Abstract
Background
Improving inhaled corticosteroid (ICS) adherence should improve asthma outcomes.
Objective
In a randomized controlled trial we tested whether an individualized problem-solving intervention improves ICS adherence and asthma outcomes.
Methods
Adults with moderate or severe asthma from clinics serving inner-city neighborhoods were randomized to problem-solving (PS) (defining specific barriers to adherence, proposing/ weighing solutions, trying the best, assessing, and revising) or standard asthma education (AE) for 3 months, then observed for 3 months. Adherence was monitored electronically. Outcomes included: asthma control, FEV1, asthma-related quality of life, emergency department (ED) visits, and hospitalizations. In an intention-to-treat-analysis, longitudinal models using random effects and regression were employed.
Results
333 adults were randomized: 49±14 years, 72% female, 68% African American, 7% Latino, mean FEV1 66%±19%, 103(31%) with hospitalizations and 172(52%) with ED visits for asthma in the prior year. There was no difference between groups in overall change in any outcome (p>0.20). Mean adherence (61%±27%) declined significantly (p=0.0004) over time by 14% and 10% in the AE and PS groups, respectively. Asthma control improved overall by 15% (p=0.002). In both groups, FEV1 and quality of life improved: 6% (p=0.01) and 18% (p<0.0001), respectively. However, the improvement in FEV1 only occurred during monitoring but not subsequently after randomization. Rates of ED visits and hospitalizations did not significantly decrease over the study period.
Conclusion
Problem-solving was not better than asthma education in improving adherence or asthma outcomes. However, monitoring ICS use with provision of medications and attention, imposed on both groups, was associated with improvement in FEV1 and asthma control.
Clinical Trials registration
ClinicalTrials.gov number NCT00115323.
Keywords: asthma, adherence, adults, inner city asthma, problem-solving, health disparities, inhaled corticosteroids
INTRODUCTION
Asthma, a chronic treatable disease affecting 15 million US adults, is characterized by a high degree of health disparity.1 Blacks have 3 times the hospitalizations and deaths compared to whites; the rate is also disturbingly high in Latinos. Improving asthma outcomes is particularly important in these groups.
Inhaled corticosteroid (ICS) is an essential medicine for all but the mildest asthma, yet patients commonly take far less than prescribed.2–5 It is thought improving adherence will improve asthma outcomes, but there are few interventions that show this is the case.6, 7 Adherence has complicated determinants, including patients’ perception of benefits compared with adverse effects, medication costs, personal priorities, and the waxing and waning of the disease.2, 8 It may be a sign of the adequacy of patient-physician communication.6, 7, 9
Measuring adherence to ICSs is difficult;10 serum levels cannot be measured; canister weighing and patient and physician report are unreliable.4, 11–16 Electronic monitoring giving the time and date of use is the best means of directly assessing medicine-taking.10, 17 Other methods are more limited in addressing how adherence affects asthma outcomes. For example, counting prescribed or filled prescriptions does not ensure medications are taken.3, 18 Nevertheless, electronic monitoring is not without problems. Monitors can fail to record or download data and the act of monitoring---that is, observing behavior---can change behavior (Hawthorne Effect). Additionally, monitors may be able to detect when an inhaler is actuated, but not if the medication is actually inhaled. Nevertheless, measuring adherence is absolutely essential to determine if an intervention actually improves adherence and whether adherence improves outcomes.
In this study we investigated the use of a problem-solving approach to improve medication adherence in patients with moderate or severe asthma. The rationale for this approach is that interventions that involve frequent interaction with patients, take account of patients’ circumstances, and boost communication have been recommended in the past6, 7 and problem-solving is an individualized intervention successful in other settings for promoting behavior change. Problem-solving considers the unique context and special barriers of patients’ lives. It has been used to reduce psychological distress; increase self-efficacy; enhance overall coping skills,19–22 promote weight loss;23, 24 and decrease destructive behaviors such as suicide attempts,25 substance abuse,26 and unprotected sex.27 A version of this problem-solving model has been used to improve glucose control in diabetes, a chronic disease that like asthma requires significant self-management skills.28–30 We adapted a version by D’Zurilla and Nezu31 that consists of four iterative steps: 1) defining specific barriers to adherence and orientation (developing a coping perspective), 2) proposing and weighing solutions, 3) choosing the best, and 4) trying out the solution and revising.
We tested whether problem-solving compared with standard asthma education improves ICS adherence and asthma health outcomes. The subjects were adults with moderate or severe asthma recruited from medical practices serving low-income urban neighborhoods. To improve the relevance of problem-solving, participants also were asked to problem-solve a problem of their own choosing. This was to demonstrate problem-solving’s applicability to many problems; suggest that if one’s asthma is controlled, one can better address life’s other problems; and acknowledge to patients that their unique context must be considered in achieving health.
METHODS
A more detailed description of the Methods is available from the on-line repository.
Study Design
We conducted a randomized controlled trial, assigning asthmatic adults to either problem-solving (PS) or asthma education (AE). The study was approved by the institutional review boards of the University of Pennsylvania and the Philadelphia Veterans Affairs Medical Center. Potential subjects were told that the purpose of the study “is to examine better ways to treat asthma using currently recommended medications and procedures.”
Subjects
Participants were English- or Spanish-speaking adults with moderate or severe persistent asthma according to National Heart Lung and Blood Institute Expert Panel Report 3 guidelines.32 Inclusion criteria were designed to identify patients with sufficiently severe and reversible asthma that were likely to benefit from ICS therapy. Specific criteria were: 1) age ≥ 18 years; 2) physician’s diagnosis of asthma; 3) prescription for an ICS-containing medication for asthma; 4) evidence of reversible airflow obstruction--- (i) an increase ≥ 15% and 200 ml in FEV1 with asthma treatment over the previous 3 years or (ii) an increase in FEV1 or FVC ≥ 12% and 200 ml in FEV1 within 30 minutes of inhaled albuterol. Smokers were included. Excluded were patients with severe psychiatric problems such as obvious mania or schizophrenia that would make it impossible for them to understand or carry out problem-solving.
Subjects were recruited from primary care and asthma specialty practices serving low income inner-city neighborhoods with high prevalence of asthma morbidity. Charts or electronic medical records were pre-screened for patients with a diagnosis of asthma who were prescribed an ICS. Potential subjects were then approached by telephone or at the time of a clinic visit and asked to sign consent for further screening. Those satisfying all enrollment criteria were then asked to sign a second informed consent to participate in the 26-week study.
