Abstract
Objective
Evaluate electrophysiologic findings in incidental Lewy Body disease (ILBD).
Methods
ILBD, Control, and Parkinson's disease (PD) subjects had electrophysiological evaluation within two years prior to autopsy. Data analyzed included surface electromyography (EMG) of upper extremity muscles during rest and muscle activation, and electroencephalography (EEG) recording at rest. For EMG, gross tracings and spectral peaks were analyzed. EEG measures analyzed were background frequency and power in delta, theta, alpha, and beta bands.
Results
Three of ten ILBD subjects (30%) showed unilateral rhythmic EMG discharges at rest without a visually apparent rest tremor. The ILBD resting EMG frequency was lower than in the Control group with no overlap (P=0.03) and close to that of the PD group. The ILBD group had significantly lower background rhythm frequency than the Control group (P=0.001) but was greater than the PD group (P=0.01).
Conclusions
The electrophysiologic changes in ILBD cases are between those of Control and PD, suggesting that these findings may reflect changes correlating with ILBD as a possible precursor to PD.
Significance
Electrophysiologic changes in ILBD may assist with the identification of a preclinical stage for Lewy body disorders and help the development of a therapeutic agent for modifying Lewy body disease progression.
Keywords: Lewy body, Electromyography, Electroencephalography, Pathology, Parkinson's disease, Tremor
Introduction
The Lewy body is the pathological hallmark of Parkinson's disease (PD) and dementia with Lewy bodies (DLB). Lewy bodies have been reported in 10–30% of undiagnosed elderly persons (Gibb and Lees, 1988; Adler et al., 2010). “Incidental Lewy Body Disease” (ILBD) refers to the presence of Lewy bodies at autopsy in the absence of a clinical diagnosis. Our group recently found decreased striatal tyrosine hydroxylase (TH) in subjects with ILBD, and this finding was interpreted to possibly suggest that ILBD represents preclinical PD and DLB (Beach et al., 2008a). Other investigators have confirmed that other striatal dopamine markers are depleted in ILBD to levels intermediate between those of PD and control (Dickson et al. 2008; Delledonne et al., 2008). Thus, these findings and those of other groups suggest that such “incidental Lewy bodies” may be a precursor to the eventual clinical onset of PD or DLB (Fujishiro et al., 2008; Frigerio et al., 2009; Markesbery et al., 2009).
We have reported that ILBD cases in our brain bank do not have parkinsonian signs, other movement problems, or cognitive findings that are out of proportion to similarly assessed age-matched cases without Lewy bodies at autopsy (Frigerio et al., 2009). Both olfactory dysfunction and constipation have been reported to be associated with ILBD (Abbott et al., 2001; Ross et al., 2006). Such study of pre-mortem correlates of ILBD yields valuable information regarding the putative preclinical state. Moreover, preclinical correlates will have potential usefulness in clinical trials and other studies of preclinical disease. Our group and others have reported that electroencephalographic (EEG) changes are progressive in PD and differ according to cognitive state (Caviness et al., 2007a). In this study, we report pre-mortem surface electromyography (EMG) and quantitative EEG (QEEG) findings in subjects that were found at autopsy to have ILBD.
Methods
Pre-mortem clinical evaluation
The Banner Sun Health Research Institute (SHRI) Brain and Body Donation Program is an autopsy program that enrolls volunteer subjects from the community, as well as targeted patients with PD and Alzheimer's disease (AD), for standardized longitudinal pre-mortem clinical assessments until death (Adler et al., 2002). Data for this study were obtained from the 11 June 2010 version of the Banner Sun Health Research Institute Brain and Body Donation Program database. All subjects signed informed consent approved by the SHRI and Mayo IRBs. Subjects underwent prospective pre-mortem standardized longitudinal movement disorder, behavioral neurology, and neuropsychological assessments as well as biennial electrophysiological assessments with EEG and surface EMG recordings. Unified Parkinson's Disease Rating Scale Part III motor (UPDRS III) and Hoehn and Yahr measures were recorded on PD subjects. The neuropsychological battery for all subjects included Folstein Mini-Mental Examination (MMSE), Auditory Verbal Test A7 (AVLT-A7), Stroop Interference, Controlled Word Association Test (COWAT), Judgment of Line Orientation (JLO), and Trails Making Test-B (TMT-B). Levodopa equivalents were calculated as Hobson et al (Hobson et al., 2002). At time of death, subjects received final movement disorder and cognitive diagnoses by consensus conference among a movement disorder neurologist (CHA), behavioral neurologist (MNS), and neuropsychologist per previously published criteria that include DSM-IV (Hughes et al., 1992; Hughes et al., 1993; Caviness et al., 2007b). Exclusion criteria for this study included dementia, electrophysiologic evaluation only greater than two years prior to death, or if they were taking anticonvulsant or benzodiazepine medication because of possible alteration of EEG activity with these agents. For subjects with multiple electrophysiological assessments available, the study within two years of autopsy was used for the analysis.
