Abstract
Unlike breast and prostate cancers, the nature and sequence of critical genetic and epigenetic events involved in the initiation and progression of melanoma is not well understood. A contributing factor to this dilemma, especially given our current understanding of the importance of UV light in melanoma etiology, is the lack of quality UV-inducible melanoma animal models. In this study we elaborate on the capability of UV light to induce cutaneous malignant melanomas (CMM) in Xiphophorus fishes, which were previously found to develop melanomas after acute neonatal UVB irradiation. In two separate tumorigenesis experiments, we exposed adult Xiphophorus hybrids to either acute UVB irradiations (5 consecutive daily treatments) or chronic solar irradiations (continuous UVA/UVB treatment for 9 months). Acute adult UVB irradiation resulted in the significant induction of melanomas, and moreover, this induction rate is equivalent to that of animals exposed to acute neonatal UVB irradiation. This study represents the first evidence that acute adult UVB irradiation, in the absence of any early life exposures, induces CMM. Similar to the findings conducted on other divergent melanoma models, including HGF/SF transgenic mice and Monodelphis domestica, prolonged chronic solar UV was not a factor in melanomagenesis.
Keywords: Melanoma, ultraviolet radiation, UVB, Xiphophorus, chronic, acute
1. Introduction
Malignant melanoma remains one of the few cancers that is increasing in prevalence as well as mortality rate (Jemal et al., 2007). The prognosis for metastatic melanoma patients is extremely poor, in part because there is a relative lack of understanding regarding the canonical steps that underlie melanomagenesis. A primary reason for this is decades of melanoma studies (in-vitro, in-vivo, clinical) that have often yielded conflicting and/or inconclusive results (Elwood et al., 1997; Chang et al., 2003; Tucker, 2003; Schuchter, 2004). Most agree that UV exposure is the primary modifiable cause of melanoma; however, surprisingly melanomas frequently occur on non-sun exposed skin and indoor workers have a high incidence rate (Rivers, 2004). In 1998, Whiteman and colleagues provided an explanation for such enigmatic melanoma research and epidemiology by positing distinct pathways to melanoma formation that coincide with their anatomical location (divergent pathway hypothesis). Recent reports confirm that melanomas occurring on different anatomical locations are biologically distinct, having different mutation spectra in key secondary messengers, protein expression profiles and underlying etiologies (Curtin et al., 2005; Hacker et al., 2008). Therefore, the current ideology is that repeated UV exposure is the primary risk factor for malignancies on sun-exposed skin (‘head and neck melanomas’) whereas genetic factors and predispositions are key factors in the etiology of the disease on non-sun exposed skin with isolated events of UV exposure perhaps contributing to the potential for neoplasms (‘truncal melanomas’; Walker, 2008).
There are, surprisingly, few good sunlight inducible melanoma animal models, especially given our current understanding of the importance of UV in melanoma etiology. This is in stark contrast to chemical carcinogenesis of melanoma. Numerous models in evolutionary divergent taxa have been established including: Sinclair miniature swine (Misfeldt et al., 1994), Camargue horses (Fleury et al., 2000), Syrian hamsters (Homburger, 1969), Xiphophorus fishes (Schwab et al., 1981; Rahn et al., 2009), and a number of murine transgenics (for review see Larue et al., 2007). With respect to sunlight induced models of melanoma, three primary models have experimentally demonstrated the importance of sunlight in the initiation of melanoma [reviewed by Ley, 2002: Monodelphis domestica (a South American opossum), Xiphophorus hybrids (livebearing fishes), and a transgenic mouse that overexpresses hepatocyte growth factor/scatter factor (HGF/SF mice)]. As with any animal model, there are pros and cons to the utility of all three models in studying the underlying causes of melanoma formation (Table 1). However, of the three models, we believe the opossum bears the least resemblance, and thus is least applicable, to the pathology and genetic determinants of human melanomagenesis. Unlike the other two models, M. domestica possesses only dermal melanophores and melanomas do not extend into the epidermis and rarely metastasize (Kusewitt et al., 1991), therefore, the pathology of sunlight induced melanomas in this system are markedly different from that in humans (Ley, 2002). In light of this, we will focus our efforts in this article on presenting new data regarding sunlight induced melanomas in Xiphophorus hybrids and drawing comparisons to the published UV studies conducted on the HGF/SF mice.
Table 1.
Experimental Animal Models of UV-induced melanomagenesis.
| Model Organism | Advantages | Disadvantages |
|---|---|---|
| Monodelphis domestica (Gray short-tailed opossum) | Marsupial; intermediate phylogenetic distance Capable of photoreactivation; experimental removal of UV-induced DNA damage Whole genome sequenced |
Not amenable to inbreeding; outbreed stocks only Dermal melanophores only, no epidermal melanocytes Dissimilar histopathology to humans; melanoma of dermal origin only that rarely metastasize |
| HGF-SF mice | Mammal; least phylogenetic distance Excellent laboratory breeder; easy husbandry Broad distribution of melanocytes; located in the dermis, dermal-epidermal junction, basal epidermis Similar histopathology to humans; dermal-epidermal melanomas and metastatic melanomas Whole genome sequenced |
Not capable of photoreactivation Transgenic animal Spontaneous melanomas are of dermal origin only; do not resemble the human condition |
| Sp-Couchianus backcross fish (Xiphophorus hybrid) | Capable of photoreactivation Easy to obtain and maintain large sample sizes for experimentation Melanocytes found in the epidermis and at the dermal-epidermal junction Similar histopathology to humans; dermal-epidermal melanomas and highly invasive melanomas Well characterized genetics; including the involvement of RTKs and CDKs in melanomagenesis |
Teleost fish; great phylogenetic distance Internal fertilization; Not amenable to genetic manipulation Whole genome not sequenced |
Wildtype (unmanipulated) mice do not develop cutaneous melanomas as they lack epidermal skin pigmentation and only possess melanocytes at the base of their hair follicles. In the late 1990s, Takayama and colleagues (Takayama et al., 1997) developed and described the HGF/SF mice that develops neoplasms in a wide variety of tissues (mammary, skeletal muscle, liver, epithelial, mesenchymal) due to the inappropriate expression and paracrine signaling of HGF/SF and it’s tyrosine kinase receptor; c-Met. Soon thereafter, the HGF/SF mice became a promising model for UV induction of skin carcinogenesis. Noonan and colleagues (2000) first demonstrated that these mice have a strong propensity to develop a variety of skin cancers after the chronic UVB/UVA irradiation of adult animals; however, melanoma induction was not observed in this experiment. Subsequently, it was discovered that a single dose of UVB/UVA in early developmental stages (3.5 days old) does result in malignant melanoma formation as well as an increase in tumor multiplicity if an additional single dose is administered to 6 week old adult animals (Noonan et al., 2001). However, the same single dose administered to 6 week old adults (without neonatal irradiation) does not induce cutaneous melanomas (Noonan et al., 2001; Table 2).
