Abstract
Background
Asthma in obese individuals is poorly understood, these patients are often refractory to standard therapy.
Objectives
To gain insights into the pathogenesis and treatment of asthma in obese individuals by determining how obesity and bariatric surgery affect asthma control, airway hyperresponsiveness and markers of asthmatic inflammation.
Methods
A prospective study of (i) asthmatic and non-asthmatic bariatric surgery patients compared at baseline, and (ii) asthmatic patients followed for 12 months after bariatric surgery.
Results
We studied 23 asthmatic and 21 non-asthmatic patients undergoing bariatric surgery. At baseline, asthmatics had lower FEV1 and FVC, and lower levels of lymphocytes in bronchoalveolar lavage.
Following surgery, asthmatic participants experienced significant improvements in asthma control (asthma control score 1.55 to 0.74, p < 0.0001) and asthma quality of life (4.87 to 5.87, p < 0.0001). Airways responsiveness to methacholine improved significantly (PC20 3.9 to 7.28, p = 0.03). There was a statistically significant interaction between IgE status and change in airways responsiveness (p for interaction term = 0.01), improvement in AHR was significantly related to change in BMI in those with normal IgE (p = 0.02, R2 = 0.46). The proportion of lymphocytes in bronchoalveolar lavage and production of cytokines from activated peripheral blood CD4+ T cells increased significantly.
Conclusions
Bariatric surgery improves airway hyperresponsiveness in obese asthmatics with normal serum IgE. Weight loss has dichotomous effects on airway physiology and T cell function typically involved in the pathogenesis of asthma, suggesting that obesity produces a unique phenotype of asthma that will require a distinct therapeutic approach.
Keywords: Obesity, asthma, bariatric surgery, weight loss, airway hyperreactivity, CD4 T cell
Introduction
Obesity is an important risk factor for being diagnosed with asthma 1, 2. There has been an alarming increase in the prevalence of obesity, and in the United States it is estimated that approximately 250,000 obese people are being newly diagnosed with asthma each year3. Further compounding the impact of obesity on asthma is the fact that obese asthmatics are difficult to treat effectively, as they tend to have worse asthma control 4, 5 and do not respond as well to standard therapy as lean asthmatics 6–8. The reasons for this are not known as the pathogenesis of asthma in obesity is poorly understood.
It has been argued that a number of factors could contribute to respiratory symptoms in obesity (e.g. deconditioning, gastroesophageal reflux, sleep apnea, and increased ventilatory requirements9), and that obesity does not actually cause asthma, but only symptoms that are misdiagnosed as asthma. As asthma is a syndrome characterized by airway hyperresponsiveness (AHR), inflammation and clinical symptoms, a key question is whether obesity causes AHR and inflammation, in addition to clinical symptoms. Many clinical studies suggest that obesity contributes to asthma symptoms, but few studies have addressed how obesity contributes to AHR and inflammation characteristic of asthma.
A central effector cell mediating inflammation in asthma is the CD4 lymphocyte10. CD4 cells produce Th2 cytokines (e.g. Interleukins 5, 6, 13) and Th17 cytokines (such as Interleukin 17, also increased in obesity11)12 which are implicated in the pathogenesis of asthma. Th1 cytokines (such as interferon γ and TNFα) may counterbalance this process, though recent data suggest that Th1 cytokines may also contribute to disease activity13, 14. CD4 cells elaborate cytokines leading to airway cellular inflammation. If obesity actually causes asthma, it would seem reasonable to postulate that obesity would increase markers of lymphocytic inflammation and increase AHR, and that weight loss would have the opposite effect.
To address the pathogenesis of asthma in obesity, and gain insights into potential treatment for these patients, we designed a study to test the hypotheses that (i) obese asthmatics would have evidence of increased markers of asthmatic inflammation compared to obese non-asthmatics; and (ii) bariatric (weight-loss) surgery would lead to improved AHR, asthma control, and reduced makers of inflammation. We performed a cross-sectional study of asthmatic and non-asthmatic patients before bariatric surgery, and a longitudinal study of asthmatics before, and 12 months after, bariatric surgery.
METHODS
Participant selection
Participants undergoing evaluation for bariatric surgery were invited to participate in this study. The study was reviewed by the local institutional review board and written informed consent was obtained from all participants. Asthmatic participants had physiological evidence of asthma with either a positive methacholine challenge (PC20 less than16 mg/ml) or a positive response to a bronchodilator (improvement in FEV1 and/or FVC of at least 12% and 200 ml). Non-asthmatic participants had no diagnosis of asthma and did not respond to methacholine or bronchodilator. Participants with greater than 20 pack years of smoking were excluded; participants who had smoked within the prior six months were also excluded. Participants were excluded if they had FEV1 less than 60% predicted, had been treated with systemic steroids during the prior six weeks, had active pulmonary disease other than asthma (those with obstructive sleep apnea were not excluded) or had significant disease that in the opinion of the investigator would interfere with study participation. Participants on thiazolidinedione anti-diabetic medication (which may affect adipokines and inflammation) were also excluded.
Study design
This was a study of asthmatic and non-asthmatic participants undergoing bariatric surgery with (i) a cross-sectional study of obese asthmatics and non-asthmatics prior to bariatric surgery, and (ii) a prospective observational study of obese asthmatics before and 12 months after bariatric surgery.
Asthmatic and control participants answered baseline questionnaires regarding demographics, medical history and asthma control and quality of life 15,16 and underwent baseline lung function testing. Blood was drawn for Immunoglobulin E (IgE), adipokine levels and lymphocyte isolation. Participants underwent bronchoscopy with bronchoalveolar lavage at the time of bariatric surgery.
Asthmatic participants returned at 3, 6, 9 and 12 months after surgery. At 3, 6, 9 and 12 months they performed spirometry and completed medical and asthma questionnaires. At 12 months they underwent repeat methacholine challenge and blood draw. Consenting asthmatic participants had a bronchoscopy 12 months after surgery under conscious sedation (n = 17).
Outcome measures
The primary outcome measures were change in methacholine reactivity and asthma control in asthmatic participants 12 months after bariatric surgery. Methacholine reactivity was expressed as the concentration of inhaled methacholine causing a 20% reduction in FEV1 (PC20). Methacholine challenge was measured in participants with an FEV1 that was 70% or more of the predicted value. Challenge was performed according to American Thoracic Society guidelines using the 5-breath dosimeter method 17. Asthma control was quantified by the Asthma Control Questionnaire 15.
