : C, 47.43; H, 3.86; N, 12.13.
5.5. Synthesis of 1-(4-azido-2,5-dideoxy-2-fluoro-5-iodo-β-D-arabino-furanosyl)uracil (7)
To a suspension of NaN3 (1.38 g, 21.3 mmol) in THF (50 mL) at 0 °C was added ICl (2.31 g, 14.2 mmol). After 10 minutes, a solution of compound 6 (1.62 g, 7.10 mmol) in THF (16 mL) was added dropwise to the mixture and the reaction mixture was stirred at room temperature overnight. Aqueous Na2SO3 solution was added and the mixture extracted with EtOAc (3×250 mL). The combined organic solution was dried over Na2SO4, filtered, and concentrated. The residue was purified by silica gel column chromatography (1–5% MeOH in DCM) to afford compound 7 (1.82 g, 64.6%). IR KBr): 2119 cm−1(N3); 1H NMR (300 MHz, MeOH-d4) δ 7.68 (1H, dd, J= 8.1 6, 1.87 Hz, H-6), 6.46 (1H, dd, J= 16.07 , 4.39 Hz, H-1′), 5.74(1H, d, J= 8. 12 Hz, H-5), 5.22 (1H, ddd, J = 52.93, 4.36, 3.30 Hz, H-2′), 4.65 (1H, dd, J = 18.67, 3.31 Hz, H-3′), 3.69 (2H, m, H-5′); ESI-MS: m/z 420 [M+23]+; Anal. calcd (C9H9FIN5O4): C, 27.22; H, 2.28; N, 17.64;found : C, 27.09; H, 2.23; N, 17.73.
5.6. Synthesis of 1-(4-azido-2,5-dideoxy-2-fluoro-3-O-benzoyl-5-iodo-β-D-arabinofuranosyl)uracil (8)
To a solution of compound 7 (1.17 g, 2.95 mmol) in DCM (130 mL) were added Et3N (0.82 mL, 5.90 mmol) and a catalytic amount of 4-dimethylaminopyridine. The reaction mixture was cooled to 0° C and benzyl chloride (0.41 mL, 3.54 mmol) was added. After 20 min, water and 2M K2CO3 were added, and the mixture was extracted with EtOAc (400 mL). The organic layer was washed with brine (2×50 mL), dried over Na2SO4, filtered, and concentrated. The residue was purified by silica gel column chromatography (0.1–1% MeOH in DCM) to afford compound 8 (1.2 g, 81.2%). IR (KBr): 2118 cm−1(N3); 1H NMR (300 MHz, DMSO-d6) δ 11.63 (1H, br s, NH), 7.57–8.09 (6H, m, Ar-H and H-6), 6.52 (1H, dd, J = 18.69, 5.52 Hz, H-1′), 6.07 (1H, dd, J = 21.57, 4.05 Hz, H-3′), 5.82 (1H, ddd, J = 52.21, 5.32, 4.38 Hz, H-2′), 5.78 (1H, d, J = 8.14 Hz, H-5), 3.99 (2H, q, J = 11.66 Hz, H-5′); ESI-MS: m/z 524 [M+23]+; Anal. calcd (C16H13FIN5O5): C, 38.34; H, 2.61; N, 13.97;found : C, 38.26; H, 2.48; N, 13.85.
