Abstract
Environmental stress has been demonstrated to increase susceptibility for mood and anxiety disorders, and hyperactivity of the hypothalamic-pituitary adrenal (HPA) axis, the primary endocrine response to stress, is often observed in these patients. HPA axis activation is initiated by corticotropin-releasing factor (CRF) from the hypothalamus, leading to the hypothesis that hypothalamic CRF overexpression contributes to HPA axis hyperactivity in psychiatric patients. In addition, elevated CRF in cerebrospinal fluid is observed in mood and anxiety disorder patients, suggesting that CRF is also being overproduced from extrahypothalamic sources such as the central amygdala (CeA) and overactivity of the amygdala in neuroimaging studies is a consistent finding in anxiety and depression patients. Due to the importance of CRF and the amygdala in the etiology of stress-sensitive psychiatric disorders, the present study sought to further dissect the impact of CRF overexpression (OE) in the amygdala on downstream behavioral, endocrine, and gene-expression changes typically associated with chronic stress. To test the hypothesis that elevated CRF output from the amygdala would reproduce HPA axis hyperactivity and behavioral symptoms of chronic stress, we developed a lentiviral vector in which 3.0Kb of the CRF promoter drives overexpression of CRF (LVCRFp3.0CRF). In adult male rats, Experiment-1 examined behavioral consequences of chronic CRF overexpression from the amygdala; the dexamethasone (Dex) /CRF test was used to measure HPA axis reactivity. Experiment-2 focused on HPA axis disruptions; the dexamethasone-suppression and CRF-stimulation tests as well as the Dex/CRF test were used. In both experiments, expression of HPA-axis related transcripts were assessed.
Keywords: Amygdala, Anxiety, Corticotropin-Releasing Factor (CRF), Depression, Hypothalamic Pituitary Adrenal (HPA) Axis, Lentivirus
INTRODUCTION
HPA axis reactivity in humans can be assessed using highly sensitive endocrine challenge tests. In the dexamethasone suppression test (DST), administration of the synthetic glucocorticoid (GC), dexamethasone (Dex), suppresses plasma concentrations of adrenocorticotropic hormone (ACTH) and cortisol in healthy subjects. In the CRF stimulation test, intravenously administered CRF elevates plasma ACTH and cortisol concentrations by stimulating CRF-type 1 receptors (CRF1) in the anterior pituitary. The Dex/CRF combination test is considered by many to be the most sensitive measure of HPA axis activity, reflecting both feed-back and feed-forward mechanisms (Heuser et al, 1994) reviewed in (Ising et al, 2005).
Hyperactivity of the HPA axis in patients with major depressive disorder (MDD), demonstrated by elevated plasma ACTH and cortisol concentrations at baseline, DST non-suppression, and a blunted ACTH response in the CRF stimulation test, is one of the most consistent findings in all of biological psychiatry (Gillespie and Nemeroff, 2005; Holsboer et al, 1984). HPA axis hyperactivity is also a consistent finding among patients with anxiety disorders including panic disorder (e.g. Abelson et al, 2007), social anxiety disorder (e.g.Condren et al, 2002) and, to a lesser extent, generalized anxiety disorder (reviewed in Martin et al, 2009; Risbrough and Stein, 2006).
In these patients, DST non-suppression is attributed to insufficient negative feedback, potentially resulting from glucocorticoid receptor (GR) insensitivity following chronically elevated plasma GC concentrations. A blunted response in the CRF stimulation test is attributed to decreased CRF1 expression in the anterior pituitary secondary to chronic hypersecretion of CRF from the hypothalamus and/or negative feedback of cortisol on the corticotroph. Elevated plasma ACTH and cortisol concentrations in the Dex/CRF test suggests that both GC insensitivity and CRF overexpression contribute to HPA axis hyperactivity (Kunugi et al, 2006)(Holsboer, 2000; Steckler et al, 1999).
HPA axis regulation involves many neurotransmitter systems in addition to CRF and GR. Arginine vasopressin (AVP) is colocalized with CRF in the paraventricular nucleus of the hypothalamus (PVN) and synergizes with CRF to activate the HPA axis. The hippocampus is involved in GR-mediated negative feedback to the PVN and in tonic inhibition of the HPA axis mediated by activation of hippocampal mineralocorticoid receptors (MR), which are over 80% occupied under basal conditions (Reul and de Kloet, 1985; Reul and Holsboer, 2002). Under conditions of chronic stress, HPA axis output may be indirectly increased by brain derived neurotrophic factor (BDNF). Stress-induced decreased hippocampal BDNF expression may limit the ability of the hippocampus to provide HPA axis inhibition, and stress-induced increase in PVN BDNF expression may promote activation of the HPA axis (Givalois et al, 2004; Murakami et al, 2005; Schaaf et al, 1998; Smith et al, 1995).
In addition to HPA axis hyperactivity, a second well replicated finding associated with symptoms of depression and anxiety is overactivity of the amygdala in functional imaging studies (Drevets, 2003; Pfleiderer et al, 2007; Rauch et al, 2003). The amygdala is known to play a role in fear and aggression and is a primary extrahypothalamic source of CRF; whether CRF from the amygdala plays a role in increased amygdala activation in these patients remains unknown. Evidence for this possibility includes elevated CRF concentrations in cerebrospinal fluid (CSF) from psychiatric patients (Banki et al, 1992; Fossey et al, 1996; Nemeroff et al, 1984) and elevated amygdalar CRF transcript in animal models following chronic stress. However, chronic stress paradigms in animal models produce vast disruptions throughout the brain and do not examine the specific impact of CRF overexpression on downstream behavioral and endocrine tests.
We hypothesize that a primary contributor to development and onset of depression symptoms is CRF overexpression (CRF-OE) in the central amygdala (CeA) and that much of the clinical endocrine data observed in subjects with MDD is driven by CRF-OE in the amygdala. To test this hypothesis, we developed a CRF-OE lentivirus using 3.0Kb of the mouse CRF promoter to drive CRF expression (LVCRFp3.0CRF). The following experiments utilize LVCRFp3.0CRF to assess gene-expression and endocrine consequences of chronic CRF-OE within the CeA. We performed two experiments. Experiment-1 was designed to assess behavioral consequences of chronic CRF overexpression from the amygdala; the Dex/CRF test was used to measure HPA axis reactivity. Experiment-2 was designed to dissect the HPA axis disruptions; the DST and CRF-stimulation tests as well as the Dex/CRF test were used. In both experiments, expression of HPA-axis related transcripts were assessed using in situ hybridization.
METHODS
Animals
Animal protocols were approved by the Emory University Institutional Animal Care and Use Committee (IACUC). Adult male Wistar rats were pair housed. In compliance with Emory University biosafety requirements for lentiviral vectors, Experiment-1 rats were housed in the animal BSL-2 quarantine facility for the duration of the 10-week trial. Due to changes in biosafety requirements, Experiment-2 rats were held in the quarantine facility for three-days following LV infusion surgery then transferred to the Psychiatry Department facility. In the ABSL-2 quarantine facility, the cage rack was enclosed in one cubicle within the room. In the Psychiatry Department facility, several cage racks are housed together in a larger room. Both facilities were maintained on a 0700/1900 light/dark cycle with food and water available ad libitum.
