Summary
Over the past 20 years there has been a concerted effort in the United States to reduce morbidity related to chronic disease including asthma. Attention was initially directed towards asthma in response to the recognition that asthma mortality was increasing and that the burden of disease was significant. These efforts to address asthma mortality led to many new initiatives to develop clinical practice guidelines, implement the asthma guidelines into clinical practice, conduct research to fill the gaps in the guidelines, and to continuously revise the asthma guidelines as more information became available. An assessment of our progress shows significant accomplishments in relation to reducing asthma mortality and hospitalizations.
Consequently, we are now at a crossroads in asthma care. Although we have recognized some remarkable accomplishments in reducing asthma mortality and morbidity, the availability of new tools to monitor disease activity, including biomarkers and epigenetic markers, along with information technology systems to monitor asthma control hold some promise in identifying gaps in disease management. These advances should prompt the evolution of new strategies and new treatments to further reduce disease burden. It now becomes imperative to continue a focus on ways to further reduce the burden of asthma and prevent its onset.
Keywords: Asthma, childhood asthma, asthma therapy, asthma statistics, asthma management, asthma guidelines, asthma disease management, inhaled corticosteroids, long-acting β-adrenergic agonists, leukotriene receptor antagonists, omalizumab, asthma surveillance, asthma mortality, asthma hospitalizations, asthma exacerbations, asthma progression, personalized medicine, public health
Introduction
Over the past 20 years there has been a concerted effort in the United States to reduce morbidity related to chronic disease including asthma. Attention was initially directed towards asthma in response to the recognition that asthma mortality was increasing and that the burden of disease was significant. These efforts to address asthma mortality led to many new initiatives to develop management guidelines, implement the asthma guidelines into clinical practice, conduct research to fill the gaps in the guidelines, and to continuously revise the asthma guidelines as more information became available.
The Journal has also participated in this effort to improve asthma care by publishing numerous original articles, along with review articles and editorials to draw attention to the advances in asthma research and to discuss current controversies. For the past 10 years, the Journal has also published annual reviews on advances in childhood and adult asthma in order to summarize the key publications from each year, especially from the Journal (1, 2).
Since the initial publication related to increased asthma mortality in New Zealand (3), approximately 30 years ago, many changes have occurred to organize asthma care within countries and globally. The first version of asthma guidelines in the United States was introduced in 1991. Therefore, twenty years later is a good time to appraise where we are in asthma care and what steps need to be taken to further improve asthma management in children and adults. Indeed, an assessment of our progress shows significant accomplishments and it now becomes imperative to continue a focus on ways to further reduce the burden of asthma and prevent its onset.
Pathway to success
Reports in the early 1980s brought attention to increasing mortality and this was a wakeup call to redirect asthma management (3). Over the next ten years, studies utilizing bronchoscopy and biopsy indicated that asthma was more than acute inflammation secondary to allergen exposure or simply bronchospasm related to asthma triggers, such as an environmental irritant or exercise. These studies indicated that asthma was also a chronic inflammatory disease. The approach to asthma management transitioned quickly from treatment that utilized predominantly bronchodilator-based management (theophylline) to one directed at anti-inflammatory (inhaled corticosteroid) therapy. National guidelines were introduced in several countries in order to consolidate principles of asthma care and transition the treatment approach to a base of anti-inflammatory therapy.
Asthma Guidelines
During the 1990s, a number of important developments occurred including the introduction of asthma guidelines to translate research findings into clinical practice, more effective medications, and the introduction of managed care. The first publication of asthma guidelines from the National Asthma Education and Prevention Program (NAEPP) was introduced in the United States in 1991 (4), followed by the development of International Guidelines in 1992 (5), and consequently the Global Initiative for Asthma (GINA) guidelines in 1995 (6). This was an attempt to unify treatment within countries and then to collaborate on a uniform approach to asthma management in countries with a similar socioeconomic base. The global guidelines took into account the varying economies and access to medication. Since that time, there have been continuing revisions of the asthma guidelines with the most recent version of the United States guidelines released in 2007 (7). These revised guidelines emphasized asthma control and put forth two domains of control, impairment to assess current symptoms and risk to assess potential for future exacerbations, asthma progression and adverse medication effects (7).
New Medications
Many new medications for the management of asthma were introduced in the United States during the 1990s. These medications included more potent inhaled corticosteroids (budesonide, fluticasone), long-acting β-adrenergic agonists [LABA] (salmeterol, formoterol), leukotriene modifiers (zafirlukast, zileuton, montelukast), and subsequently combination therapy with inhaled corticosteroids (ICS) and LABA in a single delivery device. The efficacy of these new medications was remarkable, especially combination ICS/LABA therapy, and physicians increasingly recognized the ability of these new medications to reduce symptoms, exacerbations and systemic steroid requirements. Concern has been raised regarding potential increased risk of severe asthma-related exacerbations with LABA therapy and attempts have been made to limit use of LABA therapy (8). The only new medication that has been introduced in the past 10 years has been omalizumab, anti-IgE. This medication, although very effective in a proportion of patients with severe asthma, has limited application due to high cost.
Ongoing Management
Along with the introduction of guidelines and new asthma medications, the concept of asthma management consisting of a multi-faceted, ongoing approach to prevent exacerbations was introduced and implemented during the 1990s and dramatically changed the landscape for medical care. This period of time saw the merging of single physician practices into multiple physician practices, and subsequently to multi-specialty clinics. A major goal was to reduce inpatient care and thus to reduce hospital costs. In the past ten years, increased attention has been placed on the management of asthma in children. This was prompted by the recognition that inhaled corticosteroids as compared to as-needed short-acting β-agonists had a significant effect on improving asthma control (9). In addition, adverse effects of inhaled corticosteroids were limited to a small reduction in growth velocity during the first year of treatment (10). This effect appears to be persistent but not progressive (10). In regards to the efficacy of ICS in asthma management, they have been shown to adequately control symptoms during the course of treatment but symptoms recur when treatment is stopped. However, ICS did not significantly alter the course of the disease based on this study (9).
With the recognition that management with asthma needs to be an ongoing rather than an intermittent effort, support programs have been developed to assist patients in symptom monitoring and to provide asthma education through various resources. The certification of asthma educators has also established some consistency in information that is provided to patients including features of environmental control. With the introduction of new medications and new insights in disease development, efforts have now been enhanced to increase awareness of asthma and provide asthma education for clinicians and patients. Community programs have emerged to support health care advances including school programs. In addition, efforts have been made to expand information related to work related asthma (11, 12). Healthy People 2000 goals made asthma a national priority (13). The objectives of Healthy People 2000 included: reduction in asthma hospitalizations (objective 11.1) and reduction to no more than 10% the proportion of people with asthma who experience activity limitation (objective 17.4) (14). Asthma-specific Healthy People goals for 2010 were expanded considerably to promote and track adoption of appropriate asthma care as established in the NAEPP Expert Panel Report guidelines and self-management education (15). The asthma objectives have been expanded and updated for the recently released Healthy People 2020 goals (http://www.healthypeople.gov/2020/about/default.aspx).