Procedures
Questionnaires on socio-demographics, present and past asthma status, and comorbidities were completed. Spirometry was obtained.33 Participants estimated their adherence over the last 3 months with the Inhaler Adherence Scale.34, 35 An electronic monitor was attached to participants’ ICS-containing inhaler. Participants were informed that the monitor recorded time and date of inhaler actuation and that data would be downloaded at each of eight study visits. Two weeks later (Visit 2), subjects were randomized according to a computer-generated algorithm in 1:1 ratio to either PS or AE. Subjects met with research coordinators monthly for four sessions (Visits 2–5) of either PS or AE, spirometry, and downloading monitor data. The need for urgent medical care since the last visit was queried. Subjects then continued to meet monthly with research coordinators for three additional months (Visits 6–8) to download monitor data but no PS or AE occurred at Visits 6–8.
Subjects received $20 for the first visit, $15 for Visits 2–5, $10 for short Visits 6–7, and $50 for completing Visit 8. ICS was supplied for subjects without any insurance coverage for ICS. For subjects with a copayment, this sum was reimbursed if all visits were completed and medication receipts were submitted.
Interventions
Problem-solving (PS) comprised four 30-minute sessions. The individualized intervention involved four interactive steps, usually one per research session. For the 158 participants who reported missing doses of ICS, the goal was to improve adherence. For the seven participants who declared adherence to the prescribed regimen, the goal was to maintain adherence. The first PS step consisted of defining the problem: improving or preserving adherence to ICS within the patient’s unique context and orientation. Problem orientation facilitated the adoption of a rational, positive, and constructive appraisal of how to achieve adherence, with nonadherence being presented as a problem to be solved. PS was presented as a means of coping with problems more generally, and modifying attitudes or beliefs that inhibit or interfere with attempts to solve problems. It was a motivational technique to help the participant view the occurrence of problems as inevitable, normal, and solvable. This first step involved breaking problems into small achievable pieces. The second step was brainstorming for alternative solutions. The third step was choosing the best solution by weighing the consequences, both desirable and undesirable, of each candidate solution. Between the 3rd and 4th meeting the solution was tried. For the 4th step, the chosen solution was evaluated and revised. As part of this intervention, downloaded data from monitored ICS was shared with the participant in a nonjudgmental fashion at each visit. At these sessions subjects followed the same problem-solving steps for addressing an additional problem of their own choosing, e.g. increasing physical activity. The problems were sometimes interrelated; e.g., a father wants to play sports with his child and improving asthma management makes this easier.
Asthma education (AE), like PS, comprised four 30-minute sessions, each about an asthma patient education topic unrelated to self-management, adherence, or ICS therapy. The topics covered, one at each session, were: 1) the proper technique for using an albuterol-rescue metered dose inhaler and a dry powder inhaler or spacer, depending on the patient’s medications; 2) the use of peak flow meters; 3) common asthma triggers; and 4) the pathophysiology of asthma. These sessions did not involve discussion of problem-solving or adherence, only didactic presentation of health information.
Research coordinators
Subjects worked with the same research coordinator for all visits, who delivered either PS or AE by script. Research coordinators were college graduates interested in health-related or education careers or further schooling, committed to working with patients and a research experience. They were diverse in race/ethnicity similarly to patients.
Outcomes
Adherence to ICS Regimen Prescribed by Participant’s Physician
We electronically monitored date and time of ICS actuation. The electronic monitor can record multiple actuations over a short time-period and thus can detect medication “dumping,” multiple actuations of an ICS unaccompanied by inhalation.13, 36, 37
Only two electronic monitors that measure time and date of ICS use were available at the time of the study. Although no commercial monitor was available for fluticasone-salmeterol, the most frequently prescribed ICS to subjects during the study period, we were able to use the Diskus Adherence Logger or DAL, the research monitor developed by a team member (DKB).17 Fluticasone and beclomethasone by metered dose inhalers were the next most frequently prescribed; for these we used a commercial monitor, MDILog (Life Link Monitoring, Inc, Kingston, NY). Approximately 90% of WIN participants were prescribed an ICS monitorable with DAL or the MDILog. Fourteen patients were initially prescribed inhaled mometasone but they were switched to a monitorable medication, fluticasone, during the study period with their physician’s permission.
Daily ICS adherence was calculated as (# actuations downloaded/# prescribed) ×100.2, 38 We truncated adherence at 100% for each monitoring period to control for multiple actuations over a very short period of time, and provide a better measure of adherence.2, 17, 37–39
Asthma outcomes
Asthma-related quality of life (AQOL) was measured at Visits 1, 5, and 8 with the Mini-Asthma Quality of Life Questionnaire (AQLQ).40–42 This 15-item questionnaire, reflecting well-being over the past two weeks, has a 7-point response scale that provides a mean summary score. A 0.5-unit change is considered clinically meaningful.42 The AQLQ has been shown to be a useful indicator of AQOL in low-income adults.43 Asthma control was measured at each visit using the 7-item version of the Asthma Control Questionnaire.44–46 It asks about symptoms over the past week. The score is the mean of all responses (0=total control, 6=extremely uncontrolled). The minimal important clinical difference is 0.5. A score >1.5 is considered inadequate control.47 Spirometry was obtained using American Thoracic Society procedures for FEV1 and FVC.33 At each research visit participants reported hospitalizations and ED visits for asthma or any cause that had occurred since the previous meeting. Participants were queried about satisfaction with and benefits of the study at its end.
Independent variables (baseline data)
Demographic characteristics—age, race, ethnicity, educational attainment, and household income—were participant-reported. Household income was ascertained in categories to make responses by participants more acceptable and feasible: < $30,000/year, $30,000–$49,999/year, $50,000–$99,999/year, and > $100,000/year. Baseline depressive symptoms were measured with the CES-D.48 Self-efficacy was obtained at baseline with a 14-item questionnaire reported previously.38, 49, 50 Literacy was measured by the Short Test of Functional Health Literacy in Adults51 and the Asthma Numeracy Questionnaire.52 Exposure to community violence was queried: “In the past 6 months, did you witness any violence in your neighborhood (yes/no)?”53 Social support was assessed at baseline using the MOS Social Support Survey.54 The Inhaler Adherence Scale asked for participant report of nonadherence at baseline.34, 35 It is a 6-item scale, each item scored 1 (nonadherence) or 0 (adherence). The range is 0–6, with 0 being optimal adherence.