Stereotaxic Surgery
Rats were anesthetized with ketamine, xylazine, and acepromazine maleate (Welberg et al, 2006). 1μl of control (LVCMVGFP) or CRF-overexpressing (LVCRFp3.0CRF) virus was injected bilaterally into the CeA. Additional details on the production of LVCRFp3.0CRF are included in the Supplemental Material. Virus used in the following experiments reached at least 1×107 infectious units/μl. Cagemates received the same virus. Coordinates from Bregma: A/P −2.1; M/L+/− 4.0; D/V −8.0 (Paxinos and Watson, 2005).
Behavior
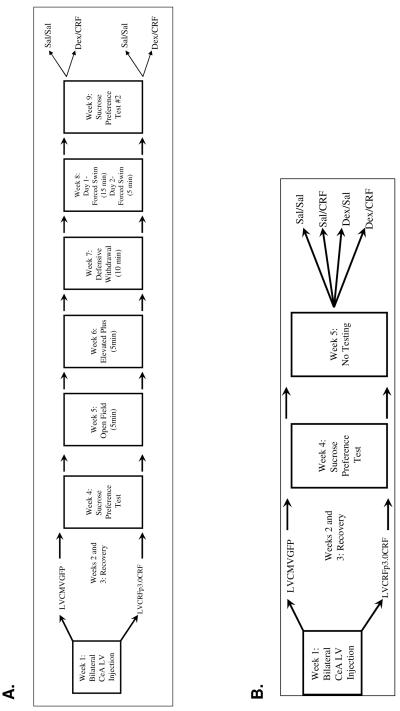
Experiment-1 behavioral tests were performed in the ABSL-2 room under red light beginning at 2100hrs. Video recordings were scored by two reviewers blind to treatment conditions. Because lentiviral vectors reach maximum expression 7-10 days after lentivirus infusion, behavioral testing began at least 14 days after lentivirus-infusion surgery. To minimize effects of multiple testing, one test was performed per week, tests were ordered from least to most stressful, and cagemates were tested simultaneously or on consecutive days (McIlwain et al, 2001; Paylor et al, 2006). Detailed methods for the open field (OF), elevated plus maze (EPM), defensive withdrawal (DW) and forced swim test (FST) are included as supplemental material. Experiment-2 rats were exposed to the sucrose-preference test (SPT) in the fourth week after lentivirus infusion but no additional behavioral testing was performed. The timelines for Experiments 1 and 2 are shown in Figure 1.
Figure 1.
Timelines for Experiments 1 (top) and 2 (bottom)
Endocrine Challenge Tests
Trunk blood was collected in cold polypropylene centrifuge tubes for corticosterone and glass EDTA-treated tubes for ACTH (BD Vacutainer®). Quantifications of ACTH and corticosterone were performed by the Emory University Hospital Core and Toxicology Laboratory. ACTH was measured by immunoradiometric assay according to the manufacturer’s instructions (Dia Sorin, Stillwater, MN). Corticosterone was measured using ImmuChemTM Double Antibody Radioimmunoassay (MP Biomedicals, Orangeburg, NY).
Experiment-1
From each cage, one rat was randomly assigned to the Dex/CRF condition and the other the Sal/Sal condition. At 1200hrs, rats received an intraperitoneal injection of 20μg/Kg dexamethasone (at a concentration of 40μg/ml) or an equivalent volume of saline. 90min later, rats received an IV (tail vein) injection of 0.5μg/Kg rat/human CRF, diluted to 2μg/ml, or an equivalent volume of saline. Tail vein injections were performed by trained personnel; the rats were not anaesthetized, but were lightly restrained. 25min later, rats were killed by decapitation; trunk blood was collected and brains were frozen on dry ice.
Experiment-2
To assess more completely the HPA axis dysregulation following CeA CRF-OE, animals were randomly assigned to one of four groups.
Saline+ Saline
Dexamethasone-Suppression Test: Dexamethasone (20μg/kg, 40μg/ml)+ Saline
CRF-Stimulation Test: Saline+ CRF (0.5μg/Kg, 2μg/ml)
Dex/CRF test: Dexamethasone+ CRF
Drug injections, blood, and brain collection proceeded as in Experiment-1. In Experiment-2 adrenal glands were also removed, cleaned of fat and weighed. However, no differences were observed.
Gene-Expression Analysis
Riboprobe and oligoprobe in situ hybridization were performed and analyzed as previously described (Nishimori et al, 1996; Paxinos et al, 1980; Skelton et al, 2000).
Statistical Analysis
Statistical analysis was performed with GraphPad Prism 5 (GraphPad Software). Unpaired Student’s t-test or analysis of variance (ANOVA) was used where appropriate. The Grubbs test for outliers was performed; data points reaching outlier criteria are identified in the text but are not eliminated from analysis due to the high degree of real individual variability. Specific analyses are described in individual figure captions.
RESULTS
Gene-Expression
Experiment-1
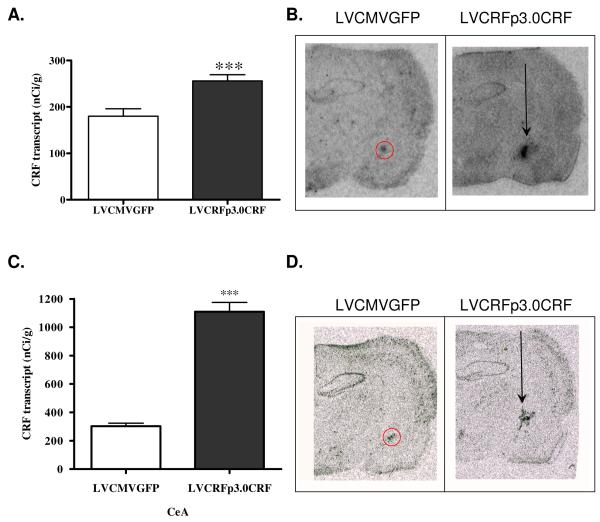
CRF transcript was significantly elevated in the CeA of rats infused with LVCRFp3.0CRF compared to control virus (In nano-curies per gram of tissue (nCi/g), LVCMVGFP= 180.15+/−16.01, LVCRFp3.0CRF= 255.75+/− 13.59; p<0.001, one-tailed Student’s t-test, Figure 2A-B). Histological analysis verified correct placement of lentivirus in 12 of the 18 rats infused with LVCRFp3.0CRF. For both experiments, only animals with verified infusion placement were included in data analysis.
Figure 2. CeA CRF Transcript is significantly elevated by LVCRFp3.0CRF in Experiment-1 and Experiment-2.
Elevated CRF transcript (nCi/g) in rats overexpressing CeA CRF in Experiment-1 (A) and Experiment-2 (C); data are displayed as mean +/− SEM; p-values reflect results of one-tailed T-test analysis (* p < 0.05; ** p < 0.01; *** p < 0.001). Representative example of lentiviral-vector mediated CRF-OE in the CeA in Experiment-1 (B) and Experiment-2 (D). Some injection tract labeling is observed.
