Abstract
Background
A common polymorphism, rs4644, coding for Pro64 or His 64 of the carbohydrate-binding protein galectin-3, influences susceptibility of galectin-3 to cleavage by matrix metalloproteinases and is associated with breast cancer incidence. Since forced expression of galectin-3 in a galectin-3-null breast cancer cell line confers sensitivity to TNF-Related Apoptosis-inducing Ligand (TRAIL), we sought to determine whether the His64/Pro64 polymorphism of galectin-3 affects the sensitivity to TRAIL.
Methods
Genomic DNA of breast cell lines was analyzed for SNP rs4644, and cytotoxicity was determined with MTT assay.
Results
When a collection of 9 breast cell lines that express galectin-3 was examined for LGALS3 genotype and sensitivity to doxorubicin and TRAIL, doxorubicin sensitivity was not related to LGALS3 genotype. In contrast, 0/5 cell lines that were homozygous for Pro64 galectin-3 were TRAIL sensitive, but 2/2 homozygous His 64 cell lines and 1/2 heterozygous cell lines were sensitive to TRAIL. Forced expression of galectin-3 of defined genotype in galectin-3 null cells was used to more directly test the effect of the P64H mutation on TRAIL sensitivity. High level expression of His64 galectin-3 rendered BT549 cells sensitive to TRAIL and resistant to doxorubicin, but cells expressing Pro64 galectin-3 remained TRAIL-resistant and doxorubicin sensitive.
Conclusion
These results indicate that the naturally occurring P64H mutation in galectin-3 increases sensitivity to death receptor-mediated apoptosis. The conclusion could be relevant to disparities in breast cancer outcomes across population groups, and could guide design of future clinical trials of TRAIL-based therapies.
Keywords: Galectin-3, Single Nucleotide Polymorphism, TNF-Related Apoptosis-Inducing Ligand, breast cancer, apoptosis
INTRODUCTION
Galectin-3 is an endogenous carbohydrate-binding protein that has been shown to have functions in a number of cellular processes (1,2). Galectin-3 can either increase or decrease apoptosis, depending on cell type and stimulus. Overexpression of galectin-3 in breast carcinoma cells renders them resistant to chemotherapeutic drugs (3,12), but inactivates Akt and sensitizes them to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis (4,5). The gene for galectin-3, LGALS3, is polymorphic in human populations. It has been reported that a common galectin-3 polymorphism, rs4644, influences susceptibility of galectin-3 to cleavage by matrix metalloproteinases (6). The protein with His 64 is cleaved by MMP-2 and MMP-9, but the form of the protein with Pro64 is resistant to cleavage. The rs4644 polymorphism (P64H mutation) is also related to breast cancer development. Genotype analyses have indicated that the His64 genotype is associated with increased breast cancer incidence (7).
TRAIL, a member of the tumor necrosis factor family, transmits death signals through death domain-containing receptors (8,9). In addition to its direct apoptosis-inducing effect (4), TRAIL also plays an important role in immune surveillance against tumor initiation, development, and metastasis, suggesting a potential application to cancer therapy (10,11). Because TRAIL selectively induces apoptosis in a variety of transformed cells, but not in most normal cells, this pathway is being investigated as a target for therapies, including phase 1 clinical trials of recombinant soluble TRAIL, anti-DR4 antibody, anti-DR5 antibody. Resistance to TRAIL mediated apoptosis in cancer cells may, however, limit the successes of TRAIL therapy. Earlier results from this laboratory showed that forced expression of galectin-3 in a galectin-3-null breast cancer cell line conferred sensitivity to TRAIL via a novel mechanism that involves upregulation of PTEN (4). The purpose of this study was to determine whether the His64/Pro 64 polymorphism of galectin-3 affects the sensitivity of breast cancer cells to TRAIL.
METHODS
Cell culture and transfection. Human breast carcinoma cells were maintained in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum, L-glutamine (2 mM), penicillin (100 units/ml), and streptomycin (50 units/ml) in a 95% air/5% CO2 incubator at 37 °C. BT549 cells were obtained from Dr. E.W. Thompson. Breast cancer cell lines Gl101A, BT474 and BT 20 were obtained from Dr. Janet E. Price, M.D. Anderson Cancer Center. Additional breast cancer cell lines were from ATCC.
