Abstract
The first transmissions of human prion diseases to rodents used guinea pigs (Gps, Cavia porcellus). Later, transgenic (Tg) mice expressing human or chimeric human/mouse PrP replaced Gps, but the small size of the mouse limits some investigations. To investigate the fidelity of strain-specific prion transmission to Gps, we inoculated “type 1” and “type 2” prion strains into Gps: we measured the incubation times and determined the strain-specified size of the unglycosylated, protease-resistant (r) PrPSc fragment. Prions passaged once in Gps from cases of sporadic (s) Creutzfeldt–Jakob disease (CJD) and Gerstmann-Sträussler-Scheinker (GSS) disease caused by the P102L mutation were used as well as human prions from a variant (v) CJD case, bovine prions from bovine spongiform encephalopathy (BSE), and mouse-passaged scrapie prions. Variant CJD and BSE prions transmitted to all the inoculated Gps with incubation times of 367 ± 4 d and 436 ± 28 d, respectively. On second passage in Gps, vCJD and BSE prions caused disease in 287 ± 4 d and 310 ± 4 d, while sCJD and GSS prions transmitted in 237 ± 4 d and 279 ± 19 d, respectively. Although hamster Sc237 prions transmitted to 2 of 3 Gps after 574 and 792 d, mouse-passaged RML and 301V prion strains, the latter derived from BSE prions, failed to transmit disease to Gps. Those Gps inoculated with vCJD or BSE prions exhibited “type 2” unglycosylated, rPrPSc (19 kDa) while those receiving sCJD or GSS prions displayed “type 1” prions (21 kDa), as determined by Western blotting. Such strain-specific properties were maintained in Gps as well as mice expressing a chimeric human/mouse transgene. Gps may prove particularly useful in further studies of novel human prions such as those causing vCJD.
Keywords: BSE, vCJD, sCJD, GSS, prions, guinea pig
In the course of studies designed to develop sensitive assays for prions causing Creutzfeldt-Jakob disease (CJD) of humans, we asked if novel strains of human prions might be transmitted to large rodents such as guinea pigs (Gps, Cavia porcellus). Gps were shown previously to be susceptible to prions causing sporadic (s) CJD (1-3) and Gerstmann-Sträussler-Scheinker disease (GSS) (4).
After the initial reports of the transmission of sCJD to monkeys and apes, Gps were the first rodent to be shown to be susceptible to human prions. In 1975, two Gps were reported to have succumbed to an acute, rapidly progressively neurological disease at 422 and 512 days after combined intracerebral (ic) and intraperitoneal (ip) inoculation (2). An inoculum was prepared from the brain of one of the Gps and injected into 10 Gps, all of which developed disease with a mean incubation time of 216 d. On third passage, the mean incubation time for 27 Gps was 231 d (5).
The biological diversity of prions is among their most remarkable features; this diversity is enciphered by PrPSc, the sole component of the prion. Strains of prions were initially identified in sheep with scrapie and later studied extensively in mice (6-8). For many years, the existence of prion strains was the most convincing argument that prions had to contain at least a short, specific sequence of nucleic acid; yet, none was ever found. Whether the auxiliary effect of RNA on prion formation observed in vitro has an analogous result in vivo is currently debated (9, 10). Recent studies showed that numerous strains of prions can be prepared without RNA and polyanions, and that modifying the stability of the amyloid polymers in the inocula gave rise to different synthetic prion strains (11-14). Additionally, modifying amyloid fibrils of PrP resulted in changes to the protease-resistant core of the protein (15).
The identification of “atypical” BSE prions in Japan, the U.S., and Europe has enlarged the spectrum of BSE strains and forced a reconsideration of the issues on the origin of the British BSE epidemic (16-20). A report of a BSE-like strain in goats suggested that BSE transmission to other ungulates might not be a mere theoretical possibility (21); furthermore, the identification of a genetic case of BSE (22) demonstrates that the full extent of the BSE epidemic is still unknown.
The biological characteristics and host range of prion strains are determined by the conformation of PrPSc in the inoculum and the sequence of PrPC of the host (11, 23-27). Studies with Tg mice (28-33) established that sequence similarity between host PrPC and inoculum PrPSc is an important factor in modulating susceptibility to infection. However, studies on ungulate and human prions demonstrated that some prion strains may elude the transmission barrier imposed by sequence differences (34, 35). In cases of chimeric or mutated transgenes, susceptibility to prion infection may depend largely on the strain-specific conformation of PrPSc in the inoculum (34, 36, 37).
Although studies of genetically engineered mice have provided a wealth of information about prions, the small size of these animals limits studies of prions in blood, and brain imaging. To circumvent these problems, we examined Gps, which have blood volumes of 35–50 ml (38) and relatively large brains. To investigate the range of prion susceptibility, we inoculated Gps with variant (v) CJD, BSE, sCJD, GSS(P102L) and several rodent prion strains. Here, we report that vCJD and BSE prions transmitted efficiently to Gps, with incubation times of 350–450 days. Gps inoculated with BSE and vCJD prions showed similar patterns of spongiform degeneration in their brains that were distinct from those seen with inocula from patients who died of sCJD or the inherited disorder GSS(P102L). Our findings argue that prion transmission barriers are determined by a complex set of parameters involving species differences in PrP sequences as well as the conformation of PrPSc that enciphers strain-specific traits. Gps may prove to be a valuable model for the study of prion diversity.
MATERIALS AND METHODS
Ethics statement
All studies presented here were reviewed and approved by the UCSF Institutional Animal Care and Use Committee.
Guinea pigs and transgenic mice
Weanling Hartley guinea pigs (strain code 051) were purchased from Charles River Laboratories, Inc. (Wilmington, MA). Transgenic (Tg) mice expressing HuPrP and chimeric MHu2M(M111V,M165V,E167Q) on the PrP-knockout background, respectively denoted Tg(HuPrP)440/Prnp0/0 and Tg(MHu2M,M111V,M165V,E167Q)1014/Prnp0/0 mice, have been previously reported (31, 39).
Prion isolates and transmission studies
The brain sample of a vCJD case, which was diagnosed by immunohistochemistry and Western immunoblotting, was kindly provided by Robert Will and James Ironside, National CJD Surveillance Unit, University of Edinburgh, UK. BSE isolate BBP12/92 was obtained from the Central Veterinary Laboratory, Weybridge, UK, and titrated in different laboratories by bioassays in cows, wt and Tg mice (40, 41). The titer of BSE prions obtained by endpoint titration in Tg mice expressing bovine PrP was 7.1–7.7 log ID50 units/g (40). Both sCJD and GSS prions, passaged first in Gps, were kindly provided by Jun Tateishi, Neurological Institute, Kyushu University, Fukuoka, Japan. sCJD prions were originally isolated from a 52-year-old female patient; GSS prions were originally isolated from a 61-year-old male carrying the PrP(P102L) mutation. The Sc237 strain was originally obtained form Richard Marsh (42) and was passaged repeatedly in golden Syrian hamsters (LVG:Lak, Charles River Laboratories); Sc237 appears to be indistinguishable from strain 263K (43). The RML prion strain was derived from the Chandler isolate (44) passaged in CD-1 mice. The 301V prion strain, originally isolated from a cow infected with BSE, was obtained from H. Fraser (45) and maintained by serial passaging in B6.I mice (46).
Brain homogenates for bioassay were prepared in PBS, pH 7.4, by three 75-s cycles in a reciprocal homogenizer Mini-BeadBeater-8 (BioSpec Products, Inc., OH). Weanling Gps were inoculated with 100 μl of a 1% (wt/vol) homogenate in sterile PBS without Ca2+ or Mg2+ by using a 27-gauge, disposable hypodermic needle inserted into the right parietal lobe. Following inoculation, the status of the Gps was monitored daily while the neurological status was assessed three times per week. The clinical criteria used for diagnosis of scrapie in mice and hamsters as previously described (28, 47, 48) were also used in scoring Gps.