Statistical analysis
Pre-enrollment power calculations based on an intention to treat (ITT) framework estimated that enrollment of 390 with a final sample size of 330 would detect a 10% effect size with a power of .80. STATA 11.0 (STATA Corporation, College Station, TX) and SAS V9.2 (SAS Corp, Cary, NC) were used. A descriptive analysis was performed for all variables. We compared the PS and AE groups for the adequacy of randomization, examining if covariates or baseline variables including potential moderators of the intervention were equally distributed among patient groups. The statistical tests were: t-tests or Wilcoxon rank sum tests for continuous variables, depending on the symmetry of the distributions; logistic regression for binary or ordinal variables; and Poisson log-linear regression for count data.
The analysis of PS vs. AE differences with respect to each longitudinal continuous outcome was based upon the intent-to-treat (ITT) principle, involving mixed effects linear regression with random intercepts and slopes to account for clustering by patient. These models also included separate fixed main effects for the intervention factor and each of the follow-up visits (treated as categorical), as well as separate interaction terms between the randomized intervention and each visit. The ITT test comparing the PS and AE across all visits was based on a model-based F test of all interaction-visit interaction terms. Additionally, tests of change across all visits within each group or overall were based on F-tests of corresponding combinations of model parameters. For count data across the entire follow-up period (e.g., emergency visits), we used a log-linear model with standard errors adjusted for overdispersion, and ITT tests of the intervention effect on the outcome, based on the log risk ratio parameter corresponding to the intervention in the log-linear model.
Exploratory moderation and subgroup analysis
For each of the subgroup factors, we tested if the PS vs. AE contrast in longitudinal outcomes differed between subgroup levels (moderation), based on an interaction among the intervention, time, and subgroups in the longitudinal models. We conducted exploratory subgroup analyzes to determine if certain baseline characteristics, sociodemographic or asthma severity variables, distinguished participants who were more likely to have improved outcomes (adherence, FEV1, asthma-related quality of life) as a result of PS. The groups tested were age, sex, baseline FEV1, hospitalizations or ED visits for asthma in the year before entry, baseline asthma related quality of life, household income, minority status, baseline depressive thoughts, literacy, baseline self-efficacy concerning adherence to taking ICS, social support, and exposure to community violence. We dichotomized these subgroups according to the median. For each of these subgroups we tested if asthma outcomes differed by intervention assignment.
RESULTS
Recruitment
We prescreened more than 49,000 patient charts. These patients were either scheduled to have a physician’s appointment in participating general or specialty clinics within the following 2 weeks or were admitted to the ED for asthma (Figure E-1). Charts were reviewed more than once if the patient had more than one appointment. This prescreening process identified approximately 7000 appointments for patients 18 years or older with a doctor’s diagnosis of asthma who were prescribed an ICS. Some patients had multiple appointments. After counting patients only once and screening for the other enrollment criteria, we identified 585 eligible patients. Of these, 397 completed the surveys for this study (Visit 1). Of the 188 who declined, 70 stated they were too busy, 57 did not come for appointments scheduled with researchers, 39 thought the travel time for appointments too burdensome, and 18 did not consider the research likely to be beneficial to themselves or others. In addition, another 4 eligible patients declined, 1 for each of the following reasons: “concerns about research,” “concerns about data privacy/protection of personal medical information,” patient’s doctors believed the study was not likely to be beneficial, and patient was unable to switch to an inhaled steroid for which we had a monitor. 333 returned for Visit 2 and were randomized.
Patient characteristics
The 333 subjects were mostly female, African-American, and from households earning less than $30,000/year (Table 1). Baseline mean CES-D scores were high, suggesting a prevalence of depressive symptoms frequently seen in poor urban populations.55 Likewise, quality of life scores were low.40, 41
Table 1.
Baseline characteristics of 333 randomized patients according to Study Group
| Characteristic | Total N=333 |
Problem- solving N=165 |
Asthma education N=168 |
p value§ |
|---|---|---|---|---|
| Sociodemographics | ||||
| Age (years)* | 49 ± 14 | 49 ± 13 | 49 ± 14 | .85 |
| Female | 241 (72%) | 122 (74%) | 119 (71%) | .53 |
| Race | .32 | |||
| Black/African American | 228 (68%) | 118 (72%) | 110 (65%) | |
| White | 67 (20%) | 30 (18%) | 37 (22%) | |
| Other** | 9 (3%) | 5 (3%) | 4 (2%) | |
| No response or declined to answer | 29 (9%) | 12 (7%) | 17 (10%) | |
| Ethnicity: Hispanic/Latino | 23 (7%) | 10 (6%) | 13 (8%) | .56 |
| Household income | .34 | |||
| < $30,000/year | 176 (53%) | 84 (51%) | 92 (55%) | |
| $30,000 – $49,999/year | 83 (25%) | 49 (30%) | 34 (20%) | |
| $50,000 to $99,999 | 46 (14%) | 22 (13%) | 24 (14%) | |
| $100,000 or more | 18 (5%) | 7 (4%) | 11 (7%) | |
| No response or declined to answer | 10 (3%) | 3(2%) | 7 (4%) | |
| High school graduate | 275 (83%) | 144 (87%) | 131 (78%) | .19 |
| Asthma numeracy questionnaire score*,# | 2.3 ± 1.2 | 2.3 ± 1.2 | 2.2 ± 1.2 | .69 |
| Reading comprehension score*,## | 31.2 ± 7.3 | 31.1 ± 7.6 | 31.4 ± 7.0 | .68 |
| Depression*, ¶ | 17.5 ± 11.5 | 17.4 ± 11.3 | 17.3 ± 12.2 | .66 |
| Social Support*,¶¶ | 73 ± 22 | 72 ± 22 | 73 ± 22 | .75 |
| Self-efficacy*, ¶¶¶ | 53 ± 7 | 52 ± 7 | 54 ± 7 | .09 |
| Never smoked | 141 (42%) | 68 (41%) | 73 (43%) | .49 |
| Number of years smoked | 11 ± 15 | 11 ± 15 | 11 ± 14 | .51 |
| Total witnessing violence in the last 6 mos | 67 (20%) | 33 (20%) | 34 (20%) | .98 |
| Asthma severity at baseline | ||||
| FEV1 (percent predicted)* | 66 ± 19 | 66 ± 19 | 64 ± 19 | .40 |
| # with ≥ 1 ED visit for asthma in past yr | 172 (52%) | 86 (52%) | 86 (51%) | .93 |
| # with ≥ 1 hospitalization for asthma in past yr | 103 (31%) | 52 (32%) | 51 (30%) | .82 |
| Asthma-related quality of life 41,42* | 4.0± 1.4 | 4.0± 1.5 | 4.0± 1.4 | .83 |
| Asthma control* | 1.67 ± 1.10 | 1.68 ± 1.09 | 1.65 ± 1.11 | .91 |
| # Comorbidities | ||||
| Hypertension | 172 (52%) | 83 (50%) | 89 (53%) | .63 |
| Diabetes | 73 (22%) | 37 (22%) | 36 (21%) | .83 |
| BMI† | 33.4 ± 8.8 | 34.2 ± 8.6 | 32.6 ± 8.9 | .07 |
mean ± sd
Comparing PS and AE groups
Other = American Indian/Alaskan Native, Asian, Native Hawaiian/Pacific Islander
Center for Epidemiologic Studies Depression (CES-D) Scale, range 0 – 60, with scores ≥ 16 suggestive of depressive symptoms or general distress.