The establishment of a stable neo resistant control vector-transfected BT549 clone (BT549/V), two His64 galectin-3-transfected BT549 clones (gal25A and gal25B) was previously described (4). A full-length galectin-3 cDNA encoding Pro64 galectin-3 was obtained by RT-PCR from BT549 cells and subcloned into an expression plasmid. A second cDNA encoding His64 galectin-3 was generated by site-directed mutagenesis, and the mutations were confirmed by DNA sequencing.
Genotype analysis. Galectin-3 genotype was determined by PCR-SSCP and PCR-based RFLP of genomic DNA isolated from cultured breast cancer cells. The rs4644 assay (ss2990728) uses the forward primer TTGATCAGCTCCACATGGTTG and the reverse primer GAGAAGTGGCTCTAAGCAGCAG) (35 cycles with 95°C for 0.5 min, 60°C for 0.5 min, 72°C for 3.0 min). Sequencing of the 1040nt product in both directions provided the genotype at the rs4644 as well as the rs4652 locus and the common intronic SNP rs2075598.
Western blotting. Total cell lysate extractions and Western blots were performed as previously described (4). Protein concentrations were measured using a DC protein assay reagent (Bio-Rad). Equal amounts of protein were separated by 12% SDS-PAGE and electrophoretically transferred to a nitrocellulose membrane. The membrane was probed with monoclonal anti-galectin-3, anti-PTEN, anti-Akt and anti-phospho-Akt (4). Anti-beta-actin (Sigma) was used to monitor equal loading in each lane. Immunoreactivity was detected by sequential incubation with horseradish peroxidase-conjugated secondary antibody and enhanced chemiluminescence (ECL) reagents using the ECL detection system (Amersham Biosciences).
Cytotoxicity assays. Cell viability was assessed by the MTT (3-(4; 5-dimethylthiazol-2-yl) -2, 5-diphenyl- tetrazolium bromide) assay (Sigma). The cells (10,000/well) were seeded into 96-ul plates and grown overnight. After 4h treatment with 100 ng/ml TRAIL or 16 h treatment with 5 ug/ml doxorubicin, MTT solution was added to a final concentration of 1 mg/ml for 3 h at 37°C, followed by 200 ul of dimethyl sulfoxide (Fisher Biotech, Fairlawn, NY). Colorimetric reading at 570 mm was performed using an MRX Revelation microplate reader (Dynex, Chantilly, VA). The results are expressed as mean percent cytotoxicity from triplicate cultures.
RESULTS
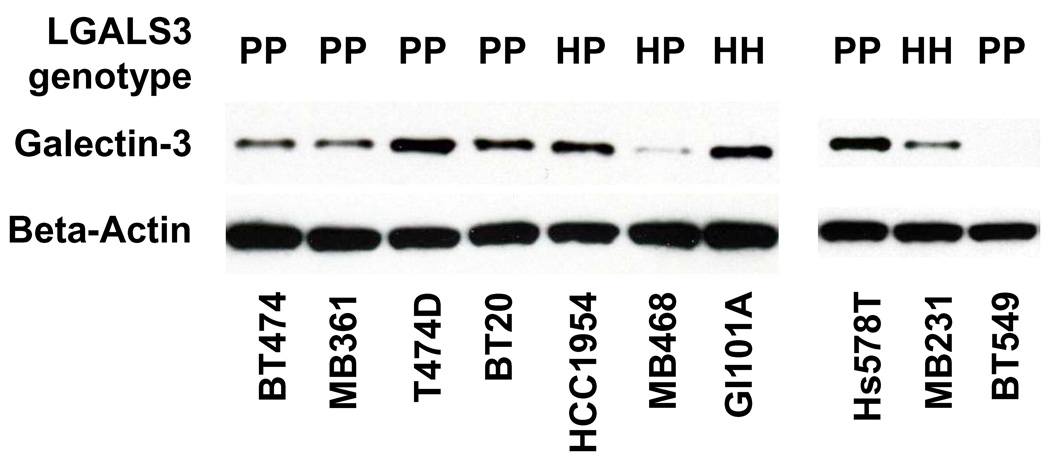
Galectin-3 genotype and expression. When a collection of breast cell lines was examined for LGALS3 genotype, 6 of 10 were homozygous for Pro64 galectin-3, 2 of 10 were homozygous for His64 galectin-3, and 2 of 10 were heterozygous (Figure 1). Galectin-3 protein was detected in lysates of 9 of the 10 cell lines. BT549 (homozygous Pro64 genotype) was galectin-3 null.
