Abstract
We report a monomeric PSmOrange protein that is initially orange (excitation and emission at 548 and 565 nm) but becomes far-red (excitation and emission at 636 and 662 nm) after irradiation with blue-green light. Compared to its parental orange proteins, PSmOrange has greater brightness, faster maturation, higher photoconversion contrast, and better photostability. The red-shifted spectra of both forms of PSmOrange enable its simultaneous use with cyan-to-green photoswitchable proteins to study four intracellular populations. Photoconverted PSmOrange has, to date, the most far-red excitation peak, provides diffraction-limited and super-resolution imaging in far-red range, is optimally excited with common red lasers, and can be photoconverted subcutaneously in a mouse. PSmOrange photoswitching occurs via a two-step photo-oxidation process, which causes cleavage of the polypeptide backbone. The far-red fluorescence of photoconverted PSmOrange results from a novel chromophore containing N-acylimine with a coplanar carbon-oxygen double bond.
Photoconvertible fluorescent proteins (FPs) are widely used to optically highlight spatial-temporal dynamics of intracellular molecules, organelles and whole cells1. There are two types of irreversibly photoconvertible FPs: photoactivatable and photoswitchable. Photoactivatable FPs (PAFPs) are activated from a non-fluorescent (dark) state to a fluorescent state. This group includes PAGFP2, PAmCherry3, and PATagRFP4. Photoswitchable FPs (PSFPs) switch between two different fluorescent colors. Most PSFPs, including Dendra25, mEos26, Kaede7, KikGR8, ClavGR29 and their derivatives, change color from green to red. The only available non-red PSFP is the cyan-to-green PSCFP2 protein5. Most photoconvertible FPs require irradiation with UV-violet light to become photoconverted. Dendra2 can be also photoconverted with blue light but this is less effective and requires high intensity illumination.5
Several super-resolution microscopy techniques, such as PALM (photoactivated localization microscopy)10 and STORM (stochastic optical reconstruction microscopy)10 use photoactivatable or photoswitchable molecules. PALM techniques utilize both PAFPs and PSFPs. Super-resolution techniques can also utilize reversibly switchable FPs in a PALMIRA mode11 and conventional FPs in GSDIM mode12. Multicolor PALM provides information about the spatial and temporal heterogeneity of several types of molecules within a cell, but compatible photoconvertible FPs are currently limited to green and red3–4. Adding a third color would be beneficial, since the relative intracellular localization of three proteins can then be compared directly, and cell-to-cell variability is less of a problem.
Non-invasive imaging of animals requires the development of genetically-encoded fluorescent probes with excitation and emission spectra in the near-infrared region, which has the lowest hemoglobin, melanin and water absorbance13. The tissue absorbance in this optical window drops so substantially that even dim far-red FPs perform better than bright green FPs14. There are several conventional far-red FPs with excitation and emission maxima up to 611 and 670 nm, respectively15–19. However, there are as yet no far-red PAFPs and PSFPs.
It has been shown that a conventional fluorescent protein mOrange photoconverts from an orange to a far-red form after irradiation with 458 nm or 488 nm light20. However, low photostability and low far-red brightness limit the use of mOrange as a photoswitchable tag.
An aerobic effect, called photooxidative redding, occurs in green fluorescent proteins of different origins in the presence of oxidants21. Interestingly, photobleaching of mOrange occurs substantially faster in the presence of oxygen than in oxygen-free conditions22 suggesting that mOrange photoconversion may be affected by intracellular oxidants.
It would advance various imaging techniques to have a PSFP, which is photoswitchable with visible light, exhibits fluorescent colors distinct from those of existing PSFPs, has far-red photoswitched color for deep-tissue imaging, and is optimized for the redox conditions of live cells. In this paper, we explored the ability of mOrange to photoswitch into the far-red form and the effect of oxidant agents on the photoconversion of FPs. We developed a bright monomeric orange probe with high-contrast photoswitching from an orange to a far-red state using oxidative conditions matching those of mammalian cells.
RESULTS
Development of photoswitchable orange fluorescent protein
We used the mOrange gene as a template to develop a variant that photoswitches from orange to far-red. First, Q63H and F100Y substitutions (numbering is based on alignment with an enhanced green fluorescent protein (EGFP)), responsible for high photostability of mOrange222, were introduced into mOrange. The resulting mOrange/Q63H/F100Y gene was then subjected to nine rounds of random mutagenesis, using an error-prone polymerase chain reaction. After nine rounds of molecular evolution, the S21T/H63L/F100Y/L125M/K166R/P192S variant was selected and named PSmOrange (Supplementary Fig. 1). Before photoswitching, PSmOrange exhibited orange fluorescence with excitation and emission peaks at 548 and 565 nm (Table 1). After photoswitching with blue-green light, it showed far-red fluorescence with excitation and emission maxima at 636 and 662 nm (Fig. 1a). Irradiation with violet light had a ~17-fold lower rate of PSmOrange photoswitching and resulted in a 7.2-fold lower maximal far-red fluorescence than with blue light of the same intensity (Supplementary Fig. 2a and 2b).
Table 1.
Properties of the orange and far-red forms of the PSmOrange purified protein.
| Protein | mOrange | mOrange2 | PSmOrange | |||
|---|---|---|---|---|---|---|
| Orange form | Far-red form | Orange form | Far-red form | Orange form | Far-red form | |
| Absorbance/Excitation (nm) | 546 | 631 | 549 | 632 | 548 | 634/636 |
| Emission (nm) | 562 | 662 | 565 | 663 | 565 | 662 |
| Extinction coefficient (M−1 cm−1) | 71,000 | 17,200 | 58,000e | ND | 113,300 | 32,700 |
| Quantum yield | 0.69e | 0.19 | 0.6e | ND | 0.51 | 0.28 |
| Brightness relative to EGFP (%) | 148 | 10 | 105 | ND | 176 | 28 |
| Photoswitching t0.5 (s) a | 12 ± 6 | 8.0 ± 0.4 | 15 ± 3 | |||
| Far-red increase (fold) | 190 | 240 | 560 | |||
| Photoswitching contrast (fold) b | 2,800 ± 200 | ND | 10,700 ± 500 | |||
| Photobleaching t0.5 (s) c | 0.65 ± 0.39 | 17.0 ± 3.7 | 7 ± 3 | ND | 15 ± 5 | 48.5 ± 8.2 |
| pKa d | 6.5e | ND | 6.5e | ND | 6.2 ± 0.1 | 5.6 ± 0.1 |
| Maturation half-time at 37°C (h) | 2.5 | 4.5e | 1.6 | |||
Determined at 1,050 W cm−2 at the sample. Error, s.d. (n = 10).