5.7. Synthesis of 1-(4-azido-2-deoxy-2-fluoro-3-O-benzoyl-5-O-(3-chlorobenzoyl)-β-D-arabinofuranosyl)uracil (9)
To a solution of 8 (1.10 g, 2.19 mmol) in DCM (100 mL) and water (60 mL) were added successively K2HPO4 (0.76 g, 4.38 mmol), n-Bu4NHSO4 (0.82 g, 2.41 mmol), and m-CBA (0.38 g, 2.41 mmol). The reaction mixture was cooled to 0 °C and m-CPBA (77%, 1.47 g, 6.57 mmol) was added. The reaction mixture was stirred at room temperature overnight. Na2SO3 was added and the mixture was extracted with EtOAc (500 mL). The combined organic solution was washed with Na2SO3 (5%), brine, dried over Na2SO4, filtered, and concentrated. The residue was purified by silica gel column chromatography (0.1–0.5% MeOH in DCM) to provide compound 9 (667 mg, 57.5%). IR (KBr): 2117 cm−1 (N3); 1H NMR (300 MHz, CDCl3) δ 7.41–8.10 (10H, m, Ar-H and H-6), 6.63(1H, dd, J = 18.36, 3.61 Hz, H-1′), 5.73–5.84(2H, m, H-3′ and H-5), 5.45 (1H, ddd, J = 51.37, 3.55, 2.03 Hz, H-2′), 4.86 (2H, q, J = 11.87 Hz, H-5′); ESI-MS: m/z553 [M+23] +;Anal. c alcd (C23H17FClN5O7): C, 52.14; H, 3.23; N, 13.22;found : C, 52.03; H, 3.22; N, 13.00.
5.8. Synthesis of 1-(4-azido-2-deoxy-2-fluoro)-β-D-arabinofuranosyl)cytosine (10)
To a solution of compound 9 (0.40 g, 0.75 mmol) in DCM (15 mL) was added 1,2,4-triazole (0.57 g, 8.25 mmol) followed by pyridine (0.67 mL, 8.25 mmol). The solution was cooled to 0 °C and POC13 (0.39 mL, 3.75 mmol) was added. The solution was stirred at 0 °C for 2 h then room temperature overnight. The reaction was quenched with water and the mixture was extracted with DCM (2×250 mL). The combined organic solution was dried over Na2SO4, filtered, and concentrated. The residue was dissolved in THF (40 mL) and NH4OH (33%, 40 mL) was added. The reaction mixture was stirred at room temperature for 1 h. Solvent was removed and the residue was dissolved in MeOH (40 mL) and 7N NH3 in MeOH (40 mL) was added. The solution was stirred at room temperature overnight. The solution was concentrated to dryness under reduced pressure and the residue was purified by silica gel column chromatography (1–5% MeOH in DCM) to give compound 10 (92 mg, 41.1%) as a white solid. mp: 86–87 °C; IR (KBr): 2118 cm−1 (N3); 1H NMR (300 MHz, MeOH-d4) δ 7.80 (1H, d, J = 7.57Hz, H-6), 6.50 (1H, dd, J = 12.10, 4.67 Hz, H-1’), 5.97 (1H, d, J = 7.57Hz, H-5), 5.21 (1H, dt, J = 53.52, 4.48 Hz, H-2′), 4.49 (1H, dd, J = 21.63, 4.34 Hz, H-3′), 3.84 (2H, s, H-5′); 13C NMR (75.47 MHz, MeOH-d4) δ 63.4, 76.5 (J = 25.2 Hz), 84.8 (J = 17.2 Hz), 96.1, 96.2 (J = 193.4 Hz), 98.6 (J = 7.5 Hz), 143.0, 157.9, 167.8; 19F NMR (282.4 MHz, MeOH-d4): -202.5; ESI-TOF/MS for C9H11FN6O4: calcd 287.0904 [M+H]+; found: 287.0905[M+H]+; Anal. calcd (C9H11FN6O4): C, 37.77; H, 3.87; N, 29.36; found: C, 37.53; H, 3.90; N, 29.19.
5.9. Synthesis of 1-(4-azido-2-deoxy-2-fluoro)-β-D-arabinofuranosyl)cytosine hydrochloride salt (11)
Compound 10 (50 mg, 0.17 mmol) was dissolved in MeOH (1 mL) and treated with a solution of hydrogen chloride (20 mmol) in EtOAc (10 mL). The product separated as a solid and was collected by filtration to afford the hydrochloride salt of compound 10 (34 mg, 61.1%). IR (KBr): 2117 cm−1 (N3); 1H-NMR (D2O) δ 7.94 (1H, dd, J = 8.05, 1.10 Hz, H-6), 6.54 (1H, dd, J = 10.98, 5.12 Hz, H-1′), 6.26 (1H, d, J = 8.05 Hz, H-5), 5.39 (1H, dt, J = 52.69, 5.12 Hz, H-2′), 4.58 (1H, dd, J = 21.59, 4.76 Hz, H-3′), 3.97 (2H, q, J = 12.66 Hz, H-5′); ESI-MS: m/z 287 [M+H]+.