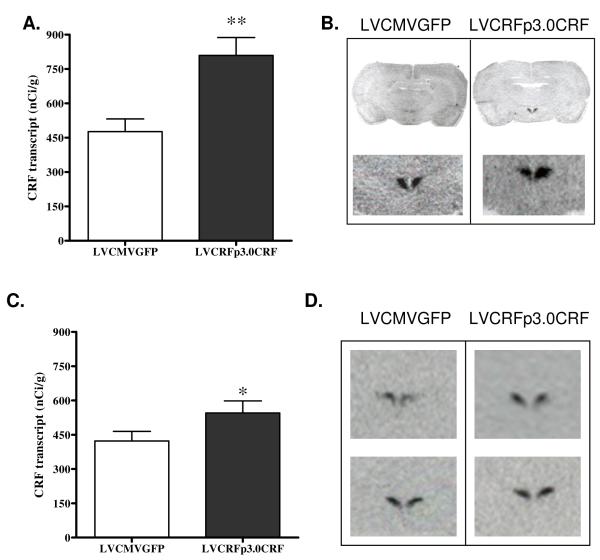
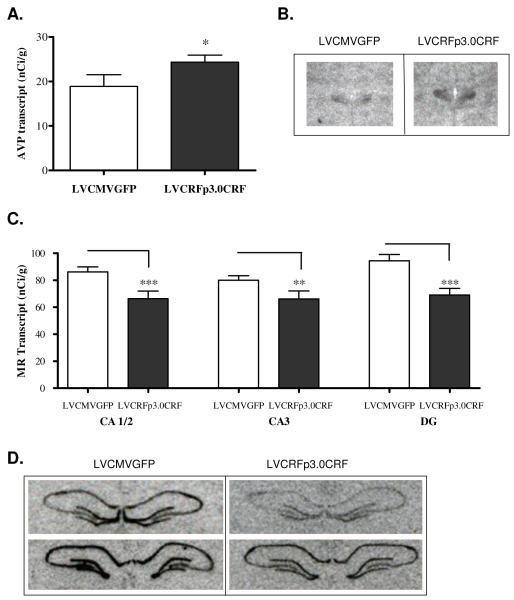
As hypothesized, CRF transcript was also significantly elevated in the PVN following 10-weeks of amygdalar CRF-OE (Experiment-1: 476.15+/− 55.46 in LVCMVGFP vs. 809.27+/− 79.01 nCi/g in LVCRFp3.0CRF subjects; p<0.01, one-tailed Student’s t-test, Figure 3A-B). Transcript for AVP was also elevated in the PVN of rats infused with LVCRFp3.0CRF (18.89+/− 2.62 nCi/g in control rats vs. 24.34+/− 1.6 in amygdalar CRF-OE subjects; p<0.05, one-tailed Student’s t-test, Figure 4A-B).
Figure 3. PVN CRF Transcript is Elevated following CeA CRF-OE in Experiment-1 and Experiment-2.
Elevated CRF transcript (nCi/g) in the PVN of rats overexpressing CeA CRF from Experiment-1 (A) and Experiment-2 (C). Data are displayed as mean +/− SEM; p-values reflect results of one-tailed T-test analysis based on the a-priori hypothesis that increased CeA CRF expression would increase hypothalamic CRF expression (* p < 0.05; ** p < 0.01; *** p < 0.001). Representative in situ hybridization of CRF transcript in the PVN from Experiment-1 (B) and Experiment-2 (D); upper panel images are anterior relative to lower panel images.
Figure 4. PVN AVP transcript is increased and Hippocampal MR transcript is decreased following CeA CRF-OE (Experiment-1).
Chronic overexpression of CeA CRF increases expression of AVP transcript (nCi/g) in the hypothalamic paraventricular nucleus. (A) Data are displayed as mean +/− SEM; p-values reflect results of one-tailed T-test analysis based on the a-priori hypothesis that increased CeA CRF expression would increase expression of AVP in the (B) Representative example of oligo in situ hybridization for AVP. MR transcript expression (nCi/g) is decreased in the hippocampus of rats overexpressing CeA CRF; upper panel images are anterior relative to lower panel images (C) Data are displayed as mean +/− SEM; p-values reflect results of two-tailed T-test analysis. (D) Representative example of riboprobe in situ hybridization for MR transcript. (* p < 0.05; ** p < 0.01; *** p< 0.001).
In situ hybridization revealed no group differences in GR expression in the hippocampus or PVN in either experiment. However, hippocampal MR expression was significantly decreased) in Experiment 1 rats overexpressing amygdalar CRF: CA1/2 (LVCMVGFP= 86.13+/− 3.81 nCi/g, LVCRFp3.0CRF= 66.33+/− 5.6; p<0.001), CA3 (LVCMVGFP= 79.95+/−3.44 nCi/g, LVCRFp3.0CRF= 66.08+/− 5.95; p<0.01) and DG (LVCMVGFP= 94.37+/− 4.63 nCi/g, LVCRFp3.0CRF= 69.02+/− 4.9; p<0.001, two-tailed t-tests Figure 4C-D.
Expression of the neurotrophic factor BDNF was hypothesized to be decreased in the hippocampus and increased in the PVN of rats overexpressing amygdalar CRF. Rats overexpressing CRF from the CeA in Experiment-1 exhibited a non-significant increase in PVN BDNF (LVCMVGFP= 38.73+/−6.0 nCi/g, LVCRFp3.0CRF= 51.22+/− 12.92; p= 0.07) and a decrease in hippocampal CA3 BDNF (LVCMVGFP= 241.97+/− 22.58 nCi/g, LVCRFp3.0CRF= 183.97+/− 24.4; p= 0.06).
Experiment-2
CRF transcript was significantly elevated in the CeA of LVCRFp3.0CRF-infused rats relative to those receiving the control virus (LVCMVGFP= 302.24+/− 22.12, LVCRFp3.0CRF= 1109.36+/− 65.06 nCi/g; p<0.0001, Figure 2C-D). Lentivirus infusion was verified in 25 of 28 rats infused with LVCRFp3.0CRF.
PVN CRF transcript was also elevated following 6-weeks of amygdalar CRF-OE (Experiment-2: 422.74+/− 41.75 in controls vs. 545.09+/− 52.96 in rats overexpressing amygdalar CRF, p<0.05, Figure 3C-D). There was a slight increase in PVN AVP that did not reach significance (LVCMVGFP= 356.41+/− 26.78, LVCRFp3.0CRF= 422.62+/− 36.98 nCi/g, p= 0.08).