Secondary clinical outcomes included spirometry, score on the asthma quality of life questionnaire 16, medication use determined at the time of study visit, and baseline comparison of asthmatic and control participants.
Adipokine levels in serum and BAL, cell counts in BAL and cytokine production from stimulated CD4+ T cells were compared between asthmatics and controls at baseline, and between asthmatics before and 12 months after bariatric surgery.
Laboratory methods
BAL
Bronchoalveolar lavage (BAL) differential cell counts were determined after BAL supernatant was separated by centrifugation at 400 × g for 10 minutes and frozen at −80°C for later analysis. Cytospins were prepared and stained with DiffQuik. Differential cell counts were determined by two observers masked to the participants’ identities. 500 cells were counted on each slide.
CD4+ T cell isolation
Whole blood was layered over Histopaque, and the buffy coat was removed after centrifugation. The cell pellet was resuspended in MACS® buffer, and the Miltenyi Biotec protocol for cell separation using positive selection with CD4 magnetic microbeads on autoMACS MS columns was followed. Cell purity was checked by flow cytometry using antibodies to CD4 and TCRαβ (BD Pharmingen), and the percentage positive for both ranged from 89% to 99%.
CD3–CD28 stimulation
Anti-CD3 antibodies (BD Pharmingen) were added to a 48 well plate at 5ug/ml and incubated 2–4 hrs at 37°C or overnight at 4°C. After incubation the media was removed, and 106 cells/ml and 1ug/ml of anti-CD28 antibody (BD Pharmingen) were added in RPMI media containing FBS and incubated @ 37°C for 24 hours. At 24 hours supernatant was collected and stored at −80°C for later cytokine measurement.
Cytokine and adipokine measurements
Cytokines in CD4 cell culture supernatant, and adipokines implicated in the pathogenesis of asthma in obesity from previous animal studies were measured in BAL and serum18, 19. Cytokines were analyzed in duplicate using the Bio-Plex suspension array system (Bio-Rad, Hercules, CA) according to the manufacturer’s instructions. Standard curves were calculated and samples were analyzed using the Bio-Plex Manager software (Bio-Rad). Millipore’s Milliplex™ Adipocyte Map Kit fluorescently labeled microsphere bead assays were used for assay of adiponectin and leptin.
Serum IgE
Serum IgE was measured at baseline and 12 months after surgery using a near-infrared particle immunoassay (NIPIA) and a Beckman Image 800 Immunochemistry Analyzer. IgE level < 100 IU/ml was defined as normal 20
Statistical Analysis
Power calculations were based on the estimated mean change in AHR and asthma control for asthmatic participants pre- and 12 months post- surgery. PC20 estimates were based on our ability to detect an improvement from 4 mg/ml at baseline to 8 mg/ml at 12 months. Assuming a Type I error rate of 0.05 and a within-person SD of 1.0 doubling doses 21, 13 subjects would provide greater than 90% power to detect a significant difference. For asthma control we estimated the score would improve from 1.5 at baseline to 1.0 at 12 months (SD 0.7) 22. Assuming a Type I error rate of 0.05, 20 participants would provide greater than 80% power to detect a significant diference. We estimated 15 % loss to follow up over the 12 months of the study, and planned to recruit a total of 23 asthmatic subjects.
We compared baseline differences between asthmatic and non-asthmatic participants, and also followed the cohort of asthmatics over time, before and after gastric-bypass surgery. Data were summarized by descriptive statistics, with average values and variation described using mean and standard deviation. We initially used a T-test to compare differences in asthmatics and non-asthmatics and Chi square analyses to examine differences in categorical variables between asthmatics and controls. Repeated measures analysis of variance was used to compare changes in measures from baseline to 12 months post surgery. Asthmatic participants who did not respond to methacholine at the 12 month visit were assigned a PC20 of 16 mg/ml for purposes of analysis. Non-normally distributed data were log-transformed prior to analysis. We performed regression analysis to control for effects of BMI when comparing asthmatics and controls at baseline, and to investigate the relationship between change in BMI and (log-transformed) change in PC20 in those with normal IgE. Spearman’s Correlation was used to investigate the relationship between change in BMI and change in PC20 in those with high IgE (as an increase in AHR produced negative values precluding log transformation).
Results
Baseline Characteristics
A total of 41 asthmatic and 35 control participants were initially enrolled. 9 out of 35 control participants were excluded as they manifested AHR with PC20 < 16 mg/ml (Figure 1). Seventeen asthmatic participants qualified for the study on the basis of AHR with PC20 < 16 mg/ml, 6 qualified with bronchodilator responsiveness. Baseline characteristics of participants proceeding to bariatric surgery are shown in Table 1. The majority of participants were female (as were 78 % of all patients undergoing bariatric surgery). There was a similar prevalence of co-morbidities such as obstructive sleep apnea, gastro-esophageal reflux disease and allergic rhinitis in asthmatics and controls. Asthmatic participants were significantly more obese than non-asthmatic participants (Table 1a).
Figure 1.
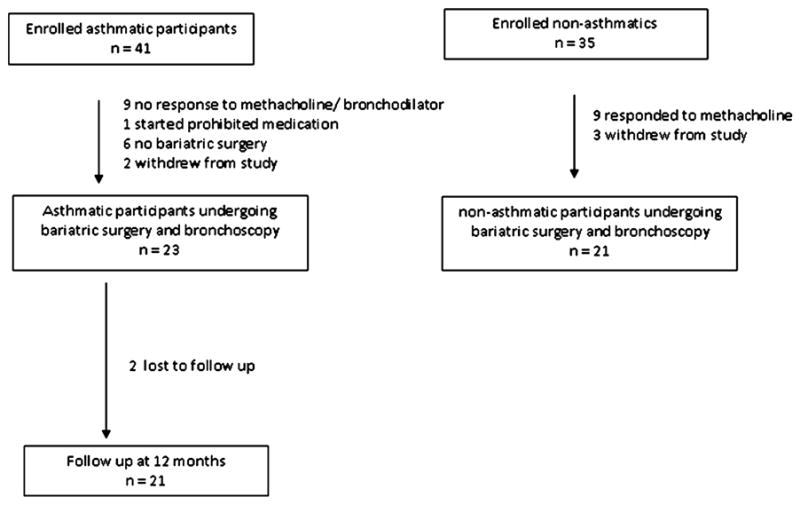
Enrollment and follow-up of study participants. Participants were initially enrolled prior to bariatric surgery, though only participants who actually went through bariatric surgery were included in the analysis. There were six asthmatics and no control participants who did not have bariatric surgery. This was related to the fact that asthmatics were enrolled at presentation to the bariatric clinic whereas control participants were only enrolled once their surgery had been scheduled.