MOS Social Support Survey54 has 19 times on a 5-point Likert scale; the overall score of the survey is given by the mean of all 19 items, rescaled to a range of 0 to 100; a higher score represents more support. These scores were in the range of a multiethnic urban sample of postpartum women attending a community health center59 and lower than a group of ovarian cancer survivors.60
Asthma Numeracy Questionnaire, 52 range 0–4.
Short Test of Functional Health Literacy in Adults,51 range 0–36, with score ≥ 23 adequate.
Self-efficacy is a 14-item questionnaire, items measured on a 5-point Likert scale with score the sum of item scores.38, 49, 50 The range is 14–70, with higher score higher self-efficacy.
BMI ≥ 30 is classified as obese where 25–29 is considered overweight.
The study population had significant asthma morbidity, with the cohort having a low mean FEV1 (66% ± 19%). More than half had had an asthma-related ED visit in the year prior to enrollment and about one-third had been hospitalized for asthma in that time interval. Comorbidities were common. For example, half had hypertension and almost a quarter diabetes. In addition, mean BMI was high (Table 1); 107 (67%) in PS group and 90 (56%) in AE group had BMI’s of at least 30.
Baseline participant report of nonadherence, as measured by the Inhaler Adherence Scale, had a mean score of 4.2 ± 1.4 and did not differ by PS/AE assignment, p= .59 (Table 2).
Table 2.
Nonadherence at baseline by patient-report, using the Inhaler Adherence Scale,1 did not differ between groups. Shown is the number of participants who endorsed an item of nonadherence of the Scale over the last 3 months.
| During last 3 months, have you … | Number of participants reporting nonadherence N=333 |
Problem- solving N=165 |
Asthma education N=168 |
p value |
|---|---|---|---|---|
| at times been careless about using your ICS?* | 168 | 83 | 85 | .96 |
| ever forgotten to use your ICS? | 115 | 52 | 63 | .25 |
| ever stopped using ICS because you felt better? | 275 | 136 | 139 | .94 |
| ever used less than the doctor prescribed because you felt better? | 245 | 118 | 127 | .35 |
| ever stopped using your ICS because you felt worse? | 321 | 159 | 162 | .98 |
| ever used your ICS more the prescribed because you felt your were having breathing problems | 286 | 143 | 143 | .69 |
Morisky et al. Med Care 1986;24:67–74. Dolce JJ et al Chest 1991;99:837–41.
ICS= inhaled corticosteroid
Fifty participants received at least one container of ICS; 25 were assigned to PS and 25 to AE. Of the 14 patients switched from mometasone to fluticasone, 7 were randomized to PS and 7 to AE. Ninety-nine participants received copayment reimbursement, 54 assigned to PS and 45 to AE.
83% of PS and 82% of AE patients had fluticasone/salmeterol monitored by the DAL. The remaining participants had a pure ICS monitored with an MDILog.
The 64 who did not return after Visit 1 to be randomized in Visit 2 differed from those that were randomized in that they were slightly younger (mean age 36 ± 13), had a slightly higher FEV1 (72% predicted ± 19%), and were slightly more likely to be African American.
Two male subjects died while enrolled due to causes unrelated to the study interventions: one of pneumonia and one of a cardiac arrest during surgery to remove a blood clot. Both were assigned to AE and had not been seen by the research team for several weeks.
Problem-solving
Participants assigned to PS were asked to identify a specific problem related to either improving or maintaining adherence. Table E-1 describes the barriers to adherence (asthma problem), solutions proposed, and the other problems the 165 participants addressed.
Outcomes
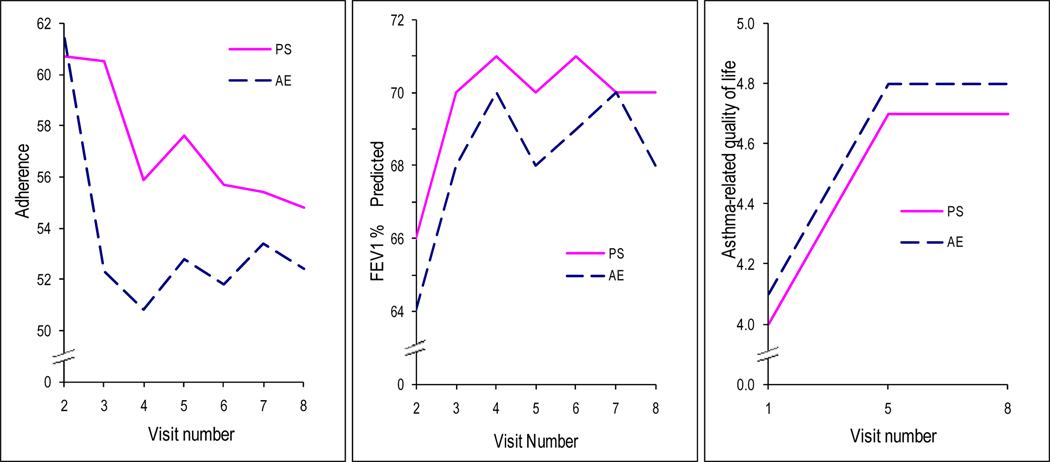
Overall mean adherence was 61% ± 27%. It declined (p=.0004) over time by 14% in the AE group and 10% in the PS group (Figure 1a). Monitor downloads failed in 380 (20%) of 2360 downloads, 18% of PS and 22% of AE. Failures were attributed to monitor failure, battery failure, and proximity to other batteries or magnets.
Figure 1.
a:Percent adherence over study period by PS versus AE. Data collected at a visit represents adherence data collected between that and the previous visit. For example, adherence point at Visit 4 represents adherence between Visits 3 and 4. The approximately 61% adherence point at Visit 2, represents adherence between Visits 1 and 2, thus, baseline adherence; adherence before the interventions. FEV1 (Figure 1b) and Asthma–related quality of life (Figure 1c) are depicted over the study period.