Brain sample preparation
Brainstem tissue slices or brain macerates weighing 250 to 350 mg were homogenized to a final 15% (wt/vol) in 4% (wt/vol) Sarkosyl in PBS, pH 7.4, by three 75-s cycles in a reciprocal homogenizer Mini-BeadBeater-8 (BioSpec Products, Inc., OH). The resulting homogenate was diluted to a final 5% (wt/vol) using PBS containing 4% (wt/vol) Sarkosyl and 5 μg/ml of proteinase K (PK; unless stated otherwise) and incubated for 60 min at 37 °C on the shaker. After a clarification spin at 500 × g for 5 min at room temperature (RT) in a drum rotor (Jouan, Winchester, VA), the samples were mixed with stock solution containing 4% sodium phosphotungstate (NaPTA) and 170 mM MgCl2, pH 7.4, to obtain a final concentration of 0.32% NaPTA. After a 1-h incubation at 37 °C on a rocking platform, the samples were centrifuged at 14,000 × g in a Jouan MR23i centrifuge for 15 to 30 min at RT. The resulting pellets were resuspended in 83 μl of H2O containing protease inhibitors [0.5 mM phenylmethylsulfonyl fluoride (PMSF); aprotinin and leupeptin, 2 μg/ml each] and assayed by Western blotting or the conformation-dependent immunoassay (CDI).
Ten-percent (wt/vol) homogenates from Tg mouse brains were prepared in PBS using a Precellys 24 bead-beater (MO BIO, Carlsbad, CA) with 2 cycles of 75 sec each at speed 6200, with a 5-min interval between runs. Samples were then treated with 100 μg/ml PK for 60 min at 37 °C, and digestion was terminated by the addition of 2 mM PMSF.
Muscle and tongue tissue preparation
For biochemical analysis only, slices from different Gp muscles or cuts of tongue weighing 250 to 350 mg were homogenized to a final 15% (wt/vol) in 4% (wt/vol) Sarkosyl in PBS, pH 7.4, containing 3 mM CaCl2 and 5 mM MgCl2, by one 75-s cycle in a reciprocal homogenizer Mini-BeadBeater-8 (BioSpec Products, Inc., OH), as described (49, 50). Resulting muscle homogenates were incubated on a rotating wheel for 3 h at 37 °C with 1 mg/ml of Collagenase type 4 (Worthington Biochemical, Lakewood, NJ), rehomogenized by six 75-s cycles, and precipitated for 1 h at 37 °C with 1.28% (wt/vol) NaPTA, as described (26, 50). After a 30-min centrifugation at 14,000 × g, the resulting pellets were resuspended in 83 μl of PBS, pH 7.4, containing a protease-inhibitor cocktail (0.5 mM PMSF and 2 μg/ml each of aprotinin and leupeptin). Tissues were then assayed by Western blotting.
Western blots
The PTA pellets precipitated from the tissue homogenates were mixed with an equal volume of SDS-loading buffer and boiled for 5 min; 30-μl samples were loaded on two 12.5% Tris-glycine SDS-PAGE Novex gels (Invitrogen, Carlsbad, CA). Each Western blot was incubated with 0.75 μg/ml of mAb 3F4 (51) for 2 h at RT. After extensive washing, the blots were developed with peroxidase-labeled, anti-mouse Fc secondary antibody and by the enhanced chemiluminescence (ECL) system, as described (36). Samples that had not been precipitated with PTA were run on precast 10% NuPAGE Novex Bis-Tris gels, and transferred using the iBlot Dry Blotting system, as previously described (39).
Histopathology
Brain tissue was either immediately frozen or immersion-fixed in 10% buffered formalin for embedding in paraffin. Eight-micrometer-thick sections were stained with hematoxylin and eosin (H&E) to evaluate vacuolation. Evaluation of reactive astrocytic gliosis was performed by GFAP immunostaining using a rabbit antiserum (Dako, Carpinteria, CA). Hydrolytic autoclaving pretreatment of the formalin-fixed tissue sections and mAb 3F4 were used to detect PrPSc, as described previously (52)
RESULTS
Primary structure of GpPrP
When we initiated transmission studies of human prions into Gps, only two fragmentary sequences of GpPrP had been deposited in the GenBank database (Accession numbers AY133039 and AF139166). Therefore, we sequenced DNA extracted from the blood of Hartley guinea pigs (strain code 051, Charles River Laboratories, Wilmington, MA) (Figure 1). Subsequently, an entire GpPrP sequence was published (53), which differed from the Hartley Gp at residue 62. Premzl et al. reported a serine encoded at position 62, while we found a glycine. This polymorphism was also observed in the two deposited fragmentary sequences: GenBank AY133039 reported serine 62 and AF139166 had glycine 62. From these sequencing data, the Gp was placed in the mammalian PrP gene tree next to the rabbit and pika (54).
Figure 1.
Sequence alignment of mature guinea pig (Gp) PrP compared to those of mouse (Mo), Syrian hamster (SHa), human (Hu), and bovine (Bo) PrP; only residues that differ from GpPrP are shown. N- and C-terminal signal sequence cleavage sites were predicted using SignalP (74) and big-PI Predictor (75), respectively. Predictions were in agreement with experimentally determined signal peptide cleavage positions. Residue numbers are given for each sequence from the N-terminus of the immature protein. Asterisks indicate residues important for the transmission of human prions to mice; polymorphic residues shown by boxes.
Additionally, GpPrP lacks 11 residues in the N-terminal region preceding the octapeptide repeats. Within the central PrP region between residues 96–167 [mouse (Mo) numbering], which has been proposed to play a major role in determining the species barrier (55), GpPrP differs from human (Hu) PrP and bovine (Bo) PrP at 6 and 4 positions, respectively. The polymorphic human codon 129 most commonly expresses methionine, as do GpPrP and BoPrP at their corresponding residue numbers.
From studies of Hu/Mo chimeric PrP genes, we learned that six human residues between positions 96 and 167 are critical for the transmission of human prions in Tg mice (37, 39). Comparing these and surrounding residues in Gp, Mo and Hu PrP, we found that five of six critical residues that feature in transmission of human prions to mice are either identical or quite similar in Gp and Hu PrP (Figure 1). At residues 108, 142 and 144, the amino acids are identical in GpPrP and HuPrP; at residue 137, the amino acid is quite similar, with leucine encoded in GpPrP and an isoleucine in HuPrP. The two PrPs are also similar at residue 154, where the aromatic amino acid tyrosine is encoded in GpPrP and another aromatic residue histidine is found in HuPrP.
Transmission of prions to Gps
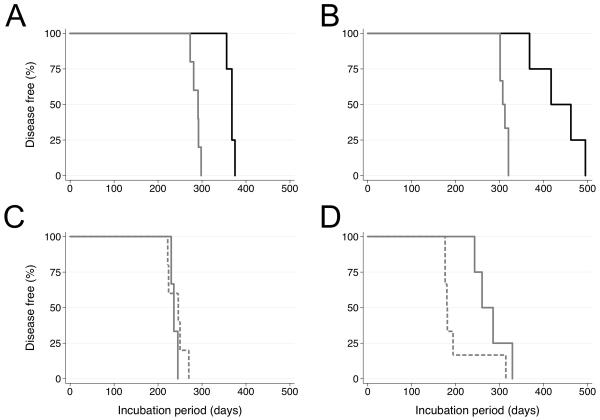
To determine the susceptibility of Gps to prion isolates from different species, we inoculated Gps intracerebrally with human vCJD and bovine BSE prions as well as sCJD and GSS prions already passaged once in Gps; in addition, Syrian hamster (SHa) Sc237 prions as well as RML and 301V mouse prions were injected into Gps (Table 1). The 301V strain was isolated in Prnpb/b mice by passaging of BSE prions (56). Variant CJD and BSE inocula were prepared as 1% homogenates from the brain tissue from an autopsied vCJD patient and from the brainstem of a BSE-infected cow, respectively. Variant CJD and BSE prions transmitted to Gps with 100% efficiency, with mean incubation times of 367 and 436 days, respectively. Upon second passage to Gps, the incubation periods for vCJD and BSE prions shortened to 287 days and 310 days, respectively (Figure 2). Similar incubation times were observed upon second and third passages of sCJD and GSS prions to Gps. In contrast, SHa Sc237 prions transmitted to 2 of 3 Gps, with incubation times of 574 and 792 days. Neither RML nor 301V mouse prions transmitted to Gps within the 850-day observation period. From these findings, we concluded that Gps are more susceptible to vCJD and BSE prions than either sCJD or GSS(P102L) prions on primary transmission. After passage in Gps, these different prion inocula gave similar incubation periods.