Table 1a.
Baseline demographic data of asthmatic and control participants*
| control | asthma | P* | |
|---|---|---|---|
| n | 21 | 23 | |
| Female (n) | 19 | 21 | 0.93 |
| Age (years) | 43 ± 7 | 43 ± 10 | 0.7 |
| Age asthma onset (years) | 29 ± 13 | ||
| Asthma exacerbation in last 12 months (n) | 7 | ||
| Sleep apnea (n) | 10 | 9 | 0.57 |
| Diabetes mellitus (n) | 6 | 9 | 0.46 |
| Gastroesophageal reflux disease (n) | 7 | 6 | 0.6 |
| Allergic rhinitis (n) | 13 | 17 | 0.39 |
| Pack year smoking (n) | 5.68 ± 8.22 | 8.3 ± 11.9 | 0.47 |
| BMI (kg/m2) | 43.48 ± 5.64 | 51.37 ± 9.71 | < 0.01 |
| Type of Surgery | 0.04 | ||
| Open Roux-en-Y | 10 | 18 | |
| Laparoscopic Roux-en-Y | 1 | 2 | |
| Laparoscopic banding | 10 | 3 |
Values shown are mean and standard deviation. p values shown for χ2 for proportions, and T-test for continuous variables.
We compared demographic characteristics of participants with normal and elevated IgE (Table 1b). Participants with normal IgE reported significantly later onset of asthma. They also tended to be older, suffer with more co-morbidities, and report more asthma exacerbations in the year prior to surgery.
Table 1b.
Baseline demographic data of asthmatic participants by IgE level*
| Low IgE | High IgE | P* | |
|---|---|---|---|
| n | 14 | 9 | |
| Serum IgE (IU/ml) | 25.2 ± 23.2 | 305.3 ± 186.9 | <0.001 |
| Female (n) | 13 | 8 | 0.74 |
| Age (years) | 46 ± 8 | 41 ± 4 | 0.27 |
| Age asthma onset (years) | 34 ± 11.5 | 21.4 ± 10.5 | 0.01 |
| Asthma exacerbation in last 12 months (n)** | 6 | 1 | 0.11 |
| Sleep apnea (n) | 7 | 2 | 0.18 |
| Diabetes mellitus (n) | 7 | 2 | 0.18 |
| Gastroesophageal reflux disease (n) | 5 | 1 | 0.19 |
| Rhinitis (n) | 10 | 7 | 0.74 |
| Pack year smoking (n) | 7.1 ± 12.8 | 9.3 ± 11.5 | 0.68 |
| BMI (kg/m2) | 51.29 ± 9.78 | 51.11 ± 9.13 | 0.97 |
| Type of Surgery | 0.08 | ||
| Open Roux-en-Y | 11 | 7 | |
| Laparoscopic Roux-en-Y | 0 | 2 | |
| Laparoscopic banding | 3 | 0 |
Values shown are mean and standard deviation. p values shown for χ2 for proportions, and T-test for continuous variables.
Asthma exacerbation defined as patient self-report of urgent health care visit for asthma and/or need for prednisone for treatment of asthma.
Baseline lung function and markers of airway inflammation are shown in Table 1c. At baseline, asthmatic participants had significantly lower FEV1 and FVC, independent of BMI. Levels of eosinophils were low and not significantly different between asthmatics and controls. Lymphocyte differentials were significantly lower in asthmatics (p = 0.03). Asthmatics tended to have lower levels of adiponectin and higher levels of leptin, though this did not reach statistical significance.
Table 1c.
Baseline pulmonary function, BAL and serum data of asthmatic and control participants*
| control | asthma | Unadjusted p | Adjusted p† | |
|---|---|---|---|---|
| n | 21 | 23 | ||
| FEV1 (% predicted) | 95.9 ± 7.3 | 82.4 ± 14.1 | < 0.001 | 0.004 |
| FVC (% predicted) | 93.6 ± 8.4 | 84.1 ± 12.9 | 0.01 | 0.04 |
| FEV/FVC (% predicted) | 102.7 ± 5.5 | 98.8 ± 14.3 | 0.02 | 0.36 |
| FEV1/FVC (absolute) | 82.6 ± 4.79 | 79.6 ± 11.3 | 0.26 | 0.24 |
| BAL macrophages (%)‡ | 85.2 ± 12.7 | 93.0 ± 4.1 | < 0.05 | 0.08 |
| BAL lymphocytes (%)‡ | 8.0 ± 9.1 | 3.5 ± 2.6 | 0.01 | 0.03 |
| BAL neutrophils (%)‡ | 6.7 ± 10.1 | 3.1 ± 3.4 | 0.6 | 0.66 |
| BAL eosinophils (%)‡ | 0.02 ± 0.11 | 0.51 ± 0.75 | 0.1 | 0.07 |
| BAL leptin (pg/ml)‡ | 23.9 ± 28.4 | 35.3 ± 65.4 | 0.99 | 0.27 |
| BAL adiponectin (pg/ml)‡ | 2502 ± 2350 | 1527 ± 1313 | 0.09 | 0.67 |
| Serum IgE (IU/ml) | 45.7 ± 72.4 | 133.3 ± 172.2 | 0.02 | 0.11 |
| Serum leptin (ng/ml) | 26.6 ± 19.2 | 30.2 ± 30.5 | 0.77 | 0.70 |
| Serum adiponectin (μg/ml) | 36.3 ± 65.9 | 13.8 ± 10.5 | 0.12 | 0.15 |
Values shown are mean and standard deviation. p values shown for T- test for continuous variables except for BAL eosinophils, where p value is shown for Kruskal Wallis test.
p value comparing control and asthmatic, adjusted for BMI.
FEV1, FVC and FEV1/FVC are % predicted from Hankinson,48.