A major public health response has been directed to actions at the state and community level to support patient- and community-level interventions and to assess the impact of the environment on asthma (16 – 18) by the Centers for Disease Control and Prevention (CDC), the National Heart, Lung and Blood Institute (NHLBI), and the Environmental Protection Agency (EPA) and other federal agency partners. The CDC National Asthma Control Program (NACP) was created in 1999 to implement evidence-based interventions at the state and local level, forge partnerships between federal agencies, state health departments and nongovernmental organizations to support asthma programs in multiple settings (hospital systems, city health departments, school systems, and local chapters of national asthma organizations), and to establish national and state asthma surveillance (19). In 2001, the Controlling Asthma in American Cities Project (CAACP) began and by 2009, the NACP supported 34 states, Washington, DC and Puerto Rico with grants and technical assistance to build and sustain programs that translate evidence-based practice into interventions. For example, the NACP in partnership with the Task Force on Community Preventive Services systematically reviewed the evidence of effectiveness of home-based multi-trigger multi-component environmental interventions in improving asthma-related morbidity. Findings indicated this approach is effective in improving overall quality of life and productivity in children with asthma, and this home-based multifaceted approach is now being implemented on the state and local level with planned evaluation of its impact.
The National Asthma Education and Prevention Program (NAEPP), sponsored by the NHLBI not only develops the clinical practice guidelines, but also supports a number of activities to enhance education of health care professionals, patients, and the public about managing asthma. The NAEPP’s National Asthma Control Initiative (NACI) (http:://naci.nhlbi.nih.gov), launched last year, is an effort to accelerate adoption of the key messages of the clinical practice guidelines in clinical practice, community settings, and among government and private insurer programs. The NACI program sponsors demonstration projects at the community level to develop educational tools, community champions, and partnership programs with professional societies and lay organizations; the website and newsletter gives up-to-date information about these projects.
The EPA supports a number of activities to enhance the integration of tailored environmental interventions to reduce exposures that worsen asthma with comprehensive medical care and community services for people who have asthma. Their website, www.AsthmaCommunityNetwork.org <http://www.AsthmaCommunityNetwrok.org>, offers community based asthma programs and health practitioners a forum for exchanging information about and share resources for improving asthma care, including Internet tools to facilitate collaboration, problem solving, and sharing program activities and news about asthma-related events. The website is sponsored by EPA in partnership with Allies Against Asthma and the Merck Childhood Asthma Network.
Surveillance
Population level surveillance has also developed in an attempt to identify trends in asthma mortality and morbidity at both the national and local level. The national surveillance program has also been created to systematically track prevalence, morbidity, health care use and mortality. The first asthma surveillance summary was published in a Morbidity and Mortality Weekly Report (MMWR) supplement series (20) in 1998. It brought together in one publication existing national data on asthma prevalence, morbidity and mortality. At that time there was no state level data on asthma and minimal national level data. State and local level surveillance was initiated in 1999 (19).
In 1999, the Behavioral Risk Factor Surveillance System (BRFSS) added questions about asthma control and medication use. In 2001 additional detail on adult asthma control characteristics were obtained through a BRFSS optional module. Another BRFSS optional module with child prevalence questions was introduced in 2005. The National Asthma Survey (NAS), piloted in 2003 in four states and a national sample provided extensive additional detail on asthma management. Use of the detailed NAS questionnaire has been expanded through its implementation as a BRFSS Asthma Call-back Survey (ACBS) since 2005. The number of states conducting the ACBS has increased each year to 39 states in 2010. There has also been an increase in publications utilizing national and state surveillance data. Two additional national surveillance summaries were published in 2003 and 2007.
State level data is readily available on the NACI Program website (http://www.cdc.gov/asthma/asthmadata.htm). Asthma surveillance data has also been published in several journals (http://www.cdc.gov/asthma/links_data.html). These efforts are intended to prompt a proactive approach to asthma management by monitoring population level changes in asthma morbidity and mortality at the national, state and local level.
Asthma research
Asthma research expanded over the past 30 years to include several new areas of investigation including, better methods to conduct clinical trials and to test interventions, effectiveness research to understand real-world application of clinical trials information, and pharmacogenetics discovery to identify new methods to individualize asthma management.
During the 1980s, studies related to asthma were mainly directed by pharmaceutical industries and largely regulatory in nature. During the 1990s, there was a move towards evidence-based medicine and the introduction of NIH asthma networks to address gaps in asthma management including inner city asthma (21 – 28). With the emergence of key asthma network studies, it was possible to identify comparative efficacy of medications, explore variability in treatment response and obtain information on areas of need including, inner city asthma, severe asthma, and early development of asthma (21 – 29). Also, studies were reported that demonstrated the role of intermittent inhaled corticosteroid in place of continuous daily dosing of this medication in order to minimize the risk of adverse effects (30–32).
In addition, information from key asthma cohorts emerged to show the variable course of asthma (33 – 40). Also, there is increasing recognition of the importance of comparative effectiveness research to assess the benefits of medications in “real world” settings through trials conducted outside the academic research setting and in the community settings, and to evaluate the uptake and implementation of guideline-based care [41].
In summary, the recognition of increased asthma mortality in the early 1980s prompted an ongoing effort to reduce mortality, reduce asthma exacerbations and improve overall asthma control. In the 1990s there was a dramatic transition away from bronchodilator therapy as core management to anti-inflammatory therapy as the preferred long-term controller. National and global guidelines emerged to help consolidate information, change the focus of management from episodic to ongoing disease management, and identify gaps in management. Concerted efforts were developed to provide a mechanism to conduct therapeutic research beyond the regulatory requirements for medication approval. Systems were also developed to monitor asthma not only for asthma related mortality but also asthma morbidity. This information also identified health disparities in mortality and morbidity. All of this has paved the way for defining methods to improve health care by recognizing the gaps in management and finding better interventions, especially for disadvantaged populations.
Current status of asthma care
Overall, we can feel a sense of accomplishment in reducing asthma mortality and hospitalizations due to asthma in the United States. Measurement of asthma prevalence changed with the redesign of the National Health Interview Survey (NHIS) in 1997 [Figure 1]. There was a rapid increase from 1980 to 1996 of asthma 12-month period prevalence by an average of 3.8% per year. After the redesign, the most comparable measure, current asthma prevalence, was introduced in 2001 and still demonstrates increasing prevalence, although at a lower rate, an average of 1.4% per year. The growing proportion of the U.S population with current asthma, 8.2% (24.6 million people) in 2009, presents a continuing challenge to adapt and improve effective prevention and disease management strategies.