Table 1.
Transmission of prions to guinea pigs.
| Inoculum | Mean incubation time ± SEM (days) |
n/n 0 a |
|---|---|---|
| vCJD | 367 ± 4 | 4/4 |
| vCJD > Gp (2nd passage) | 287 ± 4 | 5/5 |
| BSE | 436 ± 28 | 4/4 |
| BSE > Gp (2nd passage) | 310 ± 4 | 6/6 |
| sCJD | — | 5/12b |
| sCJD > Gp (2nd passage) | 237 ± 4 | 3/3 |
| sCJD > Gp > Gp (3rd passage) | 242 ± 9 | 5/5 |
| GSS | — | 1/23c |
| GSS > Gp (2nd passage) | 279 ± 19 | 4/4 |
| GSS > Gp > Gp (3rd passage) | 204 ± 22 | 6/6 |
| Sc237 | 683 ± 109 | 2/3d |
| RML | >850 | 0/3 |
| 301V | >850 | 0/4 |
n, number of ill Gps; n0, number of Gps under observation.
Aggegate data from multiple studies. The five ill animals developed signs of neurologic dysfunction between 381 and 652 days after inoculation (1-3).
Each of four cases of GSS was inoculated in 4–9 guinea pigs. Only 1 animal developed illness, but the incubation period was not reported (4).
Two Gps became ill at 574 and 792 dpi; the third Gp showed no signs of disease at 850 days postinoculation, when the experiment was terminated.
Figure 2.
Serial passage of (A) vCJD, (B) BSE, (C) sCJD and (D) GSS prions in Gps. Weanling Gps were inoculated with 100 μl of a 1% (wt/vol) homogenate in sterile PBS without Ca2+ or Mg2+ by using a 27-gauge, disposable hypodermic needle inserted into the right parietal lobe. Following inoculation, the status of the Gps was monitored daily, while the neurological status was assessed three times per week. Kaplan-Meier survival curves were drawn from first (solid black), second (solid gray), and third (dashed gray) passages.
Evidence for propagation of different prion strains in Gps
After passage in Gps, human vCJD, sCJD and GSS(P102L) prions as well as bovine BSE prions were analyzed by Western immunoblotting. Passage of vCJD and BSE prions in Gps produced type 2 rPrPSc as demonstrated by the ~19-kDa, unglycosylated PrP band (Figure 3, lanes 2 and 4). In contrast, passage of sCJD and GSS in Gps yielded type 1 rPrPSc as shown by the ~21-kDa, unglycosylated PrP bands (Figure 3, lanes 6 and 8).
Figure 3.
Human and bovine prions passaged in Gps retained their strain type. Western blot of Gp brain homogenate before (odd lanes) or after (even lanes) treatment with 10 μg/ml of PK for 1 h at 37 °C. First passage of vCJD (lanes 1 and 2) and BSE (lanes 3 and 4) prions in Gps resulted in type 2 strains characterized by a ~19-kDa unglycosylated band. In contrast, second passage of sCJD (lanes 5 and 6) and GSS (lanes 7 and 8) prions in Gps resulted in type 1 strains, characterized by ~21-kDa unglycosylated bands. Apparent molecular masses based on protein standards are shown in kilodaltons.
Neuropathology of prion disease in Gps
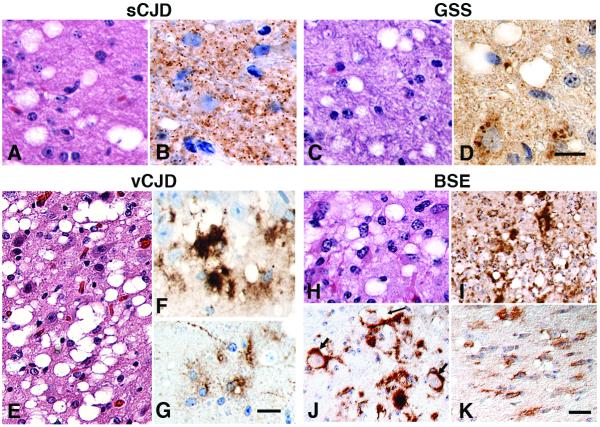
Neuropathologic analysis of brains from prion-infected Gps demonstrated that Gps replicate many of the neuropathologic features of humans with sCJD, GSS, and vCJD as well as those found in cattle with BSE. In the brains of Gps inoculated with Gp-passaged sCJD prions, vacuolation and finely granular PrPSc deposition were seen, similar to humans with sCJD (Figure 4A and B). Inoculation with Gp-passaged GSS(P102L) prions resulted in delicate gray matter vacuolation and granular PrPSc deposits in Gp brain (Figure 4C and D), a neuropathologic phenotype resembling that of CJD, as previously observed when GSS prions were passaged to non-human primates (57). Transmission of vCJD to Gps produced a phenotype similar to vCJD in humans, with large multilobulated vacuoles (Figure 4E) and large, coarse PrPSc deposits (Figure 4F). Additionally, PrP immunostaining of astrocytes, unique to vCJD and BSE infection, were found in Gps inoculated with vCJD and BSE prions (Figure 4G and K). In Gps inoculated intracerebrally with BSE prions, large clusters of vacuoles were found (Figure 4H). Both finely granular and large, coarse PrPSc deposits were found in the neocortex, hippocampus, diencephalon and brainstem (Figure 4I). Similar to BSE in cattle (58, 59), PrPSc accumulated in the cell bodies of neurons in the caudal pons and medulla in BSE-infected Gps (Figure 4J). Neuropathologic changes in Gps infected with BSE prions were severe in multiple cerebral hemisphere and brainstem regions, which contrasts with cattle orally infected with BSE prions, where neuropathologic changes are generally concentrated in the brainstem. Differences in the anatomical distribution of PrPSc might be due to different ports of entry of BSE prions (60).
Figure 4.
Transmission of human-derived sCJD (A, B), GSS (C, D) and vCJD (E–G) prions, and cattle-derived BSE prions (H–K) to Gps accurately reproduces the vacuolation and PrPSc deposition phenotypes observed in humans and cattle. Brain sections stained with H&E show vacuolation from all inocula (A, C, E, H). GSS (C) and BSE (H) prions resulted in vacuolation profiles similar to that from sCJD prions (A). Vacuoles from the vCJD inoculum were large and multiloculated (E). Immunohistochemistry using the 3F4 antibody revealed that PrPSc deposits were finely granular for sCJD (B); both fine (3–6 μm) and large (30–80 μm) for BSE prions (I); and coarse for vCJD prions (F); the coarse PrPSc deposits did not bind thioflavin S, indicating they were not mature amyloid plaques (data not shown). Following BSE inoculation, many neuronal cytoplasmic PrPSc inclusions (short arrows) and intraneuronal vacuoles (long arrow) (J) were seen in the reticular formation of the pons. Astrocytes were immunopositive for PrPSc in the pontine white matter after BSE transmission (K) and in the white matter after vCJD inoculation (G). Bar in D represents 20 μm and also applies to B. Bar in G represents 20 μm and also applies to A, C, F, H. Bar in K represents 40 μm and also applies to E, I, J.