5.10. Single-crystal X-ray diffraction analysis of hydrochloride salt 11
C9H11FN6O4·HCl, fw = 322.70, Monoclinic P2(1), a = 6.5738(13) Å, b = 14.309(3) Å, c = 7.3271(15) Å, α = γ = 90°, β = 99.68(3)°, V = 679.4(2) Å3,Z = 2, ρcalcd = 1.577 Mg/m3, λ(Mo-Kα)= 0.71073Å, μ = 0.320 mm−1, F(000) = 332, T = 293(2). A colorless 0.22 × 0.18 × 0.14 mm3 crystal was used for data collection. The theta range for data collection was 3.14 to 26.01° with 2633 reflections collected.
5.11. Anti-HIV (wt) activity assay
Vesicular stomatitis virus glycoprotein (VSV-G) plasmid was co-transfected with env-deficient HIV vector, pNL4-3.luc.R−E− [24, 25], into 293 cells by using modified Ca3(PO4)2 method.26 Briefly, 293 cells (100 mm plate) were transfected with 8 μg HIV vector alone or with 3 μg VSVG DNA. After 16 h, plates were washed with phosphate-buffered saline, and fresh medium was added into the plates. After post-infection for 48 h, the supernatant was harvested and filtered through a 0.45 μm filter. The supernatant contains VSVG/HIV pseudotyped virions and can be used directly or stored at −80 °C.
One hour prior to infection, MT-2 cells were plated on 24-well plates at the density of 1.2×105 cells per well. Compound was incubated with target cells for 15 minutes prior to adding VSVG/HIV-1 (0.5 mL/well) for infection. The same amount of solvent alone was used as control. After post-infection for 48 h, cells were spin down and supernatant was discarded. Cells were lysed in 50 μl Cell Lysis Reagent (Promega). Luciferase activity of the cell lysate was measured by a FB15 luminometer (Berthold Detection System) according to the manufacturer’s instructions. Compounds 10 and 11 showed dose-dependent inhibition activity on HIV-1 replication with EC50 values of 0.3 nM and 0.13 nM, respectively, and thus, were more potent than AZT (EC50: 47 nM).
5.12. Cell viability assay
Compounds 10 and 11 were evaluated for cell viability by MTT methods [27]. MTT assay was performed in MT-2 cell line. Both compounds had no significant effect on cell growth or any cytotoxic effect on MT-2 cells at a final concentration of 100 μM.
5.13. Anti-HIV replication assay against NL4-3 (K101E) and RTMDR strains in TZM-bl cell lines
A previously described HIV-1 infectivity assay was used in the experiments [28]. A 96-well cell culture plate was used to set up the virus replication screening sassy. NL4-3 (K101E) or RTMDR at a multiplicity of infection (MOI) of 0.001 was used to infect TZM-bl cells. Culture supernatants were collected on day 4 post-infection for a p24 assay using an ELISA kit from PerkinElmer.
Acknowledgments
J. Chang thanks the National Natural Science Foundation of China (#20672030; #30825043) for financial support. Thanks are also due to a grant AI-33066 from the National Institute of Allergy and Infectious Diseases (NIAID) awarded to K.H. Lee.