HPA Axis Reactivity
Experiment-1
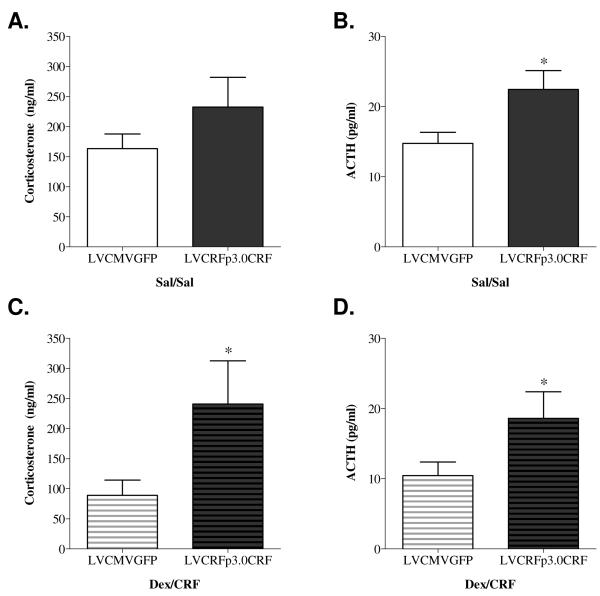
As hypothesized, ten weeks of CeA CRF-OE resulted in HPA axis hyperactivity (Figure 5A-D). Rats infused with LVCRFp3.0CRF exhibited elevated plasma ACTH concentrations both in the Sal/Sal (LVCMVGFP= 14.74+/−1.56, LVCRFp3.0CRF=22.44+/−2.68 pg/ml; p<0.05, one-tailed t-test) and in the Dex/CRF group (LVCMVGFP=10.43+/− 1.94, LVCRFp3.0CRF=18.60+/−3.78 pg/ml; p<0.05, one-tailed t-test). Plasma corticosterone concentration was also increased in rats overexpressing CeA CRF in the Dex/CRF group (LVCMVGFP= 88.80+/− 25.47, LVCRFp3.0CRF= 240.76+/− 71.93ng/ml; p<0.05) but not in the Sal/Sal group.
Figure 5. HPA Axis Hyperactivity following CeA CRF-OE (Experiment-1).
LVCRFp3.0CRF led to an increase in plasma ACTH concentrations in the Sal/Sal group (B) and the Dex/CRF group) (D). LVCRFp3.0CRF also produced a significant increase in plasma corticosterone in the Dex/CRF group (C) but not the Sal/Sal group (A). (One-tailed T-test analysis based on the a-priori hypothesis that increased CeA CRF would increase HPA axis reactivity * p < 0.05; ** p < 0.01; *** p< 0.001).
Experiment-2
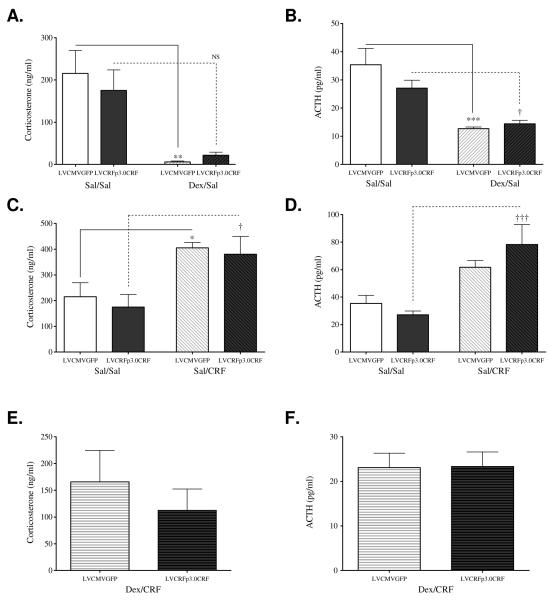
In the DST (Figure 6A-B), a two-factor ANOVA revealed a main effect of dexamethasone on plasma corticosterone (F(1, 18)= 16.49, p<0.001) and ACTH (F(1,21)= 29.44, p<0.0001) concentrations. Bonferroni tests revealed significant decreases in corticosterone (p<0.01) and ACTH (p<0.001) concentrations following dexamethasone administration to LVCMVGFP-infused rats. Chronic amygdalar CRF-OE was hypothesized to produce DST non-suppression. Among LVCRFp3.0CRF-treated subjects, dexamethasone administration failed to significantly suppress corticosterone concentration (p>0.05), though there was one significant outlier (594.60, z-score 1.7866; p<0.01) and a trend for suppression was apparent. ACTH concentration was significantly suppressed by dexamethasone (p<0.05). In the CRF stimulation test (Figure 6C-D), two-factor ANOVA identified a significant main effect of I.V. CRF peptide injection, but not of virus, on plasma corticosterone (F(1,21)= 14.61, p= 0.001) and ACTH (F(1,23)= 20.58, p<0.001) concentrations. Within the LVCMVGFP group, I.V. administration of CRF peptide significantly increased plasma corticosterone (p<0.05) and, nonsignificantly, ACTH concentrations (p>0.05). There was one significant outlier in the corticosterone data set (812.4, z-score 1.9792, p<0.01). Six weeks of amygdalar CRF-OE was hypothesized to produce a blunted ACTH response in the CRF-stimulation test. However, I.V. CRF peptide administration stimulated both corticosterone (p<0.05) and ACTH (p<0.001) in LVCRFp3.0CRF subjects and did not differ from LVCMVGFP-infused rats. Six weeks of amygdalar CRF-OE was hypothesized to produce HPA axis hyperactivity observed in Experiment-1; however no significant differences were observed between the LVCMVGFP and LVCRFp3.0CRF groups in either the Sal/Sal or Dex/CRF test (Figure 6E-F).
Figure 6. HPA Axis Reactivity following CeA CRF-OE (Experiment-2) is similar to that in control subjects.
In the DST, two-factor ANOVA identified a significant main effect of dexamethasone injection on plasma corticosterone (A) and ACTH (B). Post-hoc Bonferroni tests demonstrated that within the LVCMVGFP group, both plasma corticosterone and ACTH concentrations were significantly decreased. Notably, in rats overexpressing CeA CRF, dexamethasone administration failed to significantly suppress corticosterone. ACTH concentration was significantly suppressed by dexamethasone. In the CRF-Stimulation Test, two-factor ANOVA identified a significant effect of CRF injection, but not of virus, on plasma corticosterone (C) and ACTH (D) concentrations. Post-hoc Bonferroni analysis revealed that within the LVCMVGFP group, exogenous administration of CRF significantly increased plasma corticosterone concentration but not ACTH concentration. In rats chronically overexpressing CeA CRF, exogenous CRF administration stimulated corticosterone and ACTH concentrations. The Dex/CRF Test revealed no significant differences in plasma corticosterone (E) or ACTH (F) concentration between the LVCMVGFP and LCRFp3.0CRF groups in either the Sal/Sal condition or in the more sensitive Dex/CRF test. (* = significant difference from LVCMVGFP Sal/Sal; p < 0.05; ** p < 0.01 † = significant difference from LVCRFp3.0CRF Sal/Sal; p < 0.05; ††, p < 0.01; ††† p < 0.001)
Behavior
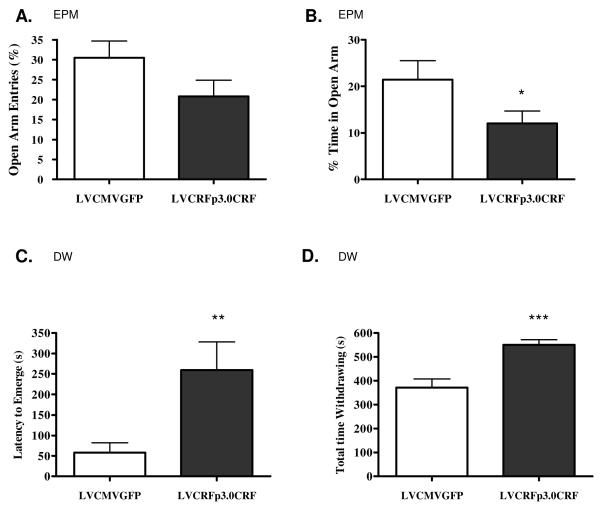
In the OF, rats overexpressing amygdalar CRF were hypothesized to spend less time exploring the center of the arena. At week five, rats overexpressing CRF from the amygdala exhibited decreased total locomotion (total # squares crossed= 103.78+/− 7.86 in control rats vs. 67.78+/− 9.66 in amygdalar CRF-OE subjects; p<0.01) and vertical locomotion (23.36+/− 1.52 vs. 17.5+/− 2.08 p<0.05), but time-in-center and number of center squares crossed were unaltered. In the EPM, CRF-OE within the CeA was hypothesized to decrease open-arm exploration; one-tailed t-test revealed a significant decrease in percentage of time in the open arm at week 6 following LV infusion surgery (LVCMVGFP= 21.43+/− 4.08%, LVCRFp3.0CRF= 12.06+/− 2.64%; p<0.05). There was also a non-significant decrease in the percentage of open arm entries (LVCMVGFP= 30.50+/− 4.19%, LVCRFp3.0CRF= 20.85+/− 4.01% p= 0.06, Figure 7A-B). The number of closed arm entries and number of rears did not differ between groups, suggesting that variance in open arm activity is not due to altered locomotion.