Figure 1.
Genotype and expression levels of galectin-3 in breast cancer cell lines. LGALS3 genotype at the rs4644 locus was determined by sequence analysis. PP denotes homozygous Pro64; HH, homozygous His64; and HP, heterozygous. Expression of the galectin-3 protein was determined by Western analysis.
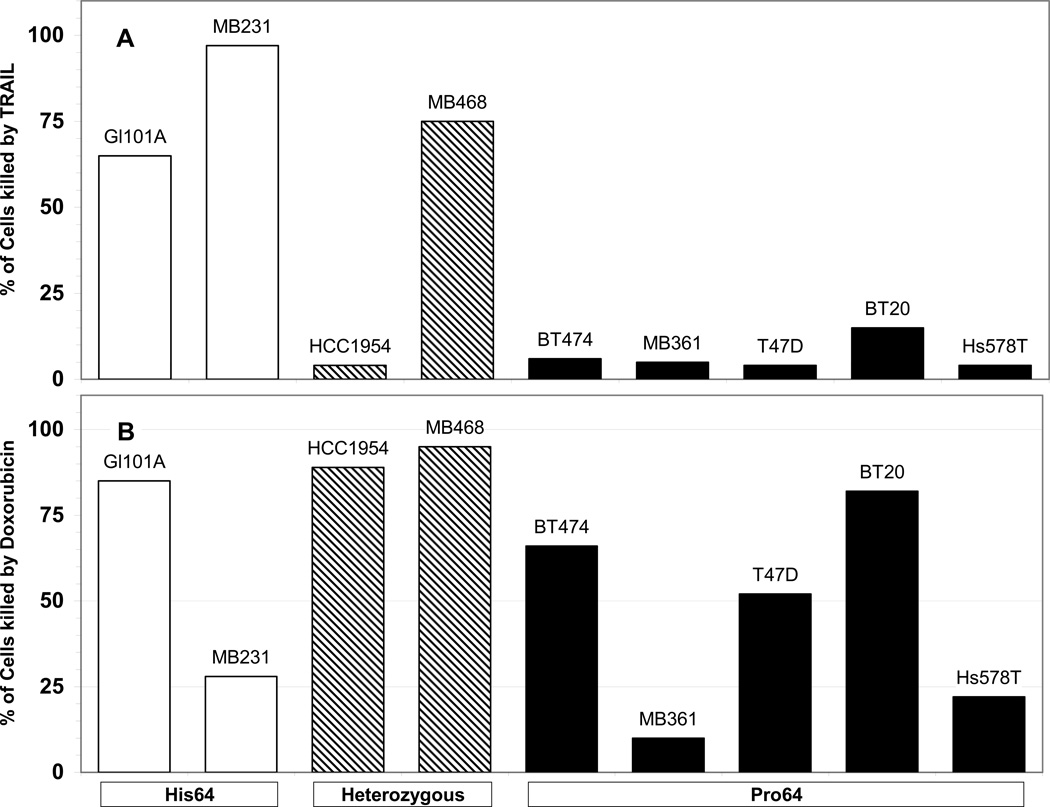
TRAIL sensitivity of breast cancer cell lines expressing His64 galectin-3. The 9 breast cancer cell lines that express galectin-3 were assayed for sensitivity to TRAIL (Figure 2A). None of the homozygous Pro64 cell lines was TRAIL sensitive, but both of the homozygous His 64 cell lines and one of two heterozygous cell lines were sensitive to TRAIL. These results indicate that TRAIL resistance is associated with the homozygous Pro64 genotype (p<0.05 by Fisher’s exact test.) Among the 9 breast cancer cell lines tested, sensitivity to doxorubicin was not dependent on galectin-3 genotype (Figure 2B). Two of 5 Pro64 homozygous cell lines were relatively doxorubicin-resistant, while 1 of 4 cell lines that are heterozygous or homozygous for His64 galectin-3 is relatively doxorubicin-resistant.
Figure 2.
