Abstract
Key aspects of lysosomal function are affected by the ionic content of the lysosomal lumen and, therefore, by the ion permeability in the lysosomal membrane. Such functions include regulation of lysosomal acidification, a critical process in delivery and activation of the lysosomal enzymes, release of metals from lysosomes into the cytoplasm and the Ca2+-dependent component of membrane fusion events in the endocytic pathway. While the basic mechanisms of lysosomal acidification have been largely defined, the lysosomal metal transport system is not well understood. TRPML1 is a lysosomal ion channel whose malfunction is implicated in the lysosomal storage disease Mucolipidosis Type IV. Recent evidence suggests that TRPML1 is involved in Fe2+, Ca2+ and Zn2+ transport across the lysosomal membrane, ascribing novel physiological roles to this ion channel, and perhaps to its relatives TRPML2 and TRPML3 and illuminating poorly understood aspects of lysosomal function. Further, alterations in metal transport by the TRPMLs due to mutations or environmental factors may contribute to their role in the disease phenotype and cell death.
Introduction
Lysosomes are components of the endocytic pathway responsible for storage and processing of digestive enzymes and for terminal degradation as well as absorption of the endocytosed material [1–3]. Lysosomes also digest the cellular material delivered to autophagosomes during cell renewal and cell death [4–6]. In addition to digestive enzymes, lysosomes contain a system of transporters that play several important roles. These include: i) establishing controlled acidic pH in the lysosomes, ii) absorption of the products of digestion, iii) the release of Ca2+ from the lysosomal lumen that drives the fusion of lysosomes with late endosomes, and iv) transport of the metals bound to endocytosed proteins across the lysosomal membrane into the cytoplasm [1–3]. This list of lysosomal ion transport activity is incomplete and it is limited by the available information on the lysosomal transport pathways and by the scope of this review. TRPML channels appear to be prominent lysosomal metal transporters. Several lines of evidence associate the lysosomal ion channel TRPML1 and its relative TRPML3 with these functions and their possible role in cell death. This review aims to summarize this evidence and delineate the remaining questions.
TRPML1 was identified in a search for genetic determinants of the lysosomal storage disease (LSD) Mocolipidosis type IV (MLIV) [7, 8]. TRPML1 is a member of the TRP superfamily of ion channels, and a founding member of the mucolipin subfamily. The main structural features of the mucolipins are in line with other TRP channels with the exception of a large extracytoplasmic loop (luminal for TRPML1 localized in lysosomes and for TRPML2 and TRPML3 when in intracellular organelles) connecting the 1st and 2nd transmembrane domains and the presence of intracellular targeting signals in their C- and N-terminals responsible for localization of these channels in the vesicles comprising the endocytic pathway [9–12]. There are 3 mucolipins in mammals, coded by 3 genes: MCOLN1, MCOLN2 and MCOLN3. The presence of splice variants has been reported in mammals [13, 14], but their function and regulation is unknown at present. Drosophila and C. elegans appear to contain a single gene coding for a mucolipin channel [15–17]. The genetics and the pathogenesis of MLIV as well as expression and regulation of the mucolipins have been the subject of several recent reviews [9, 18, 19] and will not be discussed here further. Instead, we will focus on the recent evidence suggesting that TRPML1 is a lysosomal divalent metal transporter and the possible role of this activity in lysosomal function.
The localization of TRPML1 in the lysosomes and the clear LSD phenotype associated with TRPML1 downregulation suggest a prominent role of this ion channel in lysosomal digestive activity, biogenesis, and/or access to endocytosed material. This general division of lysosomal functions lists the three main topics for which experimental data were previously obtained as being altered in MLIV and thus assumed to be TRPML1 functions. Some insight pertaining to TRPML1 function can also be gleaned from the comparative analysis of localization and activity of TRPML1 and its close relative TRPML3. Whether and how TRPML1 affects lysosomal digestive activity, biogenesis, and/or access to endocytosed material to digestive enzymes is still scarcely delineated. Recent results suggest exciting new possibilities and developments in the mucolipins’ role in the endocytic pathway.
TRPML1 as a lysosomal Ca2+ channel in membrane trafficking
The evidence that TRPML1 functions as a lysosomal Ca2+ release channel relies on the findings that the TRPML channels are permeable to Ca2+ with higher selectivity for Ca2+ than for monovalent cations [20] and that TRPML1 is a lysosomal protein [11, 12, 21]. Membrane trafficking deficits [16, 22], as well as impaired fusion of lysosomes with autophagosomes in TRPML1 deficient cells [23] support the role of TRPML1-mediated Ca2+ release in the Ca2+ dependent membrane fusion along the endocytic pathway. Limitations of the chronic lysosomal storage disease models as experimental systems for studying membrane traffic have been discussed before [24, 25], including the fact that the same delays in membrane traffic were seen in LSDs whose pathogenesis is entirely enzymatic and has little direct relevance to membrane fusion or fission [26, 27]. The post-lysosomal lipid translocation defect observed in the acute TRPML1 knockdown model [24] can be interpreted as a post-lysosomal trafficking defect, or a degradation deficit. Nonetheless, the TRPML1 relative TRPML3 was shown to conduct Ca2+ [28–33] and TRPML3 downregulation by siRNA induced trafficking deficits in two model systems [28, 34]. It is important to note that TRPML3 function is inhibited, while TRPML1 function is potentiated [30, 31, 35] by the low pH typical of lysosomes. TRPML3 localizes in the upper (earlier), relative to TRPML1, endocytic pathway compartments, whose acidity is lower than the lysosomal acidity. Taken together, the Ca2+ permeability through TRPML1 and TRPML3 and their pH-dependence, matching their localization, support the idea that these channels regulate membrane fusion in specific compartments of the endocytic pathway.