Figure 7. Behavioral Anxiety following CeA CRF-OE (Experiment-1).
Chronic CeA CRF-OE increases anxiety-like behavior in the EPM. There was a trend towards a decrease in the percentage of open arm entries out of total arm entries (A), and a significant decrease in the percentage of time spent in the open arm during the 5min trial (B). Rats overexpressing CRF from the CeA emerge from the withdrawal box later than the control animals (C) and spend more total time withdrawing (D). Data are displayed as mean +/− SEM; p-values reflect results of one-tailed T-test analysis based on the a priori hypotheses that increased CeA CRF expression would decrease exploration in the open arm of the EPM and in the center of the open arena in the DW test (* p < 0.05; ** p < 0.01; *** p < 0.001)
In the DW test, performed seven weeks following LV infusion surgery, rats overexpressing amygdalar CRF exhibited a significant increase in latency to emerge from the withdrawal box (LVCMVGFP= 57.92+/− 24.0s, LVCRFp3.0CRF= 259.17+/− 69.11s; p<0.01, one-tailed t-test) and increased total time withdrawing (LVCMVGFP = 371.32+/− 36.65s, LVCRFp3.0CRF = 550.72+/− 21.24s p<0.001, one-tailed t-test, Figure 7C-D). Total locomotion was unaltered and the decrease in center squares crossed as a percentage of the total squares crossed during the time exploring did not reach significance (LVCMVGFP= 3.82+/− 1.56%, LVCRFp3.0CRF= 1.09+/− 0.73%, one-tailed t-test; p= 0.08).
Chronic amygdalar CRF-OE was hypothesized to increase anhedonia as measured by a decreased preference for sucrose solution. In the first SPT, performed in week-4, there was a slight decrease in the percentage of 1% sucrose solution consumed per total volume, but the difference was not statistically significant. No differences were observed in the second SPT, which was carried out in week 9 following LV infusion surgery. Within subjects there was also no difference between SPT #1 and #2. Results from the SPT in Experiment-2, performed at week 4 in that study, also revealed no group differences.
No group differences were observed in the FST (Supplemental Table 1). Additional behavioral results are shown in Supplemental Table 1.
DISCUSSION
The goals of these experiments were to determine whether a chronic increase in the availability of amygdalar CRF is sufficient to duplicate the behavioral consequences of chronic stress and sufficient to induce HPA axis hyperactivity similar to that observed in human psychiatric patients. Compared to daily injections of CRF-peptide over a series of days (e.g. (Buwalda et al, 1997; Buwalda et al, 1998) or CRF-OE over the lifetime of the animal (e.g. (Stenzel-Poore et al, 1994), the timeframe and mechanism of CRF overexpression using lentiviral vectors could more closely duplicate CRF overexpression in humans.
Expression of CRF transcript in the amygdala was significantly increased following LVCRFp3.0CRF lentivirus infusion in Experiment-1 and Experiment-2. The differences in the magnitude of CRF-OE (125% of control versus 300% of control in experiments 1 and 2, respectively) may be the result of differences in time following lentivirus infusion, in exposure to behavioral testing, in housing or handling, or some combination of these. Although unlikely, it is also possible that components of the virus solution could have conferred different infectivity and incorporation between Experiments 1 and 2 (Torashima et al, 2006).
In Experiment-1, chronic amygdalar CRF-OE also increased expression of CRF and AVP in the PVN. CRF and AVP synergistically activate the HPA axis and the increase in their expression is consistent with the HPA axis hyperactivity also seen in this group. Although there are few direct connections between the CeA and PVN, numerous indirect connections could mediate this effect; activation of CRFergic inhibitory cells connecting the lateral to the medial CeA (Crane et al, 2003), and activation of CRFergic excitatory cells connecting the CeA to the lateral septum (Gallagher et al, 2008) could both contribute to PVN disinhibition. In contrast, PVN CRF transcript but not AVP transcript was significantly elevated in CRF-OE rats in Experiment-2.
Expression of BDNF was hypothesized to be decreased in the hippocampus and increased in the hypothalamus of rats overexpressing amygdalar CRF (Givalois et al, 2004; Murakami et al, 2005; Schaaf et al, 1998; Smith et al, 1995). However, the decrease in hippocampal BDNF and increase in hypothalamic BDNF transcript did not reach significance; the physiological significance of these results remains to be seen.
Ten weeks of amygdalar CRF-OE in Experiment-1 decreased hippocampal MR transcript, potentially resulting in HPA axis disinhibition and contributing to HPA axis hyperactivity. These results are consistent with previous studies showing a decrease in MR transcript following central injection of CRF peptide (Hugin-Flores et al, 2003) or following elevations in circulating glucocorticoids (Sterlemann et al, 2008). These data also argue for continued research on MR agonists as adjunctive treatment for MDD (Otte et al, 2010).
In Experiment-1, amygdalar CRF-OE increased plasma ACTH concentrations under baseline (Sal/Sal) conditions relative to LVCMVGFP controls, and increased ACTH and corticosterone concentrations in the Dex/CRF test. That corticosterone was unaltered by amygdalar CRF-OE in the Sal/Sal group likely reflects the enhanced sensitivity of the Dex/CRF test and not a less disrupted HPA axis in Sal/Sal subjects compared to those in the Dex/CRF group.
In the expanded endocrine tests of Experiment-2, chronic amygdalar CRF-OE was hypothesized to decrease negative feedback as measured by non-suppression in the DST, and to decrease anterior pituitary sensitivity to CRF administration as measured by a blunted response to the CRF stimulation test. Rats infused with LVCMVGFP exhibited significant suppression of plasma corticosterone and ACTH concentration in the DST and significant elevation of corticosterone in the CRF stimulation test. Consistent with dexamethasone non-suppression, the DST revealed no significant decrease in plasma corticosterone concentrations from rats infused with LVCRFp3.0CRF. However, plasma corticosterone and ACTH concentrations did not differ significantly from that of LVCMVGFP subjects, rats overexpressing amygdalar CRF did not exhibit a blunted response in the CRF stimulation test, and there were no group differences in the Dex/CRF test.