Figure 1.
Asthma prevalence in the United States, 1980–2009
Source: National Health Interview Survey; National Center for Health Statistics, Centers for Disease Control and Prevention
Coupled to this observation of increasing prevalence of asthma is the current estimate of substantial costs related to asthma. Barnett and Nurmagambetov (42) evaluated data from the Medical Expenditure Panel Survey and reported that over the years 2002–2007, the incremental cost of asthma was $3,259 per person per year. For 2007, the total incremental cost of asthma to society was $59 billion, with productivity losses due to morbidity accounting for $3.8 billion and productivity losses due to mortality accounting for $2.1 billion.
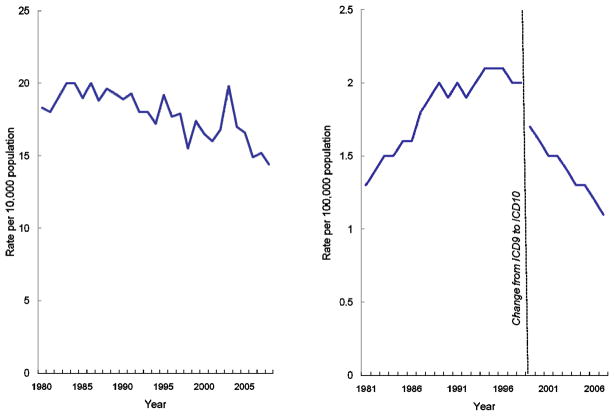
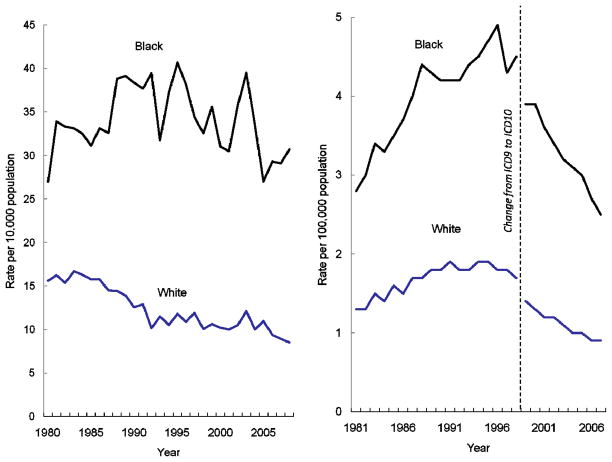
Reductions in both asthma hospitalization and death rates have occurred over the past decade despite the challenge of increasing prevalence [Figure 2]. However, racial disparities in hospitalization and mortality remain a challenge. Asthma hospitalization rates among the black population remain consistently higher than among the white population [Figure 3a]. In 2008, hospitalization rates were 3.6 times as high among black versus white populations, the largest disparity during the period shown from 1980 to 2008.
Figure 2.
Figure 2a. Asthma hospitalizations per 10,000 population, United States, 1980–2008
Figure 2b. Asthma deaths per 100,000 population, United States 1981–2007
Source: National Hospital Discharge Survey, Mortality Component of the National Vital Statistics System; National Center for Health Statistics, Centers for Disease Control and Prevention
Note: Asthma hospitalizations are first-listed using ICD-9CM code 493; Asthma deaths include those with underlying cause coded as ICD9 code 493 from 1981–1998, and ICD10 codes J45-J46 from 1999–2007.
Figure 3.
Figure 3a. Asthma hospitalizations per 10,000 population, by race, United States, 1980–2008
Figure 3b. Asthma deaths per 100,000 population, by race, United States 1981–2007
Source: National Hospital Discharge Survey, Mortality Component of the National Vital Statistics System; National Center for Health Statistics, Centers for Disease Control and Prevention
Note: Asthma hospitalizations are first-listed using ICD-9CM code 493; Asthma deaths include those with underlying cause coded as ICD9 code 493 from 1981–1998, and ICD10 codes J45-J46 from 1999–2007.
While asthma death rates among the black population are also much higher, the relative disparity has decreased from a high of 3.1 times higher death rates among black compared to whites in 2004 to 2.8 in 2007 [Figure 3b]. These striking disparities post a challenge for implementing effective asthma control measures among disparate populations with likely different needs and obstacles.
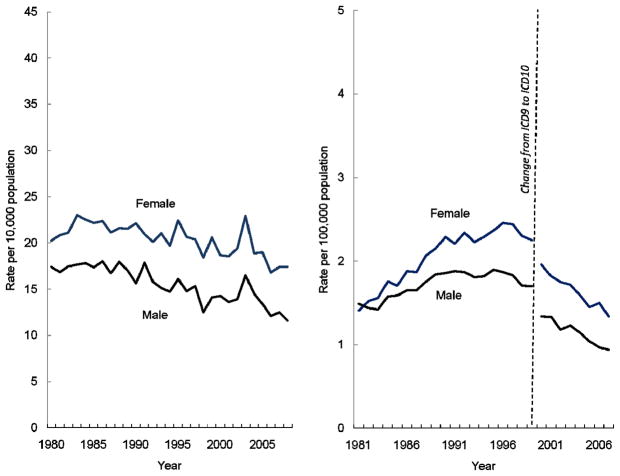
Although less dramatic, gender disparities also exist. In 2008, asthma hospitalizations were 1.5 times higher among females than males [Figure 4a]. The rise in asthma death rates in the 1980s was disproportionately borne among females with relative disparities reaching 1.4 times higher rates among females compared to males, a difference that has persisted despite declining death rates during the last decade [Figure 4b].
Figure 4.
Figure 4a. Asthma hospitalizations per 10,000 population, by sex, United States, 1980–2008
Figure 4b. Asthma deaths per 100,000 population, by sex, United States 1981–2007
Source: National Hospital Discharge Survey, Mortality Component of the National Vital Statistics System; National Center for Health Statistics, Centers for Disease Control and Prevention
Note: Asthma hospitalizations are first-listed using ICD-9CM code 493; Asthma deaths include those with underlying cause coded as ICD9 code 493 from 1981–1998, and ICD10 codes J45-J46 from 1999–2007.
Although this information has not been quantified in full publications, clinicians have recognized a reduction in the number of patients receiving oral glucocorticoid therapy as part of the maintenance schedule. This has significantly reduced the number of patients suffering the consequences of adverse effects of systemic steroid therapy including osteoporosis, cataracts, and growth suppression. This observation has been particularly remarkable for children and has changed the spectrum of the disease. This has been largely attributed to the introduction of new medications, organization of treatment into guidelines, and better systems of management.