Some of the requirements for TRPML1 to function as a Ca2+ release channel that triggers the membrane fusion in the endocytic pathway include: a) an activation signal that is relevant to endosomal function, b) the dependence of lysosomal Ca2+ content on TRPML1 status and c) perhaps changes in cellular Ca2+ signaling as a function of TRPML1 status since lysosomes have been shown to contribute to the receptor dependent Ca2+ signaling in several tissues [36–40]. Ca2+ signaling in human [41] and mouse [42] cells lacking TRPML1 appears normal with regard to Ca2+ release from internal stores and Ca2+ influx across the plasma membrane, with the exception of aberrant mitochondrial function [43]. However, aberrant mitochondrial function in TRPML1 deficient cells is likely secondary to the inhibited lysosomal function as the same Ca2+ phenotype have been reported in other models of lysosomal storage diseases [36, 37]. The reports on lysosomal Ca2+ content in TRPML1 deficient cells are sparse. Our data on MLIV fibroblasts showed no changes in the Ca2+ pool that is released into the cytoplasm by bursting the lysosomes with osmotic shock [43]. It is important to note that lysosomal Ca2+ changes have been reported in Niemann-Pick [36, 37], a disease not directly related to ion channel activity. Thus, such changes can be attributed to the effects of storage accumulation, which may bind Ca2+ and thus interpretations of such results should be done with caution.
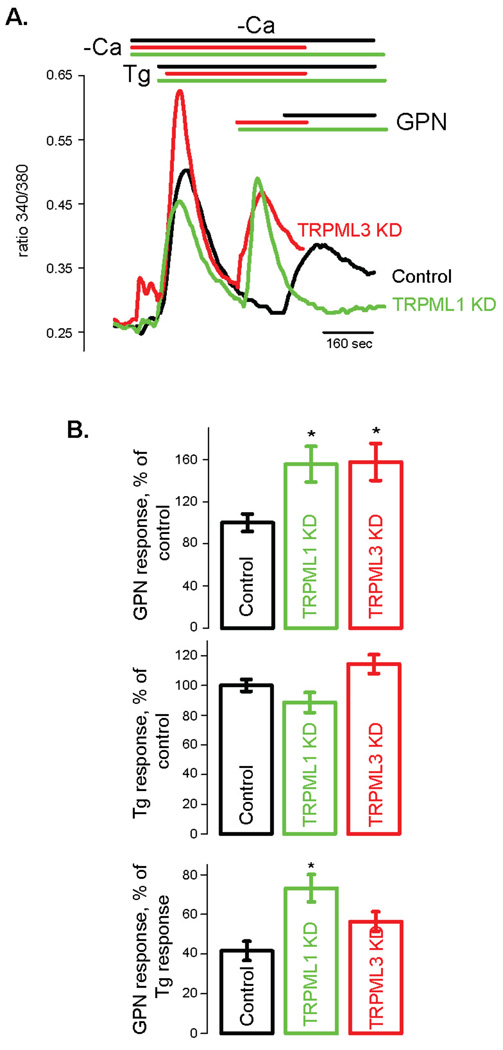
In order to test lysosomal Ca2+ homeostasis in a system that is free of artifacts due to clonal variations and effects of storage material, we directly measured lysosomal Ca2+ while manipulating TRPML1 function. Fig. 1 shows an assay of lysosomal Ca2+ content based on Ca2+ released by glycyl-L-phenylalanine 2-naphthylamide (GPN) [44, 45] into the cytoplasm of acutely TRPML1 deficient HeLa cells. The cells were treated with TRPML1 siRNA for 48 hours. In a previous study we show that such timeframe is associated with minimal buildup of storage bodies [24]. The results show a statistically significant increase in a GPN-releasable Ca2+ pool in TRPML1 deficient HeLa cells. Recently, it was reported that the endosomal TRPML3 [28] may participate in Ca2+ homeostasis of intracellular organelles [34]. Interestingly, Fig. 1 shows that cells deficient in TRPML3 have increased lysosomal Ca2+ levels comparable to TRPML1 deficient cells. However, when considered relative to the size of total endoplasmic reticulum Ca2+ pool in the given cell type, only TRPML1 deficient cells show an increase in the lysosomal Ca2+ pool. This would suggest that depletion of TRPML3 results in expansion of the entire intracellular Ca2+ pool, including the ER pool. It is unclear, at present, why the ER Ca2+ pool is larger in TRPML3 deficient cells considering that TRPML3 is not constitutively expressed in the ER [28, 34].
Figure 1. Lysosomal Ca2+ content is increased in TRPML1 deficient HeLa cells.
HeLa cells were transfected with TRPML1 siRNA as described before [79] and cytoplasmic Ca2+ was measured 48 hours later using Fura 2AM. A) Ca2+ traces (cytoplasmic Ca2+ is proportional to the ratio of Fura 2AM fluorescence ratio measured at 340 and 380 nm excitation light). Lysosomes were burst using exracellular application of glycyl-L-phenylalanine 2-naphthylamide (GPN, 100 µM) [44]. Thapsigargin (Tg, 1 µM) was applied before GPN in order to deplete Ca2+ in the endoplasmic reticulum and remove its contribution to the GPN-induced Ca2+ release. Data represent 3–10 experiments and are expressed as mean ± S.E.M. * denotes p<0.05. B) Statistical analysis of Ca2+ measurements. Top: amplitudes of GPN-induced Ca2+ release expressed as a percentage of Ca2+ release in control cells. Middle: Tg-induced Ca2+ release expressed as a percentage of Ca2+ release in control cells. Bottom: the ratios of GPN-induced Ca2+ release to Tg-induced Ca2+ release in control, TRPML1 and TRPML3 deficient cells.
Recent work clearly identified the two-pore-channels (TPCs) as organellar Ca2+ channels, where TPC2 functions as a lysosomal Ca2+ channel that is activated by the second messenger NAADP [38, 46–49]. This topic is covered by several reviews in the recent Special Issue of Cell Calcium and the reader is referred to these reviews. It is important to note that the TRP activation by NAADP suggests a clear paradigm of lysosomal Ca2+ signaling, where oscillations in the cytoplasmic NAADP content facilitate TPC activation and, therefore, Ca2+ release followed by fusion. Up until recently, such a paradigm was not demonstrated for TRPML1 or other TRPML channels. Hence, although manipulation of TRPML1 (Fig. 1) and TRPML3 [34] affect lysosomal storage of Ca2+, active Ca2+ release from the lysosomes due to activation of any TRPML channel was not demonstrated, as was shown for the TPCs and their activation by NAADP. Nevertheless, if the TRPMLs function as organellar Ca2+ channels in the endocytic pathway, they may mediate distinct steps than are different than those regulated by the TPCs. It is also possible that the TRPMLs functions mainly in the endocytic pathway while the TPCs mediate fusion events in the exocytic pathway. It is also possible that the TRPMLs may have additional functions that are not related to Ca2+ homeostasis.