Transmission of Gp-passaged prions to transgenic mice
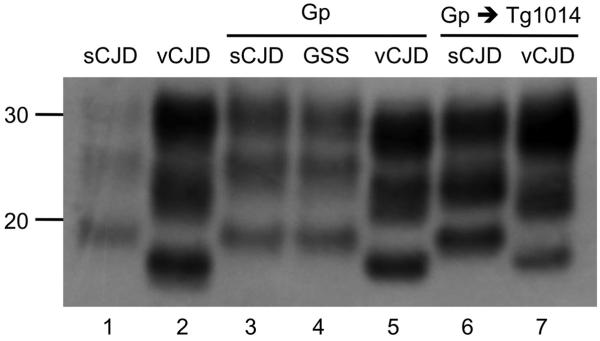
Western blots of samples from Gp brains were analyzed to determine the electrophoretic mobility of protease-resistant PrPSc fragments (PrP 27-30). As shown in Figure 5 (lanes 3–5), the unglycosylated rPrPSc fragment from Gp brains inoculated with Gp-passaged sCJD and GSS prions migrated at a higher molecular weight (type 1), and that from Gp brains inoculated with vCJD prions migrated at a lower molecular weight (type 2). These electrophoretic mobilities mirrored those found in human brains (Figure 5, lanes 1 and 2).
Figure 5.
Western blot shows that the strain type of human prions was retained upon passage to Gps and transgenic mice. vCJD and sCJD prions before (lanes 1 and 2) and after transmission to Gps (lanes 3 and 5); homogenates from ill Gps were then transmitted to Tg(MHu2M,M111V,M165V,E167Q)1014 mice (lanes 6 and 7). Human GSS prions were also transmitted to Gps (lane 3). Type 1 PrPSc from sCJD and type 2 PrPSc from vCJD were maintained upon passage to Gps and Tg mice. Apparent molecular masses based on migration of protein standards are shown in kilodaltons.
To determine whether the strain-specific characteristics of human prions were retained after passage in Gps, we inoculated samples into Tg(HuPrP)440/Prnp0/0 and chimeric Tg(MHu2M,M111V,M165V,E167Q)1014/Prnp0/0 mice. In both Tg lines, incubation periods for Gp-passaged sCJD prions were increased by ~50% (Table 2). The type 1 rPrPSc phenotype (21-kDa PrP band) found in the human sCJD brain and after passage in Gps was also observed after passage in Tg1014 mice (Figure 5). Although vCJD prions transmitted to Tg1014 mice, they did not produce disease in the Tg440 mice. Similar to sCJD prions passaged in Gps and then to Tg1014 mice, vCJD prions passaged in Gps also produced longer incubation periods in Tg1014 mice (Table 2). As shown in Figure 5, the type 2 rPrPSc phenotype (19-kDa PrP band) found in the human vCJD brain and after passage in Gps was also observed after serial transmission to Tg1014 mice. Moreover, while vCJD produced both type 1 and type 2 rPrPSc in Tg1014 mice depending on the incubation period (39), 7 of 7 Tg1014 mice inoculated with Gp-passaged vCJD exhibited type 2 rPrPSc.
Table 2.
Transmission of Gp-passaged sCJD and vCJD prions to Tg(MHu2M,M111V,M165V,E167Q)1014/Prnp0/0 and Tg(HuPrP)440/Prnp0/0 mice.
First passage of GSS(P102L) prions in Gps produced the type 1 unglycosylated rPrPSc phenotype (21-kDa PrP band), which was found in ~70% of GSS cases (Figures 3 and 5) (61). Unfortunately, the original human GSS prion sample used in primary passage to Gps was not available for direct comparison (4). From the results of the foregoing studies, we concluded that strain-specified conformations of human prions were maintained during passage in Gps.
Relative PrPSc levels in brain, skeletal muscle, and tongue of Gps
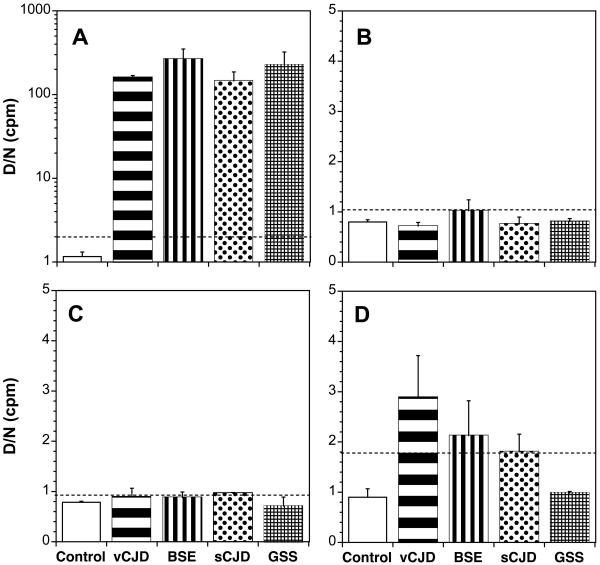
Using Western immunoblotting and the CDI, we determined the relative PrPSc levels in the brains, skeletal muscles and tongues of Gps inoculated with human vCJD prions and BSE prions as well as sCJD and GSS(P102L) prions passaged in Gps. In the brains of Gps displaying neurological dysfunction, similar levels of PrPSc were found regardless of the inoculum (Figures 3 and 5). No PrPSc was found in the skeletal muscles of Gps infected with vCJD, BSE, or Gp-passaged sCJD or GSS prions as determined by CDI (Figure 6). Western blotting was inconclusive with traces of PrPSc-like signals sometimes seen in skeletal muscles (data not shown).
Figure 6.
CDI measurements of PrPSc in (A) brains, (B) forelimb muscles (C) hindlimb muscles and (D) tongues of Gps after first passage of vCJD and BSE prions or second passage of sCJD and GSS prions. Gps were inoculated intracerebrally with 1% brain homogenates prepared from human brain infected with vCJD prions, bovine brain with BSE, or Gp brain infected with either sCJD or GSS(P102L) prions. As a control, tissues from an age-matched, noninfected Gp were analyzed. The concentration of PrPSc was measured using recFab HuM D18 to capture and Eu-labeled mAb 3F4 to detect GpPrP. Muscle homogenates were incubated with 1 mg/ml of collagenase for 3 h at 37 °C. Samples were subjected to limited digestion with 10 μg/ml of PK for 1 h at 37 °C, then precipitated with PTA. The dashed lines indicate the upper normal limit (cut-off values) calculated as [mean + (3*S.D.)] from tissues taken from age-matched, control Gps. The results obtained with samples taken from three Gps are expressed as the mean ± S.D.; each sample was tested two or four times by the CDI. The concentration of PrPSc is proportional to the (D/N) value, measured as the time-resolved fluorescence in counts per minute (cpm) from denatured (D) and native (N) samples (26, 49); values exceeding the cut-off indicate the presence of PrPSc.
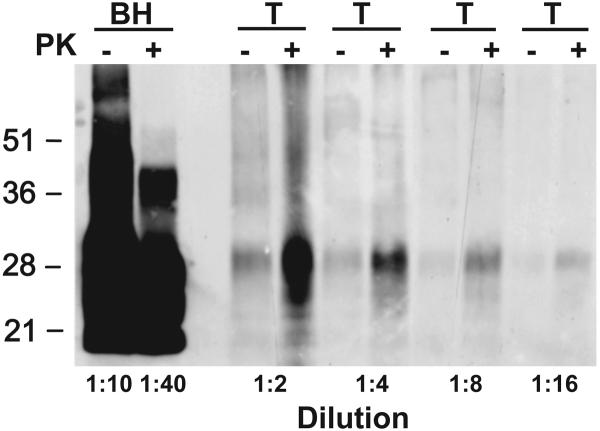
In the tongues of terminal Gps, those inoculated with either vCJD or BSE prions gave low CDI signals that were above background; the respective CDI values were 140-fold and 220-fold lower than those in brain (Figure 6, compare panels D and A). To determine if the low CDI signals in tongue were PrPSc, we performed Western blotting. As shown in Figure 7, no shift in the molecular size of PrPSc after limited PK digestion was found, arguing that bioassays will need to be performed in order to determine the level, if any, of prions in Gp tongue.