Determined as the product of the far-red fluorescence increase and the orange fluorescence decrease after photoswitching. Error, s.d. (n = 3).
Determined at 1,130 W cm−2 at the sample for the orange form and at 870 W cm−2 at the sample for the far-red form. Error, s.d. (n = 5).
Error, s.d. (n = 4).
Ref. 22. ND, not determined.
Figure 1. Characterization of purified PSmOrange in vitro.
(a) Absorbance and emission spectra of PSmOrange before and after photoswitching with 489 nm LED array. (b) PSmOrange far-red brightness and photostability are plotted against the concentration of the oxidant K3Fe(CN)6. (c) Photoswitching half-times for PSmOrange is plotted against the concentration of K3Fe(CN)6. Half-time at 0.25 mM oxidant is shown by dashed line. (d) Formation of the far-red form over time is plotted for purified PSmOrange protein (in 0.25 mM K3Fe(CN)) and for cytoplasmic PSmOrange inside the indicated mammalian cells. The half-time for the purified protein is indicated by dashed line. (e–f) Photoswitching kinetics for orange (e) and far-red (f) forms of the indicated proteins. (g) PSmOrange initial photoswitching rate is plotted against power of the photoswitching 480/40 nm light. (h, i) Photobleaching half-times for the orange (h) and far-red (i) forms of the indicated fluorescent proteins at different power densities Inset: zoomed in bars for 1,130 mW cm−2 power. The power densities were estimated at the sample. The photobleaching data (h–i) were normalized to the spectral output of the lamp, transmission profile of the filter and dichroic mirror, and absorbance spectra of the proteins. Error bars, s.d. (n = 10 (b, c) and n = 5 (g, h, i)).
Properties of PSmOrange
When PSmOrange and other mutants we obtained in the process of molecular evolution were expressed in mammalian cells, their kinetics of photoswitching were faster than that of the purified proteins, suggesting that the intracellular redox environment might affect the process. Therefore, we examined the effect of oxidants on the PSmOrange purified protein. It has been shown that potassium ferricyanide, K3Fe(CN)6, performed as an optimal model oxidant in the oxidative redding of various green fluorescent proteins in vitro, mimicking their photoswitching in mammalian cells21. We observed that brightness and photostability of the PSmOrange far-red form was almost independent of K3Fe(CN)6 concentration over a wide range (Fig. 1b) but that higher oxidant concentrations substantially decreased the PSmOrange photoswitching half-time (Fig. 1c). The photoswitching half-time of PSmOrange in live mammalian cells corresponded to that observed with the purified protein at K3Fe(CN)6 concentration of 0.25 mM (Fig. 1d). In order to match the effect of intracellular redox agents on PSmOrange behavior, we further characterized purified PSmOrange in the presence of 0.25 mM K3Fe(CN)6.
At a high intensity of photoswitching light, PSmOrange exhibited a 1.25-fold longer photoswitching half-time (Fig. 1e,f). The photoswitching half-time of mOrange2 was even shorter than that for mOrange, and its maximal far-red fluorescence was 6.1-fold and 2.4-fold dimmer compared to PSmOrange and mOrange, respectively. The far-red form was generated with mono-exponential kinetics whereas switching off kinetics of the orange form was bi-exponential suggesting that switching off is a complex photochemical process involving at least two pathways, of which the photoconversion is a major one.
The initial rate of PSmOrange photoswitching using both blue and green light depended on the light intensity in a non-linear fashion (Supplementary Fig. 2c,d). On the logarithmic scale, the initial rate had a linear dependence on the light intensity with a slope of 2.01 (Fig. 1g), suggesting that PSmOrange photoswitching requires two photons. We concluded that PSmOrange photoswitching is a two-step photochemical process.
PSmOrange was 1.2-fold brighter in the orange state and 2.8-fold brighter in the far-red state, as compared to parental mOrange (Table 1). The photoswitching orange-to-far-red fluorescence contrast achieved with purified PSmOrange protein was ~4-fold higher than for mOrange. PSmOrange had pKa values of 6.2 and 5.6 before and after photoswitching, respectively (Supplementary Fig. 3a). Maturation of PSmOrange at 37°C was 1.6-fold faster compared to mOrange (Supplementary Fig. 3b). Similarly to mOrange and mEGFP, purified PSmOrange exhibited monomeric behavior (Supplementary Fig. 4).
At a high light intensity, photobleaching of the orange and far-red fluorescence signals of PSmOrange decreased 23-fold and ~3-fold compared to parental mOrange (Supplementary Fig. 3c), although this difference in photostability decreased with the decreasing power of the photobleaching light (Fig. 1h,i). The orange form of PSmOrange was ~2-fold more photostable than that of mOrange2 at a high light intensity and had the same photostability at a low light intensity (Fig. 1h). Photostability of the mOrange2 far-red form was not studied because of an inefficient mOrange2 photoswitching (Fig. 1f).
We calculated the fluorescence brightness of the far-red form of PSmOrange above 650 nm at 633 nm excitation, as a criterion for a practical application with common HeNe and red diode lasers15,17,18. The brightness of photoswitched PSmOrange in the far-red region was ~6-fold greater than that of the most far-red shifted FPs, such as mNeptune18, TagRFP65715, and eqFP67019 (Supplementary Table 1). We also compared PSmOrange and several far-red FPs expressed in bacterial cells, using FACS equipped with a standard 638 nm excitation laser and a 660/20 nm emission filter. The mean fluorescence brightness of cells with the photoswitched PSmOrange was substantially higher than that of cells expressing the other FPs (Supplementary Fig. 5).
Behavior of PSmOrange in live mammalian cells
We fused PSmOrange to several cellular proteins and examined expression patterns in HeLa cells. PSmOrange fusions with α-tubulin, vimentin, keratin, paxillin, myosin, and histone H2B properly localized in live cells and did not affect cell division (Supplementary Fig. 6).