Neuropathology
Methods using a standardized series of brain regions (including olfactory bulb) stained with H&E as well as immunohistochemically for α-synuclein have been previously described (Beach et al., 2008b; Beach et al., 2009). The DLB Consortium staging system and the Unified Staging System for Lewy Body Disorders were used to describe stages of Lewy related pathology (Lewy bodies and Lewy neurites) (McKeith et al., 2005; Beach et al., 2009). Brain regions examined included medulla, pons, substantia nigra, nucleus basalis of Meynert, limbic regions including the amygdala, transentorhinal and cingulate cortices, and frontal, parietal, and temporal neocortical regions. Lewy-related pathology scores using the third DLB consensus conference methods were used to compare those subjects with and without rest EMG discharges within the ILBD group (McKeith et al., 2005). The “Total Score” equaled the sum of scores from all sampled areas. “Brainstem Total” equaled the sum of scores from the medulla, pons, and substantia nigra, and “Neocortical Total” equaled the sum of frontal, temporal, and parietal neocortex.
Definition of three subject groups
The ILBD and Control group criteria were the same as in our report on ILBD clinical findings and included not meeting clinical criteria for the diagnosis of PD, and the absence of pre-mortem clinical or post-mortem pathological evidence of dementia or movement disorder secondary to neurodegenerative diseases. In addition, the ILBD group had Lewy-related synucleinopathy present (Adler et al., 2010). PD criteria were as previously published along the lines of UK brain bank criteria (Hughes et al., 1992; Hughes et al., 1993; Caviness et al., 2007b). We included only PD without dementia cases, as dementia was an exclusion criterion for all groups. Subjects were consecutively recruited and all Control, ILBD, and PD cases were considered if electrophysiological data were available.
Electrophysiology recording
Surface EMG recording of upper extremity muscles and simultaneous EEG was acquired at rest and then postural wrist muscle activation using the Neuroscan System (Sterling, VA) at 1,000 Hz, bandpass 1 to 200 Hz. The same EMG and EEG electrode locations were used as in previous electrophysiological studies, including using Fz as reference and right mastoid region as ground (Caviness et al., 2003; Caviness et al., 2007a). EMG recordings were made from bilateral pectoralis major, deltoid, biceps, triceps, wrist flexor group, wrist extensor group, abductor pollicis brevis, and first dorsal interossei. The subjects were coached to have relaxed muscles for the rest condition and this was confirmed by surface EMG. The rest condition was recorded for four minutes with one minute of eyes open quiet and relaxed, one minute of eyes closed counting backward, and two minutes of eyes closed quiet and relaxed. An EMG-EEG technician and electrophysiologist (JNC) were present during the entire recording session to monitor behavioral state, on-line signal quality, and adequate performance. During the two minutes of eyes closed quiet and relaxed, subjects were asked every minute if they were awake and monitored for EEG evidence of drowsiness. When muscle, eye movement/blink, or other artifacts were identified, the subject was coached until a 100–120 second artifact-free recording was obtained. For postural wrist muscle activation, data were collected during 20-second periods of only right wrist extension (60 degrees) against gravity, alternating with 20-second periods of rest for 10 minutes. Adequacy of performance was assessed during a practice period. For both of the rest and muscle activation states, the subjects were observed for tremor by the electrophysiologist and movement disorder expert (JNC).
Raw data processing
Data were processed off-line using Neuroscan © software (EL Paso, TX, USA). Both resting and muscle activation continuous files were divided into 4096-point (4095 ms for 0.24Hz resolution) epochs that were consecutive and nonoverlapping. Epochs were inspected for artifacts, but artifact rejection was rare due to close monitoring during acquisition. Subjects were excluded if 20% or more of the traces had visible artifact. Further processing was performed as below separately for surface EMG and quantitative EEG (QEEG).
Surface EMG analysis (rest and muscle activation)
In addition to gross inspection for rhythmic modulation and/or discharges, tracings were processed with EMG signal rectification. 50–60 epochs were created during the resting condition (using whole resting file) to maximize the detection of low amplitude activity, and another 50–60 (to match same number at rest) epochs were created during the muscle activation condition with resting periods rejected. These epochs were passed through a 10% cosine window, and then processed with a fast Fourier transform (FFT) and averaged to produce an averaged FFT power spectrum of EMG activity for both the resting and muscle activation for each subject. The presence or absence of clearly distinct peaks in the FFT spectrum was assessed both for bilateral upper extremity muscles in the resting state and for the right wrist extensor muscle activation task.
QEEG analysis
Tracings obtained only during the resting state with eyes closed quiet and relaxed were processed as in a previous study (Caviness et al., 2007a). Individual files yielded 25–30 epochs. These epochs were passed through a 10% cosine window, and then processed with a FFT and averaged to produce an averaged FFT power spectrum for each subject. The dominant posterior background rhythm frequency was defined as the peak background frequency assessed by using the spectra at P3, P4, and Oz. The global relative EEG bandpower for each frequency band was calculated using all electrodes (except Fp1, Fp2, A1, A2) as a percentage of overall summed EEG power across all designated frequency bands (delta, theta, alpha, beta) and averaged for each subject. We designated frequency bands in hertz (Hz) as follows: delta: 1.5–3.9 Hz; theta: 4–7.9 Hz; alpha: 8–12.9 Hz; beta: 13–30 Hz. A cutoff of 1.5 Hz was used to minimize interference from movement artifact.