Currently, there is a focus on asthma control and emerging interest in prevention, initially on preventing symptoms and then moving towards preventing progression. However, available research and surveillance show a continued underutilization of evidence-based management strategies to control asthma and thus there is room for improvement. This is an important step for addressing disparities in health care. National data exists for persons with asthma who report receiving components of self-management education and show that only a low to moderate percentage have received the basic elements of asthma education [Table 1] (43).
Table 1.
Percent of people with current asthma* reporting receipt of self-management education, National Health Interview Survey, 2008
| Total Percent (95% CI) | Children 0–17 yrs† Percent (95% CI) | Adults 18 yrs+ Percent (95% CI) | |
|---|---|---|---|
| Given an action plan | 34.2 (31.1–35.9) | 44.3 (40.0–48.8) | 29.9 (27.2–32.8) |
| Taken a class to learn how to manage their asthma | 12.2 (10.7–13.8) | 12.5 (10.0–15.7) | 12.0 (10.3–13.9) |
| Taught to recognize early signs and symptoms of an asthma attack | 59.9 (57.3–62.5) | 72.1 (68.1–75.8) | 54.8 (51.5–58.0) |
| Taught how to respond to an asthma attack | 68.1 (65.6–70.6) | 78.3 (74.2–82.0) | 63.8 (60.8–66.8) |
| Taught how to use a peak flow meter | 42.2 (39.7–44.7) | 49.4 (45.1–53.6) | 39.2 (36.2–42.2) |
| Given advice on environment control | 49.3 (46.6–52.0) | 50.6 (45.9–55.2) | 48.8 (45.6–51.9) |
| Followed “most or all” advice about environment | 60.7 (57.1–64.2) | 81.2 (76.2–85.3) | 51.6 (47.0–56.2) |
Persons reporting ever having been diagnosed with asthma and still having asthma at the time of the survey.
Percent parents reporting receiving this management education for their child’s asthma.
CI=confidence interval
Source: Centers for Disease Control and Prevention, Morbidity and Mortality Weekly Report, May 6,2011:60(7):547–552
Steps have been taken to achieve better monitoring systems for asthma including new tools such as electronic medical records and health care provider surveillance systems. This level of systematic monitoring also has practice level implications that strive to improve quality of care by tracking outcomes.
Along with the changes in practice management, the concept of personalized medicine has also emerged and early indicators suggest that this could significantly advance asthma management since there are variable responses to asthma treatment. While clinicians readily use a personalized approach to selecting a medication, dose and delivery system to relieve and prevent asthma symptoms, they do not currently use patient characteristics, biomarkers, and genetics to select and adjust treatment. Generally, treatment is still based most often on symptom presentation. Even basic tools, such as spirometry, are not readily incorporated in assessing asthma control, monitoring the course of asthma, and adjusting therapy accordingly. While spirometry is incorporated in asthma care conducted by most subspecialists, it has not been readily adapted in the course of asthma management in primary care.
There are several other interesting trends that will impact asthma management in the near future. There are increasing public health concerns regarding the impact of the environment on respiratory health and measures are being taken to institute control procedures and alert the public to hazards, for example publication of pollen counts and to incorporate asthma friendly environmental policies into public housing as well as school structure and rehabilitation. Current targets have been set to reduce hospitalizations and urgent care utilization by developing a systematic approach to managing chronic diseases including asthma by health care providers. The concept of a medical home has been introduced in order to help coordinate medical care, self-management education, and programs to link families to the services they need to support the treatment plan.
Unfortunately, there are no new asthma medications on the horizon. However, observations have been made regarding the beneficial effects of medications that were not directly approved for asthma management, including vitamin D and tiotropium (44–46). Steps are also being taken to obtain better insight on the origins of asthma, the natural history of asthma, the relationship of genetics to the risk of developing asthma and to the variations in response to treatment, and identification of easily measured biomarkers that can be used to predict response to medications and monitor disease activity.
In summary, we are now at a crossroads in asthma care. We have recognized some remarkable accomplishments in reducing asthma mortality and morbidity and closing some gaps in health disparities with the introduction of new medications and management systems. The availability of new tools to monitor disease activity, including biomarkers and epigenetic markers, along with information technology systems to monitor asthma control hold some promise in identifying gaps in disease management and should prompt the evolution of new strategies and new treatments to further reduce disease burden.
Challenges for the next 10 years
How do we go beyond measures of asthma mortality and hospitalizations?
Efforts are now being made to provide precise definitions and methods for measuring key asthma outcomes based on work of the NIH Asthma Outcomes Task Force held in 2010 (report in preparation). This will allow comparison across data in studies and facilitate pooling of data for meta-analysis.
While it is important to continue monitoring critical asthma outcome measures, it would also be useful to assess the impact of asthma on individual lives of all asthma patients. Some health care systems are already taking steps to monitor asthma control in their patients and prompt treatment adjustments to their clinicians. Indeed, disease severity is a combination of the level of asthma control along with the number and dose of medications used to achieve that control. Useful tools for health care providers to assess the impact of asthma and the proportion of patients that are considered severe and systemic-steroid requiring will be helpful. Identifying those patients that are significantly impacted by asthma and refractory to conventional treatment could direct them to studies that evaluate the efficacy of new treatment strategies, such as immunomodulator therapy.
Will techniques of “personalized medicine” move from the bench to the clinic to the home setting?
Currently, risk of disease is gauged by family history. Symptom expression is used to assess asthma severity and control. Utilization of asthma action plans and education programs have helped patients manage their own disease. The asthma self-management concept has expanded. However, many patients with asthma still do not benefit from a comprehensive management program and despite application of such a program there are still unpredictable and severe exacerbations that lead to urgent care.
Combining a system of careful follow-up and communication through electronic medical records along with periodic measurements of pulmonary function, there is the capability of monitoring disease activity and the natural history of the disease in individual patients including, remission, progression, and relapse (36, 38). However, these are still prominent features of the disease and perhaps the identification of more subtle monitors of disease activity, such as biomarkers and epigenetic markers, could prompt a more effective treatment strategy to actually prevent exacerbations and progression. There is a continuing debate on whether chronic obstructive pulmonary disease (COPD) might reflect the evolution of a certain phenotype of asthma. Identifying these patients early in the course of the disease, therefore, could theoretically lead to more effective early intervention strategies to prevent COPD. The definition of asthma phenotypes and identification of specific phenotype-related interventions will also be important.
To date, exhaled nitric oxide and sputum eosinophils have been prototype markers of disease activity and targets of therapeutic intervention. Strategies to reduce sputum eosinophils via methods to monitor and adjust glucocorticoid therapy have been shown to significantly reduce asthma exacerbations (47). However, use of sputum eosinophils has been limited to clinics with readily available procedures. Exhaled nitric oxide, on the other hand, is easier to measure, although still requiring special training, equipment and maintenance. It may be used in the clinic setting to obtain on-site results for clinical decisions. The results of studies incorporating periodic measures of exhaled nitric oxide have been less impressive in reducing exacerbations, as compared to sputum eosinophils (48, 49).