Figure 7.
Western immunoblotting of tongue (T) from a BSE prion-infected Gp. PrPSc in brain homogenate (BH) of a BSE-infected Gp is shown for comparison. Brain and tongue tissues were serially diluted with SDS sample buffer at the indicated ratios to allow the same film exposure. Samples were either subjected to limited digestion with PK (+) or left undigested (−), then precipitated with PTA as described in Figure 6. PTA pellets were resuspended in 150 μl of 2× sodium dodecyl sulfate (SDS) sample buffer (76). Equal volumes of undigested and digested samples were boiled for 5 min prior to electrophoresis. SDS gel electrophoresis and Western blotting were performed as previously described (77). PrP was detected with the mAb 3F4 and developed with the enhanced chemiluminescent detection system (Amersham Biosciences). Apparent molecular masses based on migration of protein standards are shown in kilodaltons.
DISCUSSION
Maintenance of prion strain characteristics upon transmission of human prions to guinea pigs
The susceptibility of Gps to vCJD prions was unexpected due to the poor transmission of human prions to most rodents. Initial attempts to transmit kuru and CJD prions to guinea pigs were unsuccessful (1). Subsequent studies succeeded in infecting two guinea pigs, 422 and 512 days after inoculation with a biopsy sample from a patient who later died of CJD (2). Two additional cases of CJD were inefficiently transmitted to Gps, with long incubation periods: for case one, 1 of 4 animals died at 652 d and for case two, 2 of 4 animals succumbed at 381 and 382 d (3). However, it is notable that these transmission studies were performed before PrPSc was discovered and human prion strains were appreciated: the genotype and strain type of these inocula are unknown, making it difficult to relate the results to our findings. Transmission of GSS(P102L) prions to Gps was even less efficient than CJD, with only 1 of 23 animals, inoculated with 1 of 4 GSS cases, succumbing to disease (4). Notably, the GSS phenotype and molecular characteristics of PrPSc vary considerably; to date, transmitted GSS cases harbor the P102L mutation and type 1 PrPSc (62-64). Here, we report that human vCJD prions transmitted to Gps approximately 350 days after inoculation. Upon serial passage, vCJD prions had similar incubation periods to sCJD and GSS prions, of approximately 250 d (Figure 2). We attempted to transmit two different strains of mouse prions to Gps, but were not successful (Table 1).
Western immunoblotting showed that the molecular size of the unglycosylated PrP 27-30 fragment after limited proteolysis was maintained upon passage of sCJD, GSS and vCJD prions through Gps (Figures 3 and 5). Although the Gp-passaged CJD upon transmission to Tg1014 mice mirrored the glycosylation and type 1 PrPSc pattern observed with the primary inoculum of type 1 sCJD, neither the full PRNP sequences nor Western blots of the original GSS and sCJD cases were available for comparison because the neuropathological diagnosis was performed in Japan 30 years ago. Modern neuropathological classification of sCJD and GSS based on full sequencing of the PRNP gene, immunohistochemistry for PrPSc, and patterns of PrPSc on high-resolution Western blots evolved gradually over the past ~10 years (65). Since the original human tissues were not available for these analyses and re-isolation, these proof-of-principle experiments will have to be refined with other cases of GSS and sCJD characterized by these modern methods. In addition, many features of human PrPSc immunostaining and neuropil vacuolation were maintained on passage through Gps (Figure 4). Gp-passaged human prions also had similar transmission characteristics to the original strains (Tables 1 and 2, Figure 5). All Tg1014 mice inoculated with Gp-passaged vCJD had a type 2 strain analogous to vCJD, in contrast to a mixture of type 1 and type 2 strains, which was observed after inoculation of vCJD in Tg1014 mice (39). These observations suggest that the GpPrP sequence may stablilize the type 2 conformation.
GpPrP sequence
The degree of identity among the Gp, Hu and Bo PrP sequences within residues 96 and 154, which are thought to be critical for the transmission of human prions to Tg mice, is strikingly similar (Figure 1). In studies of mice expressing chimeric Mo/Hu PrP transgenes, we found that the incubation times for transmission of sCJD prions could be reduced from over 200 to ~110 days by reverting Hu residues 165 and 167 to Mo (37); we designated those mice as Tg(MHu2M,M165V,E167Q) mice. In recent studies, we found that reverting residue 111 in Tg(MHu2M,M111V,M165V,E167Q) mice shortened the incubation period for human prions to ~80 days (39). Residue 111 is methionine in both Gp and Hu PrP, but valine in Mo and Bo PrP.
Inocula prepared from the brains of one vCJD and one BSE case showed 100% transmission rates and mean incubation times of 367 to 436 days, respectively. On second passage, the incubation time decreased to 287 days for vCJD prions and to 310 days for BSE prions (Figure 2). This shortening of the incubation time suggests some species-barrier effect probably due to the differences in the PrP sequences.
Interestingly, Gps were resistant to two different strains of mouse prions, RML and 301V (Table 1). The RML strain was propagated in Prnpa/a mice that encode leucine at residue 108, and 301V prions were replicated in Prnpb/b mice that specify phenylalanine at this position. In contrast, Gp, Bo and Hu PrP express methionine at residue 108. Whether the poor transmission of mouse prions to Gps can be ascribed to residue 108 remains to be determined. Since BSE prions transmitted efficiently to Gps, a finding that has been independently confirmed by another group (66), the lack of 301V prion transmission underscores the argument that this strain is a poor model for BSE (67). Of note, SHaPrP also expresses methionine at position 108 (Figure 1), but SHa prions transmitted to 2 of 3 Gps after longer incubation periods; however, SHaPrP differs from Mo, Gp, Bo and Hu PrP at many other positions.
Prion disease neuropathology in Gps
Neuropathological analysis demonstrated that the human prion strains maintained many, but not all, of their characteristics after passage in Gps (Figure 4). The fine granular staining of PrPSc deposits observed in Gps inoculated with sCJD is not exclusively specific for MM1 or MV1 sCJD but is also present in some other prion diseases (65). Frank amyloid plaques that are characteristic of human vCJD and GSS were absent in infected Gps, as determined by thioflavin S staining. However, large PrPSc deposits, reminiscent of plaques, were found (Figure 4). Whether GpPrPSc is less amyloidogenic than HuPrPSc remains to be determined (68). Both the neuropathology and progressive shortening of the incubation time indicate an adaption process, in which some of the phenotypic characteristics of the original inocula changed.
Prions in the tongue
Because of several reports of prions in the tongues of experimentally infected animals (69-73), we measured the levels of PrPSc in the tongues of Gps. Although PrPSc was readily detectable in the brains of infected Gps by Western blotting, the protease-resistant PrP signal in tongue was ambiguous because a shift in the molecular size of PrPSc was not observed (Figure 3). One possibility is the extended collagenase digestion used to liberate PrP from skeletal muscle also cleaved the N-terminal residues of GpPrPSc prior to limited proteolysis with PK (Figure 7). Detection of prions in tongue by bioassays would support this suggestion.
Concluding remarks
Our findings suggest that Gps may be a useful model for the study of some aspects of human prion diseases. Unexpectedly, vCJD and BSE prions transmitted efficiently to Gps in contrast to earlier primary transmission studies of sCJD and GSS prions. The large deposits of PrPSc surrounded by spongiform degeneration in the brains of Gps infected with vCJD prions were reminiscent of the neuropathological changes seen in the brains of vCJD patients. Currently, Gps probably provide the best animal model for studies of vCJD; whether Gps have detectable levels of prions in their lymphoid tissues requires further investigation.