It has been shown that some FPs are cytotoxic when expressed at high levels using transient expression23. We compared cytotoxicity of PSmOrange with mEGFP as a non-cytotoxic control23 using transient transfection of HeLa cells (Supplementary Fig. 7a). Relative changes in mean fluorescence intensity of the PSmOrange-expressing cells over time were similar to those of the mEGFP-expressing cells. Next, to test PSmOrange cytotoxicity over longer periods, we made stable preclonal mixtures of MTLn3 cells expressing PSmOrange, mEGFP or mKate217. The brightest fluorescent cell population for each reporter was collected by FACS 18 days after transfection (Supplementary Fig. 7b), further cultured for 19 more days, and then again FACS analyzed. Mean fluorescence intensities of PSmOrange- and mEGFP-expressing cells changed slightly in 19 days of culture, whereas mKate2-expressing cells showed a large number of cells with decreased mean fluorescence. Lastly, we tested whether PSmOrange is cytotoxic in an animal. PSmOrange- or GFP-expressing MTLn3 cells were injected into mouse mammary glands, and tumor growth was observed by monitoring orange and green fluorescence over time (Supplementary Fig. 7c). Fluorescence of the GFP-expressing tumor reached a plateau after 33 days while fluorescence of the PSmOrange-expressing tumor increased up to 46 days. Overall, these data demonstrate that, similarly to mEGFP or GFP, PSmOrange is noncytotoxic.
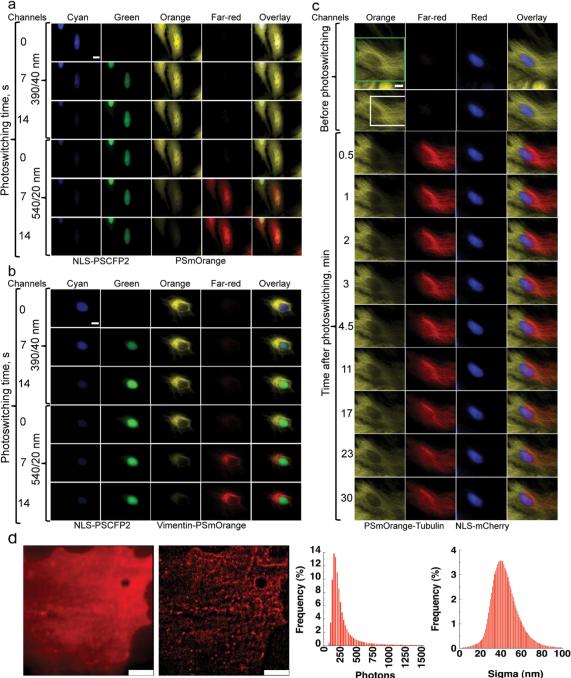
The red-shifted spectra of both forms of PSmOrange, as compared to other red PSFPs, could allow for the simultaneous intracellular use of PSmOrange with the photoswitchable cyan-to-green PSCFP2. To test this, we co-expressed nuclear-targeted NLS-PSCFP2 with either untagged PSmOrange (Fig. 2a) or with a vimentin-PSmOrange fusion protein (Fig. 2b). The initial and the photoconverted fluorescence signals of both PSFPs were well separated spectrally in the cells. Photoswitching of one PSFP did not cause substantial photobleaching of the other PSFP. Thus, using two spectrally compatible PSFPs, it is possible to simultaneously image four distinct intracellular populations.
Figure 2. Imaging of PSmOrange in mammalian cells.
The micrographs show HeLa cells expressing NLS-PSCFP2 in the nucleus with (a) cytoplasmic PSmOrange. and (b) vimentin-PSmOrange. Photoswitching of PSCFP2 and PSmOrange was performed with 390/40 nm and 540/20 nm light, respectively. (c) The micrographs show dynamics of PSmOrange-tubulin co-expressed with NLS-mCherry in live HeLa cells. Photoswitching of PSmOrange was performed with 480/40 nm light for 60 s. The zoomed area is marked as a green square in the first row and is shown in all subsequent rows. The area of the PSmOrange photoconversion is indicated as a white square. See Online Methods for filter set information. Scale bars are 10 μm. (d) The micrographs show a TIRF (left) and a PALM (right) image of EGFR-PSmOrange in fixed COS-7 cells. The histograms show distributions of photons and localization uncertainties. The mean number is 337 and the mean molecular localization uncertainty (sigma) is 45 nm. Data are from 576,290 molecules collected from 5 cells. Scale bars are 5 μm.
To demonstrate PSmOrange use to study intracellular dynamics together with common red FPs, we next co-expressed PSmOrange-α-tubulin with NLS-mCherry (Fig. 2c). A good spectral resolution between the orange and far-red forms of PSmOrange and red fluorescence of mCherry allows using these two proteins simultaneously for multicolor imaging. As expected24, the fast α-tubulin dynamics resulted in the complete replacement of the nonconverted orange PSmOrange-α-tubulin with the photoconverted far-red PSmOrange-α-tubulin, and vice versa, within 30 min after photoswitching.
Evaluation of PSmOrange as a PALM probe
We fused PSmOrange to epidermal growth factor receptor (EGFR), expressed the construct in COS-7 cells, and imaged the cells with PALM11, using total internal reflection fluorescence (TIRF) illumination with 488 nm photoconversion and 640 nm excitation. The punctate distribution of the EGFR-PSmOrange chimera throughout the plasma membrane was similar to that seen previously4 (Fig. 2d). The PALM images are notably more detailed when compared with the TIRF images. Photon statistics of the photoconverted far-red PSmOrange molecules showed that they have properties sufficient for PALM. Taken together, these tests indicate that PSmOrange can be used in PALM imaging. Furthermore, PSmOrange can be activated by blue rather than violet light, providing opportunities for PALM experiments on instruments lacking 405 nm lasers.
Imaging of PSmOrange in mouse models
To assess PSmOrange for use in vivo we injected equal amounts of purified mKate2, mNeptune, E2-Crimson16, TagRFP657, eqFP670, and photoswitched PSmOrange subcutaneously (~2 mm deep) into a mouse, as has been previously performed25. mKate2 and mNeptune exhibited the highest fluorescent signal at the 605/30 nm excitation channel (Supplementary Fig. 8a,c,f) while photoconverted PSmOrange was detected in both 605/30 nm and 640/30 nm channels but exhibited several-fold higher fluorescence comparing to far-red FPs when excited using the 640/30 nm channel (Supplementary Fig. 8b,d,g). These data suggest that photoswitched PSmOrange can be used as a second far-red color complementary to mKate2/mNeptune (Supplementary Fig. 8e).
To explore how a deeper-tissue location could affect brightness of far-red FPs and photoswitched PSmOrange, we imaged the same amount of the purified proteins at 7.0 mm and 18.1 mm depth inside of a mouse phantom (Supplementary Fig. 9). The results were similar to those observed with subcutaneously injected FPs (Supplementary Fig. 8). At both 7.0 mm and 18.1 mm, photoconverted PSmOrange was brightest in the 640/30 nm excitation channel while mKate2 and mNeptune were brightest in the 605/30 nm channel (Supplementary Fig. 9).