Statistical Analysis
The ILBD group was compared to both the Control group and the PD group. Comparisons for continuous measures were made using the pairwise contrasts from an analysis of variance model. Adjusted comparisons were made using the pairwise contrasts from a general linear model with terms for group, age, and sex. First order interactions were also tested. Comparisons for dichotomous measures were made using the Fisher exact test. In the ILBD group, subjects with rest EMG discharges were compared to those without by using the two-sample t-test. Significance was set at P<0.05 for all comparisons.
Results
Subjects
The database search identified the following subjects for screening: 11 ILBD; 32 Control; and 7 PD. Two subjects in the Control group were excluded because of excessive artifact in electrophysiologic recordings by the above criteria. One subject in the ILBD group and two subjects in the Control group were excluded due to use of anticonvulsant or benzodiazepine medication at the time of the EEG examination. All PD subjects were on dopaminergic treatment at the time of testing, and if there was a history of treatment response fluctuation, the recording was in the “ON” condition.
Age, sex, clinical, and electrophysiology measures of the three groups and statistical data are given in table 1. Asterisks for statistical significance for the pairwise comparisons are shown in table 1 when the overall F test or chi-square test is significant. In the analysis of variance model, no interactions were detected for any comparisons. Mean age was slightly older in the ILBD group than in the PD group. Two subjects in the PD group had Hoehn and Yahr score 2, one had score 2.5, three had score 3, and one had score 4. Levodopa equivalents in the PD group ranged from 300 to 1050 mg, with a mean of 520 mg (SD 290).
Table 1.
Subject demographics, electrophysiology, and clinical results.
| ILBD versus | ||||||
|---|---|---|---|---|---|---|
| Overall | PD | Control | ||||
| PD | ILBD | Control | P | P | P | |
| N | 7 | 10 | 28 | |||
| Age at EEG (y); mean (SD) | 81.3 (4.9) | 89.4 (4.4) | 86.3 (5.4) | .01 | .003* | .11 |
| Female | 4 (57%) | 3 (30%) | 14 (50%) | .20 | .35 | .46 |
| Rest EMG Discharge | 4 (57%) | 3 (30%) | 4 (14%) | .05 | .35 | .35 |
| Rest EMG Discharge Peak Frequency (Hz); mean (SD) | 4.09 (0.61) | 4.07 (0.74) | 8.1 (2.3) | .009 | .99 | .008* |
| Rest EMG Discharge Peak Frequency <5.5 Hz | 4/4 (100%) | 3/3 (100%) | 0/4 (0%) | .004 | >.99 | .03* |
| Activation EMG Discharge | 7 (100%) | 4 (40%) | 17 (61%) | .04 | .03* | .29 |
| Activation EMG Discharge Peak Frequency (Hz); mean (SD) | 8.2 (3.5) | 11.2 (3.1) | 9.3 (3.9) | .44 | .20 | .37 |
| Rest or Activation EMG discharge | 7 (100%) | 7 (70%) | 18 (64%) | .18 | .10 | >.99 |
| Background Rhythm Frequency (Hz); mean (SD) | 7.25 (0.67) | 8.32 (0.80) | 9.40 (0.83) | <.001 | .01* | .001* |
| Adjusted for Age and Sex; adjusted mean | 7.0 | 8.5 | 9.4 | <.001 | .001* | .004* |
| Delta BP(%); mean (SD) | 15.3 (5.9) | 18 (11) | 14.7 (7.5) | .62 | .56 | .33 |
| Theta BP(%); mean (SD) | 37 (14) | 18.4 (7.9) | 19 (12) | .002 | .002* | .88 |
| Alpha BP(%); mean (SD) | 29 (11) | 34 (15) | 41 (13) | .07 | .44 | .15 |
| Beta BP(%); mean (SD) | 19.2 (8.2) | 29 (11) | 25 (11) | .22 | .09 | .39 |
| UPDRS III; mean (SD) | 30 (15), 5 | 9.2 (3.5) | 8.0 (5.4) | <.001 | <.001 | .62 |
| MMSE; mean (SD) | 28.33 (0.82) | 27.9 (1.3) | 28.6 (1.5) | .48 | .55 | .23 |
| AVLT A7; mean (SD) | 5.4 (2.7) | 7.9 (3.6) | 7.8 (4.0) | .43 | .28 | .98 |
| Stroop Interference; mean (SD) | −9.7 (9.9) | −6.3 (4.5) | −6.5 (6.6) | .64 | .44 | .95 |
| COWA; mean (SD) | 44.2 (9.0) | 29 (11) | 40 (12) | .06 | .03 | .04 |
| JLO; mean (SD) | 17.0 (5.6) | 20.9 (4.0) | 24.0 (4.1) | .03 | .20 | .12 |
| TMT-B; mean (SD) | 198 (79) | 209 (63) | 116 (57) | .002 | .76 | .002 |
ILBD=Incidental Lewy body disease; PD=Parkinson's disease without dementia. Rest EMG Discharge=rhythmic EMG discharges at rest; Activation EMG Discharge=rhythmic EMG modulation during postural muscle activation. BP=EEG bandpower.
Significant P values
UPDRS score was considerably higher for the PD group than in the ILBD group (P<.001). The COWA was significantly decreased for the PD group (P<.03) but increased for the Control group (P<.04) when compared to the ILBD group. For the TMT-B score, the ILBD group had a higher score than the Control group (P<.002).