The susceptibility of Gps to human prions is particularly interesting in view of the development of Tg(MHu2M,M111V,M165V,E167Q) mice that express a chimeric Hu/Mo PrP transgene and have an incubation period for human sCJD prions of approximately 80 days (39). Certainly, the creation of Tg(GpPrP) mice as well as mice expressing various chimeric Gp/Mo PrP transgenes will be of interest. Whether Tg mice expressing GpPrP or some chimeric construct will exhibit incubation times more abbreviated than those found in Tg(MHu2M,M111V,M165V,E167Q) mice inoculated with sCJD(MM1) prions remains to be determined.
ACKNOWLEDGMENTS
We are grateful to Robert Will and James Ironside (National CJD Surveillance Unit, University of Edinburgh, UK) for brain samples of the vCJD patient, and to Jun Tateishi (Neurological Institute, Kyushu University, Fukuoka, Japan) for brain samples from the first passage of sCJD and GSS prions to Gps. This work was supported by a contract (NS02328) and by grants (AG02132, AG010770, and AG021601) from the National Institutes of Health.
Abbreviations
- Bo
bovine
- BSE
bovine spongiform encephalopathy
- CJD
Creutzfeldt-Jakob disease
- Gps
guinea pigs
- GSS
Gerstmann-Sträussler-Scheinker
- Hu
human
- ic
intracerebral
- ip
intraperitoneal
- Mo
mouse
- PrP
prion protein
- sCJD
sporadic Creutzfeldt-Jakob disease
- SHa
Syrian hamster
- Tg
transgenic
- vCJD
variant Creutzfeldt-Jakob disease
Footnotes
Conflict of interest: none declared.
REFERENCES
- 1.Gibbs CJ, Jr., Gajdusek DC. Experimental subacute spongiform virus encephalopathies in primates and other laboratory animals. Science. 1973;182:67–68. doi: 10.1126/science.182.4107.67. [DOI] [PubMed] [Google Scholar]
- 2.Manuelidis EE. Transmission of Creutzfeldt-Jakob disease from man to the guinea pig. Science. 1975;190:571–572. [PubMed] [Google Scholar]
- 3.Tateishi J, Sato Y, Koga M, Doi H, Ohta M. Experimental transmission of human subacute spongiform encephalopathy to small rodents. I. Clinical and histological observations. Acta Neuropathol (Berl) 1980;51:127–134. doi: 10.1007/BF00690454. [DOI] [PubMed] [Google Scholar]
- 4.Tateishi J, Kitamoto T, Hoque MZ, Furukawa H. Experimental transmission of Creutzfeldt-Jakob disease and related diseases to rodents. Neurology. 1996;46:532–537. doi: 10.1212/wnl.46.2.532. [DOI] [PubMed] [Google Scholar]
- 5.Manuelidis E, Kim J, Angelo J, Manuelidis L. Serial propagation of Creutzfeldt-Jakob disease in guinea pigs. Proc Natl Acad Sci USA. 1976;73:223–227. doi: 10.1073/pnas.73.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pattison IH, Millson GC. Scrapie produced experimentally in goats with special reference to the clinical syndrome. J Comp Pathol. 1961;71:101–108. doi: 10.1016/s0368-1742(61)80013-1. [DOI] [PubMed] [Google Scholar]
- 7.Pattison IH. The relative susceptibility of sheep, goats and mice to two types of the goat scrapie agent. Res Vet Sci. 1966;7:207–212. [PubMed] [Google Scholar]
- 8.Dickinson AG, Meikle VMH, Fraser H. Identification of a gene which controls the incubation period of some strains of scrapie agent in mice. J Comp Pathol. 1968;78:293–299. doi: 10.1016/0021-9975(68)90005-4. [DOI] [PubMed] [Google Scholar]
- 9.Deleault NR, Harris BT, Rees JR, Supattapone S. Formation of native prions from minimal components in vitro. Proc Natl Acad Sci USA. 2007;104:9741–9746. doi: 10.1073/pnas.0702662104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang F, Wang X, Yuan C-G, Ma J. Generating a prion with bacterially expressed recombinant prion protein. Science. 2010;327:1132–1135. doi: 10.1126/science.1183748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Legname G, Nguyen H-OB, Peretz D, Cohen FE, DeArmond SJ, Prusiner SB. Continuum of prion protein structures enciphers a multitude of prion isolate-specified phenotypes. Proc Natl Acad Sci USA. 2006;103:19105–19110. doi: 10.1073/pnas.0608970103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanaka M, Collins SR, Toyama BH, Weissman JS. The physical basis of how prion conformations determine strain phenotypes. Nature. 2006;442:585–589. doi: 10.1038/nature04922. [DOI] [PubMed] [Google Scholar]
- 13.Colby DW, Giles K, Legname G, Wille H, Baskakov IV, DeArmond SJ, et al. Design and construction of diverse mammalian prion strains. Proc Natl Acad Sci USA. 2009;106:20417–20422. doi: 10.1073/pnas.0910350106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colby DW, Wain R, Baskakov IV, Legname G, Palmer CG, Nguyen H-OB, et al. Protease-sensitive synthetic prions. PLoS Pathog. 2010;6:e1000736. doi: 10.1371/journal.ppat.1000736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bocharova OV, Makarava N, Breydo L, Anderson M, Salnikov VV, Baskakov IV. Annealing prion protein amyloid fibrils at high temperature results in extension of a proteinase K-resistant core. J Biol Chem. 2006;281:2373–2379. doi: 10.1074/jbc.M510840200. [DOI] [PubMed] [Google Scholar]
- 16.Buschmann A, Gretzschel A, Biacabe AG, Schiebel K, Corona C, Hoffmann C, et al. Atypical BSE in Germany--proof of transmissibility and biochemical characterization. Vet Microbiol. 2006;117:103–116. doi: 10.1016/j.vetmic.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 17.Biacabe AG, Laplanche JL, Ryder S, Baron T. Distinct molecular phenotypes in bovine prion diseases. EMBO Rep. 2004;5:110–115. doi: 10.1038/sj.embor.7400054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Casalone C, Zanusso G, Acutis P, Ferrari S, Capucci L, Tagliavini F, et al. Identification of a second bovine amyloidotic spongiform encephalopathy: Molecular similarities with sporadic Creutzfeldt-Jakob disease. Proc Natl Acad Sci USA. 2004;101:3065–3070. doi: 10.1073/pnas.0305777101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamakawa Y, Hagiwara K, Nohtomi K, Nakamura Y, Nishijima M, Higuchi Y, et al. Atypical proteinase K-resistant prion protein (PrPres) observed in an apparently healthy 23-month-old Holstein steer. Jpn J Infect Dis. 2003;56:221–222. [PubMed] [Google Scholar]
- 20.Richt JA, Kunkle RA, Alt D, Nicholson EM, Hamir AN, Czub S, et al. Identification and characterization of two bovine spongiform encephalopathy cases diagnosed in the United States. J Vet Diagn Invest. 2007;19:142–154. doi: 10.1177/104063870701900202. [DOI] [PubMed] [Google Scholar]
- 21.Eloit M, Adjou K, Coulpier M, Fontaine JJ, Hamel R, Lilin T, et al. BSE agent signatures in a goat. Vet Rec. 2005;156:523–534. doi: 10.1136/vr.156.16.523-b. [DOI] [PubMed] [Google Scholar]