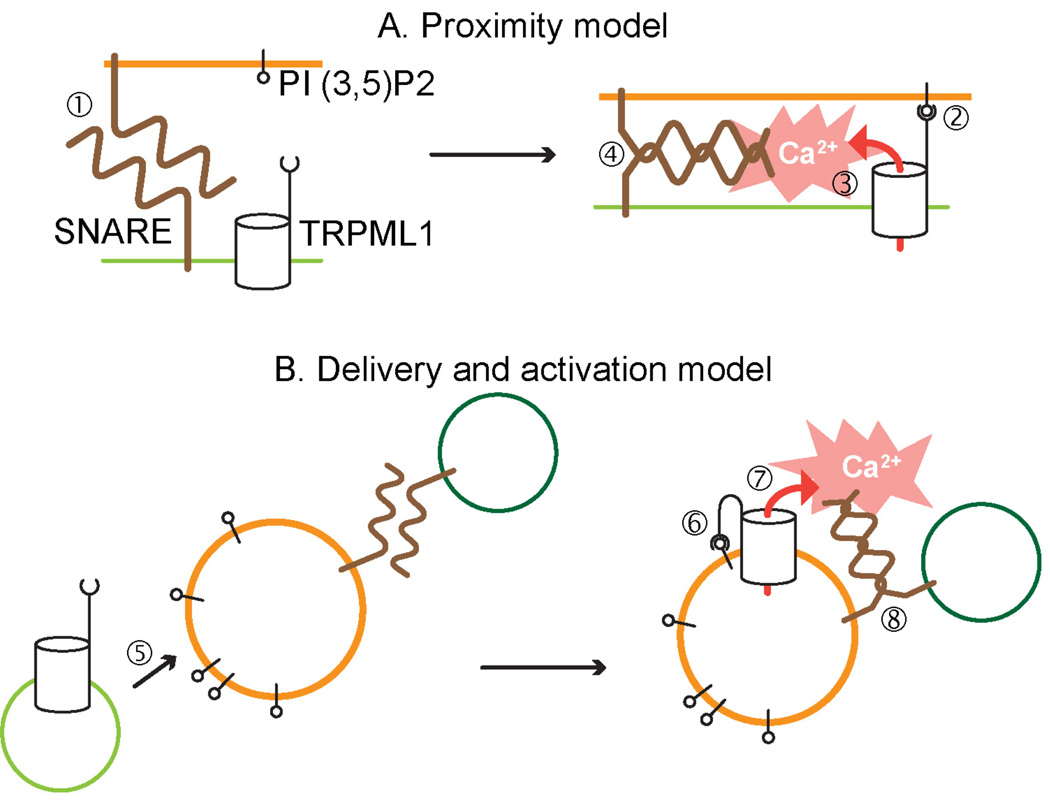
The recent finding that TRPML1 is activated by PI(3,5)P2 [50] suggests exciting new possibilities to explain the TRPML1 function in the endocytic pathway. Adapting the whole cell patch clamp technique to patching lysosomes enlarged by vacuolin, revealed that recombinant as well as native TRPML1 channels are activated by PI(3,5)P2 added to the cytoplamic side of the lysosomes. PI(3,5)P2 appears to effect TRPML1 activation through the TRPML1 C-terminal, since removal of the C-terminus abolishes the TRPML1 activation. A crucial point is that PI(3,5)P2 is localized in the distal portion of the endocytic pathway, the site of TRPML1 localization. Activation of TRPML1 by PI(3,5)P2 lends support to the proposed function of TRPML1 in membrane fusion in the distal portion of the endocytic pathway. It poses questions regarding the mechanics of PI(3,5)P2 effect on TRPML1 within the context of membrane interaction within the endocytic pathway. It is tempting to suggest that TRPML1 binding to PI(3,5)P2 in the opposing membrane of the interacting organelle triggers Ca2+ release, that actuates the SNARE-mediated fusion machinery. In this sense, the C-terminus of TRPML1 may work as a proximity sensor for the fusing organelles (Fig. 2). It is also possible, and has been recently proposed [51], that TRPML1 is activated by PI(3,5)P2 present within the same membrane. In this case, PI(3,5)P2 may work as a potentiating agent, which assures TRPML1 activation once it reaches the proper (PI(3,5)P2 enriched) membrane compartment.
Figure 2. Models of the role of the activation of TRPML1 by PI(3,5)P2 in the endocytic pathway.
A) TRPML1 as an organellar proximity sensor: TRPML1 activation by PI(3,5)P2 in the opposing membrane promotes organellar fusion. The lack of Ca2+ release through TRPML1 in the absence of PI(3,5)P2 binding precludes conformational change in SNARE that promotes fusion (step 1). The proximity of TRPML1-containing and target membranes promotes TRPML1 interaction with PI(3,5)P2 (step 2), Ca2+ release through TRPML1 (step 3) and membrane fusion (step 4). B) TRPML1 activation by delivery to PI(3,5)P2-rich compartments. TRPML1 is inactive in the delivery vesicles, but its activation is promoted by its delivery to PI (3,5)P2-rich organelles (steps 5 and 6). This is followed by Ca2+ release through TRPML1 (step 7) and membrane fusion (step 8).
The fact that TRPML1 down-regulation does not induce gross inactivation of membrane fusion in the endocytic pathway indicates that the proposed Ca2+ release through TRPML1 is responsible for only a subset of the endocytic fusion events. It is, therefore, possible that mucolipins, TPC, and, perhaps, yet unidentified channels cooperate to ensure the membrane flow through the endocytic pathway. Additionally, the recent set of data implicating TRPML channels in transition metal (TM) transport raises the possibility that TRPML channels combine functions of membrane fusion and TM uptake. We will argue here that a potentially central function of the TRPML channels is transport of TM that are byproducts of endocytic capture and degradation, for their transport out of the lysosomes and clearance by the cells.