BAL is bronchoalveolar lavage.
n=21 for asthmatics and 21 for controls
Baseline lung function and markers of airway inflammation in asthmatics with normal and high IgE are shown in the online supplement. BAL eosinophils tended to be higher in those with high IgE (0.22 ± 0.40 versus 0.93 ± 0.96, p = 0.11). Otherwise there were no differences in measure of lung function, BAL cell counts or adipokine levels between those with normal and high IgE (see Table E1 in the Online Repository).
Effect of bariatric surgery on asthma control
BMI changed significantly from 51.4 ± 9.7 at baseline to 37.5 ± 7.8 after 12 months (p < 0.0001, Table 2). We measured the effect of bariatric surgery on asthma control, quality of life and medication use. The study demonstrated clinically and statistically significant improvements in asthma control and quality of life scores. Participants reported using significantly less short acting beta agonist 12 months after surgery. Participants reported no asthma exacerbations following bariatric surgery (defined as urgent health care visit for asthma or prednisone treatment for asthma). Asthma control and quality of life improved in those with both normal and high IgE levels (data not shown).
Table 2.
Asthma symptoms and medication use pre and 12 months following bariatric surgery*
| visit 0 | visit 12 | p† | |
|---|---|---|---|
| BMI (kg/m2) | 51.37 ± 9.71 | 37.51 ± 7.76 | < 0.0001 |
| ACQ 6‡ | 1.64 ± 1.06 | 0.63 ± 0.97 | < 0.0001 |
| AQLQ§ | 4.87 ± 1.11 | 5.87 ± 1.70 | < 0.01 |
| Inhaled corticosteroid | 16 | 10 | 0.21 |
| Fluticasone dose (μg/day) | 331 ± 381 | 238 ± 329 | 0.16 |
| Long acting β agonist | 11 | 8 | 0.29 |
| Leukotriene modifier | 4 | 2 | 0.89 |
| Short acting β-agonist | 22 | 9 | 0.001 |
Values shown are mean plus–minus standard deviation for symptom scores and fluticasone dose, and n for medication use. ACQ is Juniper Asthma Control Questionnaire, AQLQ denotes Asthma Quality of Life Questionnaire
p values are for repeated measures analysis of variance for ACQ and AQLQ, Fisher’s exact test for medication use
Scores on the ACQ range from 0 to 6, with lower scores indicating better asthma control and 0.5 as the minimal clinically important difference, score > 1.5 suggestive of poorly controlled asthma, score < 0.75 suggestive of well controlled asthma. ACQ6 omits last item, which is score for FEV1 (which is known to improve with weight loss), ACQ7 is full 7 item questionnaire.
scores on the AQLQ range from 1 to 7, with higher scores indicating better quality of life and 0.5 as the minimal clinically important difference
Effect of bariatric surgery on AHR
Airway hyperresponsiveness improved significantly 12 months after bariatric surgery ( p = 0.03, Table 3) even though our data underestimate the absolute change in AHR (participants without AHR at 12 months were assigned a value of 16 mg/ml for analysis purposes).
Table 3.
Lung function and markers of inflammation in asthmatic participants pre- and 12 months post bariatric surgery*
| Visit 0 | Visit 12 | p | |
|---|---|---|---|
| PC20 (mg/ml methacholine)† | 3.90 ± 3.59 | 7.28 ± 6.50 | 0.03 |
| FEV1 (% predicted) | 82.4 ± 14.1 | 90.4 ± 14.2 | < 0.01 |
| FVC (% predicted) | 84.1 ± 12.9 | 93.9 ± 15.5 | < 0.001 |
| FEV/FVC (% predicted) | 98.8 ± 14.3 | 96.8 ± 8.2 | 0.74 |
| BAL macrophages (%)‡ | 93.0 ± 4.1 | 90.3 ± 6.0 | 0.13 |
| BAL lymphocytes (%)‡ | 3.5 ± 2.6 | 7.9 ± 5.7 | < 0.01 |
| BAL neutrophils (%)‡ | 3.1 ± 3.4 | 1.6 ± 1.5 | 0.18 |
| BAL eosinophils (%)‡ | 0.51 ± 0.75 | 0.23 ± 0.61 | 0.27 |
| BAL leptin (pg/ml)‡ | 35.3 ± 65.4 | 29.4 ± 30.0 | 0.63 |
| BAL adiponectin (pg/ml)‡ | 1527 ± 1313 | 4530 ± 6168 | 0.01 |
| Serum IgE (IU/ml) | 133.3 ± 172.2 | 133.9 ± 150.1 | 0.95 |
| Serum leptin (ng/ml) | 30.2 ± 30.5 | 17.1 ± 13.4 | 0.096 |
| Serum adiponectin (μg/ml) | 13.8 ± 10.5 | 25.2 ± 16.3 | 0.003 |
Values shown are mean plus-minus standard deviation. FEV1, FVC and FEV1/FVC are % predicted from Hankinson 48 BAL is bronchoalveolar lavage. p values are shown repeated measures analysis of variance comparing controls and asthmatics before and 12 months after bariatric surgery. For BAL eosinophils, p value is shown for Kruskal Wallis test.
n=17 at visit 0 and 12
n=21 at visit 0, n = 17 at visit 12.
As obesity is particularly a risk factor for non-atopic asthma2, we compared change in AHR in asthmatics with a normal IgE to those with high IgE in a post-hoc analysis. We found highly significant improvements in AHR in those with a normal IgE (p = 0.001, n = 11), but not in those with an elevated IgE (p = 0.89, n = 6). A significant interaction exists between IgE status and change in PC20 (p=0.01 for interaction term). Indeed, change in AHR was significantly related to change in BMI in those with normal IgE (R2 = 0.46, p = 0.02, Figure 2). This suggests that IgE status significantly affected the change in AHR in response to surgery.
Figure 2.