Abbreviations
- IR
infrared
- NMR
nuclear magnetic resonance
- HRMS
high resolution mass spectrometry
- RT
reverse transcriptase
- RTMDR
reverse transcriptase multi-drug resistant
- NRTI
nucleoside reverse transcriptase inhibitor
- NNRTI
non-nucleoside reverse transcriptase inhibitor
- HIV
human immunodeficiency virus
- AIDS
acquired immunodeficiency syndrome
- dN
2′-deoxynucleoside
- ddN
2′,3′-dideoxynucleoside
- 4′sdN
4′-C-substituted-2′-deoxynucleoside
- DCM
dichloromethane
- THF
tetrahydrofuran
- m-CPBA
m-chloroperbenzoic acid
- m-CBA
m-chlorobenzoic acid
- EFV
efavirenz
- AZT
zidovudine
- HMDS
hexamethyldisilazane
- VSV-G
vesicular stomatitis virus glycoprotein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.De Clercq E. Nat Rev Drug Discov. 2007;6:1001–1018. doi: 10.1038/nrd2424. [DOI] [PubMed] [Google Scholar]
- 2.Sharma PL, Nurpeisov V, Hernandez-Santiago B, Beltran T, Schinazi RF. Curr Top Med Chem. 2004;4:895–919. doi: 10.2174/1568026043388484. [DOI] [PubMed] [Google Scholar]
- 3.Brinkman K, ter Hofstede HJ, Burger DM, Smeitink JA, Koopmans PP. AIDS. 1998;12:1735–1744. doi: 10.1097/00002030-199814000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Chen CH, Cheng YC. J Biol Chem. 1989;264:11934–11937. [PubMed] [Google Scholar]
- 5.Shulman N, Winters M. Curr Drug Targets Infect Disord. 2003;3:273–281. doi: 10.2174/1568005033481024. [DOI] [PubMed] [Google Scholar]
- 6.Lewis W, Day BJ, Copeland WC. Nat Rev Drug Discov. 2003;2:812–822. doi: 10.1038/nrd1201. [DOI] [PubMed] [Google Scholar]
- 7.Ohrui H, Kohgo S, Kitano K, Sakata S, Kodama E, Yoshimura K, Matsuoka M, Shigeta S, Mitsuya H. J Med Chem. 2000;43:4516–4525. doi: 10.1021/jm000209n. [DOI] [PubMed] [Google Scholar]
- 8.Watanabe KA, Reichman U, Hirota K, Lopez C, Fox JJ. J Med Chem. 1979;22:21–24. doi: 10.1021/jm00187a005. [DOI] [PubMed] [Google Scholar]
- 9.Hertel LW, Kroin JS, Misner JW, Tustin JM. J Org Chem. 1988;53:2406–2409. [Google Scholar]
- 10.Marquez VE, Tseng CKH, Mitsuya H, Aoki S, Kelley JA, Ford H, Jr, Driscoll JS. J Med Chem. 1990;33:978–985. doi: 10.1021/jm00165a015. [DOI] [PubMed] [Google Scholar]
- 11.Pai SB, Liu SH, Zhu YL, Chu CK, Cheng YC. Antimicrob Agents Chemother. 1996;40:380–386. doi: 10.1128/aac.40.2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chun BK, Schinazi RF, Cheng YC, Chu CK. Carbohydr Res. 2000;328:49–59. doi: 10.1016/s0008-6215(99)00312-2. [DOI] [PubMed] [Google Scholar]
- 13.Klumpp K, Kalayanov G, Ma H, Pogam SL, Leveque V, Jiang WR, Inocencio N, Witte AD, Rajyaguru S, Tai E, Chanda S, Irwin MR, Sund C, Winqist A, Maltseva T, Eriksson S, Usova E, Smith M, Alker A, Najera I, Cammack N, Martin JA, Johansson NG, Smith DB. J Biol Chem. 2008;283:2167–2175. doi: 10.1074/jbc.M708929200. [DOI] [PubMed] [Google Scholar]
- 14.Schaerer OD, Verdine GL. J Am Chem Soc. 1995;117:10781–10782. [Google Scholar]
- 15.Minakawa N, Takeda T, Sasaki T, Matasuda A, Ueda T. J Med Chem. 1991;34:778–786. doi: 10.1021/jm00106a045. [DOI] [PubMed] [Google Scholar]
- 16.Siddiqui MA, Hughes SH, Boyer PL, Mitsuya H, Van QN, George C, Sarafinanos SG, Marquez VE. J Med Chem. 2004;47:5041–5048. doi: 10.1021/jm049550o. [DOI] [PubMed] [Google Scholar]
- 17.Graul A, Rabasseda X, Castaner J. Drugs Future. 1998;23:133–141. [Google Scholar]
- 18.Maag H, Rydzewski RH, McRberts MJ, Crawford-Ruth D, Verheyden JPH, Prisbe EJ. J Med Chem. 1992;35:1440–1451. doi: 10.1021/jm00086a013. [DOI] [PubMed] [Google Scholar]
- 19.Chang J, Bao X, Wang Q, Guo X, Wang W, Qi X. Preparation of 2′-fluoro-4′-substituted nucleoside analogs as antiviral agents. 20070807. Chinese Patent Application No: CN 2007-10137548. Chinese Patent No: CN 101177442A, 20080514.