Transition metal absorption through mucolipins
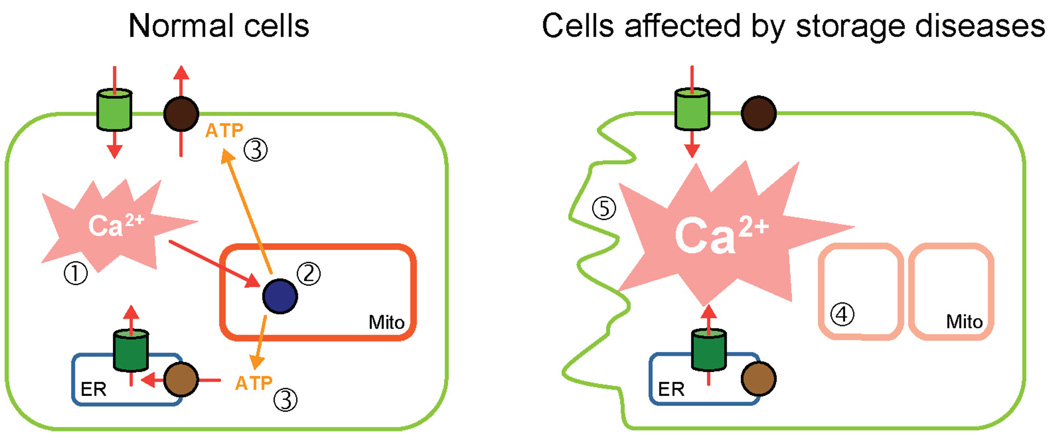
When cells engulf external medium during endocytosis, they capture extracellular macromolecular complexes that are almost inevitably bound to metal ions, including transition metals (TM), a group that contains chemically active elements such as Cu2+, Fe2+, Fe3+, Zn2+ and Co2+. Under normal conditions, the absolute majority of TMs are bound to plasma proteins such as albumin, transferrin and ceruloplasmin [52–54]. Degradation of these proteins in the endocytic pathway releases the metals into the lysosomal lumen. The active chemical environment of the lysosomal lumen promotes Fenton-like chemical reactions resulting in generation of free radicals, which are damaging to the lysosomal membrane and result in the production of the inert chemical compound called lipofuscin [55, 56]. The acute exposure to high concentrations of TMs has been linked to lysosomal storage phenotypes [57–59] and to lysosomal permeabilization [60, 61]. The long-term exposure to transition metals, e.g. in postmitotic cells, has been linked to buildup of lipofuscin, which inhibits lysosomal function and autophagy [62, 63]. Autophagy deficits have been shown in several models of LSDs [17, 23, 43, 64–70] and in TMs overload [71, 72]. According to the lysosomal-mitochondria axis models of aging and cell death in lysosomal storage diseases, the suppression of autophagy results in buildup of dysfunctional organelles, specifically mitochondria [62, 63]. We suggested that the loss of mitochondrial Ca2+ buffering capacity and of the Ca2+-driven ATP production positive feedback loop makes cells vulnerable to the pro-apoptotic effects of stimulation with Ca2+ mobilizing agonists (neurotransmitters, hormones, growth factors) (Fig. 3) [43, 69].
Figure 3. A model depicting mitochondrial deterioration and cell death in storage diseases.
In the normal cells (left) Ca2+ fluxes induced by hormones and neurotransmitters (step 1) are buffered by the energized mitochondria. The Ca2+ dependent components of oxidative phosphorylation chain (step 2) respond by producing more ATP. The resulting spike in ATP promotes Ca2+ extrusion and prevents pro-apoptotic effects of Ca2+. In cells with lysosomal storage diseases (right), the general mitochondrial function is impaired due to buildup of dysfunctional mitochondria (step 4), resulting in the loss of Ca2+/ATP-driven feedback loop, which leads to pro-apoptotic effects of Ca2+ and cell death.
The detrimental effects of transition metals in the lysosomes, combined with the cellular requirements for transition metals necessitate extraction of the metals from the lysosomal lumen into the cytoplasm, where their effects can be mitigated by chelating proteins or by extrusion out of the cell [52, 54]. Existence of such mechanisms has been shown before. Specifically, SLC11A1 (NRAMP1) and SLC11A2 (NRAMP2, DMT1) are ion transporters responsible for divalent cation uptake by cells [53, 54]. The intracellular localization of SLC11A2 and its apparent role in export of divalent cations from the lumen of lysosomes led to the suggestion that SLC11A2 is, or is part of, the endocytic divalent cation absorption pathway [53, 54]. As discussed above, it is possible that a range of ion channels may be involved in the endocytic function. The recently published data suggest that NRAMP may not be the only TMs absorption mechanism in the endocytic pathway [73, 74].
At the time of publication of the original report, the TRPML1 activation pathway has not been known and therefore activating mutations have been introduced into TRPML1 in order to detect current carried by this channel. The mutations mimic the mutation causing the varitint-waddler phenotype in TRPML3 [29–33, 35, 75], which is a spontaneously arisen gain-of-function mutation in mice leading to pigmentaiton defects and hearing loss [76]. Using this approach, TRPML1 was found to be permeable to Fe2+ [74]. TRPML1 was proposed to function as a lysosomal Fe2+ leak channel that exports Fe2+ from the lysosomal lumen into the cytopasm to prevent Fenton reactions and the buildup of lipofuscin catalysed in the lysosomes by Fe2+. The previously demonstrated buildup of auto-fluorescent material in MLIV patient skin fibroblasts was interpreted as lipofuscin and byproduct of abnormally high lysosomal Fe2+ levels [74]. MLIV patient fibroblasts were shown to contain higher levels of Fe2+ than matched controls, that is presumably present in the lysosomes [74].