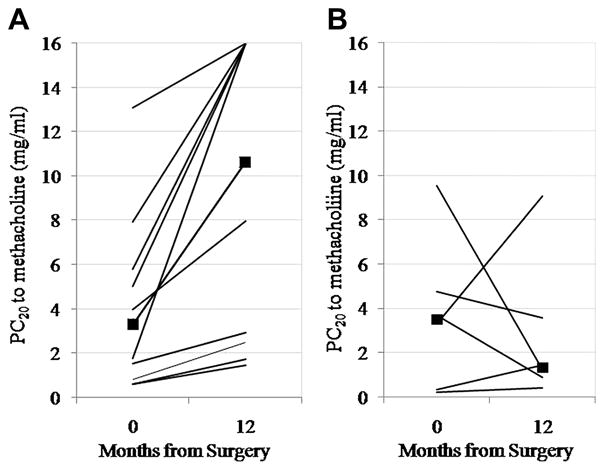
Change in AHR in patients with normal IgE (A) (P = .001), n = 11 and elevated IgE (B) (P = .89). Squares represent median values. A significant interaction term exists between IgE status and change in AHR (P = .01 for interaction term).
There was no significant interaction between any other measure of lung function and IgE status.
Effect of bariatric surgery on markers of airway cellular and metabolic inflammation
Changes in markers of airway inflammation are also shown in Table 3. The percentage of lymphocytes increased 12 months post surgery. Since this could be related to change in medication, analyses were performed only in participants reporting use of inhaled corticosteroids both at the time of surgery and 12 months after with similar results. Serum IgE levels did not change. BAL and serum adiponectin increased significantly with weight loss. There was a trend towards a positive correlation between BAL adiponectin and PC20, (p = 0.12, Spearman’s rho = 0.30), but no correlation between serum adiponectin and PC20. There was no correlation between leptin levels and AHR (data not shown).
Effect of bariatric surgery on markers of lymphocytic inflammation
We determined the effect of bariatric surgery on stimulated CD4+ T cells to determine if bariatric surgery affects lymphocyte function pertinent to asthma (Figure 3). We found that there was a significant increase in interferon-γ, TNF-α, interleukin 5, interleukin 6, interleukin 13, and interleukin 17 twelve months after surgery.
Figure 3.
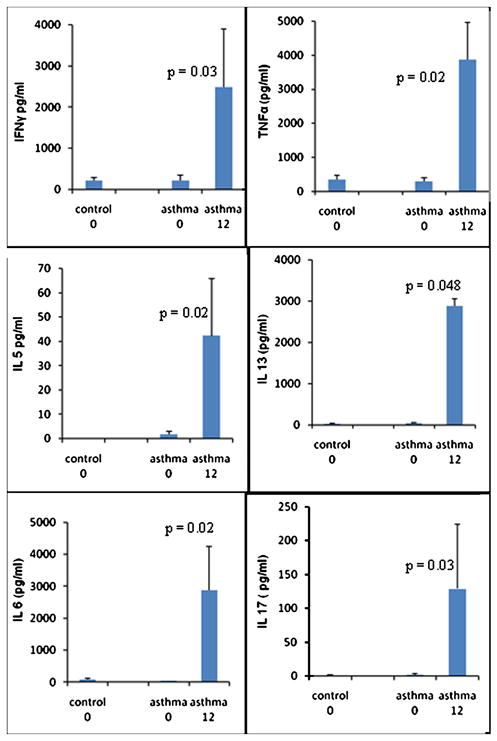
Cytokine production from CD3–CD28 stimulated CD4+ T lymphocytes before, and 12 months after, bariatric surgery in controls (n=15) and asthmatics (n=10). P values shown for comparison of asthmatics at 0 and 12 months, no significant differences between asthmatics and control at visit 0.
Discussion
This study shows that bariatric surgery produces clinically and statistically significant improvements in asthma control. Previous studies have suggested that bariatric surgery might improve asthma symptoms23–26, although these studies have been criticized for not objectively diagnosing asthma in their study populations. Airway hyperresponsiveness improves with bariatric surgery in individuals with normal IgE, and the change in AHR is significantly related to change in BMI in this group. These improvements were not related to decreased inflammation and CD4+ lymphocyte function; indeed CD4+ T cell responses increased after bariatric surgery. Our study suggests that bariatric surgery should be considered for treatment of poorly controlled asthma in patients with extreme obesity, and this should be studied in larger clinical trial. Our study also provides important insights into the pathogenesis of asthma in obesity.
One singularly important insight is the relationship of atopy with asthma in obesity. Recent publications suggest that obesity is particularly a risk factor for non-atopic asthma2, 27, and so in this study we performed a post-hoc analysis to determine if atopic status (measured by IgE level in our study cohort) affected results of this study. Non-atopic asthmatics reported significantly later onset of asthma than atopic asthmatics. They tended to have more co-morbidities and more exacerbations that atopic obese asthmatics before surgery. This suggests that the pathogenesis of asthma in the non-atopic obese group may differ from that in the atopic group, particularly as it leads to airway disease later in life.
The atopic status of participants also affected the change in AHR following bariatric surgery. There was a statistically significant interaction between IgE status and change in AHR, and change in AHR was significantly related to change in BMI in individuals with normal IgE. These observations suggest that atopic status does indeed significantly affect the AHR response to surgery.
No previous study has determined the effects of bariatric surgery on AHR. We are aware of only one previous study that quantified change in AHR in response to weight loss, and in that case diet-induced weight loss did not affect AHR28. We do not know the reason for the difference between the results from the bariatric surgery and dietary interventions, but speculate that it could be related to differences in the amount of weight lost or the atopic status of participants (which was not reported in the dietary intervention study)28. The finding that a marker of atopy was related to change in AHR with weight loss may also explain conflicting reports on the relationship between AHR and BMI, since these studies often do not differentiate on atopic status 29, 30. Studies in mice are also pertinent in this regard - obese mice have increased airway reactivity compared with lean mice, even in the absence of allergen challenge31. Obesity may lead to AHR even in the absence of allergic inflammation, suggesting that a non-allergic mechanism leads to AHR in obesity. These data have important clinical and scientific implications: weight loss may be a critical intervention to improve AHR in non-atopic individuals, and obese individuals with minimal allergic inflammation likely represent a distinct phenotype of asthma more common in older female asthmatics32, 33.
With respect to markers of allergic inflammation, airway eosinophilia did not change with weight loss, nor were there differences between obese asthmatics and controls. Since the initiation of our study, cross-sectional studies have been published suggesting that airway eosinophilia does not increase, but may actually decrease, with increasing BMI34–36. Animal models of allergic asthma suggest decreased airway eosinophilia in obese compared with lean mice.37 Our current findings provide strong support for these previous cross-sectional and animal studies. Bariatric surgery does not reduce airway eosinophilia even though symptoms and AHR improve.