- 22.Heaton MP, Keele JW, Harhay GP, Richt JA, Koohmaraie M, Wheeler TL, et al. Prevalence of the prion protein gene E211K variant in U.S. cattle. BMC Vet Res. 2008;4:25. doi: 10.1186/1746-6148-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bessen RA, Marsh RF. Distinct PrP properties suggest the molecular basis of strain variation in transmissible mink encephalopathy. J Virol. 1994;68:7859–7868. doi: 10.1128/jvi.68.12.7859-7868.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bessen RA, Kocisko DA, Raymond GJ, Nandan S, Lansbury PT, Caughey B. Non-genetic propagation of strain-specific properties of scrapie prion protein. Nature. 1995;375:698–700. doi: 10.1038/375698a0. [DOI] [PubMed] [Google Scholar]
- 25.Telling GC, Parchi P, DeArmond SJ, Cortelli P, Montagna P, Gabizon R, et al. Evidence for the conformation of the pathologic isoform of the prion protein enciphering and propagating prion diversity. Science. 1996;274:2079–2082. doi: 10.1126/science.274.5295.2079. [DOI] [PubMed] [Google Scholar]
- 26.Safar J, Wille H, Itri V, Groth D, Serban H, Torchia M, et al. Eight prion strains have PrPSc molecules with different conformations. Nat Med. 1998;4:1157–1165. doi: 10.1038/2654. [DOI] [PubMed] [Google Scholar]
- 27.Peretz D, Williamson RA, Legname G, Matsunaga Y, Vergara J, Burton D, et al. A change in the conformation of prions accompanies the emergence of a new prion strain. Neuron. 2002;34:921–932. doi: 10.1016/s0896-6273(02)00726-2. [DOI] [PubMed] [Google Scholar]
- 28.Scott M, Foster D, Mirenda C, Serban D, Coufal F, Wälchli M, et al. Transgenic mice expressing hamster prion protein produce species-specific scrapie infectivity and amyloid plaques. Cell. 1989;59:847–857. doi: 10.1016/0092-8674(89)90608-9. [DOI] [PubMed] [Google Scholar]
- 29.Prusiner SB, Scott M, Foster D, Pan K-M, Groth D, Mirenda C, et al. Transgenetic studies implicate interactions between homologous PrP isoforms in scrapie prion replication. Cell. 1990;63:673–686. doi: 10.1016/0092-8674(90)90134-z. [DOI] [PubMed] [Google Scholar]
- 30.Race RE, Priola SA, Bessen RA, Ernst D, Dockter J, Rall GF, et al. Neuron-specific expression of a hamster prion protein minigene in transgenic mice induces susceptibility to hamster scrapie agent. Neuron. 1995;15:1183–1191. doi: 10.1016/0896-6273(95)90105-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Telling GC, Scott M, Mastrianni J, Gabizon R, Torchia M, Cohen FE, et al. Prion propagation in mice expressing human and chimeric PrP transgenes implicates the interaction of cellular PrP with another protein. Cell. 1995;83:79–90. doi: 10.1016/0092-8674(95)90236-8. [DOI] [PubMed] [Google Scholar]
- 32.Manson JC, Jameison E, Baybutt H, Tuzi NL, Barron R, McConnell I, et al. A single amino acid alteration (101L) introduced into murine PrP dramatically alters incubation time of transmissible spongiform encephalopathy. EMBO J. 1999;18:6855–6864. doi: 10.1093/emboj/18.23.6855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barron RM, Thomson V, Jamieson E, Melton DW, Ironside J, Will R, et al. Changing a single amino acid in the N-terminus of murine PrP alters TSE incubation time across three species barriers. EMBO J. 2001;20:5070–5078. doi: 10.1093/emboj/20.18.5070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scott MR, Peretz D, Nguyen H-OB, DeArmond SJ, Prusiner SB. Transmission barriers for bovine, ovine, and human prions in transgenic mice. J Virol. 2005;79:5259–5271. doi: 10.1128/JVI.79.9.5259-5271.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Castilla J, Gonzalez-Romero D, Saa P, Morales R, De Castro J, Soto C. Crossing the species barrier by PrPSc replication in vitro generates unique infectious prions. Cell. 2008;134:757–768. doi: 10.1016/j.cell.2008.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tremblay P, Ball HL, Kaneko K, Groth D, Hegde RS, Cohen FE, et al. Mutant PrPSc conformers induced by a synthetic peptide and several prion strains. J Virol. 2004;78:2088–2099. doi: 10.1128/JVI.78.4.2088-2099.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Korth C, Kaneko K, Groth D, Heye N, Telling G, Mastrianni J, et al. Abbreviated incubation times for human prions in mice expressing a chimeric mouse—human prion protein transgene. Proc Natl Acad Sci USA. 2003;100:4784–4789. doi: 10.1073/pnas.2627989100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ancill RJ. The blood volume of the normal guinea-pig. J Physiol. 1956;132:469–475. doi: 10.1113/jphysiol.1956.sp005539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giles K, Glidden DV, Patel S, Korth C, Groth D, Lemus A, et al. Human prion strain selection in transgenic mice. Ann Neurol. 2010;68:151–161. doi: 10.1002/ana.22104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buschmann A, Groschup MH. Highly bovine spongiform encephalopathy-sensitive transgenic mice confirm the essential restriction of infectivity to the nervous system in clinically diseased cattle. J Infect Dis. 2005;192:934–942. doi: 10.1086/431602. [DOI] [PubMed] [Google Scholar]
- 41.Wells GA, Konold T, Arnold ME, Austin AR, Hawkins SA, Stack M, et al. Bovine spongiform encephalopathy: the effect of oral exposure dose on attack rate and incubation period in cattle. J Gen Virol. 2007;88:1363–1373. doi: 10.1099/vir.0.82421-0. [DOI] [PubMed] [Google Scholar]
- 42.Marsh RF, Kimberlin RH. Comparison of scrapie and transmissible mink encephalopathy in hamsters. II. Clinical signs, pathology, and pathogenesis. J Infect Dis. 1975;131:104–110. doi: 10.1093/infdis/131.2.104. [DOI] [PubMed] [Google Scholar]
- 43.Kimberlin R, Walker C. Characteristics of a short incubation model of scrapie in the golden hamster. J Gen Virol. 1977;34:295–304. doi: 10.1099/0022-1317-34-2-295. [DOI] [PubMed] [Google Scholar]
- 44.Chandler RL. Encephalopathy in mice produced by inoculation with scrapie brain material. Lancet. 1961;277:1378–1379. doi: 10.1016/s0140-6736(61)92008-6. [DOI] [PubMed] [Google Scholar]
- 45.Bruce M, Chree A, McConnell I, Foster J, Pearson G, Fraser H. Transmission of bovine spongiform encephalopathy and scrapie to mice: strain variation and the species barrier. Philos Trans R Soc Lond B Biol Sci. 1994;343:405–411. doi: 10.1098/rstb.1994.0036. [DOI] [PubMed] [Google Scholar]
- 46.Carlson GA, Ebeling C, Yang S-L, Telling G, Torchia M, Groth D, et al. Prion isolate specified allotypic interactions between the cellular and scrapie prion proteins in congenic and transgenic mice. Proc Natl Acad Sci USA. 1994;91:5690–5694. doi: 10.1073/pnas.91.12.5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prusiner SB, Cochran SP, Groth DF, Downey DE, Bowman KA, Martinez HM. Measurement of the scrapie agent using an incubation time interval assay. Ann Neurol. 1982;11:353–358. doi: 10.1002/ana.410110406. [DOI] [PubMed] [Google Scholar]
- 48.Carlson GA, Kingsbury DT, Goodman PA, Coleman S, Marshall ST, DeArmond S, et al. Linkage of prion protein and scrapie incubation time genes. Cell. 1986;46:503–511. doi: 10.1016/0092-8674(86)90875-5. [DOI] [PubMed] [Google Scholar]