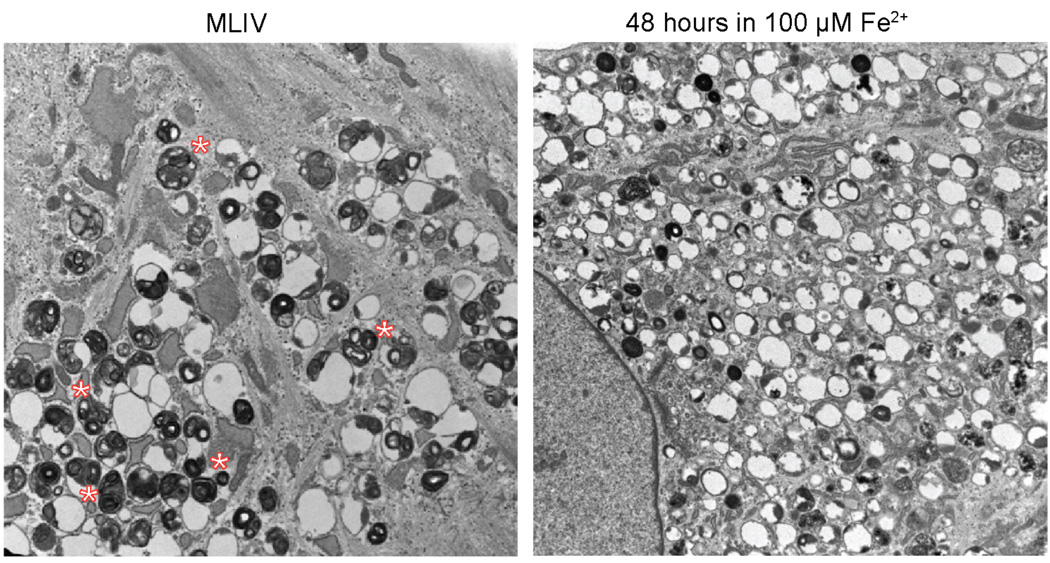
The central paradigm of the Fe2+-transport model of TRPML1 function and MLIV pathogenesis is that TRPML1 is a lysosomal Fe2+ leak pathway, in the absence of which lysosomes become overloaded with Fe2+, leading to the loss of lysosomal function. One would expect then that the lysosomal phenotype of TRPML1 loss should be the same as the lysosomal phenotype of Fe2+ overload. Fig. 4 shows electron micrographs of fibroblasts obtained from MLIV patient and of fibroblasts obtained from a heterozygous relative that were treated with 100 µM of Fe2+ for 48 hours. Close analysis of storage bodies in the two samples shows that morphology of the inclusions present in Fe2+ treated cells is different from those in present MLIV knockdown cells. Therefore, the effects of TRPML1 loss and lysosomal Fe overload do not completely overlap.
Figure 4. Analysis of inclusion bodies in MLIV fibroblasts and fibroblasts treated with Fe2+.
Left: electron micrograph of an MLIV fibroblast (clone WG0909 from the McGill University cell line collection). Right: electron micrograph of a control fibroblast (clone WG0987 from the McGill University cell line collection, which is a heterozygous relative of WG0909) treated with 100 µM Fe2+ for 48 hours. Note the difference in inclusion morphology: the presence of large numbers of inclusions containing enfolded membranes in MLIV cells (designated by *), which are rare or absent in Fe2+-treated cells.
The second line of evidence for TRPML1 acting as a lysosomal heavy metal transporter comes from recent demonstration of Zn2+ accumulation in MLIV fibroblasts [73]. Using a combination of electrophysiology and chemical analysis, Zn2+ permeability through TRPML1 was observed, alongside with an increase in total cellular and lysosomal Zn2+ content. In these studies, the mutant, “activated”, form of TRPML1 was used as well. It is unclear exactly how well the TRPML1 “activated” by introducing the varitint-waddler mutations approximates the function of the wild type channel. In fact at least some of the aspects of TRPML physiology, such as regulation by pH, seem to dramatically change in the “activated” forms [35]. In TRPML3, the activation mutation changes the pore properties of the channel and its selectivity to divalent ions [77]. Demonstrating Fe2+ and Zn2+ permeability in wild type channel activated by PI(3,5)P2 would help resolved this question. Another question that remains to be resolved is whether the net increase in the metals associated with storage bodies can be explained by their binding to the undigested material. It is also important to note that the cellular response to TRPML1 loss may involve changes in expression of proteins that handle TMs. Microarray analyses of MLIV fibroblasts revealed a large number of genes whose activity changed, ostensibly as a result of TRPML1 loss [78]. It is possible and, indeed, likely, that some of these changes may affect transport of TMs across the endocytic membranes, or their retention by the storage bodies.
Despite of these questions, the findings discussed above open an interesting possibility for existence of a novel metal export pathway from the lysosomes that their aberrant function in MLIV and perhaps other LSDs play a critical role in the disease phenotype. Further studies, including comparative analysis of SLC11A1 and TRPML1 localization together with analysis of Fe2+ buildup in different tissues will be important for solidifying this exciting model.
Summary
Since its identification in 2000, TRPML1 provided an exciting opportunity to learn about lysosomal function as well as the function of the entire cell. Further advances in understanding TRPML1 transport and activation properties are likely to facilitate our understanding of such central cellular processes as metal handling and the fusion of organelles in the endocytic pathway. If TRPML1 functions not as a Ca2+ channel but as a heavy metals channel that have important role in fusion events associated with membrane trafficking, it will be of paramount interest to find out how aberrant heavy metals transport by the lysosomes leads to defective endocytosis. The central paradigms learned in this process are likely to extend beyond TRPML1 and MLIV pathogenesis, and to other biological processes and molecules. Furthermore, since a number of system-wide processes such as growth factor signaling and antigen handling depend on lysosomal function, TRPML1 activation and transport mechanisms are likely to affect research beyond the storage disease area.
Among other important questions raised by the TRPML1 ion transport and activation mechanism described above are the cell wide responses to TRPML1 loss and TRPML1 interaction with other transport molecules and regulatory proteins. As discussed above, the relative contribution of SLC11A2 and TRPML1 in divalent metals export from the lysosomes is unclear and comparative analysis of their localization would be extremely useful in delineating their roles. Similarly, recent identification of the TPCs as lysosomal Ca2+ channels raises the question of the role sharing between these two types of channels. Answering these and other questions pertaining to the activity of the lysosomal ion transport pathways will unquestionably improve our understanding of the lysosomal function and the cell- and organism-level activities that depend on it.