To demonstrate that PSmOrange can be used as a second far-red color complementary to mKate2 or mNeptune in live mouse tissues, we applied a test recently used to compare far-red FPs in cells in mice19. HEK293T cells transiently expressing mKate2 or PSmOrange after photoconversion were injected intramuscularly into gluteal regions of a mouse. PSmOrange was detectable in two channels, 605/30 nm and 640/30 nm. mKate2 cells were brighter in 605/30 nm channel while PSmOrange cells were brighter in 640/30 nm channel (Fig. 3a,b).
Figure 3. Imaging of PSmOrange in vivo.
(a) The photographs show whole body images of a mouse injected intramuscularly with 106 cells expressing mKate2 (left flank) or photoconverted PSmOrange (right flank). Right column: images presented in the left column in 605/30 nm and 640/30 nm excitation channels are shown with green and red pseudocolors, respectively. (b) Bar graphs of the total radiant efficiency corresponding to panel (a). Total radiant efficiency of mKate2 was set as 100% in 605/30 nm excitation channel, and total radiant efficiency of PSmOrange was set as 100% in 640/30 nm excitation channel. Error bars, s.d. (n = 3). (c) Photoconversion of PSmOrange in mammary tumor xenograft in a mouse. The photographs show whole body images at the indicated excitation and emission wavelengths before and after photoconversion. (d) Bar graphs of the total radiant efficiency corresponding to panel (c). Maximal total radiant efficiency in each channel was accepted as 100%. Error bars, s.d. (n = 4). In (a–d) the first and second numbers in 535 nm/580 nm, 605 nm/660 nm, and 640 nm/680 nm indicate excitation and emission wavelengths, respectively. In (a, c) black scale bars are 1 cm. Color scale bars represent the radiant efficiency in p s−1 sr−1 μW−1.
To utilize an advantage of the substantially lower in vivo absorbance of blue light compared to violet light26 we tested the possibility of PSmOrange photoswitching directly in a mouse. Subcutaneously injected PSmOrange sample was photoconverted through the skin (Supplementary Fig. 10). The photoconverted far-red form of PSmOrange was clearly detectable in both 605/30 nm and 640/30 nm excitation channels. To test whether we can photoconvert PSmOrange in cells inside of mice, we grew MTLn3 adenocarcinoma cancer cells in mouse mammary gland. Before photoconversion, the PSmOrange-expressing tumor was only detectable in the 535/30 nm excitation channel (Fig. 3c,d). After the photoconversion using a 489 nm LED array, the tumor was clearly detected in the far-red 605/30 nm and 640/30 nm excitation channels.
PSmOrange far-red chromophore structure
To elucidate the chemical nature of the far-red chromophore of PSmOrange, we measured absorbance spectra of the denatured protein. The orange and far-red PSmOrange forms exhibited the same absorbance maxima at 387 nm after denaturation in acid and at 450 nm after denaturation in alkali. These peaks are characteristic of an N-acylimine-containing DsRed-like chromophore. Thus, both PSmOrange forms possibly contain an N-acylimine C=N group, which, similarly to the DsRed-like chromophore is hydrated upon denaturation.
PSmOrange and mOrange polypeptides in the orange state did not exhibit polypeptide chain cleavage in SDS-PAGE after boiling (Fig. 4a). After photoswitching, however, we observed cleavage with formation of 19 kDa and 9 kDa fragments, suggesting that this cleavage may occur in the PSmOrange far-red chromophore. The PSmOrange far-red form was less stable with time than the orange form, with a half-life time of 69 h (Supplementary Fig. 11). We further used mass spectrometry to analyse both forms of PSmOrange. The mass spectrum of PSmOrange chymotrypsin-digested fragments before photoswitching revealed a monoisotopic mass of 1211.39 Da, corresponding to the chromopeptide. The mass was as expected from the cyclization (loss of H2O) and double oxidation (loss of 2H2) of the chromophore inside the SPLFTYGSKAY fragment (cleavage after Leu60 and Tyr71). Further MS/MS fragmentation showed that the dehydrogenation sites were located between nitrogen and α-carbon of Thr65 and between Cα and Cβ atoms of Tyr66 (Fig. 4b). Thus, the data are consistent with the formation of two double bonds inside of the orange chromophore of PSmOrange, similar to the mOrange-like chromophore27.
Figure 4. Mass spectrometry analysis of the PSmOrange chromophore.
(a) The gel shows SDS-PAGE of PSmOrange samples before and after photoswitching. (b, c) The chromophore-bearing peptides and structures of chromophores for PSmOrange before (b) and after (c) photoswitching are shown. (b) m/z ratio calculated (first number) and m/z ratio observed (second number) are as following: y3, 381.21 and 381.21; y4, 468.25 and 468.29; y6, 696.38 and 696.32; y8, 914.45 and 914.35; b2, 185.09 and 185.13; b3, 298.18 and 298.18; b4, 445.24 and 445.26; b7, 744.38 and 744.31; b8 831.41 and 831.42; b9, 959.50 and 959.47; b10, 1030.54 and 1030.45. (c) m/z ratio calculated (first number) and m/z ratio observed (second number) are as following: y3, 381.21 and 381.22; y4, 468.25 and 468.26; b4, 413.17 and 413.16; b5, 541.27 and 541.23; b6 612.30 and 612.28. (d) A proposed scheme for the PSmOrange photoconversion is depicted. Asterisk indicates the position 2 of the GFP-like chromophore core. [Ox] and [OxH] represent an oxidant molecule and a reduced oxidant molecule, respectively.
In the mass spectrum of photoswitched PSmOrange, we observed a new monoisotopic mass of 793.3 Da, 4 Da heavier than the theoretical mass of the TYGSKAY fragment. The mass increase suggested a modification of the chromopeptide that could result from cleavage between the dihydrooxazole ring and CH group in Phe of the SPLFTYGSKAY fragment. MS/MS fragmentation of the TYGSKAY peptide (Fig. 4c) revealed that the far-red chromophore includes the C=N bond in the third dihydrooxazole ring of the mOrange-like chromophore, and that the new carbonyl group substitutes for the hydroxyl group in this ring (Fig. 4d). The observed cleavage of the PSmOrange far-red form in SDS-PAGE is consistent with the suggested far-red chromophore structure based on mass spectrometry.