Sensitivity of breast cancer cells to TRAIL and doxorubicin. Cells of homozygous His64 genotype (open bars), heterozygous (hashed bars) or homozygous Pro64 (solid bars) were treated 4h with 100 ng/ml TRAIL (A) or overnight with 5 ug/ml doxorubicin (B) and cell viability was determined with the MTT assay.
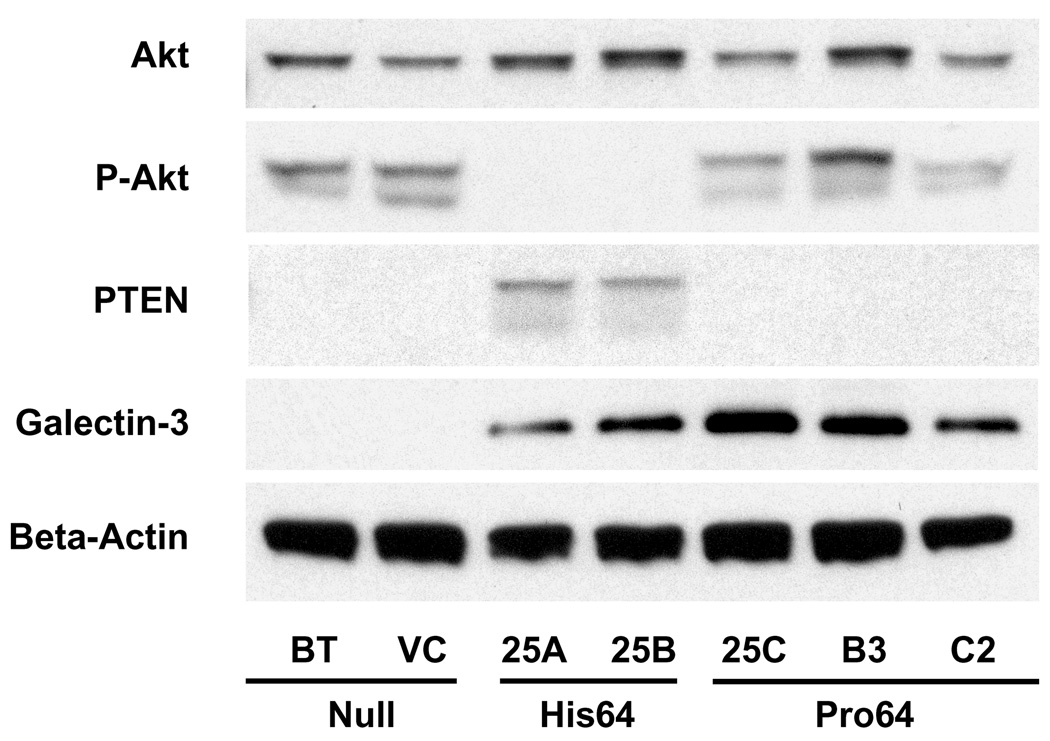
Forced expression of galectin-3 of defined genotype in galectin-3 null cells. To more directly test the effect of the P64H mutation of galectin-3 on TRAIL sensitivity, galectin-3-null cells were engineered to express either His64 galectin-3 or Pro64 galectin-3. Western analysis (Figure 3) showed that parental BT549 cells have undetectable levels of galectin-3 protein, but multiple clones express high levels of His64 galectin-3 (clones 25A and 25B) or Pro64 galectin-3 (clones 25C, B3, and C2). Previous results have shown that TRAIL sensitivity in two His64 galectin-3-transfected BT549 clones (25A and 25B) results from the induction of PTEN, which inactivates the PI3K/Akt survival pathway (4). In contrast, expression of Pro64 galectin-3 (25C, B3, and C2) is not associated with PTEN expression, and does not lead to a reduction in Akt phosphorylation (Figure 3).
Figure 3.
Forced expression of galectin-3 of defined genotype in BT549 galectin-3 null breast cancer cells. Western blot detection of galectin-3, PTEN, Akt, and phospho-Akt in total cell lysates of stable transfectants. BT, BT549 parental cells; VC, control containing vehicle plasmid; gal25A and gal25B, His64 galectin-3-expressing clones; 25C, B3, and C2 Pro64 galectin-3-expressing clones. Beta-actin expression is shown as loading control.