There were no differences between groups with respect to overall change in any outcome (p>.20). Table 3 presents outcomes at baseline, the end of the intervention (Visit 5) and the end of the observation period (Visit 8). Asthma control measured by the Asthma Control Questionnaire improved significantly (p=0.002) for both groups: 20% (AE) and 11% (PS), but there was no significant statistical or clinical difference between groups. In both groups, FEV1% and quality of life improved from baseline: 6% (p=.01) and 18% (p<0.0001), respectively for AE and PS (Figures 1b and 1c). However, the improvement in FEV1 occurred only between Visits 1 and 2; changes in FEV1 for subsequent visits were not significant. There was no association between adherence and asthma outcomes (p > .50) (data not shown).
Table 3. Adherence and asthma outcomes at baseline, end of the intervention (Visit 5), and end of observation (Visit 8).
There was no significant statistical or clinical difference between groups (p>.20). The p-value indicates change from baseline to the end of the intervention: decline in adherence but improvement in asthma control, quality of life, and FEV1.
| Outcome | Baseline | Visit 5 | Visit 8 | p-value of change from baseline |
|||
|---|---|---|---|---|---|---|---|
| PS | AE | PS | AE | PS | AE | ||
| Adherence (%)* | 61 ± 26 | 61 ± 28 | 58 ± 28 | 53 ± 27 | 55 ± 29 | 52 ± 28 | 0.0004 |
| Asthma Control** | 1.7 ± 1.1 | 1.7 ± 1.1 | 1.6 ± 1.3 | 1. 5 ± 1.1 | 1.5 ± 1.2 | 1.3 ± 1.1 | 0.002 |
| Quality of life# | 4.0 ± 1.4 | 4.1 ± 1.4 | 4.7 ± 1.4 | 4.8 ± 1.4 | 4.7 ± 1.3 | 4.8 ± 1.4 | <0.0001 |
| FEV1% predicted | 66 ± 19 | 64 ± 19 | 70 ± 18 | 68 ± 20 | 70 ± 19 | 68 ± 18 | 0.01 |
| ED for asthma## | 4.27 | 4.76 | 6.21 | 4.23 | 7.30 | 2.96 | p>.20 |
| ED for any cause## | 9.09 | 8.33 | 9.66 | 9.15 | 13.87 | 7.41 | p>.20 |
| Hospitalizations for asthma## | 1.82 | 2.98 | 2.76 | 2.82 | 1.46 | 0.74 | p>.20 |
| Hospitalizations for any cause## | 3.03 | 2.98 | 6.90 | 6.34 | 3.65 | 5.93 | p>.20 |
ICS adherence was calculated from the date-time record of the ICS data downloaded from the monitors as the mean of daily adherence where daily adherence is (# actuations/# prescribed) ×100.2, 38 Adherence was truncated at 100% for each monitoring period to control for multiple actuations over a very short period of time, and thus provides a better measure of adherence.2, 17, 37–39
Asthma Control Questionnaire. The score is the mean of all responses (0=total control, 6=extremely uncontrolled). The minimal important clinical difference is 0.5. A score > 1.5 is considered inadequate control.47
Asthma Quality of Life Questionnaire. A change of .5 in individual patients is considered clinically meaningful.
Percent of participants reporting an ED visit or hospitalization occurring since last visit, e.g., at Visit 5, the percent of participants reporting an event since Visit 4.
There was no difference between PS and AE groups with respect to ED visits for asthma (p=.51) or any cause (p=.30). Likewise there was no difference in hospitalizations for asthma (p =.79) or any cause (p=.95). There also was no statistically significant change in rate of these events across the study period (p>.20) (Table 3).
In end of study questionnaires (See complete online Methods) given to the last 95 enrollees, 68/95 thought they had learned about asthma and 40/59 thought PS was helpful. Interestingly, of the 26/36 in AE who thought the study was worthwhile, 11/26 specifically thought they learned about benefits of adherence.
Within the exploratory moderation/subgroup analysis, we found those patients with low baseline social support (p=0.003), more exposure to violence (p=0.007), and low income (p=0.03) had better adherence if randomized to PS compared to the AE group. Those with low CES-D scores were more likely to have improved asthma-related quality of life (p=0.007) if randomized to PS. Those with low numeracy (p=0.007) and low asthma-related quality of life (0.03) were more likely to have higher FEV1 if assigned to PS. There was no difference in outcomes between PS and AE in subgroups defined by age, gender, hospitalizations or ED visits in year prior to enrollment, baseline FEV1, low self-efficacy, or low educational attainment. There was no change in self-efficacy over the observation period (p>0.1).
DISCUSSION
To study whether an individualized intervention focused on problem-solving could improve adherence for vulnerable patients, we conducted a randomized controlled trial in adults from low-income inner city neighborhoods with moderate or severe asthma. Even with a relatively large sample size, a long observation period, and the use of electronic monitoring rather than self-report, we could not detect any differences in outcomes between the group that received PS and the group that received standard AE. Adherence declined over the 6-month monitoring period, but both groups demonstrated improved quality of life, FEV1, and asthma control. These observed improvements could have been due to monitoring, attention, provision of medications, or regression to the mean. Additionally, there were no statistically detectible differences or changes over time with respect to ED visits or hospitalizations, either overall or asthma-related. To isolate the factors most responsible for the favorable outcomes, future work should explore the contribution to outcomes from the common elements in both treatment groups: attention, electronic monitoring, and the provision of medications, keeping in mind that adherence declined.
Despite an intervention aimed at adherence, there was no difference between PS and AE in asthma outcomes, nor was there a difference in the decline of adherence. It is noteworthy that adherence was already relatively good at baseline and remained so throughout the observation period at a mean of 61%.7 This is especially remarkable considering a recent study by Williams et al56 which measured ICS adherence of less than 30% using prescription fill data for asthma patients in southeastern Michigan. Actual total adherence was likely lower than their estimates because not all filled medications are taken.56
In our study we monitored both groups from the beginning, informing participants that monitor use was recorded which could have resulted in higher adherence than would have occurred had medicine-taking behavior not been monitored (Hawthorne Effect). Additionally, the relatively high adherence found in both groups may help explain the improvements in quality of life and asthma control observed for both PS and AE.6 However, hospitalizations and ED visits did not decline. It is possible that within the context of our particular sample, while attention, monitoring and providing medications can improve asthma status to some degree, they are not sufficient to mitigate the use of other medical resources, such as hospitalizations, over the 6 month period. This may be a result of the vulnerability of our sample of adults who, in addition to their unusually severe asthma (mean baseline FEV1= 66%), are high baseline users of hospitalizations and ED visits, have many comorbidities, have significant tobacco exposure, and have limited financial and social resources.