Rhythmic EMG Activity during Rest
Three of the ten subjects (30%) with ILBD showed rhythmic EMG discharges at rest with corresponding spectral peaks. An example of the surface EMG polygraphy and frequency spectrum is shown in figure 1. None of the ILBD subjects showing these discharges had a visually apparent rest tremor, all had unilateral discharges, and none had muscle activation tremor or peaks in their muscle activation spectra. In contrast, only four of 28 Control subjects (14%) had rhythmic EMG discharges at rest. None of these four Control subjects had a visually apparent rest tremor, three of the four had bilateral discharges, and all had accompanying visually apparent muscle activation tremor with peaks in their muscle activation spectra. The most distinguishing characteristic between the resting EMG discharges between these two groups was that ILBD rest EMG frequency was lower than in the Control group with no overlap (P=0.03). One of the three ILBD cases with rhythmic EMG discharges at rest had three electrophysiological exams (biennial) before death, another had two exams, and the third case had a single exam. All exams for these three ILBD subjects showed the rhythmic EMG discharges at rest, revealing that low-frequency subclinical rest EMG discharges in these three ILBD cases were present for 5.1, 3.3, and 1.7 years before the incidental Lewy bodies were detected on autopsy.
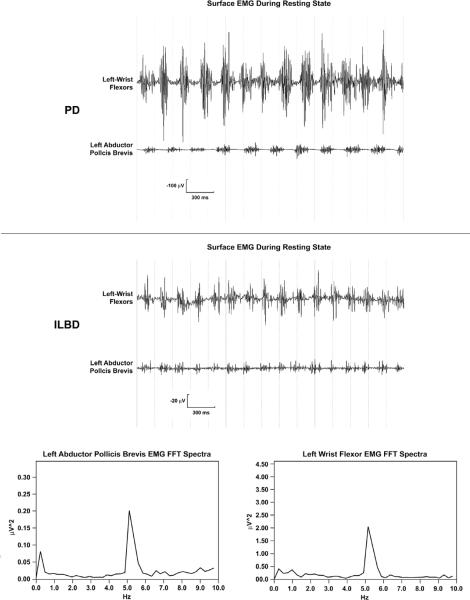
Figure 1.
Surface EMG rest discharges from a PD subject (top), and surface EMG rest discharges and their Fast-Fourier Transform (FFT) averaged spectra from an ILBD subject (bottom).
Top-Surface EMG polygraphy of left wrist flexors and left abductor pollicis brevis from a PD subject. Bottom-Surface EMG polygraphy of left wrist flexors and left abductor pollicis brevis and their FFT averaged spectra show a distinct at about 5 hertz for both muscles from an ILBD Subject. Note that the PD rest tremor discharges are much larger than for the ILBD rest tremor discharges that have no visible rest tremor.
Within the ILBD group, all three cases with rhythmic EMG discharges showed an agonist-only activation pattern. These three cases showed relatively low-amplitude EMG activity with a mean 80 μV (range 60–110) for during maximal sustained amplitude of the positive-negative deflection in the EMG polygraphy. The mean theta power for the ILBD cases with rhythmic EMG discharges trended higher than those without (P<.04). All other QEEG measure comparisons were not significant.
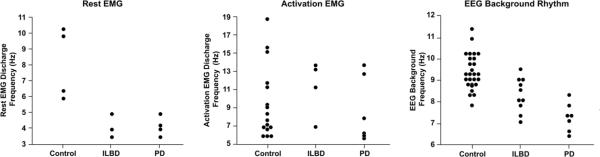
In the PD group, four of seven subjects (57%) showed unilateral rhythmic EMG discharges at rest, and all four of these subjects had a visually apparent rest tremor. Three of the four with rest tremor showed a reciprocal agonist-antagonist pattern and one subject showed a co-contraction agonist-antagonist pattern. No rest tremor was observed in other locations. The mean resting EMG discharge frequency was almost identical to that of the ILBD group (Table 1). Figure 2 (left panel) shows the frequency values for those demonstrating rest EMG discharges/spectral peaks among the three groups. However, the PD mean for during maximal sustained amplitude of the positive-negative deflection for the rest tremor in the EMG polygraphy was higher (mean=387.5 μV, range 270–510) with no overlap when compared to the ILBD group.
Figure 2.
Frequency value plots among Control, ILBD, and PD for rest and activation EMG discharges and EEG background frequency.
Rhythmic EMG Activity during Postural Activation
Four of the ten subjects (40%) with ILBD showed bilateral rhythmic EMG modulation with spectral peaks during muscle activation. These subjects did not have rest EMG discharges. Only one of these four subjects had a small visually perceptible tremor (grade 1). Seventeen of 28 Control subjects (61%) showed rhythmic EMG modulation with spectral peaks during muscle activation. Eleven of these 17 Control subjects showed a small bilateral visually perceptible tremor (grade 1). All seven PD subjects (100%) showed bilateral rhythmic EMG modulation with spectral peaks during muscle activation. Two PD subjects demonstrated a grade 2 activation tremor, three had a grade 1 activation tremor, and two showed no visually perceptible tremor. Rhythmic EMG modulation with spectral peaks during muscle activation was more common in PD than ILBD subjects (P=.03). The frequency of the muscle activation discharges was not different between the three groups. In all groups, muscle activation rhythmic frequencies at or above the physiologic range (>9 Hz) were not associated with visually perceptible tremor. Figure 2 (middle panel) shows the frequency values for those demonstrating muscle activation EMG discharges/spectral peaks among the three groups.