DISCUSSION
PSmOrange has spectral advantages over conventional green-to-red PSFPs. First, PSmOrange is red-shifted, enabling its utilization for simultaneous multicolor imaging with green and blue FPs and PSCFP2. Second, the orange and far-red forms of PSmOrange and mCherry can be spectrally separated, so that it is also possible to use PSmOrange simultaneously with red FPs and PAFPs. Third, when excited using standard red laser lines or red-shifted excitation filters, photoconverted PSmOrange is brighter than conventional far-red FPs (Fig. 3a, Supplementary Figs. 5, 8, 9).
Chromophore structures of most FPs with a tyrosine residue in the chromophore-forming tripeptide include the 4-(p-hydroxybenzylidene)-5-imidazolone heterocyclic core, which is the green fluorescent protein-like (GFP-like) chromophore28. The C=N substituent transforms the GFP-like chromophore into the mOrange-like chromophore, exhibiting a red shift of ~35–55 nm. The C=O group-substituent leads to a red shift of ~80–100 nm that is observed in the asFP595 protein29. An N-acylimine group, which consists of two double bonds (C=N-C=O), transforms the GFP-like chromophore into the DsRed-like chromophore. The N-acylimine should provide a bathochromic shift substantially larger than that of single C=N or C=O groups, but this is not the case when compared to the C=O substituent. The reason is that in all crystal structures of FPs having the DsRed-like chromophore, the C=O group is out of the chromophore plane27. This means its conjugation with the chromophore core and hence the red shift are substantially reduced.
Mass-spectrometry data indicate that the far-red PSmOrange chromophore contains an N-acylimine substituent in the GFP-like core. Its C=N bond is involved in the five-member dihydrooxazole ring, which is characteristic of the mOrange-like chromophore. Light-induced oxidation causes polypeptide chain cleavage and formation of a C=O group linked to the five-member ring. Thus, the involvement of the N-acylimine functionality in the dihydrooxazole ring results in all its bonds being in the chromophore plane and hence in a more efficient conjugation of the C=O group, as compared to the DsRed-like chromophore.
Using the resolved structures of the orange and far-red chromophores, we can suggest the chemical scheme of the PSmOrange photoswitching (Fig. 4d). Since photoswitching accelerates in the presence of oxidant (Fig. 1c) and requires two photons of blue-green light (Fig. 1g), the orange to far-red transformation should include two steps of a photo-oxidative polypeptide cleavage. A hydroxyl group of the orange chromophore is transformed into a carbonyl group of the far-red chromophore. We suggest that this reaction is similar to a two-step oxidation of a hydroxyl group of alcohols to a carbonyl group of aldehydes and ketones in the presence of oxidants30.
METHODS
Mutagenesis and screening of libraries
We amplified an mOrange31 gene as a BglII-EcoRI fragment, using a polymerase chain reaction, and inserted into a pBAD/His-B vector (Invitrogen). A site-specific mutagenesis of the mOrange gene was performed using a QuickChange Mutagensis Kit (Stratagene). For simultaneous mutagenesis at several positions, we applied an overlap-extension approach32. A random mutagenesis was performed using a GeneMorph II Random Mutagenesis Kit (Stratagene) or a Diversity PCR Random Mutagenesis Kit (Clontech) under conditions resulting in a mutation frequency of up to 16 mutations per 1,000 base pairs. After mutagenesis we electroporated a mixture of the mutated genes into LMG194 bacterial host cells (Invitrogen).
Libraries of 107–108 independent clones of the mOrange mutants were photoswitched with a custom built 489 nm LED array (adjusted to 93 mW cm−2) and screened using a MoFlo (Dako) fluorescence-activated cell sorter (FACS), followed by colony visualization, using a Leica MZ16F fluorescence stereomicroscope, as previously described3. After each round of FACS screening, typically 10–20 best photoswitchable candidate clones were sequenced, purified, and characterized before the next round of mutagenesis.
Characterization of purified proteins
We expressed mOrange31, mOrange222 (contained Q63H/F100Y mutations comparing to mOrange), mNeptune18, and PSmOrange mutant proteins with polyhistidine tags in LMG194 bacteria grown in an RM medium supplemented with 0.002% arabinose for 24–48 h at 37°C and then purified using a Ni-NTA agarose (Qiagen). For spectroscopy, photoswitching of purified proteins was performed with the 489 nm LED array (adjusted to 280 mW cm−2) in 1.5 ml transparent Eppendorf tube on ice. Excitation and emission spectra of recombinant proteins were measured with a FluoroMax-3 spectrofluorometer (Jobin Yvon). For absorbance measurements, a Hitachi U-3010 spectrophotometer was used.
To determine extinction coefficients of orange forms, we measured the mature chromophore concentration, as previously described for mOrange31. For this, the purified mOrange and PSmOrange proteins were alkali-denatured. It is known that the extinction coefficient of the GFP-like chromophore is 44,000 M−1cm−1 at 447 nm in 1 M NaOH33. Based on the absorbance of the native and denatured proteins, molar extinction coefficients for the native states were calculated. To determine extinction coefficients of far-red forms, we photoswitched purified proteins with the 489 nm LED array (adjusted to 280 mW cm−2) for 10 min on ice in the presence of 5 mM K3Fe(CN)6. Under this oxidant concentration the maximally achievable far-red fluorescence was not dependent on the 489 nm light intensity. Extinction coefficients were calculated based on a comparison between the absorbance decrease of orange forms (at 548 and 546 nm for PSmOrange and mOrange, respectively) and the absorbance increase of far-red forms (at 634 and 631 nm for PSmOrange and mOrange, respectively). To determine quantum yields, we compared the fluorescence intensities of orange and far-red forms at pH 8.5 to the fluorescence intensities of equally absorbing amounts of mOrange in the orange form (quantum yield is 0.6931) and mNeptune (quantum yield is 0.218), respectively.
Equilibrium pH titrations were performed using a series of buffers (100 mM NaOAc, 300 mM NaCl for pH 2.5–5.0, and 100 mM NaH2PO4, 300 mM NaCl for pH 4.5–10.0).
Photobleaching kinetics was measured using purified proteins in phosphate buffered saline (PBS) at 1 mg ml−1 in aqueous drops in oil using Olympus IX81 inverted microscope equipped with a 200 W metal halide arc lamp (Prior), a 100× 1.4 NA oil immersion objective lens (UPlanSApo, Olympus), 540/20 nm excitation and 575/30 nm emission filters for orange forms, and 605/40 nm excitation and 640LP nm emission filters for far-red forms. The microscope was operated with SlideBook 4.2 software (Intelligent Imaging Innovations). Light power densities were measured at a rear focal plane of the objective lens, and light power at the sample was estimated. Photoswitching kinetics was measured using the above conditions with a 480/40 nm filter for photoswitching. The data were normalized to a spectral output of the lamp, transmission profiles of the filters and dichroic mirror, and absorbance spectra of the respective proteins.