Bariatric surgery did affect lymphocyte percentage in BAL and peripheral blood lymphocyte function. Obese mouse models of allergic asthma also report decreased BAL lymphocyte counts in obese compared with lean mice37, consistent with the current findings. We also found that stimulated CD4+ T cells isolated from the peripheral blood of asthmatics had significantly increased cytokine production 12 months after surgery. Participants were using less inhaled anti-inflammatory medication at 12 months, but even high dose inhaled corticosteroids do not affect response of T cells to stimulation38. Changes in other medications may be affecting T cell responses, this will require further investigation. However, this finding of altered lymphocyte function in obesity is consistent with previous publications. Tanaka et al found that mitogenic and cytokine responses in T cell subsets are reduced in obese individuals compared with lean individuals 39, 40, and Farooqi et al found that leptin deficiency (obesity is characterized by leptin resistance) was associated with reduced circulating CD4 T cells, and impaired T cell proliferation and cytokine production 41. The changes in CD4+ T cell cytokine release and airway lymphocytes suggest that asthma in obesity is likely not related to enhanced lymphocyte driven inflammation. Future studies which phenotype lymphocytes in BAL and blood during weight loss may provide important insights into how obesity and asthma interact to affect lymphocyte function in obese asthmatics. The important clinical implication of this observation is that anti-inflammatory therapies that target lymphocyte-driven inflammation are likely to have limited efficacy in obese asthmatics, which may help explain the reduced steroid responsiveness that has been reported in these patients6, 7.
Contrary to our original hypothesis, AHR in obesity is dissociated from lymphocytic and eosinophilic inflammation. However, there are other factors associated with obesity that change with weight loss and may lead to AHR. These include the mechanical effects of breathing at low lung volumes, altered metabolic factors and co-morbidities such as sleep apnea.
Breathing at low lung volumes (which occurs in obesity) is known to lead to AHR in normal volunteers, possibly through biophysical effects on airway smooth muscle42, 43. Breathing at low lung volumes unloads airway smooth muscle, allowing it to shorten excessively when activated. In addition, deep breaths are potent bronchodilators, this bronchodilatory effect may be compromised by breathing at low lung volumes.42
Altered metabolic factors may contribute to airway hyperresponsiveness. Studies in mice suggest that factors produced by adipose tissue may also affect AHR. For example, adiponectin decreases AHR in a mouse model of asthma19. The results of our study show that BAL and serum adiponectin increase significantly with weight loss, and there may be a modest correlation between BAL adiponectin levels and AHR, although our study was not powered to determine this. There are multiple other mediators such as TNFα, plasminogen activator inhibitor-1 and interleukin 6 that are also altered in obesity and could contribute to airway disease44. Future studies of the role of these mechanical and metabolic factors, and co-morbidities such as sleep apnea may provide important information on the pathogenesis of asthma in obesity.
Other interesting insights into the relationship between asthma and obesity are suggested by certain baseline characteristics of our study population. Asthmatic participants were significantly heavier than controls. We do not know the reasons for this, although a recent report from a bariatric surgery consortium found that asthma prevalence increased with increasing BMI; in fact over 30% of individuals with a BMI over 60 reported a diagnosis of asthma45. The fact that our asthmatic participants had higher BMIs may reflect the fact that heavier patients are more likely to have asthma. Another interesting observation was the high prevalence of asymptomatic AHR in controls. The prevalence of asymptomatic AHR has been reported as 8 – 12 % in normal weight populations46, 47. It has been suggested that obesity may be risk factor for symptomatic AHR. Our findings suggest that asymptomatic AHR may in fact be very common in severely obese patients. It seems likely that the same factors causing asthma in obesity could be contributing to asymptomatic AHR in these severely obese patients. These observations likely warrant further investigation.
In summary, we found that bariatric surgery led to significant improvements in AHR, particularly in individuals with normal IgE levels. These improvements were not associated with detectable changes in airway inflammation but somewhat paradoxically were associated with significant increases in CD4+ T cell cytokine responses and airway lymphocytes. These data suggest that AHR and asthma symptoms in obese asthmatics are not associated with the typical lymphocyte-dependent pathways that drive asthma in lean allergic individuals. There are likely to be at least two distinct phenotypes of obese asthmatics: (i) one group with non-atopic late onset asthma with AHR that will improve with weight loss, and (ii) a group with early onset atopic disease. The former category likely have asthma developing due to obesity, the latter likely have early onset atopic asthma which is complicated by the co-existence of obesity. Future studies and interventions for obese asthmatics need to treat the subgroup of obese asthmatics with little evidence of allergic inflammation as having a distinct phenotype of asthma; therapies that target lymphocytic inflammatory pathways are likely to have limited efficacy in this population.
Supplementary Material
Key Messages.
Bariatric surgery produces significant improvements in airway hyperresponsiveness, particularly in asthmatics with normal IgE levels
Improvements in airway hyperresponsiveness following bariatric surgery are paradoxically associated with increases in markers of lymphocyte inflammation.
The obese state produces a unique phenotype of asthma
Acknowledgments
Sources of Support: Supported by NIH NCRR grants: P20 RR15557, RR019965
The authors would like to gratefully acknowledge the time, effort and commitment of all those patients that participated in this study and Dr. Stephanie Shore (Harvard School of Public Health) for her support and advice. In addition, the authors gratefully acknowledge the invaluable support of members of the Vermont Lung Center in particular Kendall Black, Stephanie Burns Lorraine Bourassa and Joan Lippman; Department of Anesthesia; the Fletcher Allen Operating Room staff; and the faculty and staff of the Fletcher Allen Bariatric Clinic, in particular Laurie Spaulding, MD and Deborah Wachtel.