Quantitative Electroencephalography
The ILBD group had significantly lower background rhythm frequency mean than the Control group (P=.001) while having a higher background rhythm frequency than the PD group (P=.01). Figure 2 (right panel) shows the background rhythm frequency values for the three groups. Theta bandpower was higher in the PD group than for ILBD subjects (P=.003). No other frequency bands showed a significant difference among the three groups. When adjusting for age and sex, the differences remained statistically significant (Table 1).
Lewy Related Pathology Scores in ILBD cases
Lewy related pathology staging and scores in selected brain regions are given in table 2 for the ILBD cases. All ILBD cases with rhythmic rest EMG discharges had Lewy related pathology in the substantial nigra (subjects 1, 4, 7 in table 2), but two cases (subjects 5 and 10) had grade 1 Lewy-related pathology in the substantia nigra but no rhythmic rest EMG discharges. Comparisons of selected areas and total scores are given in table 3. The mean Total Score of all brain regions for ILBD cases with rhythmic rest EMG discharges was 15.0 (N=3, SD 7.8) versus only 4.3 (N=7, SD 3.0) for those without rhythmic rest EMG discharges (P=.01). The Brainstem Total Lewy related pathology score was 7.7 (N=3, SD=1.5) among subjects with rest tremor discharges versus only 1.71 (N=7, SD=0.95) among those without rest EMG discharges (P<.001).
Table 2.
Lewy body scores (0–4 points) in selected areas for subjects with ILBD.
| Case | DLB III Stage | Unified Lewy Body Stage | O B & T | Substantia Nigra | Amygdala | Cingulate gyrus | Frontal gyrus |
|---|---|---|---|---|---|---|---|
| 1 | Brainstem | II A | 1 | 3 | 1 | 1 | 0 |
| 2 | Unclassified* | II A | 2 | 0 | 0 | 0 | 0 |
| 3 | Brainstem | II A | 0 | 0 | 0 | 0 | 0 |
| 4 | Brainstem | II A | 1 | 1 | 2 | 0 | 0 |
| 5 | Brainstem | III | 0 | 1 | 1 | 0 | 0 |
| 6 | Brainstem | II A | 0 | 0 | 0 | 0 | 0 |
| 7 | Limbic | III | 2 | 2 | 4 | 2 | 1 |
| 8 | Brainstem | II A | 3 | 0 | 0 | 0 | 0 |
| 9 | Unclassified* | II B | 3 | 0 | 3 | 0 | 0 |
| 10 | Brainstem | III | 3 | 1 | 1 | 1 | 0 |
ILBD cases with rest EMG discharges are in bold. DLB III refers to the third DLB consensus conference methods (McKeith et al., 2005). Unified Lewy Body Staging system as per Beach et al., 2009. OB&T=Olfactory bulb and tract.
Cases with Lewy related pathology in the olfactory tract and bulb only or olfactory tract and bulb and amygdala only do not have a stage defined in the DLB-III consensus criteria.
Table 3.
Lewy body scores among subjects with and without rest tremor discharges in the group with ILBD.
| Rest Tremor | |||
|---|---|---|---|
| Yes | No | P | |
| N | 3 | 7 | |
| Olfactory Bulb (0–4 points); mean (SD) | 1.33 (0.58) | 1.6 (1.5) | .80 |
| Brainstem Sum (0–12 points); mean (SD) | 7.7 (1.5) | 1.71 (0.95) | <.001 |
| Neocortical Sum (0–12 points); mean (SD) | 1.0 (1.7) | 0.0 (0.0) | .30 |
| Total Sum (0–40 points); mean (SD) | 15.0 (7.8) | 4.3 (3.0) | .01 |
Discussion
Our results suggest that electrophysiological changes in surface EMG activity and EEG spectra can occur even when Lewy bodies present at autopsy are “incidental.” All these ILBD cases were prospectively examined by movement disorder experts and neuropsychologists pre-mortem and did not receive a PD or dementia diagnosis. In this study, both the low-frequency surface EMG activity at rest and spectral EEG changes in the ILBD cases were between those of autopsied confirmed Control and PD cases. Both findings support the concept that ILBD cases may possibly represent a preclinical stage of Lewy body disorders rather than a variant of normal aging. Moreover, these electrophysiological findings were detected in the absence of clinically significant findings on pre-mortem assessment when compared to a similarly assessed Control group. Similar to other studies of ILBD, we can not predict what proportion of our ILBD cases would have progressed to PD or DLB. Finally, we suggest that these electrophysiology correlates have potential usefulness in the study of early PD and DLB.