To study protein maturation, LMG194 bacteria transformed with the mOrange or PSmOrange genes were grown in an RM medium supplemented with ampicillin at 37°C overnight. The next morning, we diluted bacterial cells to optical density 1.0 at 600 nm, and 0.2% arabinose was added. Upon induction of protein expression, bacterial cultures were grown at 37°C in 50 ml tubes filled to the brim and tightly sealed to restrict oxygen supply. After 2 hours, the cultures were centrifuged in the same tightly closed tubes. After opening the tubes, we purified the proteins using the Ni-NTA resin within 30 min, with all procedures and buffers at or below 4°C. Protein maturation occurred in PBS at 37°C. Orange fluorescence signal of the proteins was monitored using the FluoroMax-3 spectrofluorometer.
Mammalian plasmids and cell culture
To construct pPSmOrange-C1, pPSmOrange-α-Tubulin and pPSmOrange-Myosin plasmids, the PSmOrange gene was swapped with the EGFP gene or the mTagBFP gene in the pEGFP-C1, pEGFP-α-Tubulin and pmTagBFP-Myosin vectors (Clontech), respectively. To design a pH2B-PSmOrange plasmid, the PSmOrange gene was swapped with the PAmCherry1 gene in the pH2B-PAmCherry1 plasmid3. To construct pVimentin-PSmOrange, pKeratin-PSmOrange and pPaxillin-PSmOrange plasmids, the PSmOrange gene was swapped with the mTagBFP gene in the pVimentin-mTagBFP, pKeratin-mTagBFP and pPaxillin-mTagBFP vectors, respectively. To construct a pPSmOrange-N1 plasmid, the PSmOrange gene was PCR amplified as a AgeI-NotI fragment and swapped with the EGFP gene in a pEGFP-N1 vector (Clontech). To generate pNLS-PSCFP2 plasmid, AgeI-NotI fragment containing the PSCFP2 gene was cut out from the pPSCFP2-N1 plasmids and was swapped with the ECFP gene in a pNLS-ECFP plasmid. To construct a pEGFR-PSmOrange plasmid, a SacII-BsrGI fragment containing the PSmOrange gene was PCR amplified and swapped with the mRFP1 gene in the pEGFR-mRFP1 plasmid34.
HeLa and COS-7 cells were grown in a Dulbecco's Modified Eagle Medium (DMEM) containing 10% fetal bovine serum and 2 mM glutamine (all from Invitrogen). MTLn3 cells were grown in alpha-Modified Eagle Medium containing 5% fetal bovine serum and 2 mM glutamine (all from Invitrogen). Cells were grown in #1.5 glass bottom culture dishes (MatTek Corporation). Plasmid transfections were performed either with an Effectene (Qiagen) or a FuGENE (Roche) reagents. Live HeLa cells were photoswitched and imaged in a dye-free DMEM medium without serum (Invitrogen). COS-7 cells were incubated at 37°C for 36 hours before fixing with 4% paraformaldehyde (Electron Microscopy Sciences).
Imaging of mammalian cells
Wide-field epifluorescence imaging of live mammalian cells was performed 48–72 h after transfection. Cells were imaged using the Olympus IX81 inverted microscope described above. A 480/40 nm (1,050 W cm−2, here and below the light intensities are estimated at the sample) or 540/20 nm (1,130 W cm−2) filters were used for photoswitching of mOrange and PSmOrange, and a 390/40 nm filter (900 W cm−2) was used for photoswitching of PSCFP2. The 540/20 nm excitation and 575/30 nm emission filters (126–468 W cm−2) were used to image orange forms, and the 605/40 nm excitation in combination with 640LP nm emission filters (360 W cm−2) or 622/36 nm excitation in combination with 680LP emission filters (340 W cm−2) to image far-red forms. The 390/40 nm excitation and 460/40 nm emission filters (360 W cm−2) were used to image the cyan form of PSCFP2, and the 480/40 nm excitation and 530/40 nm emission filters (432 W cm−2) were applied to image the green form of PSCFP2. The 570/30 nm excitation and 615/40 nm emission filters (120 W cm−2) were used to image mCherry. All filter sets were from Chroma.
PALM imaging of single molecules was performed on an Olympus IX71 microscope using a 60× 1.45 NA PlanApoN TIRF objective (Olympus), as previously described35,36. The lasers were a 100 mW 640 nm (Coherent) and a 50 mW 488 nm (Oxxius). Photoswitched EGFRPSmOrange single molecules were imaged using 640 nm laser light. Data were collected in 100 msec frames over 12,000 frames with a pulse of 488 nm laser light every 10 frames. Power levels measured near the rear aperture of the objective lens were < 2 mW (estimated < 80 W cm−2 at the sample) for the 640 nm laser and ranged from ~65 μW to ~8 mW (estimated 2.6-320 W cm−2 at the sample) for the 488 nm laser. The dichroic mirror was designed for use with 4 laser lines (Di01-R405/488/561/635-25×36, Semrock) and fluorescence was detected through a long pass far-red filter (BLP01-635R-25, Semrock) with an iXon EMCCD camera (Andor Technology). Image analysis was performed as previously described35. Coverslips were cleaned with piranha wash as previously described37. Fidudicary markers were 25 nm gold nanorodz (Nanopartz). The fluorescence was collected during the 12,000 frames for a diffraction-limited TIRF image. The position and uncertainty from 183,089 molecules were plotted as Gaussian-normalized spots to form a PALM image.
Imaging of purified proteins in mouse models
Bacterial plasmids encoding several far-red FPs were kindly provided by B. Glick (University of Chicago, USA) and D. Chudakov and K. Lukyanov (both from Institute of Bioorganic Chemistry, Russia). We expressed and purified recombinant mKate2, mNeptune, E2-Crimson, TagRFP657, and eqFP670 proteins as described above for PSmOrange. The purified FPs were diluted in PBS to the equal concentrations calculated based on the extinction coefficients at the chromophore absorbance maxima. All procedures with mice were conducted in accordance with NIH regulations, and approved by the Albert Einstein College of Medicine Animal Use Committee. Albino C57BL/6 6–8 weeks old female mice (Jackson Laboratory) were used. Isoflurane (Aerrane) was used to sedate the mice during short imaging sessions. Belly fur was removed using a depilatory cream, and 10 μl volume of each fluorescent protein (100 μM) was injected subcutaneously into mice. Imaging of mice was performed at 37°C using an IVIS Spectrum instrument in the epifluorescence mode (Caliper Life Sciences). The fluorescent signals of different far-red FPs and photoswitched PSmOrange were compared as total radiant efficiencies. A radiant efficiency is equal to the radiance of the object divided by the illumination intensity. When ROI measurements are made, a total radiant efficiency within the ROI is the efficiency per pixel integrated over the ROI area, so the resulting units of the total radiant efficiency is area or cm2.