Abbreviations
- AHR
airway hyperresponsiveness
- BAL
bronchoalveolar lavage
- FEV1
forced expiratory volume in 1 second
- FVC
forced vital capacity
- IgE
immunoglobulin E
- PC20
concentration of methacholine producing 20 % fall in FEV1 of FVC
References
- 1.Camargo CA, Jr, Weiss ST, Zhang S, Willett WC, Speizer FE. Prospective study of body mass index, weight change, and risk of adult-onset asthma in women. Arch Intern Med. 1999;159:2582–8. doi: 10.1001/archinte.159.21.2582. [DOI] [PubMed] [Google Scholar]
- 2.Chen Y, Dales R, Jiang Y. The association between obesity and asthma is stronger in nonallergic than allergic adults. Chest. 2006;130:890–5. doi: 10.1378/chest.130.3.890. [DOI] [PubMed] [Google Scholar]
- 3.Beuther DA, Sutherland ER. Overweight, Obesity, and Incident Asthma: A Meta-analysis of Prospective Epidemiologic Studies. Am J Respir Crit Care Med. 2007;175:661–6. doi: 10.1164/rccm.200611-1717OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vortmann M, Eisner MD. BMI and health status among adults with asthma. Obesity (Silver Spring) 2008;16:146–52. doi: 10.1038/oby.2007.7. [DOI] [PubMed] [Google Scholar]
- 5.Mosen DM, Schatz M, Magid DJ, Camargo CA., Jr The relationship between obesity and asthma severity and control in adults. J Allergy Clin Immunol. 2008;122:507–11. e6. doi: 10.1016/j.jaci.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 6.Peters-Golden M, Swern A, Bird SS, Hustad CM, Grant E, Edelman JM. Influence of body mass index on the response to asthma controller agents. Eur Respir J. 2006;27:495–503. doi: 10.1183/09031936.06.00077205. [DOI] [PubMed] [Google Scholar]
- 7.Boulet LP, Franssen E. Influence of obesity on response to fluticasone with or without salmeterol in moderate asthma. Respir Med. 2007 doi: 10.1016/j.rmed.2007.06.031. [DOI] [PubMed] [Google Scholar]
- 8.Sutherland ER, Goleva E, Strand M, Beuther DA, Leung DY. Body mass and glucocorticoid response in asthma. Am J Respir Crit Care Med. 2008;178:682–7. doi: 10.1164/rccm.200801-076OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shore SA. Obesity and asthma: cause for concern. Curr Opin Pharmacol. 2006;6:230–6. doi: 10.1016/j.coph.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Shalaby KH, Martin JG. Overview of asthma; the place of the T cell. Curr Opin Pharmacol. 10:218–25. doi: 10.1016/j.coph.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Winer S, Paltser G, Chan Y, Tsui H, Engleman E, Winer D, et al. Obesity predisposes to Th17 bias. Eur J Immunol. 2009;39:2629–35. doi: 10.1002/eji.200838893. [DOI] [PubMed] [Google Scholar]
- 12.Alcorn JF, Crowe CR, Kolls JK. TH17 cells in asthma and COPD. Annu Rev Physiol. 72:495–516. doi: 10.1146/annurev-physiol-021909-135926. [DOI] [PubMed] [Google Scholar]
- 13.Hayashi N, Yoshimoto T, Izuhara K, Matsui K, Tanaka T, Nakanishi K. T helper 1 cells stimulated with ovalbumin and IL-18 induce airway hyperresponsiveness and lung fibrosis by IFN-gamma and IL-13 production. Proc Natl Acad Sci U S A. 2007;104:14765–70. doi: 10.1073/pnas.0706378104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cui J, Pazdziorko S, Miyashiro JS, Thakker P, Pelker JW, Declercq C, et al. TH1-mediated airway hyperresponsiveness independent of neutrophilic inflammation. J Allergy Clin Immunol. 2005;115:309–15. doi: 10.1016/j.jaci.2004.10.046. [DOI] [PubMed] [Google Scholar]
- 15.Juniper EF, O’Byrne PM, Guyatt GH, Ferrie PJ, King DR. Development and validation of a questionnaire to measure asthma control. Eur Respir J. 1999;14:902–7. doi: 10.1034/j.1399-3003.1999.14d29.x. [DOI] [PubMed] [Google Scholar]
- 16.Juniper EF, Guyatt GH, Ferrie PJ, Griffith LE. Measuring quality of life in asthma. Am Rev Respir Dis. 1993;147:832–8. doi: 10.1164/ajrccm/147.4.832. [DOI] [PubMed] [Google Scholar]
- 17.Crapo RO, Casaburi R, Coates AL, Enright PL, Hankinson JL, Irvin CG, et al. Guidelines for methacholine and exercise challenge testing-1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med. 2000;161:309–29. doi: 10.1164/ajrccm.161.1.ats11-99. [DOI] [PubMed] [Google Scholar]
- 18.Shore SA, Schwartzman IN, Mellema MS, Flynt L, Imrich A, Johnston RA. Effect of leptin on allergic airway responses in mice. J Allergy Clin Immunol. 2005;115:103–9. doi: 10.1016/j.jaci.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 19.Shore SA, Terry RD, Flynt L, Xu A, Hug C. Adiponectin attenuates allergen-induced airway inflammation and hyperresponsiveness in mice. J Allergy Clin Immunol. 2006;118:389–95. doi: 10.1016/j.jaci.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 20.Wang TN, M-C L, Wu C-C, Leung S-Y, Huang M-S, Chaung H-Y, et al. Risk of Occupational asthmogens in atopic and non-atopic asthma: A case control study in Taiwan. American Journal of Respiratory and Critical Care Medicine. 2010;182:1369–76. doi: 10.1164/rccm.200906-0969OC. [DOI] [PubMed] [Google Scholar]
- 21.Knox AJ, Wisniewski A, Cooper S, Tattersfield AE. A comparison of the Yan and a dosimeter method for methacholine challenge in experienced and inexperienced subjects. Eur Respir J. 1991;4:497–502. [PubMed] [Google Scholar]
- 22.Dixon AE, Shade DM, Cohen RI, Skloot GS, Holbrook JT, Smith LJ, et al. Effect of obesity on clinical presentation and response to treatment in asthma. J Asthma. 2006;43:553–8. doi: 10.1080/02770900600859123. [DOI] [PubMed] [Google Scholar]
- 23.Sikka N, Wegienka G, Havstad S, Genaw J, Carlin AM, Zoratti E. Respiratory medication prescriptions before and after bariatric surgery. Ann Allergy Asthma Immunol. 2010;104:326–30. doi: 10.1016/j.anai.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 24.Reddy RC, Baptist AP, Fan Z, Carlin AM, Birkmeyer NJ. The Effects of Bariatric Surgery on Asthma Severity. Obes Surg. 2011;21:200–6. doi: 10.1007/s11695-010-0155-6. [DOI] [PubMed] [Google Scholar]