We believe that these low-frequency rest EMG discharges found in the ILBD group represent subclinical rest tremor related to presymptomatic PD or DLB. The proportion of our ILBD cases with subclinical rest tremor (3 out of 10) is smaller than the published proportion of PD patients that present with rest tremor from two published large databases (47% and 59%) (Jankovic et al., 1990; Utti et al., 2005). Thus, it may be that with more disease progression, rest EMG discharges are more likely to occur and be clinically manifest. Among our ILBD cases with rest EMG discharges, the lower frequency values distinguished the ILBD group from the Control group. Indeed, the highest rest EMG frequency value in the ILBD group was 1Hz lower than the lowest rest EMG value in the Control group. The rest tremor values in our ILBD group are within the characteristically low 3.5–5.5 Hz frequency range of PD (Hallett, 1986; Deuschl et al., 2003;). The rhythmic EMG discharges in the ILBD group were not accompanied by a visually apparent tremor. This may reflect that fact that the rest discharges were of lower amplitude than those of the PD cases, and without antagonist activation. It is known that rest tremor muscle discharges in PD may be present without a visually perceptible tremor, and spectral analysis of rectified EMG provides objective assessment of the oscillating discharges (Deuschl et al., 2003). The rest EMG discharges in the four Control cases, unlike the ILBD cases, were associated with muscle activation tremor. It is very unlikely that the rest tremor discharges in the ILBD group represents poor relaxation of muscle. First, all three subjects with rest tremor discharges in the ILBD group did not have activation tremor. Second, constant monitoring for poor muscle relaxation in all four limbs ensured a relaxed state. All these combined findings suggest that these rest EMG discharges may represent early abnormal activity that could evolve into clinically apparent rest tremor associated with Lewy body disorders. Our findings do not support strong sensitivity of rhythmic EMG discharges for detecting ILBD (30%), nor do we know with certainty that similar ILBD cases would indeed develop PD or DLB during life. Longitudinal study that follows control subjects to the eventual development of PD is needed to help answer this question. However, if confirmed, the specificity of low-frequency resting EMG discharges for ILBD could be useful in algorithms for early Lewy body disorder detection when other instruments for sensitivity are also being employed (Postuma et al., 2010).
The finding that the ILBD EEG background rhythm frequency is intermediate between Control and PD is an unexpected finding. Despite the mean background rhythm value still being within the normal alpha frequency range, the tendency toward downward spectral shift of this rhythm in ILBD may represent the first QEEG change seen in Lewy body disorders. We know that QEEG changes occur with PD, PDDementia, and DLB, but more work is needed to develop these measures as valid biomarkers (Caviness et al., 2007; Aarsland et al., 2008). Since most ILBD cases do not have cortical Lewy bodies, the question arises as to what might be the substrate for the EEG changes. One possibility is that the QEEG change in ILBD is from subcortical pathophysiology. The EEG background rhythm is known to be at least partially dependent on normal activity from subcortical centers. Abnormal subcortical influence via the cortical-basal ganglia-thalamo-cortical loop may cause the background rhythm frequency to shift lower. Decreased direct subcortical projections, such as from the nucleus basalis of Meynert, may also have such an effect, as seen in animal models (Stewart et al, 1984; Buzsaki et al., 1988). EEG spectral power partially normalizes after cholinesterase inhibitor treatment in PD-Dementia (Bosboom et al., 2009). Alternatively, intrinsic neocortical neuron dysfunction may occur before Lewy bodies are discovered on microscopic examination (Libow et al., 2009). This is supported by the finding of oxidative stress in the neocortex of ILBD regardless of neocortical Lewy body presence or absence (Dalfo et al., 2005).
A more defined picture is beginning to emerge for ILBD. Several recent studies favor the view that individuals with ILBD would develop symptoms of PD or DLB if they had lived long enough. There are several reasons for this hypothesis. The Lewy bodies in ILBD cases are found in multiple brainstem and subcortical locations along the lines of symptomatic Lewy body staging systems (Braak et al., 2003; DelleDonne et al., 2008; Beach et al., 2009). Substantia nigra involvement occurs in many ILBD cases but seemingly with less neuron loss than in PD (DelleDonne et al., 2008). The fact that markers of dopamine terminals in the striatum are decreased in ILBD cases further suggests preclinical abnormal nigrostriatal function (Dickson et al., 2008; DelleDonne et al., 2008; Fujishiro et al., 2008). Our finding that subclinical EMG discharges at rest occur in ILBD cases with more brainstem Lewy related pathology may suggest that pathology reaches a threshold in the brainstem for such EMG activity to occur. It is possible that more pathology develops before a rest tremor is seen. The EEG changes that we found suggest that mild cortical dysfunction may be present in some ILBD subjects. It is also interesting to note that within the ILBD group, the cases with rhythmic EMG discharges at rest had a trend toward more EEG theta bandpower which may suggest more subclinical progression that correlates with both electrophysiologic findings. Based on our earlier study of clinical abnormalities in ILBD cases, only Trails B, a cognitive test reflecting frontoexecutive function, was found to be decreased in ILBD (Adler et al., 2010). In another study, mild cognitive impairment was found in a proportion of PD cases at time of initial diagnosis (Aarsland et al., 2009). More study is needed to determine whether early EEG changes correlate with mild cognitive abnormalities, or whether the QEEG biomarker may be able to predict which ILBD cases could represent preclinical DLB rather than PD. Moreover, it would be important to know whether individuals with EEG changes early in their Lewy body disease course are more likely to show early dementia or MCI.