It is noteworthy that our interventions, PS and AE, are focused solely on the patient. Medical services and the patient’s larger context were not addressed. Patient concerns, detailed in their choice of the Other Problem (Table E-1), indicate that additional significant health and social problems are common. These Other Problems suggest that it is important for practices to take into account of these “external” factors which may be extremely difficult.
Some support for this argument comes from Table 2. Patients readily admitted that there were times when they did not take medications. Self-efficacy was relatively good and did not change over the course of the study suggesting that patients were not motivated to achieve better adherence. Thus, this intervention is too far “downstream”; instead motivation needs to be addressed and addressed better considering patients other priorities and problems.
The limitations of our study are informative. As noted, electronic monitoring of adherence cannot be achieved divorced from the Hawthorne Effect. Because of monitoring, many members of the AE group (66%) thought researchers were teaching about adherence. Thus, the interventions may have been perceived similarly by participants. Although the intervention was complicated and labor intensive, it focused only on patient behavior and did not consider the environment of the practice site or that of the patient’s larger social context. The study design had important elements of pragmatic research, although its complexity limits as a “real world” pragmatic intervention.57 The pragmatic elements that can inform subsequent studies are: inclusion of patients with comorbidities and those with significant tobacco exposure; utilization of a flexible individualized intervention; and delivery of PS and AE by research staff who were not trained medical personnel. The latter factor demonstrates that interventions such as attention and provision of medications could be delivered by a variety of health care workers.
In summary, our study indicates that problem-solving does not improve adherence or decrease asthma morbidity in this population. Provision of medications, monitoring, and attention are associated with improvement in some asthma outcomes but research is needed to identify interventions to alleviate the need for ED visits and hospitalizations for asthma and to improve the health of high morbidity patients living in low-income inner city neighborhoods. Such interventions likely should be incorporated into practice procedures58 and include consideration of the larger social context.
Clinical Implications/Key Summary.
Problem-solving was not better than asthma education in improving adherence or asthma outcomes in low-income inner-city adults with moderate or severe asthma.
Monitoring inhaled steroid use with provision of medication was associated with improvement in FEV1, asthma-related quality of life, and asthma control but did not reduce asthma-related hospitalizations or ED visits for these patients.
Supplementary Material
ACKNOWLEDGEMENT
We gratefully acknowledge the advice and guidance of the DSMB: Bruce G. Bender, PhD (Chair), Susan T. Reisine, PhD, and Tyra Bryant-Stephens, MD.
Source of funding:
HL070392, HL088469.
Declaration of all sources of funding:
Andrea J. Apter, MD, MSc: HL070392, HL088469, RC1HL099612. Xingmei Wang, MS, Daniel Bogen, MD, PhD, Thomas Ten Have, Rodalyn Gonzalez BA, Chantel Priolo, BA, Bariituu Adam, BA, Sabrina Geer, BA: HL070392, RC1HL099612. Daniel Polsky: HL070392
ABBREVIATIONS
- AE
Asthma Education
- BMI
body mass index
- ED
emergency department
- FEV1
forced expiratory volume in 1 second, percent predicted
- FVC
forced vital capacity, percent predicted
- ICS
inhaled corticosteroid
- PS
Problem-Solving intervention
- RCT
randomized controlled trial
REFERENCES
- 1.Moorman JE, Rudd RA, Johnson CA, King M, Minor P, Bailey C, et al. National surveillance for asthma--United States, 1980–2004. MMWR Surveill Summ. 2007;56:1–54. [PubMed] [Google Scholar]
- 2.Apter AJ, Boston R, George M, Norfleet A, Tenhave T, Coyne JC, et al. Modifiable barriers to adherence to inhaled steroids among adults with asthma: it's not just black and white. J Allergy Clin Immunol. 2003;111:1219–1226. doi: 10.1067/mai.2003.1479. [DOI] [PubMed] [Google Scholar]
- 3.Gamble J, Stevenson M, McClean E, Heaney LG. The prevalence of nonadherence in difficult asthma. Am J Respir Crit Care Med. 2009;180:817–822. doi: 10.1164/rccm.200902-0166OC. [DOI] [PubMed] [Google Scholar]
- 4.Milgrom H, Bender B, Ackerson L, Bowry P, Smith B, Rand C. Noncompliance and treatment failure in children with asthma. J Allergy Clin Immunol. 1996;98:1051–1057. doi: 10.1016/s0091-6749(96)80190-4. [DOI] [PubMed] [Google Scholar]
- 5.Williams LK, Joseph CL, Peterson EL, Wells K, Wang M, Chowdhry VK, et al. Patients with asthma who do not fill their inhaled corticosteroids: a study of primary nonadherence. J Allergy Clin Immunol. 2007;120:1153–1159. doi: 10.1016/j.jaci.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 6.Wilson SR, Strub P, Buist AS, Knowles SB, Lavori PW, Lapidus J, et al. Shared treatment decision making improves adherence and outcomes in poorly controlled asthma. Am J Respir Crit Care Med. 2010;181:566–577. doi: 10.1164/rccm.200906-0907OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haynes RB, Ackloo E, Sahota N, McDonald HP, Yao X. Cochrane Database Syst Rev. John Wiley & Sons; 2008. Interventions for enhancing medication adherence; pp. 1–157. CD000011. [DOI] [PubMed] [Google Scholar]
- 8.George M, Freedman TG, Norfleet AL, Feldman HI, Apter AJ. Qualitative research enhanced understanding of patients' beliefs: results of focus groups with low-income urban African American adults with asthma. J Alllergy Clin Immunol. 2003;111:967–973. doi: 10.1067/mai.2003.1459. [DOI] [PubMed] [Google Scholar]
- 9.Smedley BD, Stith AY, Nelson AR. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington, D.C.: Institute of Medicine. The National Academies Press; 2003. [PubMed] [Google Scholar]