- 25.Maniscalco M, Zedda A, Faraone S, Cerbone MR, Cristiano S, Giardiello C, et al. Weight loss and asthma control in severely obese asthmatic females. Respir Med. 2008;102:102–8. doi: 10.1016/j.rmed.2007.07.029. [DOI] [PubMed] [Google Scholar]
- 26.Simard B, Turcotte H, Marceau P, Biron S, Hould FS, Lebel S, et al. Asthma and sleep apnea in patients with morbid obesity: outcome after bariatric surgery. Obes Surg. 2004;14:1381–8. doi: 10.1381/0960892042584021. [DOI] [PubMed] [Google Scholar]
- 27.Visness CM, London SJ, Daniels JL, Kaufman JS, Yeatts KB, Siega-Riz AM, et al. Association of Childhood Obesity With Atopic and Nonatopic Asthma: Results From the National Health and Nutrition Examination Survey 1999–2006. J Asthma. doi: 10.3109/02770903.2010.489388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aaron SD, Fergusson D, Dent R, Chen Y, Vandemheen KL, Dales RE. Effect of weight reduction on respiratory function and airway reactivity in obese women. Chest. 2004;125:2046–52. doi: 10.1378/chest.125.6.2046. [DOI] [PubMed] [Google Scholar]
- 29.Chinn S, Jarvis D, Burney P. Relation of bronchial responsiveness to body mass index in the ECRHS. European Community Respiratory Health Survey Thorax. 2002;57:1028–33. doi: 10.1136/thorax.57.12.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schachter LM, Salome CM, Peat JK, Woolcock AJ. Obesity is a risk for asthma and wheeze but not airway hyperresponsiveness. Thorax. 2001;56:4–8. doi: 10.1136/thorax.56.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shore SA. Obesity and asthma: lessons from animal models. J Appl Physiol. 2007;102:516–28. doi: 10.1152/japplphysiol.00847.2006. [DOI] [PubMed] [Google Scholar]
- 32.Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 2010;181:315–23. doi: 10.1164/rccm.200906-0896OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haldar P, Pavord ID, Shaw DE, Berry MA, Thomas M, Brightling CE, et al. Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med. 2008;178:218–24. doi: 10.1164/rccm.200711-1754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Veen IH, Ten Brinke A, Sterk PJ, Rabe KF, Bel EH. Airway inflammation in obese and nonobese patients with difficult-to-treat asthma. Allergy. 2008;63:570–4. doi: 10.1111/j.1398-9995.2007.01597.x. [DOI] [PubMed] [Google Scholar]
- 35.Lessard A, Turcotte H, Cormier Y, Boulet LP. Obesity and asthma: a specific phenotype? Chest. 2008;134:317–23. doi: 10.1378/chest.07-2959. [DOI] [PubMed] [Google Scholar]
- 36.Komakula S, Khatri S, Mermis J, Savill S, Haque S, Rojas M, et al. Body mass index is associated with reduced exhaled nitric oxide and higher exhaled 8-isoprostanes in asthmatics. Respir Res. 2007;8:32. doi: 10.1186/1465-9921-8-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnston RA, Zhu M, Rivera-Sanchez YM, Lu FL, Theman TA, Flynt L, et al. Allergic airway responses in obese mice. Am J Respir Crit Care Med. 2007;176:650–8. doi: 10.1164/rccm.200702-323OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sharma KC, Stevens D, Casey L, Kesten S. Effects of high-dose inhaled fluticasone propionate via spacer on cell-mediated immunity in healthy volunteers. Chest. 2000;118:1042–8. doi: 10.1378/chest.118.4.1042. [DOI] [PubMed] [Google Scholar]
- 39.Tanaka S, Isoda F, Ishihara Y, Kimura M, Yamakawa T. T lymphopaenia in relation to body mass index and TNF-alpha in human obesity: adequate weight reduction can be corrective. Clin Endocrinol(Oxf) 2001;54:347–54. [PubMed] [Google Scholar]
- 40.Tanaka S, Inoue S, Isoda F, Waseda M, Ishihara M, Yamakawa T, et al. Impaired immunity in obesity: suppressed but reversible lymphocyte responsiveness. Int J Obes Relat Metab Disord. 1993;17:631–6. [PubMed] [Google Scholar]
- 41.Farooqi IS, Matarese G, Lord GM, Keogh JM, Lawrence E, Agwu C, et al. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest. 2002;110:1093–103. doi: 10.1172/JCI15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shore SA, Fredberg JJ. Obesity, smooth muscle, and airway hyperresponsiveness. J Allergy Clin Immunol. 2005;115:925–7. doi: 10.1016/j.jaci.2005.01.064. [DOI] [PubMed] [Google Scholar]
- 43.Ding DJ, Martin JG, Macklem PT. Effects of lung volume on maximal methacholine-induced bronchoconstriction in normal humans. J Appl Physiol. 1987;62:1324–30. doi: 10.1152/jappl.1987.62.3.1324. [DOI] [PubMed] [Google Scholar]
- 44.Dixon AE, Holguin F, Sood A, Salome CM, Pratley RE, Beuther DA, et al. An official American Thoracic Society Workshop report: obesity and asthma. Proc Am Thorac Soc. 7:325–35. doi: 10.1513/pats.200903-013ST. [DOI] [PubMed] [Google Scholar]
- 45.Belle SH, Chapman W, Courcoulas AP, Flum DR, Gagner M, Inabnet WB, et al. Relationship of body mass index with demographic and clinical characteristics in the Longitudinal Assessment of Bariatric Surgery (LABS) Surg Obes Relat Dis. 2008;4:474–80. doi: 10.1016/j.soard.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roth BJ, Hammers LM, Dillard TA. Methacholine challenge testing in Reserve Officer Training Corps cadets. Chest. 2001;119:701–7. doi: 10.1378/chest.119.3.701. [DOI] [PubMed] [Google Scholar]
- 47.Malo JL, Pineau L, Cartier A, Martin RR. Reference values of the provocative concentrations of methacholine that cause 6% and 20% changes in forced expiratory volume in one second in a normal population. Am Rev Respir Dis. 1983;128:8–11. doi: 10.1164/arrd.1983.128.1.8. [DOI] [PubMed] [Google Scholar]
- 48.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–87. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