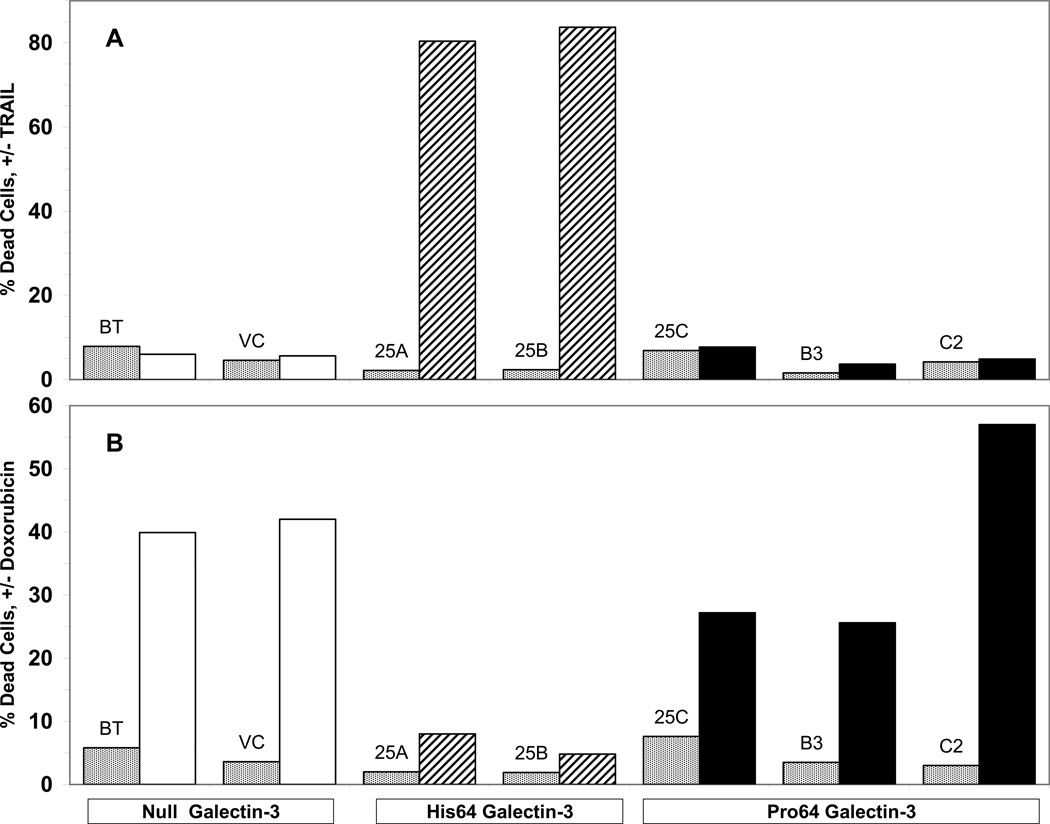
TRAIL sensitivity and doxorubicin resistance conferred by His64 galectin-3. Parental BT549 cells and vector control-transfected cells, both of which are galectin-3 null, were assayed for TRAIL-dependent cytotoxicity, and found to be TRAIL-resistant (Figure 4A). High level expression of His64 galectin-3 rendered the BT549 cells sensitive to TRAIL. In contrast, BT549 cells expressing Pro64 galectin-3 remained TRAILresistant, in spite of high levels of galectin-3 protein expression. These results indicate that the naturally occurring P64H mutation in galectin-3 increases sensitivity to death receptor-mediated apoptosis. In examining sensitivity to doxorubicin, we found that parental BT549 and cells transfected with Pro64 galectin-3 cDNA were doxorubicin sensitive (Figure 4B). In contrast, high level expression of His64 galectin-3 rendered the BT549 cells resistant to doxorubicin. Thus, in BT549 cells, His64 galectin-3 can confer TRAIL sensitivity and doxorubicin resistance, while Pro64 galectin-3 has no effect. However, the relationship of LGAS3 genotype to doxorubicin sensitivity may differ among breast cancer cell lines (Figure 2B).
Figure 4.
Sensitivity to TRAIL and resistance to doxorubicin conferred by expression of His64 galectin-3 but not Pro64 galectin-3. Parental BT549 cells and BT549 transfectants expressing vehicle plasmid (BT and VC, open bars) or transfectants expressing His64 galectin-3 (25A and 25B, hashed bars) or Pro64 galectin-3 (25C, B3, and C2, solid bars) were treated 4h with TRAIL (A) or overnight with doxorubicin (B) and cell viability was measured by the MTT assay. Stippled bars represent dead cell in the absence of TRAIL or doxorubicin.