Although, there is limited additional value of measurements of exhaled nitric oxide for monitoring and adjusting therapy over a guidelines-based approach, measures of exhaled nitric oxide can be used as a predictor of inhaled corticosteroid response in children, but not necessarily in adults (50, 51). Furthermore, a recent study using a combination of urinary leukotrienes and exhaled nitric oxide including the ratio of these two biomarkers, may help direct the selection of either inhaled corticosteroid or leukotriene receptor antagonist therapy for first-line long-term controller therapy in children (52). Exhaled nitric oxide might also be used as a measure of medication adherence for inhaled corticosteroid therapy, although further studies are needed, since exhaled nitric oxide might also be elevated due to other exposures, such as allergens in sensitized patients.
To date, monitoring daily peak flows has not been sufficiently sensitive and specific to identify impending asthma exacerbations (53), but still may be useful in assessing variability, an important indicator of unstable asthma.
Furthermore, information is emerging that the Asthma Predictive Index, identified as part of the Tucson Respiratory Study, can be useful in identifying young children with early respiratory tract illnesses suggestive of asthma or emerging asthma. A positive Asthma Predictive Index may also be associated with a higher likelihood of beneficial effects from inhaled corticosteroid therapy in young children with emerging asthma (31, 54). Further refinement of these clinical tools and their application could help direct the selection and monitoring of asthma therapy, especially in children.
Exploration of genetic markers continues in relation to clinical application for asthma management. The most well studied genetic marker to date for asthma has been associated with β-adrenergic polyporphisms. Early information suggested that the B16 Arg-Arg polymorphism therapy was associated with declining pulmonary function in those treated with regular short-acting β-agonists (55). Since this method of treatment, namely regular use of short acting β-agonits, is now rarely used and the marker has not been associated with deteriorating asthma control with combination ICS/LABA therapy, the clinical application of this genetic marker is not an essential component of asthma care. However, emerging information with filaggrin polymorphisms, suggests that this marker, when identified in patients with atopic dermatitis, may carry a prognosis for higher risk of asthma (56, 57). This combination of patient characteristics and genetics could prompt early intervention strategies that would provide improved control and perhaps lead to disease remission.
What changes can be expected from the Public Health perspective?
Continued efforts are being made to follow the epidemiology and impact of asthma through national, regional and local statistics. This information can be used to identify areas of need. In particular, community statistics can be useful in identifying hot spots of asthma prevalence or areas of increased asthma burden in order to focus resources or identify impact factors, such as the environment, socioeconomics, or a combination thereof. This is one of the direct effects of the CDC National Asthma Control Program that funds states for surveillance, coalition building and interventions (19). Recent information from the websites of the NHLBI NACI program and the EPA’s Asthma Community Networks, in addition to the CDC programs reveals increasing effort to coordinate federal programs to broaden their reach to underserved communities and strengthen their ability to help clinicians and patients improve asthma management.
With the development of a global approach to asthma care, there can be more standardization of asthma management, however, various economic factors may lead to site- and community- specific medication selections or intervention strategies. Once again, statistical information to identify areas of high asthma prevalence and morbidity will be important to channel knowledge and resolution.
On a broader scale, public health response to increased application of guideline-recommended care, could further identify and address health disparities in asthma care. There are ongoing challenges to implement a guidelines-based approach into clinical practice and to increase public awareness of asthma control and for patients to work with clinicians to achieve control. Continued health care policy changes should help to improve reimbursement for physicians to provide this level of care and for patients to benefit from it. A carefully integrated plan to understand and address emerging public health challenges, e.g., increasing exposure to traffic pollutants, housing, climate change and pollutant/allergen interactions, and reducing second hand tobacco smoke exposure, should also prove helpful in reducing asthma burden.
Can we “cure” asthma by halting progression and preventing onset?
With the continued utilization and development of data bases for clinical outcomes, biomarkers, genetics and environment to help understand disease origins and natural history, we will be able to successfully develop early intervention strategies for control and prevention. Indeed, the Getting to Healthy People 2020 strategy places an emphasis on disease prevention (58). To get to that point, it will be important to define genetic markers and environmental factors that place individuals at risk for developing asthma. If certain markers and environmental factors are associated with the development of asthma, especially severe asthma, this will prompt consideration for the use of immunomodulators at an earlier time point in management.
Methods to monitor disease activity, such as epigenetics and biomarkers, will be important to assess the risk of disease persistence and progression. Information is also developing related to potential effect of the microbiome on asthma development (59). However, it is not clear whether the organisms identified are causative or perhaps commensal due to a defect in the immune system related to asthma (60). This story will continue to develop.
Currently, our early intervention strategies center around the use of short-acting β-adrenergic agonists to relieve symptoms and glucocorticoids to address airway inflammation. However, some studies suggest that this strategy is not sufficient to alter the course of the disease once glucocorticoid treatment is discontinued (61). If this is indeed true, then more effective strategies are needed to prevent progression and to reduce the risk of asthma exacerbations. We must continue to try to understand reasons for treatment failure to shed light on potential new treatment strategies including new medications.
To date, the application of immunomodulator therapy in young children is viewed with great concern due to potential effects on growth and development including the immune system and the ability to maintain adequate host defense. However, a recent article related to omalizumab therapy and the reduction of fall exacerbations in inner city children, prompts questions related to the potential interaction of viral and allergen-induced inflammation (24, 62). Perhaps, strategies designed to target periods of allergic inflammation will also reduce the risk of viral-induced asthma exacerbations. This area merits further study. Efforts to define severe forms of asthma and identify phenotypes could also lead to a more directed approach to management (26, 27, 63).
In regards to environmental control, it is apparent that airway inflammation and consequent symptoms of airway obstruction due to inflammation can be related to allergy, virus, and air pollutants. However, there are no specific biomarkers that can be used to identify the driving force of the airway inflammation. Although exhaled nitric oxide and sputum eosinophils are often associated with allergic airway inflammation, urinary leukotrienes with tobacco smoke exposure and sputum neutrophils with infection or air pollution, it is not entirely clear on how to use these specific markers to set a direction in treatment for individual patients. If certain biomarkers could prove useful to differentiate the factors leading to airway inflammation, perhaps they could channel environmental control strategies that are more specific to the individual patient rather than generalized to all patients with asthma.
Can this effort extend to other national and global initiatives?
Indeed, asthma is considered a global public health problem. Concerted efforts within and beyond countries can help shed light on the epidemiology and management of asthma. This review has focused on information available in the United States but asthma and allergies are being addressed in various worldwide efforts. As previously mentioned, the Global Initiative for Asthma guidelines seek to address asthma management for developed and developing countries. In addition, the World Allergy Organization is preparing a white book that will address the rising prevalence of asthma and allergies to increase the visibility of this problem and to propose recommendations to improve management.