- 49.Safar JG, Scott M, Monaghan J, Deering C, Didorenko S, Vergara J, et al. Measuring prions causing bovine spongiform encephalopathy or chronic wasting disease by immunoassays and transgenic mice. Nat Biotechnol. 2002;20:1147–1150. doi: 10.1038/nbt748. [DOI] [PubMed] [Google Scholar]
- 50.Safar JG, Geschwind MD, Deering C, Didorenko S, Sattavat M, Sanchez H, et al. Diagnosis of human prion disease. Proc Natl Acad Sci USA. 2005;102:3501–3506. doi: 10.1073/pnas.0409651102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kascsak RJ, Rubenstein R, Merz PA, Tonna-DeMasi M, Fersko R, Carp RI, et al. Mouse polyclonal and monoclonal antibody to scrapie-associated fibril proteins. J Virol. 1987;61:3688–3693. doi: 10.1128/jvi.61.12.3688-3693.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Muramoto T, DeArmond SJ, Scott M, Telling GC, Cohen FE, Prusiner SB. Heritable disorder resembling neuronal storage disease in mice expressing prion protein with deletion of an α-helix. Nat Med. 1997;3:750–755. doi: 10.1038/nm0797-750. [DOI] [PubMed] [Google Scholar]
- 53.Premzl M, Gamulin V. Comparative genomic analysis of prion genes. BMC Genomics. 2007;8:1–14. doi: 10.1186/1471-2164-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Rheede T, Smolenaars MM, Madsen O, De Jong WW. Molecular evolution of the mammalian prion protein. Mol Biol Evol. 2003;20:111–121. doi: 10.1093/molbev/msg014. [DOI] [PubMed] [Google Scholar]
- 55.Telling GC, Scott M, Hsiao KK, Foster D, Yang S-L, Torchia M, et al. Transmission of Creutzfeldt-Jakob disease from humans to transgenic mice expressing chimeric human-mouse prion protein. Proc Natl Acad Sci USA. 1994;91:9936–9940. doi: 10.1073/pnas.91.21.9936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fraser H, Bruce ME, Chree A, McConnell I, Wells GAH. Transmission of bovine spongiform encephalopathy and scrapie to mice. J Gen Virol. 1992;73:1891–1897. doi: 10.1099/0022-1317-73-8-1891. [DOI] [PubMed] [Google Scholar]
- 57.Masters CL, Gajdusek DC, Gibbs CJ., Jr. Creutzfeldt-Jakob disease virus isolations from the Gerstmann-Sträussler syndrome. Brain. 1981;104:559–588. doi: 10.1093/brain/104.3.559. [DOI] [PubMed] [Google Scholar]
- 58.Wells GAH, Wilesmith JW. The neuropathology and epidemiology of bovine spongiform encephalopathy. Brain Pathol. 1995;5:91–103. doi: 10.1111/j.1750-3639.1995.tb00580.x. [DOI] [PubMed] [Google Scholar]
- 59.Wells GAH, Wilesmith JW, McGill IS. Bovine spongiform encephalopathy: a neuropathological perspective. Brain Pathol. 1991;1:69–78. doi: 10.1111/j.1750-3639.1991.tb00642.x. [DOI] [PubMed] [Google Scholar]
- 60.Foster JD, Parnham D, Chong A, Goldmann W, Hunter N. Clinical signs, histopathology and genetics of experimental transmission of BSE and natural scrapie to sheep and goats. Vet Rec. 2001;148:165–171. doi: 10.1136/vr.148.6.165. [DOI] [PubMed] [Google Scholar]
- 61.Parchi P, Chen SG, Brown P, Zou W, Capellari S, Budka H, et al. Different patterns of truncated prion protein fragments correlate with distinct phenotypes in P102L Gerstmann-Straussler-Scheinker disease. Proc Natl Acad Sci USA. 1998;95:8322–8327. doi: 10.1073/pnas.95.14.8322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brown P, Gibbs CJ, Jr., Rodgers-Johnson P, Asher DM, Sulima MP, Bacote A, et al. Human spongiform encephalopathy: the National Institutes of Health series of 300 cases of experimentally transmitted disease. Ann Neurol. 1994;35:513–529. doi: 10.1002/ana.410350504. [DOI] [PubMed] [Google Scholar]
- 63.Tateishi J, Kitamoto T, Doh-ura K, Sakaki Y, Steinmetz G, Tranchant C, et al. Immunochemical, molecular genetic, and transmission studies on a case of Gerstmann-Sträussler-Scheinker syndrome. Neurology. 1990;40:1578–1581. doi: 10.1212/wnl.40.10.1578. [DOI] [PubMed] [Google Scholar]
- 64.Tateishi J. Transmission of human prion disease to rodents. Semin Virol. 1996;7:175–180. [Google Scholar]
- 65.Gambetti P, Kong Q, Zou W, Parchi P, Chen SG. Sporadic and familial CJD: classification and characterisation. Br Med Bull. 2003;66:213–239. doi: 10.1093/bmb/66.1.213. [DOI] [PubMed] [Google Scholar]
- 66.Furuoka H, Horiuchi M, Yamakawa Y, Sata T. Predominant involvement of the cerebellum in guinea pigs Infected with bovine spongiform encephalopathy (BSE) J Comp Pathol. doi: 10.1016/j.jcpa.2010.10.004. In press. [DOI] [PubMed] [Google Scholar]
- 67.Giles K, Glidden DV, Beckwith R, Seoanes R, Peretz D, DeArmond SJ, et al. Resistance of bovine spongiform encephalopathy (BSE) prions to inactivation. PLoS Pathog. 2008;4:e1000206. doi: 10.1371/journal.ppat.1000206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.DeArmond SJ, Kretzschmar HA, Prusiner SB. Prion diseases. In: Graham DI, Lantos PL, editors. Greenfield’s Neuropathology. 7th Edition Hodder Arnold; London: 2002. pp. 273–323. [Google Scholar]
- 69.Bartz JC, Kincaid AE, Bessen RA. Rapid prion neuroinvasion following tongue infection. J Virol. 2003;77:583–591. doi: 10.1128/JVI.77.1.583-591.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thomzig A, Kratzel C, Lenz G, Kruger D, Beekes M. Widespread PrPSc accumulation in muscles of hamsters orally infected with scrapie. EMBO Rep. 2003;4:530–533. doi: 10.1038/sj.embor.embor827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.DeJoia C, Moreaux B, O’Connell K, Bessen RA. Prion infection of oral and nasal mucosa. J Virol. 2006;80:4546–4556. doi: 10.1128/JVI.80.9.4546-4556.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bartz JC, Dejoia C, Tucker T, Kincaid AE, Bessen RA. Extraneural prion neuroinvasion without lymphoreticular system infection. J Virol. 2005;79:11858–11863. doi: 10.1128/JVI.79.18.11858-11863.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mulcahy ER, Bartz JC, Kincaid AE, Bessen RA. Prion infection of skeletal muscle cells and papillae in the tongue. J Virol. 2004;78:6792–6798. doi: 10.1128/JVI.78.13.6792-6798.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Emanuelsson O, Brunak S, von Heijne G, Nielsen H. Locating proteins in the cell using TargetP, SignalP and related tools. Nat Protoc. 2007;2:953–971. doi: 10.1038/nprot.2007.131. [DOI] [PubMed] [Google Scholar]
- 75.Eisenhaber B, Bork P, Eisenhaber F. Prediction of potential GPI-modification sites in proprotein sequences. J Mol Biol. 1999;292:741–758. doi: 10.1006/jmbi.1999.3069. [DOI] [PubMed] [Google Scholar]
- 76.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T-4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 77.Scott MR, Will R, Ironside J, Nguyen H-OB, Tremblay P, DeArmond SJ, et al. Compelling transgenetic evidence for transmission of bovine spongiform encephalopathy prions to humans. Proc Natl Acad Sci USA. 1999;96:15137–15142. doi: 10.1073/pnas.96.26.15137. [DOI] [PMC free article] [PubMed] [Google Scholar]