Behavioral tests for anxiety in rodents were designed to assess the natural “conflict between fear and exploratory drive” (Lowry et al, 1996). In the present study, chronic overexpression of amygdalar CRF decreased exploration in the open arms of the EPM, increased latency to emerge from protective cover in the DW test, and increased time withdrawing in the DW test. These results suggest that overexpression of CRF released from the amygdala onto receptors in target destinations can elicit an anxiety-like response similar to that observed following chronic stress or following infusion of CRF peptide into the amygdala, activating local CRF receptors (Daniels et al, 2004). These data also support the hypothesis that the behavioral stress response initiated within the CeA is mediated by CRF, and contradict suggestions of a dissociation between CRF in the CeA and anxiety-like behavior (Merali et al, 2004; Weninger et al, 1999).
In addition to the anticipated anxiogenic effects of CeA CRF-OE, rats in the LVCRFp3.0CRF group also exhibited decreased the number of squares crossed in the OF test (supplementary Table 1). This is contrary to other studies in which intracerebroventricular (icv) CRF administration increased locomotion in rats (Sutton et al, 1982; Veldhuis and De Wied, 1984), mice (e.g. (Contarino et al, 2000), and amphibians (Lowry et al, 1996). However, in the EPM and DW tests, there were no differences in rearing behavior, in total arm entries (EPM) or total number of squares crossed per time exploring (DW), suggesting that the OF results do not demonstrate a locomotor deficit. Rather, decreased locomotion in LVCRFp3.0CRF-injected rats may represent psychomotor retardation in the novel environment.
Decreased consumption of sucrose in the SPT is interpreted as a correlate of anhedonia in depressed humans and the FST is known for its ability to screen potential antidepressant drugs. In the present study, no significant differences in sucrose preference or swimming behavior were observed. These results are contrary to previously reported effects of chronic stress (Kompagne et al, 2008; Lucca et al, 2008), or increased in amygdalar CRF expression in female rats (Keen-Rhinehart et al, 2009). However, other research has shown that central CRF administration can increase or decrease sucrose preference depending on other contextual factors and the quantity of CRF peptide injected (Heinrichs et al, 1991). Furthermore, other studies in mice have found a lack of effect of CeA CRF-OE in the FST (Regev et al, 2010), and an increase in swimming and climbing behavior in the FST has been observed in CRF-OE transgenic mice (Lu et al, 2008), in rats following icv administration of CRF (Garcia-Lecumberri and Ambrosio, 2000) or other CRF1 agonists (Tezval et al, 2004). The ambiguity of these previous reports combined with a lack of effect on SPT and FST behavior in the present study suggest a complex relationship between CRF and hedonic or coping behaviors and point to the need for the development of additional measures of depressive-like behavior in rodents.
These experiments were designed to assess the impact of chronic CRF-OE on behavior (Experiment-1) and endocrine (Experiment-2) and gene-expression (both Experiments). That Experiment-1 and Experiment-2 yielded unique effects on gene-expression and HPA axis reactivity was not anticipated. There are several explanations for these differences. First, environmental differences including exposure to behavioral tests interacted with chronic CRF overexpression to increase PVN AVP and decrease hippocampal MR in subjects from Experiment-1. Second, gene-expression changes result from secondary effects of chronic amygdalar CRF-OE which have occurred by week 10 but not week six. Both of these possibilities suggest that increased PVN AVP and decreased hippocampal MR may be required for the impact of increased CRF drive from the amygdala on HPA axis reactivity. These differences may also be downstream consequences of the different total CeA CRF-OE in the two experiments. The study by Regev et.al. (2010) also failed to identify an effect of CeA CRF-OE on HPA axis reactivity, however the total expression of CRF relative to control mice was not reported and expression of AVP and MR were not examined.
Perhaps most interesting is the possibility that the effects of chronic CRF overexpression continue to change over time. This possibility is supported by data from human studies; most MDD patients experience more than one depressive episode, each of which increases the likelihood of continued episodes and decreases the likelihood of a positive response to treatment (DSM-IV-TR 2000; Eaton et al, 2008). Additional studies must be designed to assess how time-dependent changes in gene expression may contribute to increased vulnerability to symptoms even in the absence of external stressors.
Supplementary Material
Acknowledgements
We thank Faketa Zejnelovic for assisting in slicing brains, Catie Cappello for assisting in the tail-vein injections, brain removal, and analysis of behavioral testing, Nicola Hanson for assisting in the adrenal gland removal, and Susan Plott and Swati Rogers for general technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Elizabeth I. Flandreau, Peptide Biology Laboratory The Salk Institute 10010 N. Torrey Pines Road La Jolla, CA 92037 emartin@salk.edu Office: 858-453-4100, ext. 1510 Fax: 858-558-8763.
Kerry J. Ressler, Howard Hughes Medical Institute Department of Psychiatry and Behavioral Sciences Yerkes Research Center Emory University 954 Gatewood Dr Atlanta, GA 30329 kressle@emory.edu (ph) 404-727-7739 (fax) 404-727-8070.
Michael J. Owens, Laboratory of Neuropsychopharmacology Department of Psychiatry and Behavioral Sciences Emory University Woodruff Memorial Research Building, Suite 4000 101 Woodruff Circle Atlanta, GA 30322 mowens@emory.edu
Charles B. Nemeroff, Department of Psychiatry & Behavioral Sciences, University of Miami Miller School of Medicine Clinical Research Building 1120 NW 14th Street, Room 1455 (D-21) Miami, Florida 33136 Off: 305-243-3740 Fax: 305-243-1619 cnemeroff@med.miami.edu.