The Asthma series
This review is the first in a series of Journal articles on the current status of asthma. The objective of this series is to highlight specific areas important to advancing asthma care. Each review will summarize current status of the area and future needs to advance the field. The next article in this series will focus on asthma pathophysiology. Dr. Stephen Holgate will discuss the pathophysiology of asthma and the information that has developed following the evaluation of new medications intended to interrupt various pathways of inflammation. Dr. Wiliam Busse will then discuss the advantages and limitations of our current tools and gaps that must be filled to improve on the diagnosis and treatment of asthma.
Next in this series, will be a discussion of the origins of asthma by Dr. Fernando Martinez. Dr. Martinez will summarize current knowledge on the origins and natural history and the need for further studies to define the various pathways of this disease, as it presents in children and adults. This will be followed by a comprehensive discussion of the current state of severe asthma.
Dr. Sebastian Johnston will follow with a topical review of asthma exacerbations. He will discuss our knowledge regarding the etiology of asthma exacerbations and future steps that could be taken to reduce the frequency and impact of these exacerbations or periodic loss of control.
Dr. Peter Barnes will analyze whether the pattern of severe asthma is changing and what determines whether asthma is severe. He will also discuss our current treatment strategies and the promise for new interventions.
Dr. Scott Weiss will conclude the series with a timely discussion of genetics and the application of personalized medicine. He will identify steps that could be taken now to apply these principles and steps that could be taken in the future to advance therapy and improve the asthma guidelines.
We thank all of the invited experts for the time they took to evaluate their area for review and for a comprehensive discussion of these topics. We hope that these summaries will prompt further research that will lead to continuing advances in asthma care.
Acknowledgments
Supported in part by Public Health Services Research Grants HR-16048, HL64288, HL 51834, AI-25496, HL081335, HL075416, and the Colorado Cancer, Cardiovascular and Pulmonary Disease Program. Supported in part by Colorado CTSA grant 1 UL1 RR025780 from the National Institutes of Health (NIH) and National Center for Research Resources (NCRR).
I would like to thank several colleagues for their assistance in preparing this review on the current status of asthma management. I am grateful for the enthusiastic and thoughtful insights from Lara Akinbami, Jeanne Moorman and Paul Garbe from the Centers for Disease Control, Virginia Taggart from the National Heart, Lung and Blood Institute and my close friend and colleague, Harold Nelson. I would also like to thank the Editor-In-Chief, Donald Leung, for encouraging the development of this series. I also thank Gretchen Hugen for assistance in the manuscript preparation.
Abbreviations
- ACBS
Asthma Call-Back Survey
- BRFSS
Behavioral Risk Factor Surveillance System
- CDC
Centers for Disease Control
- COPD
chronic obstructive pulmonary disease
- EPA
Environmental Protection Agency
- GINA
Global Initiative for Asthma
- ICS
inhaled corticosteroid
- LABA
long-acting β-adrenergic agonists
- MMWR
Morbidity and Mortality Weekly Report
- NACI
National Asthma Control Initiative
- NACP
National Asthma Control Program
- NAEPP
National Asthma Education and Prevention Program
- NAS
National Asthma Survey
- NHIS
National Health Interview Survey
- NHLBI
National Heart, Lung, and Blood Institute
References
- 1.Szefler SJ. Advances in pediatric asthma in 2010: Addressing the major issues. J Allergy Clin Immunol. 2011;127:102–15. doi: 10.1016/j.jaci.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Apter AJ. Advances in adult asthma diagnosis and treatment and HEDQ in 2010. J Allergy Clin Immunol. 2011;127:116–22. doi: 10.1016/j.jaci.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 3.Jackson RY, Beaglehole R, Rea HH, Sutherland DC. Mortality from asthma: a new epidemic in New Zealand. Br Med J. 1982;285:771–774. doi: 10.1136/bmj.285.6344.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Asthma Educational Program. Expert Panel Report, Guidelines for the Diagnosis and Management of Asthma. US Department of Health and Human Services, Public Health Service, National Institutes of Health; Aug, 1991. Publication No. 91-3042. [Google Scholar]
- 5.National Heart, Lung and Blood Institute. National Institutes of Health. International Consensus Reporton Diagnosis and Management of Asthma. U.S. Department of Health and Human Services; Jun, 1992. Publication No. 92-3091. [Google Scholar]
- 6.National Institutes of Health. National Heart, Lung and Blood Institute. Global Initiative for Asthma. Publication No. 95-3659. Jan, 1995. [Google Scholar]
- 7.National Institutes of Health. National Heart, Lung, and Blood Institute. National Asthma Education and Prevention Program. [accessed April 24, 2011];Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. 2007 August; NIH Publication No. 07-4051, http://www.nhlbi.nih.gov/guidelines/asthma/index.htm.
- 8.Lemanske RF, Busse WW. The US Food and Drug Administration and long-acting Beta2-agonists: the importance of striking the right balance between risks and benefits of therapy? J Allergy Clin Immunol. 2010;126:449–52. doi: 10.1016/j.jaci.2010.05.039. [DOI] [PubMed] [Google Scholar]
- 9.The Childhood Asthma Management Program Research Group. Long term effects of budesonide or nedocromil in children with asthma. N Engl J Med. 2000;343:1054–63. doi: 10.1056/NEJM200010123431501. [DOI] [PubMed] [Google Scholar]
- 10.Kelly HW, Strunk RC, Donithan M, Bloomberg GR, McWilliams BC, Szefler SJ. Growth and bone density in children with mild-moderate asthma: a cross sectional study in children entering the Childhood Asthma Management Program (CAMP) J Pediatrics. 2003;142:286–291. doi: 10.1067/mpd.2003.86. [DOI] [PubMed] [Google Scholar]
- 11.National Institute for Occupational Safety and Health. Worker Health Chartbook 2004. Chapter 2: Fatal and nonfatal injuries, and selected illnesses and conditions. NIOSH Publication No. 2004-146. http://www.cdc.gov/niosh/docs/2004-146/ch2/ch2-10.asp.htm.
- 12.Work-related lung disease (WoRLD) surveillance system. http://www2a.cdc.gov/drds/WorldReportData/
- 13.National Center for Health Statistics. Healthy People 2000 Final Review. Hyattsville, Maryland: Public Health Service; 2001. http://www.cdc.gov/nchs/data/hp2000/hp2k01.pdf. [Google Scholar]
- 14.Healthy people 2000 Midcourse Review and 1995 Revisions. http://odphp.osophs.dhhs.gov/pubs/hp2000/midcours.htm.