Acknowledgements
This work was supported by the National Institutes of Health grants HD058577 and ES01678 to Kirill Kiselyov by the intramural research funds of the NIH/NIDCR ZIA DE000735-01 to S.M. The authors would like to thank Drs. Rosa Puertollano, Emir Lloyd-Evans and Haoxing Xu for fruitful discussion.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ruivo R, et al. Molecular and cellular basis of lysosomal transmembrane protein dysfunction. Biochim Biophys Acta. 2009;1793(4):636–649. doi: 10.1016/j.bbamcr.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 2.Jentsch TJ. Chloride and the endosomal-lysosomal pathway: emerging roles of CLC chloride transporters. J Physiol. 2007;578(Pt 3):633–640. doi: 10.1113/jphysiol.2006.124719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luzio JP, et al. Membrane traffic to and from lysosomes. Biochem Soc Symp. 2005;(72):77–86. doi: 10.1042/bss0720077. [DOI] [PubMed] [Google Scholar]
- 4.Codogno P, Meijer AJ. Autophagy and signaling: their role in cell survival and cell death. Cell Death Differ. 2005;12 Suppl 2:1509–1518. doi: 10.1038/sj.cdd.4401751. [DOI] [PubMed] [Google Scholar]
- 5.Cuervo AM. Autophagy: in sickness and in health. Trends Cell Biol. 2004;14(2):70–77. doi: 10.1016/j.tcb.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Eskelinen EL, Saftig P. Autophagy: a lysosomal degradation pathway with a central role in health and disease. Biochim Biophys Acta. 2009;1793(4):664–673. doi: 10.1016/j.bbamcr.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 7.Bassi MT, et al. Cloning of the gene encoding a novel integral membrane protein, mucolipidin-and identification of the two major founder mutations causing mucolipidosis type IV. Am J Hum Genet. 2000;67(5):1110–1120. doi: 10.1016/s0002-9297(07)62941-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun M, et al. Mucolipidosis type IV is caused by mutations in a gene encoding a novel transient receptor potential channel. Hum Mol Genet. 2000;9(17):2471–2478. doi: 10.1093/hmg/9.17.2471. [DOI] [PubMed] [Google Scholar]
- 9.Colletti GA, Kiselyov K. Trpml1. Adv Exp Med Biol. 2011;704:209–219. doi: 10.1007/978-94-007-0265-3_11. [DOI] [PubMed] [Google Scholar]
- 10.Puertollano R, Kiselyov K. TRPMLs: in sickness and in health. Am J Physiol Renal Physiol. 2009;296(6):F1245–F1254. doi: 10.1152/ajprenal.90522.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vergarajauregui S, Puertollano R. Two di-leucine motifs regulate trafficking of mucolipin-1 to lysosomes. Traffic. 2006;7(3):337–353. doi: 10.1111/j.1600-0854.2006.00387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miedel MT, et al. Posttranslational cleavage and adaptor protein complex-dependent trafficking of mucolipin-1. J Biol Chem. 2006;281(18):12751–12759. doi: 10.1074/jbc.M511104200. [DOI] [PubMed] [Google Scholar]
- 13.Goldin E, et al. Transfer of a mitochondrial DNA fragment to MCOLN1 causes an inherited case of mucolipidosis IV. Hum Mutat. 2004;24(6):460–465. doi: 10.1002/humu.20094. [DOI] [PubMed] [Google Scholar]
- 14.Falardeau JL, et al. Cloning and characterization of the mouse Mcoln1 gene reveals an alternatively spliced transcript not seen in humans. BMC Genomics. 2002;3(1):3. doi: 10.1186/1471-2164-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fares H, Greenwald I. Regulation of endocytosis by CUP-5, the Caenorhabditis elegans mucolipin-1 homolog. Nat Genet. 2001;28(1):64–68. doi: 10.1038/ng0501-64. [DOI] [PubMed] [Google Scholar]
- 16.Treusch S, et al. Caenorhabditis elegans functional orthologue of human protein h-mucolipin-1 is required for lysosome biogenesis. Proc Natl Acad Sci U S A. 2004;101(13):4483–4488. doi: 10.1073/pnas.0400709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Venkatachalam K, et al. Motor deficit in a Drosophila model of mucolipidosis type IV due to defective clearance of apoptotic cells. Cell. 2008;135(5):838–851. doi: 10.1016/j.cell.2008.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng X, et al. Mucolipins: Intracellular TRPML1–3 channels. FEBS Lett. 2010;584(10):2013–2021. doi: 10.1016/j.febslet.2009.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abe K, Puertollano R. Role of TRP channels in the regulation of the endosomal pathway. Physiology (Bethesda) 26(1):14–22. doi: 10.1152/physiol.00048.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.LaPlante JM, et al. Functional links between mucolipin-1 and Ca2+-dependent membrane trafficking in mucolipidosis IV. Biochem Biophys Res Commun. 2004;322(4):1384–1391. doi: 10.1016/j.bbrc.2004.08.045. [DOI] [PubMed] [Google Scholar]
- 21.Kiselyov K, et al. TRP-ML1 is a lysosomal monovalent cation channel that undergoes proteolytic cleavage. J Biol Chem. 2005;280(52):43218–43223. doi: 10.1074/jbc.M508210200. [DOI] [PubMed] [Google Scholar]
- 22.Thompson EG, et al. Lysosomal trafficking functions of mucolipin-1 in murine macrophages. BMC Cell Biol. 2007;8:54. doi: 10.1186/1471-2121-8-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vergarajauregui S, et al. Autophagic dysfunction in mucolipidosis type IV patients. Hum Mol Genet. 2008;17(17):2723–2737. doi: 10.1093/hmg/ddn174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miedel M, et al. Membrane traffic and turnover in TRP-ML1-deficient cells: a revised model for mucolipidosis type IV pathogenesis. Journal of Experimental Medicine. 2008;205(6):1477–1490. doi: 10.1084/jem.20072194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kiselyov K, Soyombo A, Muallem S. TRPpathies. J Physiol. 2007;578(Pt 3):641–653. doi: 10.1113/jphysiol.2006.119024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pagano RE. Endocytic trafficking of glycosphingolipids in sphingolipid storage diseases. Philos Trans R Soc Lond B Biol Sci. 2003;358(1433):885–891. doi: 10.1098/rstb.2003.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zeevi DA, Frumkin A, Bach G. TRPML and lysosomal function. Biochim Biophys Acta. 2007;1772(8):851–858. doi: 10.1016/j.bbadis.