DISCUSSION
We find that TRAIL sensitivity of breast cancer cells is associated with His64 galectin-3. The finding that galectin-3 null cells, like cells expressing Pro64 galectin-3, are TRAIL resistant argues against the alternative explanation, that TRAIL resistance depends on expression of Pro64 galectin-3. It has earlier been reported that His64 galectin-3 but not Pro64 galectin-3 confers relative resistance to cisplatin-induced apoptosis in BT549 cells (7). We find a similar trend for sensitivity to doxorubicin in derivatives of BT549 cells expressing galectin-3 of defined genotype. Introduction of a galectin-3 cDNA encoding galectin-3 with His64 into BT549 galectin-3-null cells rendered the BT549 cells sensitive to TRAIL and resistant to doxorubicin. However, among the 9 galectin-3 positive cell lines tested, there was not clear association between galectin-3 genotype and doxorubicin sensitivity.
Galectin-3 may exert anti-apoptotic (3) or pro-apoptotic (4) activity depending on the cell type and the nature of the stimulus, and the present study indicates that the His64/Pro64 polymorphism can influence this balance. The mechanisms by which galectin-3 can be either anti-apoptotic or pro-apoptotic are not fully understood, but could include different contributions of the mitochondrial (intrinsic) vs. the death receptor (extrinsic) apoptosis pathway or could reflect distinct functions of galectin-3 depending on nuclear vs. cytoplasmic localization.
A number of mechanisms of resistance to TRAIL-induced apoptosis in cancer have been identified (13). These include alteration in death receptors and decoy receptors, in DISC assembly, in caspase, in inhibitors of apoptosis, and in downstream signaling. In the case of BT549, galectin-3 has been shown to play a pivotal role in promoting TRAIL sensitivity through up-regulation of PTEN and inactivation of the PI3K/Akt survival pathway (4). In contrast, nuclear PTEN can have the opposite effect, protecting against DNA damage, leading to decreased apoptosis (14). It is not yet known, however, whether the His64/Pro64 polymorphism of galectin-3 influences PTEN localization. In the present study, we find that expression of Pro64 galectin-3 in BT549 cells does not lead to expression of PTEN and loss of phospho-Akt. However, it is not known whether the same mechanism is responsible for the TRAIL resistance observed in other breast cancer cells lines that express Pro64 galectin-3.
Since the His 64 allele has been reported to be associated with increased breast cancer incidence (7), it might be expected that many breast cancer cell lines would be homozygous for His64 galectin-3. Among the 10 cell lines examined in this study, there is not a pronounced overabundance of the His64 allele. The P64H mutation in galectin-3 is common in Caucasians, but lower in incidence in African-American and Asian populations, mirroring the higher incidence of breast cancer in Caucasians. If the His64/Pro64 polymorphism in the gene for galectin-3 is associated with TRAIL sensitivity, this could be relevant to disparities in breast cancer outcomes across population groups, and could guide design of future clinical trials of TRAIL-based therapies.
Since breast cancer is a very heterogeneous disease, there is a continuing need to predict those patients who are likely to be sensitive or resistant to chemotherapy, in order to tailor treatment accordingly. Since TRAIL is preferentially cytotoxic to cancer cells, as opposed to normal cells, it is being investigated as a potential therapy. Based on the finding that the naturally occurring P64H mutation in galectin-3 increases sensitivity to TRAIL and may decrease the sensitivity of breast cancer cells to doxorubicin, it is hypothesized that the LGALS3 rs4644 polymorphism could be used to predict chemosensitivity vs. chemoresistance of breast cancers.
Acknowledgments
Supported by National Cancer Institute grant RO1CA69480 (RSB)
Footnotes
There are no conflicts of interest or financial disclosures for any authors.