- 15.US Department of Health and Human Services. Healthy People 2010 Midcourse Review: Respiratory Diseases. Washington, DC: US Government Printing Office; Dec, 2006. http://www.cdc.gov/asthma/pdfs/asthma_guide.pdf. [Google Scholar]
- 16.Centers for Disease Control and Prevention. Guide for State Health Agencies in the development of asthma programs. Atlanta, GA: Dec, 2003. http://www.cdc.gov/asthma/pdfs/asthma_guide.pdf. [Google Scholar]
- 17.US Environmental Protection Agency. Identifying Environmental Risks in Asthma. http://www.epa.gov/epahome/sciencenb/asthma/IdentifyingRisks.html.
- 18.National Institute of Environmental Health Sciences. Asthma: What NIEHS is doing on asthma. http://www.niehs.nih.gov/health/topics/conditions/asthma/index.cfm.
- 19.CDC. National Asthma Control Program: America Breathing Easier. http://www.cdc.gov/asthma/pdfs/breathing_easier_brochure.pdf.
- 20.Mannino DM, Homa DM, Pertowski CA, Ashizawa A, Nixon LL, Johnson CA, et al. Surveillance for Asthma --United States, 1960–1995. MMWR CDC Surveill Summ. 1998 April 24;47(SS-1):1–28. http://www.cdc.gov/mmwr/preview/mmwrhtml/00052262.html. [PubMed]
- 21.Drazen JM, Lenfant C, Hurd SS. Acorns and inhalers: The Asthma Clinical Research Network. J Allergy Clin Immunol. 2007;119:28–9. doi: 10.1016/j.jaci.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 22.Austen KF. The National Institute of Allergy and Infectious Diseases’ Asthma and Allergic Disease Centers: A personal commentary. J Allergy Clin Immunol. 2007;119:34–5. doi: 10.1016/j.jaci.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 23.Togias A, Fenton MJ, Gergen PJ, Rotrosen D, Fauci AS. Asthma in the inner city: the perspective of the National Institute of Allergy and Infectious Diseases. J Allergy Clin Immunol. 2010;125:540–4. doi: 10.1016/j.jaci.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 24.Busse WW. The National Institutes of Allergy and Infectious Diseases networks on asthma in inner-city children: An approach to improved care. J Allergy Clin Immunol. 2010;125:529–37. doi: 10.1016/j.jaci.2010.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Denlinger LC, Sorkness CA, Chinchilli VM, Lemanske RF. Guideline-defining asthma clinical trials of the National Heart, Lung and Blood Institute’s Asthma Clinical Research Network and Childhood Asthma Research and Education Network. J Allergy Clin Immunol. 2007;119:3–11. doi: 10.1016/j.jaci.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wenzel SE, Busse WW for the National Heart, Lung and Blood Institute’s Severe Asthma Research Program. J Allergy Clin Immunol. 2007;119:14–21. doi: 10.1016/j.jaci.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 27.Fitzpatrick AM, Higgins M, Holguin F, Brown LAS, Teague WG for the National Institutes of Health/National Heart, Lung and Blood Institute’s Severe Asthma Research Program. The molecular phenotype of severe asthma in children. J Allergy Clin Immunol. 2010;125:851–7. doi: 10.1016/j.jaci.2010.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strunk RC for the Childhood Asthma Management Program. Childhood Asthma Management Program: Lessons learned. J Allergy Clin Immunol. 2007;119:36–42. doi: 10.1016/j.jaci.2006.09.038. [DOI] [PubMed] [Google Scholar]
- 29.Martinez FD. Asthma treatment and asthma prevention: A tale of 2 parallel pathways. J Allergy Clin Immunol. 2007;119:30–33. doi: 10.1016/j.jaci.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 30.Boushey HA, Sorkness CA, King TS, Sullivan SD, Fahy JV, Lazarus SC, et al. for the National Heart, Lung and Blood Institute’s Asthma Clinical Research Network. Daily vs. as-needed corticosteroids for mild persistent asthma. N Engl J Med. 2005;352:1519–1528. doi: 10.1056/NEJMoa042552. [DOI] [PubMed] [Google Scholar]
- 31.Bacharier LB, Guilbert TW, Zeiger RS, Strunk RC, Morgan WJ, Lemanske RF, et al. for the Childhood Asthma Research and Education Network of the National Heart, Lung and Blood Institute. Episodic use of an inhaled corticosteroid or leukotriene receptor antagonist in preschool children with moderate-to-severe intermittent wheezing. J Allergy Clin Immunology. 2008;122:1127–1135. doi: 10.1016/j.jaci.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martinez FD, Chinchilli VM, Morgan WJ, Boehmer SJ, Lemanske RF, Mauger DT, et al. Use of beclomethasone dipropionate as rescue treatment for children with mild persistent asthma (TREXA): a randomized, double-blind, placebo-controlled trial. Lancet. 2011;377:650–57. doi: 10.1016/S0140-6736(10)62145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Eng J Med. 1995;332:133–8. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- 34.Morgan WJ, Stern DA, Sherrill DL, Guerra S, Holberg CJ, Guilbert TW, et al. Outcome of asthma and wheezing in the first 6 years of life: follow-up through adolescence. Am J Resp Crit Care Med. 2005;172:1253–8. doi: 10.1164/rccm.200504-525OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sears MR, Greene JM, Willan AR, Wiecek EM, Taylor DR, Flannery EM, et al. A longitudinal, population-based, cohort study of childhood asthma followed to adulthood. N Eng J Med. 2003;349:1414–22. doi: 10.1056/NEJMoa022363. [DOI] [PubMed] [Google Scholar]
- 36.Covar RA, Spahn JD, Murphy JR, Szefler SJ for the Childhood Asthma Management Program Research Group. Progression of asthma measured by lung function in the Childhood Asthma Management Program. Am J Respir Crit Care Med. 2004;170:235–241. doi: 10.1164/rccm.200308-1174OC. [DOI] [PubMed] [Google Scholar]
- 37.Strunk RC, Weiss ST, Yates KP, Tonascia J, Zeiger RS, Szefler SJ for the CAMP group. Mild to moderate asthma affects lung growth in children and adolescents. J Allergy Clin Immunology. 2006;118:1040–7. doi: 10.1016/j.jaci.2006.07.053. [DOI] [PubMed] [Google Scholar]