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 28.Kim HJ, et al. The Ca(2+) channel TRPML3 regulates membrane trafficking and autophagy. Traffic. 2009;10(8):1157–1167. doi: 10.1111/j.1600-0854.2009.00924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagata K, et al. The varitint-waddler (Va) deafness mutation in TRPML3 generates constitutive, inward rectifying currents and causes cell degeneration. Proc Natl Acad Sci U S A. 2008;105(1):353–358. doi: 10.1073/pnas.0707963105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu H, et al. Activating mutation in a mucolipin transient receptor potential channel leads to melanocyte loss in varitint-waddler mice. Proc Natl Acad Sci U S A. 2007;104(46):18321–18326. doi: 10.1073/pnas.0709096104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cuajungco MP, Samie MA. The varitint-waddler mouse phenotypes and the TRPML3 ion channel mutation: cause and consequence. Pflugers Arch. 2008;457(2):463–473. doi: 10.1007/s00424-008-0523-4. [DOI] [PubMed] [Google Scholar]
- 32.Kim HJ, et al. Gain-of-function mutation in TRPML3 causes the mouse Varitint-Waddler phenotype. J Biol Chem. 2007;282(50):36138–36142. doi: 10.1074/jbc.C700190200. [DOI] [PubMed] [Google Scholar]
- 33.Grimm C, et al. A helix-breaking mutation in TRPML3 leads to constitutive activity underlying deafness in the varitint-waddler mouse. Proc Natl Acad Sci U S A. 2007;104(49):19583–19588. doi: 10.1073/pnas.0709846104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martina JA, Lelouvier B, Puertollano R. The calcium channel mucolipin-3 is a novel regulator of trafficking along the endosomal pathway. Traffic. 2009;10(8):1143–1156. doi: 10.1111/j.1600-0854.2009.00935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim HJ, et al. A novel mode of TRPML3 regulation by extracytosolic pH absent in the varitint-waddler phenotype. Embo J. 2008;27(8):1197–1205. doi: 10.1038/emboj.2008.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lloyd-Evans E, et al. Niemann-Pick disease type C1 is a sphingosine storage disease that causes deregulation of lysosomal calcium. Nat Med. 2008;14(11):1247–1255. doi: 10.1038/nm.1876. [DOI] [PubMed] [Google Scholar]
- 37.Pelled D, et al. Inhibition of calcium uptake via the sarco/endoplasmic reticulum Ca2+-ATPase in a mouse model of Sandhoff disease and prevention by treatment with N-butyldeoxynojirimycin. J Biol Chem. 2003;278(32):29496–29501. doi: 10.1074/jbc.M302964200. [DOI] [PubMed] [Google Scholar]
- 38.Zong X, et al. The two-pore channel TPCN2 mediates NAADP-dependent Ca(2+)-release from lysosomal stores. Pflugers Arch. 2009;458(5):891–899. doi: 10.1007/s00424-009-0690-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kinnear NP, et al. Lysosomes co-localize with ryanodine receptor subtype 3 to form a trigger zone for calcium signalling by NAADP in rat pulmonary arterial smooth muscle. Cell Calcium. 2008;44(2):190–201. doi: 10.1016/j.ceca.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Umesh A, et al. Integrin ligands mobilize Ca2+ from ryanodine receptor-gated stores and lysosome-related acidic organelles in pulmonary arterial smooth muscle cells. J Biol Chem. 2006;281(45):34312–34323. doi: 10.1074/jbc.M606765200. [DOI] [PubMed] [Google Scholar]
- 41.Soyombo AA, et al. TRP-ML1 regulates lysosomal pH and acidic lysosomal lipid hydrolytic activity. J Biol Chem. 2006;281(11):7294–7301. doi: 10.1074/jbc.M508211200. [DOI] [PubMed] [Google Scholar]
- 42.Chandra M, et al. A Role for the Ca(2+) Channel TRPML1 in Gastric Acid Secretion, Based on Analysis of Knockout Mice. Gastroenterology. 2011;140(3):857–867. doi: 10.1053/j.gastro.2010.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jennings JJ, Jr, et al. Mitochondrial aberrations in mucolipidosis Type IV. J Biol Chem. 2006;281(51):39041–39050. doi: 10.1074/jbc.M607982200. [DOI] [PubMed] [Google Scholar]
- 44.Berg TO, et al. Separation of lysosomes and autophagosomes by means of glycyl-phenylalanine-naphthylamide, a lysosome-disrupting cathepsin-C substrate. Eur J Biochem. 1994;221(1):595–602. doi: 10.1111/j.1432-1033.1994.tb18771.x. [DOI] [PubMed] [Google Scholar]
- 45.Haller T, et al. The lysosomal compartment as intracellular calcium store in MDCK cells: a possible involvement in InsP3-mediated Ca2+ release. Cell Calcium. 1996;19(2):157–165. doi: 10.1016/s0143-4160(96)90084-6. [DOI] [PubMed] [Google Scholar]
- 46.Calcraft PJ, et al. NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature. 2009;459(7246):596–600. doi: 10.1038/nature08030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brailoiu E, et al. Essential requirement for two-pore channel 1 in NAADP-mediated calcium signaling. J Cell Biol. 2009;186(2):201–209. doi: 10.1083/jcb.200904073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pitt SJ, et al. TPC2 is a novel NAADP-sensitive Ca2+ release channel, operating as a dual sensor of luminal pH and Ca2+ J Biol Chem. 2010;285(45):35039–35046. doi: 10.1074/jbc.M110.156927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schieder M, et al. Characterization of two-pore channel 2 (TPCN2)-mediated Ca2+ currents in isolated lysosomes. J Biol Chem. 2010;285(28):21219–21222. doi: 10.1074/jbc.C110.143123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dong XP, et al. PI(3,5)P(2) controls membrane trafficking by direct activation of mucolipin Ca(2+) release channels in the endolysosome. Nat Commun. 2010;1(4):38. doi: 10.1038/ncomms1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abe K, Puertollano R. Role of TRP Channels in the Regulation of the Endosomal Pathway. Physiology (Bethesda) 2011;26(1):14–22. doi: 10.1152/physiol.00048.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harris ED. Cellular copper transport and metabolism. Annu Rev Nutr. 2000;20:291–310. doi: 10.1146/annurev.nutr.20.1.291. [DOI] [PubMed] [Google Scholar]