To image FPs in the XFM-2 rubber phantom mouse (Caliper Life Sciences), we placed 5 μl volume of each fluorescent protein (16 μM) 15 mm deep inside into a bore of the phantom. The bores were located at 7.0 mm and 18.1 mm distance from the imaging surface. Images were taken in different combinations of the far-red excitation and emission channels using an epifluorescence mode of the IVIS Spectrum instrument. A signal-to-background ratio was calculated for each wavelength combination for each fluorescent protein, using the phantom mouse without protein sample inside as a background reference. All quantitative measurements of fluorescence signal were performed utilizing the Living Image Software 4.0 (Caliper Life Sciences).
Imaging of cells in live mice
HEK293T cells expressing PSmOrange were illuminated with 489 nm LED array (adjusted to 200 mW cm−2) in PBS. 106 HEK293T cells containing either mKate2 or photoconverted PSmOrange were injected in 50 μl of 1:1 mixture of F12 medium and matrigel (BD Biosciences) intramuscularly into the right and left gluteal musculature of Nu:Nu mice (Charles River, Strain 088). Imaging was performed approximately 20 min after the injection using an IVIS Spectrum instrument in the epifluorescence mode.
To prepare mammary tumors in mice, we used female 5- to 6-week-old BALB/c SCID/Ncr mice. They were purchased from the National Cancer Institute and housed in a pathogen-free barrier facility until use. Mice were injected with 106 MTLn3 cells in the mammary gland. The tumor surface fur was removed using a depilatory cream. Several week old tumors were illuminated with 489 nm LED array (adjusted to 200 mW cm−2). To avoid mouse overheating the skin in the illumination area was cooled using hand-made optically-transparent cooler with flowing water. 24 h before illumination 500 μl of 5 mM K3Fe(CN)6 was injected subcutaneously around the tumor. Imaging of tumor was performed using an IVIS Spectrum instrument in the epifluorescence mode.
Mass spectrometry analysis
To run reducing SDS-PAGE, we incubated 10 μl (1 mg ml−1) proteins samples at 95°C in an electrophoresis sample buffer for 5 min before application to a 15% polyacrylamide gel.
An aliquot of 50 μg of His6-tagged PSmOrange was denatured by heating in 15 μl of 4.8 M guanidine chloride at 80°C for 3 min. Then the sample was cooled to room temperature. 30 μl of 2× chymotrypsin buffer containing 0.2 M Tris-HCl at pH 7.8 and 20 mM CaCl2, as well as 15 μl of water were added, and then chymotrypsin was added at an enzyme/protein ratio of 1:60. The digests were incubated at room temperature for 22 h and quenched with 0.1% TFA. We applied the digestion product to a reverse-phase HPLC chromatography (Agilent Technologies). The peptides were eluted by a linear gradient of acetonitrile in the same buffer. The effluent was monitored by an absorbance at 210 nm to detect peptide bonds and at 348 nm to detect denatured chromophore-containing peptides.
The chymotryptic peptides were mixed (1:1) with α-cyano-4-hydroxycinnamic acid solution (50% acetonitrile and water containing 0.1% tri-fluoroacetic acid). An aliquot of 1 μl of the mixture was put on a MALDI target and air-dried. Mass spectra were acquired on a 4800 MALDI TOF/TOF mass spectrometer (Applied Biosystems). The instrument was equipped with a Nd:YAG laser (PowerChip, JDS Uniphase) operating at 200 Hz and controlled by Applied Biosystems 4000 Series Explorer version 3.6 software. Each spectrum was accumulated with 500 shots in a positive ion mode. MS/MS were acquired in a PSD mode with mass isolation window of +/− 3 Da.
Supplementary Material
Acknowledgements
We are grateful to J. Zhang and L. Tesfa for assistance with flow cytometry, H. Xiao for help with mass-spectrometry analysis, and K. Kim and G. Filonov for assistance with mouse experiments and useful discussions. We thank M. Davidson (Florida State University, FL) for vimentin, keratin, myosin and paxillin plasmids, and B. Glick (University of Chicago, IL), D. Chudakov and K. Lukyanov (both from Institute of Bioorganic Chemistry, Russia) for plasmids of far-red fluorescent proteins. This work was supported by grants GM073913 (to V.V.V.) and CA100324 (to J.C.) from National Institutes of Health.
Footnotes
AUTHOR CONTRIBUTIONS O.M.S. developed the protein and characterized it in vitro. O.M.S. and G.H.P. characterized the protein in mammalian cells. O.M.S. and L.M.T. characterized the protein in mouse models. Tumor experiments were performed by O.M.S., Y.W. and J.C. V.V.V. designed the project and together with O.M.S. planned and discussed the project, and wrote the manuscript.
Note: Supplementary information is available on the website.
COMPETING INTEREST STATEMENT There are no competing financial interests.