- 38.Covar RA, Strunk R, Zeiger RS, Wilson LA, Liu AH, Weiss S, et al. for the Childhood Asthma Management Program Research Group. Predictors of remitting, periodic, and persistent childhood Asthma. J Allergy Clin Immunol. 2010;125:359–66. doi: 10.1016/j.jaci.2009.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gern JE. The Urban Environment and Childhood Asthma Study. J Allergy Clin Immunol. 2010;125:545–9. doi: 10.1016/j.jaci.2010.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bisgaard H, Bennelykke K. Long-term studies of the natural history of asthma in childhood. J Allergy Clin Immunol. 2010;126:187–97. doi: 10.1016/j.jaci.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 41.Krishnan JA, Schatz M, Apter AJ. A call for action: Comparative effectiveness research in asthma. J Alergy Clin Immunol. 2011;127:123–7. doi: 10.1016/j.jaci.2010.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barnett SBL, Nurmagambetov TA. Costs of asthma in the United States: 2002–2007. J Allergy Clin Immunol. 2011;127:145–52. doi: 10.1016/j.jaci.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 43.Centers for Disease Control and Prevention. Morbidity and Mortality Weekly Report. 2011 May 6;60(7):547–552. [Google Scholar]
- 44.Peters SP, Kunselman SJ, Icitovic N, Moore WC, Pascual R, Ameredes WT, et al. for the National Heart, Lung and Blood Institute’s Asthma Clinical Research Network. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. N Engl J Med. 2010;363:1715–26. doi: 10.1056/NEJMoa1008770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Searing DA, Zhang Y, Murphy JR, Hauk PJ, Goleva E, Leung DYM. Decreased serum vitamin D levels in children with asthma are associated with increased corticosteroid use. J Allergy Clin Immunol. 2010;125:995–1000. doi: 10.1016/j.jaci.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brehm JM, Schuemann B, Fuhlbrigge AL, Hollis BW, Strunk RC, Zeiger RS, et al. Serum vitamin D levels and severe asthma exacerbations in the Childhood Asthma Management Program study. J Allergy Clin Immunol. 2010;126:52–8. doi: 10.1016/j.jaci.2010.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Green RH, Brightling CE, McKenna S, Hagadon B, Parker D, Bradding P, et al. Asthma exacerbations and sputum eosinophil counts: a randomized controlled trial. Lancet. 2002;360:1715–21. doi: 10.1016/S0140-6736(02)11679-5. [DOI] [PubMed] [Google Scholar]
- 48.Szefler SJ, Mitchell H, Sorkness CA, Gergen PJ, O’Connor GT, Morgan WJ, et al. Management of asthma based on exhaled nitric oxide in addition to guideline-based treatment for inner-city adolescents and young adults: a randomized controlled trial. Lancet. 2008;372:1065–1072. doi: 10.1016/S0140-6736(08)61448-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Szefler SJ, Gergen PJ, Mitchell H, Morgan W. Achieving asthma control in the inner city: do the National Institutes of Health Asthma Guidelines really work? J Allergy Clin Immunol. 2010;125:521–6. doi: 10.1016/j.jaci.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 50.Martin RJ, Szefler SJ, King TS, Kraft M, Boushey HA, Chinchilli VM, et al. for the National Heart, Lung and Blood Institute’s Asthma Clinical Research Center. The Predicting Response to Inhaled Corticosteroid Efficacy (PRICE) trial. J Allergy Clin Immunology. 2007;119:73–80. doi: 10.1016/j.jaci.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Szefler SJ, Martin RJ. Lessons learned from variation in response to therapy in clinical trials. J Allergy Clin Immunol. 2010;125:285–92. doi: 10.1016/j.jaci.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rabinovitch N, Graber NJ, Chinchilli VM, Sorkness CA, Zeiger RS, Strunk RC, et al. for the Childhood Asthma Research and Education Network of the National Heart, Lung and Blood Institute. Urinary leukotriene E4/exhaled nitric oxide ratio and montelukast response in childhood asthma. J Allergy Clin Immunol. 2010;126:545–551. doi: 10.1016/j.jaci.2010.07.008. [erratum: J Allergy Clin Immunol 2010;126:959–60.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Covar RA, Szefler SJ, Zeiger RS, Sorkness CA, Moss M, Mauger DT, et al. for the Childhood Asthma Research and Education Network. Factors associated with asthma exacerbations during a long-term clinical trial of controller medications in children. J Allergy Clin Immunol. 2008;122:741–747. doi: 10.1016/j.jaci.2008.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bacharier LB, Guilbert TW, Zeiger RS, Strunk RC, Morgan WJ, Lemanske RF, et al. for the Childhood Asthma Research and Education Network of the National Heart, Lung and Blood Institute. Patient characteristics associated with improved outcomes with use of an inhaled corticosteroid in preschool children at risk for asthma. J Allergy Clin Immunol. 2009;123:1077–82. doi: 10.1016/j.jaci.2008.12.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kazani S, Wechsler ME, Israel E. The role of pharmacogenomics in improving the management of asthma. J Allergy Clin Immunol. 2010;125:295–302. doi: 10.1016/j.jaci.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Holloway JW, Arshad SH, Holgate ST. Using genetics to predict the natural history of asthma? J Allergy Clin Immunol. 2010;126:200–9. doi: 10.1016/j.jaci.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 57.Palmer CAN, Ismail T, Lee SP, Terron-Kwiatkowski Am, Zhao Y, Liao H, et al. Filaggrin null mutations are associated with increased asthma severity in children and young adults. J Allergy Clin Immunol. 2007;120:64–8. doi: 10.1016/j.jaci.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 58.Koh HK. A 2020 vision for healthy people. N Engl J Med. 2010;363:1653–56. doi: 10.1056/NEJMp1001601. [DOI] [PubMed] [Google Scholar]
- 59.Bisgaard H, Nothman Hermansen M, Buchvald F, Loland L, Brydensholt Halkier L, Bonnelykke K, et al. Childhood asthma after bacterial colonization of the airways in neonates. N Engl J Med. 2007;357:1487–95. doi: 10.1056/NEJMoa052632. [DOI] [PubMed] [Google Scholar]
- 60.Von Mutius E. Of attraction and rejection – Asthma and the microbial world. N Engl J Med. 2007;357:1545–7. doi: 10.1056/NEJMe078119. [DOI] [PubMed] [Google Scholar]
- 61.Guilbert TW, Morgan WJ, Zeiger RS, Mauger DT, Boehmer SJ, Szefler SJ, et al. Two year inhaled corticosteroid treatment on subsequent asthma in high-risk toddlers. N Engl J Med. 2006;354:1985–1997. doi: 10.1056/NEJMoa051378. [DOI] [PubMed] [Google Scholar]
- 62.Busse WW, Morgan WJ, Gergen PJ, Mitchell HE, Gern JE, Liu AH, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. New Eng J Med. 2011;364:1005–15. doi: 10.1056/NEJMoa1009705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bousquet J, Mantzouranis E, Cruz AA, Ait-Khaled N, Baena-Cagnani CE, Bleecker ER, et al. Uniform definition of asthma severity, control and exacerbations. J Allergy Clin Immunol. 2010;126:926–38. doi: 10.1016/j.jaci.2010.07.019. [DOI] [PubMed] [Google Scholar]