WORKS CITED
- DSM-IV-TR. Revised 4th Edition. American Psychiatric Association; Washington, DC: 2000. Vol. [Google Scholar]
- Abelson JL, Khan S, Liberzon I, Young EA. HPA axis activity in patients with panic disorder: review and synthesis of four studies. Depress Anxiety. 2007;24(1):66–76. doi: 10.1002/da.20220. [DOI] [PubMed] [Google Scholar]
- Banki CM, Karmacsi L, Bissette G, Nemeroff CB. Cerebrospinal fluid neuropeptides in mood disorder and dementia. J Affect Disord. 1992;25(1):39–45. doi: 10.1016/0165-0327(92)90091-j. [DOI] [PubMed] [Google Scholar]
- Buwalda B, de Boer SF, Van Kalkeren AA, Koolhaas JM. Physiological and behavioral effects of chronic intracerebroventricular infusion of corticotropin-releasing factor in the rat. Psychoneuroendocrinology. 1997;22(5):297–309. doi: 10.1016/s0306-4530(97)00032-2. [DOI] [PubMed] [Google Scholar]
- Buwalda B, Van Kalkeren AA, de Boer SF, Koolhaas JM. Behavioral and physiological consequences of repeated daily intracerebroventricular injection of corticotropin-releasing factor in the rat. Psychoneuroendocrinology. 1998;23(3):205–218. doi: 10.1016/s0306-4530(97)00096-6. [DOI] [PubMed] [Google Scholar]
- Condren RM, O’Neill A, Ryan MC, Barrett P, Thakore JH. HPA axis response to a psychological stressor in generalised social phobia. Psychoneuroendocrinology. 2002;27(6):693–703. doi: 10.1016/s0306-4530(01)00070-1. [DOI] [PubMed] [Google Scholar]
- Contarino A, Dellu F, Koob GF, Smith GW, Lee KF, Vale WW, et al. Dissociation of locomotor activation and suppression of food intake induced by CRF in CRFR1-deficient mice. Endocrinology. 2000;141(7):2698–2702. doi: 10.1210/endo.141.7.7653. [DOI] [PubMed] [Google Scholar]
- Crane JW, Buller KM, Day TA. Evidence that the bed nucleus of the stria terminalis contributes to the modulation of hypophysiotropic corticotropin-releasing factor cell responses to systemic interleukin-1beta. J Comp Neurol. 2003;467(2):232–242. doi: 10.1002/cne.10918. [DOI] [PubMed] [Google Scholar]
- Daniels WM, Richter L, Stein DJ. The effects of repeated intra-amygdala CRF injections on rat behavior and HPA axis function after stress. Metab Brain Dis. 2004;19(1-2):15–23. doi: 10.1023/b:mebr.0000027413.42946.61. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging abnormalities in the amygdala in mood disorders. Ann N Y Acad Sci. 2003;985:420–444. doi: 10.1111/j.1749-6632.2003.tb07098.x. [DOI] [PubMed] [Google Scholar]
- Eaton WW, Shao H, Nestadt G, Lee HB, Bienvenu OJ, Zandi P. Population-based study of first onset and chronicity in major depressive disorder. Arch Gen Psychiatry. 2008;65(5):513–520. doi: 10.1001/archpsyc.65.5.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossey MD, Lydiard RB, Ballenger JC, Laraia MT, Bissette G, Nemeroff CB. Cerebrospinal fluid corticotropin-releasing factor concentrations in patients with anxiety disorders and normal comparison subjects. Biol Psychiatry. 1996;39(8):703–707. doi: 10.1016/0006-3223(95)00197-2. [DOI] [PubMed] [Google Scholar]
- Gallagher JP, Orozco-Cabal LF, Liu J, Shinnick-Gallagher P. Synaptic physiology of central CRH system. Eur J Pharmacol. 2008;583(2-3):215–225. doi: 10.1016/j.ejphar.2007.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Lecumberri C, Ambrosio E. Differential effect of low doses of intracerebroventricular corticotropin-releasing factor in forced swimming test. Pharmacol Biochem Behav. 2000;67(3):519–525. doi: 10.1016/s0091-3057(00)00384-1. [DOI] [PubMed] [Google Scholar]
- Gillespie CF, Nemeroff CB. Hypercortisolemia and depression. Psychosom Med. 2005;67(Suppl 1):S26–28. doi: 10.1097/01.psy.0000163456.22154.d2. [DOI] [PubMed] [Google Scholar]
- Givalois L, Naert G, Rage F, Ixart G, Arancibia S, Tapia-Arancibia L. A single brain-derived neurotrophic factor injection modifies hypothalamo-pituitary-adrenocortical axis activity in adult male rats. Mol Cell Neurosci. 2004;27(3):280–295. doi: 10.1016/j.mcn.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Heinrichs SC, Britton KT, Koob GF. Both conditioned taste preference and aversion induced by corticotropin-releasing factor. Pharmacol Biochem Behav. 1991;40(4):717–721. doi: 10.1016/0091-3057(91)90075-d. [DOI] [PubMed] [Google Scholar]
- Heuser I, Yassouridis A, Holsboer F. The combined dexamethasone/CRH test: a refined laboratory test for psychiatric disorders. J Psychiatr Res. 1994;28(4):341–356. doi: 10.1016/0022-3956(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Holsboer F, Von Bardeleben U, Gerken A, Stalla GK, Muller OA. Blunted corticotropin and normal cortisol response to human corticotropin-releasing factor in depression. N Engl J Med. 1984;311(17):1127. doi: 10.1056/NEJM198410253111718. [DOI] [PubMed] [Google Scholar]
- Hugin-Flores ME, Steimer T, Schulz P, Vallotton MB, Aubert ML. Chronic corticotropin-releasing hormone and vasopressin regulate corticosteroid receptors in rat hippocampus and anterior pituitary. Brain Res. 2003;976(2):159–170. doi: 10.1016/s0006-8993(03)02585-x. [DOI] [PubMed] [Google Scholar]
- Ising M, Kunzel HE, Binder EB, Nickel T, Modell S, Holsboer F. The combined dexamethasone/CRH test as a potential surrogate marker in depression. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(6):1085–1093. doi: 10.1016/j.pnpbp.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Keen-Rhinehart E, Michopoulos V, Toufexis DJ, Martin EI, Nair H, Ressler KJ, et al. Continuous expression of corticotropin-releasing factor in the central nucleus of the amygdala emulates the dysregulation of the stress and reproductive axes. Mol Psychiatry. 2009;14(1):37–50. doi: 10.1038/mp.2008.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kompagne H, Bardos G, Szenasi G, Gacsalyi I, Harsing LG, Levay G. Chronic mild stress generates clear depressive but ambiguous anxiety-like behaviour in rats. Behav Brain Res. 2008;193(2):311–314. doi: 10.1016/j.bbr.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Kunugi H, Ida I, Owashi T, Kimura M, Inoue Y, Nakagawa S, et al. Assessment of the Dexamethasone/CRH Test as a State-Dependent Marker for Hypothalamic-Pituitary-Adrenal (HPA) Axis Abnormalities in Major Depressive Episode: A Multicenter Study. Neuropsychopharmacology. 2006;31(1):212–220. doi: 10.1038/sj.npp.1300868. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Rose JD, Moore FL. Corticotropin-releasing factor enhances locomotion and medullary neuronal firing in an amphibian. Horm Behav. 1996;30(1):50–59. doi: 10.1006/hbeh.1996.0008. [DOI] [PubMed] [Google Scholar]
- Lu A, Steiner MA, Whittle N, Vogl AM, Walser SM, Ableitner M, et al. Conditional mouse mutants highlight mechanisms of corticotropin-releasing hormone effects on stress-coping behavior. Mol Psychiatry. 2008;13(11):1028–1042. doi: 10.1038/mp.2008.51. [DOI] [PubMed] [Google Scholar]
- Lucca G, Comim CM, Valvassori SS, Pereira JG, Stertz L, Gavioli EC, et al. Chronic mild stress paradigm reduces sweet food intake in rats without affecting brain derived neurotrophic factor protein levels. Curr Neurovasc Res. 2008;5(4):207–213. doi: 10.2174/156720208786413406. [DOI] [PubMed] [Google Scholar]