References
- 1.Lukyanov KA, Chudakov DM, Lukyanov S, Verkhusha VV. Innovation: photoactivatable fluorescent proteins. Nat. Rev. Mol. Cell Biol. 2005;6:885–891. doi: 10.1038/nrm1741. [DOI] [PubMed] [Google Scholar]
- 2.Patterson GH, Lippincott-Schwartz J. A photoactivatable GFP for selective photolabeling of proteins and cells. Science. 2002;297:1873–1877. doi: 10.1126/science.1074952. [DOI] [PubMed] [Google Scholar]
- 3.Subach FV, et al. Photoactivatable mCherry for high-resolution two-color fluorescence microscopy. Nat. Methods. 2009;6:153–159. doi: 10.1038/nmeth.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Subach FV, et al. Bright monomeric photoactivatable red fluorescent protein for two-color super-resolution sptPALM of live cells. J. Am. Chem. Soc. 2010;132:6481–6491. doi: 10.1021/ja100906g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chudakov DM, Lukyanov S, Lukyanov KA. Tracking intracellular protein movements using photoswitchable fluorescent proteins PS-CFP2 and Dendra2. Nat. Protoc. 2007;2:2024–2032. doi: 10.1038/nprot.2007.291. [DOI] [PubMed] [Google Scholar]
- 6.McKinney SA, et al. A bright and photostable photoconvertible fluorescent protein. Nat. Methods. 2009;6:131–133. doi: 10.1038/nmeth.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ando R, Hama H, Yamamoto-Hino M, Mizuno H, Miyawaki A. An optical marker based on the UV-induced green-to-red photoconversion of a fluorescent protein. Proc. Natl. Acad. Sci. USA. 2002;99:12651–12656. doi: 10.1073/pnas.202320599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Habuchi S, Tsutsui H, Kochaniak AB, Miyawaki A, van Oijen AM. mKikGR, a monomeric photoswitchable fluorescent protein. PLoS One. 2008;3:e3944. doi: 10.1371/journal.pone.0003944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoi H, et al. A monomeric photoconvertible fluorescent protein for imaging of dynamic protein localization. J. Mol. Biol. 2010;401:776–791. doi: 10.1016/j.jmb.2010.06.056. [DOI] [PubMed] [Google Scholar]
- 10.Toomre D, Bewersdorf J. A new wave of cellular imaging. Annu. Rev. Cell Dev. Biol. 2010;26:285–314. doi: 10.1146/annurev-cellbio-100109-104048. [DOI] [PubMed] [Google Scholar]
- 11.Stiel AC, et al. Generation of monomeric reversibly switchable red fluorescent proteins for far-field fluorescence nanoscopy. Biophys. J. 2008;95:2989–2997. doi: 10.1529/biophysj.108.130146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Folling J, et al. Fluorescence nanoscopy by ground-state depletion and single-molecule return. Nat. Methods. 2008;5:943–945. doi: 10.1038/nmeth.1257. [DOI] [PubMed] [Google Scholar]
- 13.Lim YT, et al. Selection of quantum dot wavelengths for biomedical assays and imaging. Mol. Imaging. 2003;2:50–64. doi: 10.1162/15353500200302163. [DOI] [PubMed] [Google Scholar]
- 14.Deliolanis NC, et al. Performance of the Red-shifted Fluorescent Proteins in deep tissue molecular imaging applications. J. Biomed. Opt. 2008;13:044008. doi: 10.1117/1.2967184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morozova KS, et al. Far-red fluorescent protein excitable with red lasers for flow cytometry and superresolution STED nanoscory. Biophys. J. 2010;99:L13–L15. doi: 10.1016/j.bpj.2010.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strack RL, et al. A rapidly maturing far-red derivative of DsRed-Express2 for whole-cell labeling. Biochemistry. 2009;48:8279–8281. doi: 10.1021/bi900870u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shcherbo D, et al. Far-red fluorescent tags for protein imaging in living tissues. Biochem. J. 2009;18:567–574. doi: 10.1042/BJ20081949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin MZ, et al. Autofluorescent proteins with excitation in the optical window for intravital imaging in mammals. Chem. Biol. 2009;16:1169–1179. doi: 10.1016/j.chembiol.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shcherbo D, et al. Near-infrared fluorescent proteins. Nat. Methods. 2010;7:827–829. doi: 10.1038/nmeth.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kremers GJ, Hazelwood KL, Murphy CS, Davidson MW, Piston DW. Photoconversion in orange and red fluorescent proteins. Nat. Methods. 2009;6:355–358. doi: 10.1038/nmeth.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bogdanov AM, et al. Green fluorescent proteins are light-induced electron donors. Nat. Chem. Biol. 2009;5:459–461. doi: 10.1038/nchembio.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shaner NC, et al. Improving the photostability of bright monomeric orange and red fluorescent proteins. Nat. Methods. 2008;5:545–551. doi: 10.1038/nmeth.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strack RL, et al. A non-cytotoxic DsRed variant for whole-cell labeling. Nat. Methods. 2008;5:955–957. doi: 10.1038/nmeth.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Etienne-Manneville S. From signaling pathways to microtubule dynamics: the key players. Curr. Opin. Cell Biol. 2010;22:104–111. doi: 10.1016/j.ceb.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 25.Kuo C, Coquoz O, Troy TL, Xu H, Rice BW. Three-dimensional reconstruction of in vivo bioluminescent sources based on multispectral imaging. J. Biomed. Opt. 2007;12:024007. doi: 10.1117/1.2717898. [DOI] [PubMed] [Google Scholar]
- 26.Smith AM, Mancini MC, Nie S. Second window for in vivo imaging. Nat. Nanotechnol. 2009;4:710–711. doi: 10.1038/nnano.2009.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shu X, Shaner NC, Yarbrough CA, Tsien RY, Remington SJ. Novel chromophores and buried charges control color in mFruits. Biochemistry. 2006;45:9639–9647. doi: 10.1021/bi060773l. [DOI] [PubMed] [Google Scholar]
- 28.Ormö M, et al. Crystal structure of the Aequorea victoria green fluorescent protein. Science. 1996;273:1392–1395. doi: 10.1126/science.273.5280.1392. [DOI] [PubMed] [Google Scholar]
- 29.Yampolsky IS, et al. Synthesis and properties of the chromophore of the asFP595 chromoprotein from Anemonia sulcata. Biochemistry. 2005;44:5788–5793. doi: 10.1021/bi0476432. [DOI] [PubMed] [Google Scholar]
- 30.Tojo G, Fernández M. Oxidation of alcohols to aldehydes and ketones. Springer; New York: 2006. [Google Scholar]
- 31.Shaner NC, et al. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat. Biotechnol. 2004;22:1567–1572. doi: 10.1038/nbt1037. [DOI] [PubMed] [Google Scholar]
- 32.Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 33.Chudakov DM, et al. Photoswitchable cyan fluorescent protein for protein tracking. Nat. Biotechnol. 2004;22:1435–1439. doi: 10.1038/nbt1025. [DOI] [PubMed] [Google Scholar]
- 34.Verkhusha VV, Sorkin A. Conversion of the monomeric red fluorescent protein into a photoactivatable probe. Chem. Biol. 2005;12:279–285. doi: 10.1016/j.chembiol.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 35.Betzig E, et al. Imaging intracellular fluorescent proteins at nanometer resolution. Science. 2006;313:1642–1645. doi: 10.1126/science.1127344. [DOI] [PubMed] [Google Scholar]
- 36.Manley S, et al. High-density mapping of single-molecule trajectories with photoactivated localization microscopy. Nat. Methods. 2008;5:155–157. doi: 10.1038/nmeth.1176. [DOI] [PubMed] [Google Scholar]
- 37.Shroff H, et al. Dual-color superresolution imaging of genetically expressed probes within individual adhesion complexes. Proc. Natl. Acad. Sci. U.S.A. 2007;104:20308–20313. doi: 10.1073/pnas.0710517105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.