- 10.Riekert KA, Rand CS. Electronic monitoring of medication adherence: when is high-tech best? Clinical Psychology in Medical Settings. 2002;9:25–34. [Google Scholar]
- 11.Spector SL, Kinsman R, Mawhinney H, Siegel SC, Rachelefsky GS, Katz RM, et al. Compliance of patients with asthma with an experimental aerosolized medication: implications for controlled clinical trials. J Allergy Clin Immunol. 1986;77:65–70. doi: 10.1016/0091-6749(86)90325-8. [DOI] [PubMed] [Google Scholar]
- 12.Rand CS. Measuring adherence with therapy for chronic diseases: implications for the treatment of heterozygous familial hypercholesterolemia. Am J Cardiol. 1993;72:68D–74D. doi: 10.1016/0002-9149(93)90014-4. [DOI] [PubMed] [Google Scholar]
- 13.Nides MA, Tashkin DP, Simmons MS, Wise RA, Li VC, Rand CS. Improving inhaler adherence in a clinical trial through the use of the nebulizer chronolog. Chest. 1993;104:501–507. doi: 10.1378/chest.104.2.501. [DOI] [PubMed] [Google Scholar]
- 14.Berg J, Dunbar-Jacob J, Rohay JM. Compliance with inhaled medications: the relationship between diary and electronic monitor. Ann Behav Med. 1998;20:36–38. doi: 10.1007/BF02893807. [DOI] [PubMed] [Google Scholar]
- 15.Bosley CM, Fosbury JA, Cochrane GM. The psychological factors associated with poor compliance with treatment in asthma. Eur Respir J. 1995;8:899–904. [PubMed] [Google Scholar]
- 16.Bender BG, Bartlett SJ, Rand CS, Turner C, Wamboldt FS, Zhang L. Impact of interview mode on accuracy of child and parent report of adherence with asthma-controller medication. Pediatrics. 2007;120:e471–e477. doi: 10.1542/peds.2006-3457. [DOI] [PubMed] [Google Scholar]
- 17.Bogen D, Apter AJ. An adherence logger for a dry-powder inhaler: an new device for medical adherence research. J Allergy Clin Immunol. 2004;114:863–868. doi: 10.1016/j.jaci.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 18.Williams LK, Joseph CL, Peterson EL, Wells K, Wang M, Chowdhry VK, et al. Patients with asthma who do not fill their inhaled corticosteroids: a study of primary nonadherence. J Allergy Clin Immunol. 2007;120:1153–1159. doi: 10.1016/j.jaci.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 19.Arean P, Hegel M, Vannoy S, Fan MY, Unuzter J. Effectiveness of problem-solving therapy for older, primary care patients with depression: results from the IMPACT project. Gerontologist. 2008;48:311–323. doi: 10.1093/geront/48.3.311. [DOI] [PubMed] [Google Scholar]
- 20.Nezu AM. Negative life stress and anxiety: problem solving as a moderator variable. Psychol Rep. 1986;58:279–283. doi: 10.2466/pr0.1986.58.1.279. [DOI] [PubMed] [Google Scholar]
- 21.Nezu AM, Ronan GF. Social problem solving and depression: deficits in generating alternatives and decision making. The Southern Psychologist. 1987;3:29–34. [Google Scholar]
- 22.Nezu AM, Ronan GF. Life stress, current problems, problem solving, and depressive symptoms: an integrative model. J Consult Clin Psychol. 1985;53:693–697. doi: 10.1037//0022-006x.53.5.693. [DOI] [PubMed] [Google Scholar]
- 23.Perri MG, Nezu AM, Viegener BJ. Improving the long-term management of obesity: theory, research, and clinical guidelines. New York: Wiley Publications; 1992. [Google Scholar]
- 24.Perri MG, Limacher MC, Durning PE, Janicke DM, Lutes LD, Bobroff LB, et al. Extended-care programs for weight management in rural communities: the treatment of obesity in underserved rural settings (TOURS) randomized trial. Arch Intern Med. 2008;168:2347–2354. doi: 10.1001/archinte.168.21.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McLeavy BC, Daly RJ, Ludgate JW, Murray CM. Interpersonal problem-solving skills training in the treatment of self-poisoning patients. Suicide and Life-Threatening Behavior. 1994;24:382–394. [PubMed] [Google Scholar]
- 26.Platt JJ, Husband SD, Hermalin, Carter J, Metzger D. A cognitive problem-solving employment readiness intervention for methadone clients. Journal of Cognitive Psychology: An International Quarterly. 1993;7:21–33. [Google Scholar]
- 27.Kelly JA, Murphy DA, Washington CD, Wilson TS, Koob JJ, Davis DR, et al. The effects of HIV/AIDS intervention groups for high-risk women in urban clinics. Am J Public Health. 1994;84:1918–1922. doi: 10.2105/ajph.84.12.1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hill-Briggs F. Problem solving in diabetes self-management: a model of chronic illness self-management behavior. Ann Behav Med. 2003;25:182–193. doi: 10.1207/S15324796ABM2503_04. [DOI] [PubMed] [Google Scholar]
- 29.Hill-Briggs F, Echemendia RJ. Association of metabolic control with problem-solving skills. Diabetes Care. 2001;24:959. doi: 10.2337/diacare.24.5.959. [DOI] [PubMed] [Google Scholar]
- 30.Glasgow RE, Toobert DJ, Hampson SE. Effects of a brief office-based intervention to facilitate diabetes dietary self-management. Diabetes Care. 1996;19:835–842. doi: 10.2337/diacare.19.8.835. [DOI] [PubMed] [Google Scholar]
- 31.D'Zurilla T, Nezu AM. Problem-Solving Therapy, a Social Competence Approach to Clinical Intervention. 2nd Edition. New York: Springer Publishing Company; 1999. [Google Scholar]
- 32.Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma, Full Report 2007. U.S. Department of Health and Human Services, National Institutes of Health, National Heart, Lung and Blood Institute; 2007. [Google Scholar]
- 33.Standardization of Spirometry, 1994 Update. American Thoracic Society. Am J Respir Crit Care Med. 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 34.Dolce JJ, Crisp C, Manzella B, Richards JM, Hardin JM, Bailey WC. Medication adherence patterns in chronic obstructive pulmonary disease. Chest. 1991;99:837–841. doi: 10.1378/chest.99.4.837. [DOI] [PubMed] [Google Scholar]
- 35.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24:67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 36.Rand CS, Nides M, Cowles MK, Wise RA, Connett J. Long-term metered-dose inhaler adherence in a clinical trial. The Lung Health Study Research Group.PG - 580–8. Am J Respir Crit Care Med. 1995;152:580–588. doi: 10.1164/ajrccm.152.2.7633711. [DOI] [PubMed] [Google Scholar]