Finally, it is of great interest that the low-frequency subclinical rest EMG discharges in the ILBD cases were present for 5.1, 3.3, and 1.7 years before the incidental Lewy bodies were detected on autopsy. It would be useful to study retrospective electrophysiologic changes in individuals that eventually develop PD or DLB to determine the length of time preclinical EMG and QEEG changes are present in such individuals. The notion that there is a long preclinical stage in the onset of Lewy body disorders is profound. Such an interval offers tremendous opportunity if an effective therapeutic agent can be found to prevent or inhibit the biochemical processes associated with disease progression of Lewy body disorders.
ACKNOWLEDGEMENTS
This work was funded by the gift of Beth and Larry Johnson, Mayo Clinic Foundation for Medical Research, Michael J Fox Foundation for Parkinson's Research (Prescott Family Initiative), Arizona Disease Control Research Commission (contracts 04-800, 4001, 05-901), and Federal Grant P30 AG019610.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aarsland DM, Kurz M, Beyer M, Bronnick K, Nore SP, Ballard C. Early discriminatory diagnosis of Dementia with Lewy Bodies. Dement Geriatr Cogn Disord. 2008;25:195–205. doi: 10.1159/000113417. [DOI] [PubMed] [Google Scholar]
- Aarsland DM, Bronnick KP, Larsen JPM, Tysnes OB, Alves G, The Norwegian ParkWest Study Group Cognitive impairment in incident, untreated Parkinson's disease: the Norwegian ParkWest Study. Neurology. 2009;72:1121–1126. doi: 10.1212/01.wnl.0000338632.00552.cb. [DOI] [PubMed] [Google Scholar]
- Abbott RD, Petrovitch H, White LR, Masaki KH, Tanner CM, Curb JD, et al. Frequency of bowl movements and the future risk of Parkinson's disease. Neurology. 2001;57:456–462. doi: 10.1212/wnl.57.3.456. [DOI] [PubMed] [Google Scholar]
- Adler CH, Hentz JG, Joyce JN, Beach TG, Caviness JN. Motor Impairment in Normal Aging, clinically possible Parkinson's disease, and clinically probable Parkinson's disease: Longitudinal evaluation of a cohort of prospective brain donors. Parkinsonism Relat D. 2002;9:103–110. doi: 10.1016/s1353-8020(02)00012-3. [DOI] [PubMed] [Google Scholar]
- Adler CH, Connor DJ, Hentz JG, Sabbagh MW, Caviness JN, Shill HA, et al. Incidental Lewy Body Disease: Clinical comparison to a control cohort. Movement Disord. 2010;25(5):642–646. doi: 10.1002/mds.22971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach TG, Adler CH, Sue LI, Peirce JB, Bachalakuri J, Lue LF, et al. Reduced striatal tyrosine hydroxylase in incidental Lewy body disease. Acta Neuropathol. 2008a;115:445–451. doi: 10.1007/s00401-007-0313-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach TG, Sue LI, Walker DG, Roher AH, Lue L, Vedders L, et al. The Sun Health Research Institute Brain Donation Program: description and experience, 1987–2007. Cell Tissue Bank. 2008b;9(3):229–245. doi: 10.1007/s10561-008-9067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach TG, Adler CH, Lue L, Sue LI, Bachalakuri J, Henry-Watson J, et al. Unified staging system for Lewy body disorders: correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. Acta Neuropathol. 2009;117(6):613–634. doi: 10.1007/s00401-009-0538-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosboom JLW, Stoffers D, Stam CJ, Berendse HW, Wolters E.Ch. Cholinergic modulation of MEG resting-state oscillartory activity in Parkinson's disease related dementia. Clin Neurophysiol. 2009;120:910–915. doi: 10.1016/j.clinph.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rub U, de Vos RA, Stear JEN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Bickford RG, Ponomareff G, Thal LJ, Mandel RT, Gage FG. Nucleus basalis and thalamic control of neocortical activity in the freely moving rat. J. Neurol. Sciences. 1988;8:4007–4026. doi: 10.1523/JNEUROSCI.08-11-04007.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caviness JN, Adler CH, Sabbagh MN, Connor DJ, Hernandez JL, Lagerlund TD. Abnormal cortico-muscular coherence is associated with the small amplitude cortical myoclonus in Parkinson's disease. Movement Disord. 2003;18(10):1157–1161. doi: 10.1002/mds.10525. [DOI] [PubMed] [Google Scholar]
- Caviness JN, Hentz JG, Evidente VGH, Driver-Dunckley E, Samanta J, Mahant P, et al. Both early and late cognitive dysfunction affects the electroencephalogram in Parkinson's disease. Parkinsonism Relat D. 2007a;13:348–354. doi: 10.1016/j.parkreldis.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Caviness JN, Driver-Dunckley E, Connor DJ, Sabbagh MN, Noble B, Hentz JG, et al. Defining mild cognitive impairment in Parkinson's disease. Movement Disord. 2007b;22(9):1272–1277. doi: 10.1002/mds.21453. [DOI] [PubMed] [Google Scholar]