- 20.Chang J. 2′-Fluoro-4′-substituted nucleosides, the preparation and use. 20080627. International Application No: PCT/CN2008/001239. International Patent No: WO2009009951, 20090122.
- 21.Chang J. 2′-Fluorine-4′-substituted nucleoside analogues, preparation methods and uses thereof. US 2010/0234584A1, 20100916 US Patent.
- 22.Ma T, Pai SB, Zhu YL, Lin JS, Shanmuganathan K, Du J, Wang C, Kim H, Newton MG, Cheng YC, Chu CK. J Med Chem. 1996;39:2835–2843. doi: 10.1021/jm960098l. [DOI] [PubMed] [Google Scholar]
- 23.Jin YH, Bae M, Byun YJ, Kim JH, Chun MW. Arch Pharm Res. 1995;18:364–365. [Google Scholar]
- 24.Smith DB, Kalayanov G, Sund C, Wingvist A, Maltseva T, Leveque VJP, Rajyaguru S, Pogam SL, Najera I, Benkestock K, Zhou XX, Kaiser AC, Maag H, Cammack N, Martin JA, Swallow S, Johansson NG, Klumpp K, Smith M. J Med Chem. 2009;52:2971–2978. doi: 10.1021/jm801595c. [DOI] [PubMed] [Google Scholar]
- 25.Pharmasset Inc. 2′,4′-Substituted nucleosides as antiviral agents. WO2009067409, 20090528. International Patent. US Patent No: US 20090318380, 20091224.
- 26.Smith DB, Martin JA, Klumpp K, Baker SJ, Blomgren PA, Devos R, Granycome C, Hang J, Hobbs CJ, Jiang WR, Laxton C, Pogam SL, Leveque V, Ma H, Maile G, Merrett JH, Pichota A, Sarma K, Smith M, Swallow S, Symons J, Vesey D, Najera I, Cammack N. Bioog Med Chem Lett. 2007;17:2570–2576. doi: 10.1016/j.bmcl.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 27.He J, Choe S, Walker R, Di Marzio P, Morgan DO, Landau NR. J Virol. 1995;69:6705–6711. doi: 10.1128/jvi.69.11.6705-6711.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Connor RI, Chen BK, Choe S, Landau NR. Virology. 1995;206:935–944. doi: 10.1006/viro.1995.1016. [DOI] [PubMed] [Google Scholar]
- 29.Rong L, Bates P. J Virol. 1995;69:4847–4853. doi: 10.1128/jvi.69.8.4847-4853.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carmichael J, DeGraff WG, Gazdar AF, Minna JD, Mitchell JB. Cancer Res. 1987;47:943–946. [PubMed] [Google Scholar]
- 31.Qian K, Yu D, Chen CH, Huang L, Morris-Natschke SL, Nitz TJ, Salzwedel K, Reddick M, Allaway GP, Lee KH. J Med Chem. 2009;52:3248–3258. doi: 10.1021/jm900136j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.The solubility of compound 10 is 3.1 g in 100 g water. The solubility of compound 11 is 14.3 g in 100 g water.