- 37.Rand CS, Wise RA, Nides M, Simmons MS, Bleecker ER, Kusek JW, et al. Metered-dose inhaler adherence in a clinical trial. Am Rev Respir Dis. 1992;146:1559–1564. doi: 10.1164/ajrccm/146.6.1559. [DOI] [PubMed] [Google Scholar]
- 38.Apter AJ, Reisine ST, Affleck G, Barrows E, ZuWallack RL. Adherence with twice-daily dosing of inhaled steroids. Socioeconomic and health-belief differences. Am J Respir Crit Care Med. 1998;157:1810–1817. doi: 10.1164/ajrccm.157.6.9712007. [DOI] [PubMed] [Google Scholar]
- 39.Bender B, Wamboldt FS, O'Connor SL, Rand C, Szefler S, Milgrom H, et al. Measurement of children's asthma medication adherence by self report, mother report, canister weight, and Doser CT. Ann Allergy Asthma Immunol. 2000;85:416–421. doi: 10.1016/s1081-1206(10)62557-4. [DOI] [PubMed] [Google Scholar]
- 40.Juniper EF, Buist AS, Cox FM, Ferrie PJ, King DR. Validation of a standardized version of the Asthma Quality of Life Questionnaire. Chest. 1999;115:1265–1270. doi: 10.1378/chest.115.5.1265. [DOI] [PubMed] [Google Scholar]
- 41.Juniper EF, Guyatt GH, Cox FM, Ferrie PJ, King DR. Development and validation of the Mini Asthma Quality of Life Questionnaire. Eur Respir J. 1999;14:32–38. doi: 10.1034/j.1399-3003.1999.14a08.x. [DOI] [PubMed] [Google Scholar]
- 42.Juniper EF, Guyatt GH, Willan A, Griffith LE. Determining a minimal important change in a disease-specific Quality of Life Questionnaire. J Clin Epidemiol. 1994;47:81–87. doi: 10.1016/0895-4356(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 43.Leidy NK, Chan KS, Coughlin C. Is the Asthma Quality of Life Questionnaire a Useful Measure for Low-Income Asthmatics? American Journal of Respiratory and Critical Care Medicine. 1998;158:1082–1090. doi: 10.1164/ajrccm.158.4.9708130. [DOI] [PubMed] [Google Scholar]
- 44.Juniper EF, O'Byrne PM, Ferrie PJ, King DR, Roberts JN. Measuring asthma control. Clinic questionnaire or daily diary? Am J Respir Crit Care Med. 2000;162:1330–1334. doi: 10.1164/ajrccm.162.4.9912138. [DOI] [PubMed] [Google Scholar]
- 45.Juniper EF, O'Byrne PM, Guyatt GH, Ferrie PJ, King DR. Development and validation of a questionnaire to measure asthma control. Eur Respir J. 1999;14:902–907. doi: 10.1034/j.1399-3003.1999.14d29.x. [DOI] [PubMed] [Google Scholar]
- 46.Juniper EF, O'Byrne PM, Roberts JN. Measuring asthma control in group studies: do we need airway calibre and rescue beta2-agonist use? Respir Med. 2001;95:319–323. doi: 10.1053/rmed.2001.1034. [DOI] [PubMed] [Google Scholar]
- 47.Juniper EF, Bousquet J, Abetz L, Bateman ED. Identifying 'well-controlled' and 'not well-controlled' asthma using the Asthma Control Questionnaire. Respir Med. 2006;100:616–621. doi: 10.1016/j.rmed.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 48.Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 49.Apter AJ, Feldman HI, Boston RC, Coyne JC, George M, Norfleet AL, et al. Predictors of adherence differ among subject groups. Am Rev Respir Crit Care Med. 2003;167:A155. [Google Scholar]
- 50.Mahoney-Anaya P, Wang X, Tenhave T, Frazier C, Jennings R, Mims A, et al. Self-efficacy and situational baarriers to adherence in asthma among low-income populations of color. Am J Respir Crit Care Med. 2007;175:A547. [Google Scholar]
- 51.Baker DW, Williams MV, Parker RM, Gazmararian JA, Nurss J. Development of a brief test to measure functional health literacy. Patient Educ Couns. 1999;38:33–42. doi: 10.1016/s0738-3991(98)00116-5. [DOI] [PubMed] [Google Scholar]
- 52.Apter AJ, Cheng J, Small D, Albert C, Fein DL, Bennett IM, et al. Asthma Numeracy Skill and Health Literacy. J Asthma. 2006;43:705–710. doi: 10.1080/02770900600925585. [DOI] [PubMed] [Google Scholar]
- 53.Apter AJ, Garcia L, Boyd R, Wang X, Bogen D, Ten Have T. Exposure to Community Violence is Associated with Asthma Hospitalizations and ED Visits. J Allergy Clin Immunol. 2010;126:552–557. doi: 10.1016/j.jaci.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32:705–714. doi: 10.1016/0277-9536(91)90150-b. [DOI] [PubMed] [Google Scholar]
- 55.Bromberger JT, Harlow S, Avis N, Kravitz HM, Cordal A. Racial/ethnic differences in the prevalence of depressive symptoms among middle-aged women: The Study of Women's Health Across the Nation (SWAN) Am J Public Health. 2004;94:1378–1385. doi: 10.2105/ajph.94.8.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Williams LK, Peterson EL, Wells K, Campbell J, Wang M, Chowdhry VK, et al. A cluster-randomized trial to provide clinicians inhaled corticosteroid adherence information for their patients with asthma. J Allergy Clin Immunol. 2010;126:225–231. doi: 10.1016/j.jaci.2010.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thorpe KE, Zwarenstein M, Oxman AD, Treweek S, Furberg CD, Altman DG, et al. A pragmatic-explanatory continuum indicator summary (PRECIS): a tool to help trial designers. Cmaj. 2009;180:E47–E57. doi: 10.1503/cmaj.090523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lieu TA, Finkelstein JA, Lozano P, Capra AM, Chi FW, Jensvold N, et al. Cultural competence policies and other predictors of asthma care quality for Medicaid-insured children. Pediatrics. 2004;114:e102–e110. doi: 10.1542/peds.114.1.e102. [DOI] [PubMed] [Google Scholar]
- 59.Surkan PJ, Peterson KE, Hughes MD, Gottlieb BR. The role of social networks and support in postpartum women's depression: a multiethnic urban sample. Matern Child Health J. 2006;10:375–383. doi: 10.1007/s10995-005-0056-9. [DOI] [PubMed] [Google Scholar]
- 60.Mirabeau-Beale KL, Kornblith AB, Penson RT, Lee H, Goodman A, Campos SM, et al. Comparison of the quality of life of early and advanced stage ovarian cancer survivors. Gynecol Oncol. 2009;114:353–359. doi: 10.1016/j.ygyno.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 61.Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med. 152:726–732. doi: 10.7326/0003-4819-152-11-201006010-00232. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.