- Dalfo E, Portero-Otin M, Ayala V, Marinez A, Pamplona R, Ferrer I. Evidence of oxidative stress in the neocortex in Incidental Lewy Body Disease. J Neuropathol Exp Neurololgy. 2005;64(9):816–830. doi: 10.1097/01.jnen.0000179050.54522.5a. [DOI] [PubMed] [Google Scholar]
- Dickson DW, Fujishiro H, DelleDonne A, Menke J, Ahmed Z, Klos KJ, et al. Evidence that incidental Lewy body disease is pre-symptomatic Parkinson's disease. Acta Neuropathol. 2008;115:437–444. doi: 10.1007/s00401-008-0345-7. [DOI] [PubMed] [Google Scholar]
- DelleDonne A, Klos KJ, Fjishiro H, Ahmed Z, Parisi J, Josephs K, et al. Incidental Lewy Body Disease and preclinical Parkinson disease. Arch Neurol. 2008;65(8):1074–1080. doi: 10.1001/archneur.65.8.1074. [DOI] [PubMed] [Google Scholar]
- Deuschl G, Fietzek U, Klebe S, Volkmann J. Clinical neurophysiology and pathophysiology of parkinsonian tremor. In: Hallett M, editor. Movement Disorders, Handbook of Clinical Neurophysiology. Vol. 1. 2003. pp. 377–396. [Google Scholar]
- Frigerio R, Fujishiro H, Maraganore DM, Klos K, DelleDonne A, Heckman MG, et al. Comparison of risk factor profiles in incidental Lewy Body disease and Parkinson's disease. Arch Neurol. 2009;66(9):114–1119. doi: 10.1001/archneurol.2009.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujishiro H, Frigerio R, Burnett M, Klos K, Josephs K, Delledonne A, et al. Cardiac sympathetic denervation correlates with clinical and pathologic stages of Parkinson's disease. Movement Disord. 2008;23(8):1085–1092. doi: 10.1002/mds.21989. [DOI] [PubMed] [Google Scholar]
- Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson's disease. JNNP. 1988;51(6):745–752. doi: 10.1136/jnnp.51.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett M. Electrophysiologic evaluation of tremor and central disorders of movement. In: Aminoff MJ, editor. Electrodiagnosis in clinical neurology. 1986. pp. 385–401. [Google Scholar]
- Hobson DE, Lang AE, Martin WRW, Razmy A, Rivest J, Fleming J. Excessive Daytime sleepiness and sudden-onset sleep in Parkinson's disease: A survey by the Canadian Movement Disorders Group. JAMA. 2002;287:455–463. doi: 10.1001/jama.287.4.455. [DOI] [PubMed] [Google Scholar]
- Hughes AJ, Ben-Shlomo Y, Daniel SE, Lees AJ. What features improve the accuracy of clinical diagnosis in Parkinson's disease?: A clinicopathologic study. Neurology. 1992;42:1142–1146. doi: 10.1212/wnl.42.6.1142. [DOI] [PubMed] [Google Scholar]
- Hughes AJ, Daniel SE, Blankson S, Lees AJ. A clinicopathologic study of 100 cases of Parkinson's disease. Arch Neurol. 1993;50:140–148. doi: 10.1001/archneur.1993.00540020018011. [DOI] [PubMed] [Google Scholar]
- Jankovic J, McDermott M, Carter J, Gauthier S, Goetz C, Golbe L, et al. Variable expression of Parkinson's disease: a base-line analysis of the DATATOP cohort. The Parkinson Study group. Neurology. 1990;40(10):1529–1534. doi: 10.1212/wnl.40.10.1529. [DOI] [PubMed] [Google Scholar]
- Libow LS, Frisina PG, Haroutunian V, Perl DP, Purohit DP. Parkinson's disease dementia—A diminished role for the Lewy body. Parkinsonism Relat D. 2009;15:572–575. doi: 10.1016/j.parkreldis.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markesbery WR, Jicha GA, Liu H, Schmitt FA. Lewy Body pathology in normal elderly Subjects. J Neuropath Exp Neur. 2009;68(7):816–822. doi: 10.1097/NEN.0b013e3181ac10a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeith IG, Dickson DW, Lowe J, Emre M, O'Brien JT, Feldman DM, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65(12):1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- Postuma RB, Gagnon JF, Montplaisir J. Clinical prediction of Parkinson's disease: planning for the age of neuroprotection. JNNP. 2010;81:1008–1013. doi: 10.1136/jnnp.2009.174748. [DOI] [PubMed] [Google Scholar]
- Ross GW, Abbott RD, Petrovitch H, Tanner CM, Davis DG, Nelson J, et al. Association of olfactory dysfunction with incidental Lewy bodies. Movement Disord. 2006;21:2062–2067. doi: 10.1002/mds.21076. [DOI] [PubMed] [Google Scholar]
- Stewart DJ, MacFabe DF, Vandervwolf CH. Cholinergic activation of the electrocorticogram: role of substantia innominata and effects of atropine and quinnuclioninyl benzylate. Brain Res. 1984;322:219–232. doi: 10.1016/0006-8993(84)90112-4. [DOI] [PubMed] [Google Scholar]
- Uitti RJ, Baba Y, Wszolek ZK, Putzke J. Defining the Parkinson's disease phenotype: initial symptoms and baseline characteristics in a clinical cohort. Parkinsonism Relat D. 2005;11:139–145. doi: 10.1016/j.parkreldis.2004.10.007. [DOI] [PubMed] [Google Scholar]