- 53.Morgan EH, Oates PS. Mechanisms and regulation of intestinal iron absorption. Blood Cells Mol Dis. 2002;29(3):384–399. doi: 10.1006/bcmd.2002.0578. [DOI] [PubMed] [Google Scholar]
- 54.Andrews NC, Schmidt PJ. Iron homeostasis. Annu Rev Physiol. 2007;69:69–85. doi: 10.1146/annurev.physiol.69.031905.164337. [DOI] [PubMed] [Google Scholar]
- 55.Brunk UT, Jones CB, Sohal RS. A novel hypothesis of lipofuscinogenesis and cellular aging based on interactions between oxidative stress and autophagocytosis. Mutat Res. 1992;275(3–6):395–403. doi: 10.1016/0921-8734(92)90042-n. [DOI] [PubMed] [Google Scholar]
- 56.Terman A, Brunk UT. Lipofuscin. Int J Biochem Cell Biol. 2004;36(8):1400–1404. doi: 10.1016/j.biocel.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 57.Cisternas FA, et al. Early histological and functional effects of chronic copper exposure in rat liver. Biometals. 2005;18(5):541–551. doi: 10.1007/s10534-005-1244-1. [DOI] [PubMed] [Google Scholar]
- 58.Schneider P, Korolenko TA, Busch U. A review of drug-induced lysosomal disorders of the liver in man and laboratory animals. Microsc Res Tech. 1997;36(4):253–275. doi: 10.1002/(SICI)1097-0029(19970215)36:4<253::AID-JEMT4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 59.Eaton JW, Qian M. Molecular bases of cellular iron toxicity. Free Radic Biol Med. 2002;32(9):833–840. doi: 10.1016/s0891-5849(02)00772-4. [DOI] [PubMed] [Google Scholar]
- 60.Terman A, et al. Lysosomal labilization. IUBMB Life. 2006;58(9):531–539. doi: 10.1080/15216540600904885. [DOI] [PubMed] [Google Scholar]
- 61.Pourahmad J, Ross S, O'Brien PJ. Lysosomal involvement in hepatocyte cytotoxicity induced by Cu(2+) but not Cd(2+) Free Radic Biol Med. 2001;30(1):89–97. doi: 10.1016/s0891-5849(00)00450-0. [DOI] [PubMed] [Google Scholar]
- 62.Brunk UT, Terman A. The mitochondrial-lysosomal axis theory of aging: accumulation of damaged mitochondria as a result of imperfect autophagocytosis. Eur J Biochem. 2002;269(8):1996–2002. doi: 10.1046/j.1432-1033.2002.02869.x. [DOI] [PubMed] [Google Scholar]
- 63.Stroikin Y, et al. Inhibition of autophagy with 3-methyladenine results in impaired turnover of lysosomes and accumulation of lipofuscin-like material. Eur J Cell Biol. 2004;83(10):583–590. doi: 10.1078/0171-9335-00433. [DOI] [PubMed] [Google Scholar]
- 64.Ballabio A, Gieselmann V. Lysosomal disorders: from storage to cellular damage. Biochim Biophys Acta. 2009;1793(4):684–696. doi: 10.1016/j.bbamcr.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 65.Takamura A, et al. Enhanced autophagy and mitochondrial aberrations in murine G(M1)-gangliosidosis. Biochem Biophys Res Commun. 2008;367(3):616–622. doi: 10.1016/j.bbrc.2007.12.187. [DOI] [PubMed] [Google Scholar]
- 66.Walls KC, et al. Altered Regulation of Phosphatidylinositol 3-kinase Signaling in Cathepsin D-Deficient Brain. Autophagy. 2007;3(3) doi: 10.4161/auto.3822. [DOI] [PubMed] [Google Scholar]
- 67.Pan T, et al. The role of autophagy-lysosome pathway in neurodegeneration associated with Parkinson's disease. Brain. 2008;131(Pt 8):1969–1978. doi: 10.1093/brain/awm318. [DOI] [PubMed] [Google Scholar]
- 68.Pacheco CD, Kunkel R, Lieberman AP. Autophagy in Niemann-Pick C disease is dependent upon Beclin-1 and responsive to lipid trafficking defects. Hum Mol Genet. 2007;16(12):1495–1503. doi: 10.1093/hmg/ddm100. [DOI] [PubMed] [Google Scholar]
- 69.Kiselyov K, et al. Autophagy, mitochondria and cell death in lysosomal storage diseases. Autophagy. 2007;3(3):259–262. doi: 10.4161/auto.3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Venugopal B, et al. Neurologic, gastric, and opthalmologic pathologies in a murine model of mucolipidosis type IV. Am J Hum Genet. 2007;81(5):1070–1083. doi: 10.1086/521954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Terman A, et al. Aging of cardiac myocytes in culture: oxidative stress, lipofuscin accumulation, and mitochondrial turnover. Ann N Y Acad Sci. 2004;1019:70–77. doi: 10.1196/annals.1297.015. [DOI] [PubMed] [Google Scholar]
- 72.King TP, Bremner I. Autophagy and apoptosis in liver during the prehaemolytic phase of chronic copper poisoning in sheep. J Comp Pathol. 1979;89(4):515–530. doi: 10.1016/0021-9975(79)90043-4. [DOI] [PubMed] [Google Scholar]
- 73.Eichelsdoerfer JL, et al. Zinc dyshomeostasis is linked with the loss of mucolipidosis IV-associated TRPML1 ion channel. J Biol Chem. 2010;285(45):34304–34308. doi: 10.1074/jbc.C110.165480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dong XP, et al. The type IV mucolipidosis-associated protein TRPML1 is an endolysosomal iron release channel. Nature. 2008;455(7215):992–996. doi: 10.1038/nature07311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.van Aken AF, et al. TRPML3 mutations cause impaired mechano-electrical transduction and depolarization by an inward-rectifier cation current in auditory hair cells of varitint-waddler mice. J Physiol. 2008;586(Pt 22):5403–5418. doi: 10.1113/jphysiol.2008.156992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Di Palma F, et al. Mutations in Mcoln3 associated with deafness and pigmentation defects in varitint-waddler (Va) mice. Proc Natl Acad Sci U S A. 2002;99(23):14994–14999. doi: 10.1073/pnas.222425399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim HJ, et al. Properties of the TRPML3 channel pore and its stable expansion by the Varitint-Waddler-causing mutation. J Biol Chem. 2010;285(22):16513–16520. doi: 10.1074/jbc.M109.078204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bozzato A, Barlati S, Borsani G. Gene expression profiling of mucolipidosis type IV fibroblasts reveals deregulation of genes with relevant functions in lysosome physiology. Biochim Biophys Acta. 2008;1782(4):250–258. doi: 10.1016/j.bbadis.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 79.Miedel MT, et al. Membrane traffic and turnover in TRP-ML1-deficient cells: a revised model for mucolipidosis type IV pathogenesis. J Exp Med. 2008;205(6):1477–1490. doi: 10.1084/jem.20072194. [DOI] [PMC free article] [PubMed] [Google Scholar]