- Martin EI, Ressler KJ, Binder E, Nemeroff CB. The neurobiology of anxiety disorders: brain imaging, genetics, and psychoneuroendocrinology. Psychiatr Clin North Am. 2009;32(3):549–575. doi: 10.1016/j.psc.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlwain KL, Merriweather MY, Yuva-Paylor LA, Paylor R. The use of behavioral test batteries: effects of training history. Physiol Behav. 2001;73(5):705–717. doi: 10.1016/s0031-9384(01)00528-5. [DOI] [PubMed] [Google Scholar]
- Merali Z, Khan S, Michaud DS, Shippy SA, Anisman H. Does amygdaloid corticotropin-releasing hormone (CRH) mediate anxiety-like behaviors? Dissociation of anxiogenic effects and CRH release. Eur J Neurosci. 2004;20(1):229–239. doi: 10.1111/j.1460-9568.2004.03468.x. [DOI] [PubMed] [Google Scholar]
- Murakami S, Imbe H, Morikawa Y, Kubo C, Senba E. Chronic stress, as well as acute stress, reduces BDNF mRNA expression in the rat hippocampus but less robustly. Neurosci Res. 2005;53(2):129–139. doi: 10.1016/j.neures.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Widerlov E, Bissette G, Walleus H, Karlsson I, Eklund K, et al. Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science. 1984;226(4680):1342–1344. doi: 10.1126/science.6334362. [DOI] [PubMed] [Google Scholar]
- Nishimori K, Young LJ, Guo Q, Wang Z, Insel TR, Matzuk MM. Oxytocin is required for nursing but is not essential for parturition or reproductive behavior. Proc Natl Acad Sci U S A. 1996;93(21):11699–11704. doi: 10.1073/pnas.93.21.11699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otte C, Hinkelmann K, Moritz S, Yassouridis A, Jahn H, Wiedemann K, et al. Modulation of the mineralocorticoid receptor as add-on treatment in depression: a randomized, double-blind, placebo-controlled proof-of-concept study. J Psychiatr Res. 2010;44(6):339–346. doi: 10.1016/j.jpsychires.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 5th edn. Vol. 1. Elsevier Academic Press; Amsterdam ; Boston: 2005. (unpaged) [Google Scholar]
- Paxinos G, Watson CR, Emson PC. AChE-stained horizontal sections of the rat brain in stereotaxic coordinates. J Neurosci Methods. 1980;3(2):129–149. doi: 10.1016/0165-0270(80)90021-7. [DOI] [PubMed] [Google Scholar]
- Paylor R, Spencer CM, Yuva-Paylor LA, Pieke-Dahl S. The use of behavioral test batteries, II: effect of test interval. Physiol Behav. 2006;87(1):95–102. doi: 10.1016/j.physbeh.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Pfleiderer B, Zinkirciran S, Arolt V, Heindel W, Deckert J, Domschke K. fMRI amygdala activation during a spontaneous panic attack in a patient with panic disorder. World J Biol Psychiatry. 2007;8(4):269–272. doi: 10.1080/15622970701216673. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Wright CI. Neuroimaging studies of amygdala function in anxiety disorders. Ann N Y Acad Sci. 2003;985:389–410. doi: 10.1111/j.1749-6632.2003.tb07096.x. [DOI] [PubMed] [Google Scholar]
- Regev L, Neufeld-Cohen A, Tsoory M, Kuperman Y, Getselter D, Gil S, et al. Prolonged and site-specific over-expression of corticotropin-releasing factor reveals differential roles for extended amygdala nuclei in emotional regulation. Mol Psychiatry. 2010 doi: 10.1038/mp.2010.64. [DOI] [PubMed] [Google Scholar]
- Reul JM, de Kloet ER. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology. 1985;117(6):2505–2511. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- Reul JM, Holsboer F. Corticotropin-releasing factor receptors 1 and 2 in anxiety and depression. Curr Opin Pharmacol. 2002;2(1):23–33. doi: 10.1016/s1471-4892(01)00117-5. [DOI] [PubMed] [Google Scholar]
- Risbrough VB, Stein MB. Role of corticotropin releasing factor in anxiety disorders: a translational research perspective. Horm Behav. 2006;50(4):550–561. doi: 10.1016/j.yhbeh.2006.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaaf MJ, de Jong J, de Kloet ER, Vreugdenhil E. Downregulation of BDNF mRNA and protein in the rat hippocampus by corticosterone. Brain Res. 1998;813(1):112–120. doi: 10.1016/s0006-8993(98)01010-5. [DOI] [PubMed] [Google Scholar]
- Skelton KH, Nemeroff CB, Knight DL, Owens MJ. Chronic administration of the triazolobenzodiazepine alprazolam produces opposite effects on corticotropin-releasing factor and urocortin neuronal systems. J Neurosci. 2000;20(3):1240–1248. doi: 10.1523/JNEUROSCI.20-03-01240.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Makino S, Altemus M, Michelson D, Hong SK, Kvetnansky R, et al. Stress and antidepressants differentially regulate neurotrophin 3 mRNA expression in the locus coeruleus. Proc Natl Acad Sci U S A. 1995;92(19):8788–8792. doi: 10.1073/pnas.92.19.8788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenzel-Poore MP, Heinrichs SC, Rivest S, Koob GF, Vale WW. Overproduction of corticotropin-releasing factor in transgenic mice: a genetic model of anxiogenic behavior. J Neurosci. 1994;14(5 Pt 1):2579–2584. doi: 10.1523/JNEUROSCI.14-05-02579.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterlemann V, Ganea K, Liebl C, Harbich D, Alam S, Holsboer F, et al. Long-term behavioral and neuroendocrine alterations following chronic social stress in mice: implications for stress-related disorders. Horm Behav. 2008;53(2):386–394. doi: 10.1016/j.yhbeh.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Sutton RE, Koob GF, Le Moal M, Rivier J, Vale W. Corticotropin releasing factor produces behavioural activation in rats. Nature. 1982;297(5864):331–333. doi: 10.1038/297331a0. [DOI] [PubMed] [Google Scholar]
- Tezval H, Jahn O, Todorovic C, Sasse A, Eckart K, Spiess J. Cortagine, a specific agonist of corticotropin-releasing factor receptor subtype 1, is anxiogenic and antidepressive in the mouse model. Proc Natl Acad Sci U S A. 2004;101(25):9468–9473. doi: 10.1073/pnas.0403159101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torashima T, Yamada N, Itoh M, Yamamoto A, Hirai H. Exposure of lentiviral vectors to subneutral pH shifts the tropism from Purkinje cell to Bergmann glia. Eur J Neurosci. 2006;24(2):371–380. doi: 10.1111/j.1460-9568.2006.04927.x. [DOI] [PubMed] [Google Scholar]
- Veldhuis HD, De Wied D. Differential behavioral actions of corticotropin-releasing factor (CRF) Pharmacol Biochem Behav. 1984;21(5):707–713. doi: 10.1016/s0091-3057(84)80007-6. [DOI] [PubMed] [Google Scholar]
- Welberg LA, Kinkead B, Thrivikraman K, Huerkamp MJ, Nemeroff CB, Plotsky PM. Ketamine-xylazine-acepromazine anesthesia and postoperative recovery in rats. J Am Assoc Lab Anim Sci. 2006;45(2):13–20. [PubMed] [Google Scholar]
- Weninger SC, Dunn AJ, Muglia LJ, Dikkes P, Miczek KA, Swiergiel AH, et al. Stress-induced behaviors require the corticotropin-releasing hormone (CRH) receptor, but not CRH. Proc Natl Acad Sci U S A. 1999;96(14):8283–8288. doi: 10.1073/pnas.96.14.8283. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.