REFERENCES
- 1.Hsu DK, Liu FT. Regulation of cellular homeostasis by galectins. Glycoconj J. 2004;19:507–515. doi: 10.1023/B:GLYC.0000014080.95829.52. [DOI] [PubMed] [Google Scholar]
- 2.Barondes SH, Castronovo V, Cooper DN, Cummings RD, Drickamer K, Feizi T, Gitt MA, Hirabayashi J, Hughes C, Kasai K, Leffler H, Liu F-T, Lotan R, Mercurio AM, Monsigny M, Pillai S, Poirer F, Raz A, Rigby PWJ, Rini JM, Wang JL. Galectins: a family of animal beta-galactoside-binding lectins. Cell. 1994;76:597–598. doi: 10.1016/0092-8674(94)90498-7. [DOI] [PubMed] [Google Scholar]
- 3.Choi JH, Chun KH, Raz A, Lotan R. Inhibition of N-(4-hydroxyphenyl)retinamide-induced apoptosis in breast cancer cells by galectin-3. Cancer Biol Ther. 2004;3:447–452. doi: 10.4161/cbt.3.5.808. [DOI] [PubMed] [Google Scholar]
- 4.Mazurek N, Sun YJ, Liu KF, Gilcrease MZ, Schober W, Nangia-Makker P, Raz A, Bresalier RS. Phosphorylated galectin-3 mediates tumor necrosis factor-related apoptosis-inducing ligand signaling by regulating phosphatase and tensin homologue deleted on chromosome 10 in human breast carcinoma cells. J Biol Chem. 2007;282:21337–21348. doi: 10.1074/jbc.M608810200. [DOI] [PubMed] [Google Scholar]
- 5.Yoshii T, Fukumori T, Honjo Y, Inohara H, Kim HR, Raz A. Galectin-3 phosphorylation is required for its antiapoptotic function and cell cycle arrest. J Biol Chem. 2002;277:6852–6857. doi: 10.1074/jbc.M107668200. [DOI] [PubMed] [Google Scholar]
- 6.Nangia-Makker P, Raz T, Tait L, Hogan V, Fridman R, Raz A. Galectin-3 cleavage: a novel surrogate marker for matrix metalloproteinase activity in growing breast cancers. Cancer Res. 2007;67:11760–11768. doi: 10.1158/0008-5472.CAN-07-3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balan V, Nangia-Makker P, Schwartz AG, Jung YS, Tait L, Hogan V, Raz T, Wang Y, Yang ZQ, Wu GS, Guo Y, Li H, Abrams J, Couch FJ, Lingle WL, Lloyd RV, Ethier SP, Tainsky MA, Raz A. Racial disparity in breast cancer and functional germ line mutation in galectin-3 (rs4644): a pilot study. Cancer Res. 68:10045–10050. doi: 10.1158/0008-5472.CAN-08-3224. 2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 9.Suliman A, Lam A, Datta R, Srivastava RK. Intracellular mechanisms of TRAIL: apoptosis through mitochondrial-dependent and -independent pathways. Oncogene. 2001;20:2122–2133. doi: 10.1038/sj.onc.1204282. [DOI] [PubMed] [Google Scholar]
- 10.Takeda K, Hayakawa Y, Smyth MJ, Kayagaki N, Yamaguchi N, Kakuta S, Iwakura Y, Yagita H, Okumura K. Involvement of tumor necrosis factor-related apoptosis-inducing ligand in surveillance of tumor metastasis by liver natural killer cells. Nat Med. 2001;7:94–100. doi: 10.1038/83416. [DOI] [PubMed] [Google Scholar]
- 11.Cretney E, Takeda K, Smytha MJ. Cancer: Novel therapeutic strategies that exploit the TNF-related apoptosis-inducing ligand (TRAIL)/TRAIL receptor pathway. Int J Biochem Cell Biol. 2007;39:280–286. doi: 10.1016/j.biocel.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Takenaka Y, Fukumori T, Yoshii T, Oka N, Inohara H, Kim HR, Bresalier RS, Raz A. Nuclear export of phosphorylated galectin-3 regulates its antiapoptotic activity in response to chemotherapeutic drugs. Mol Cell Biol. 2004;24:4395–4406. doi: 10.1128/MCB.24.10.4395-4406.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang L, Fang B. Mechanisms of resistance to TRAIL-induced apoptosis in cancer. Cancer Gene Therapy. 2005;12:228–237. doi: 10.1038/sj.cgt.7700792. [DOI] [PubMed] [Google Scholar]
- 14.Planchon SM, Waite KA, Eng C. The nuclear affairs of PTEN. J Cell Sci. 2008;121:249–253. doi: 10.1242/jcs.022459. [DOI] [PubMed] [Google Scholar]