Table 2.
Comparison of UV carcinogenesis in Xiphophorus hybrid fishes and transgenic mice
| Melanoma Animal Model | Age @ exposure | UV type | UV delivery method | Total UV dose | UV-induced melanomas | Melanoma incidence (%) |
|---|---|---|---|---|---|---|
| Xiphophorus Sp-couchianus BC1 model | N/A | N/A | N/A | N/A | N/A | 18.5 |
| Neonate | UVB | Acute, multiple doses | 32 kJ/m2 (6.4 kJ/m2/day; 5 cons. days) | Yes | 44.3 | |
| Adult | UVB | Acute, multiple doses | 32 kJ/m2 (6.4 kJ/m2/day; 5 cons. days) | Yes | 38.8 | |
| Adulta | UVB/UVA | Chronic | ~721 kJ/m2 | Nob | 3.3 | |
| HGF-SF transgenic mice | N/A | N/A | N/A | N/A | N/Ac | N/A |
| Neonate | UVB/UVA | Acute, single dose | 9.8 kJ/m2 | Yes | ~ 53 | |
| Neonate, Adult | UVB/UVA | Acute, 2 single doses | 19.6 kJ/m2 | Yes | ~ 82 | |
| Adult | UVB/UVA | Acute, single dose | 19.6 kJ/m2 | No | N/A | |
| Adult | UVB/UVA | Chronic | 300–350 kJ/m2 | Nod | N/A |
Sp-couchianus F1 animals used not BC1 individuals
Background melanoma incidence is 2.3% for Sp-couchianus F1 animals
Amelanocytic melanomas occur in 14.4 % of unirradiated mice (Takayama et al., 1997)
Other skin cancers were initiated, predominately squamous cell carcinomas (Noonan et al., 2000)
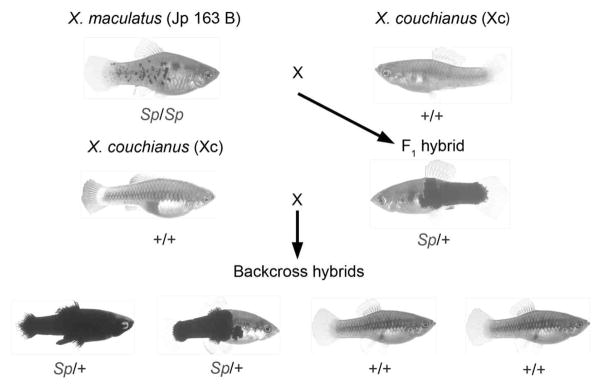
Since the 1920s, Xiphophorus fishes have been studied extensively to identify carcinogens and the genetic mechanisms underlying melanoma. The viability and fertility of hybrid animals in this system provides a powerful classical genetics approach for revealing the determinants of melanomagenesis (Nairn et al., 2001; Meierjohann and Schartl, 2006; Mitchell et al., 2007). The investigation of more than 20 hybrid crosses has led to the realization that hybrid strains have different susceptibilities to carcinogens (chemical, UV) and spontaneous rates of melanoma formation (Patton et al., 2010). Despite these differences, melanomas in Xiphophorus always result from the overexpression of a receptor tyrosine kinase (Xiphophorus melanoma receptor kinase, Xmrk) that is a mutated derivative of the fish ortholog for the human epidermal growth factor receptor (EGFR/ErbB-1). In Xmrk loss-of-function mutants, melanomas do not occur and, therefore, Xmrk is a prerequisite for melanomagenesis and acts as a dominant oncogene in this system (Schartl et al., 1999). Consistent with the activity of mammalian EGFR in melanocytes (de Wit et al., 1992; Huang et al., 1996; Volff et al., 2003), activation of the Xmrk oncoprotein leads to numerous downstream signaling cascades including, but not limited to, the Ras-Raf-Mapk and PI3-kinase-Akt signaling pathways (for review see Meierjohann and Schartl 2006). In addition to these signaling cascades, the transformed phenotype in Xiphophorus also involves participation of transcription factors (e.g., STAT5) and glycoproteins (e.g., OPN, osteopontin) that are intimately involved in cellular proliferation and anti-apoptotic responses that characterize numerous human cancers. Previously, it was demonstrated that first generation backcross (BC1) hybrids between the parentals of Xiphophorus maculatus Jp 163B strain and Xiphophorus couchianus are susceptible to UV-induced melanomagenesis (Setlow et al., 1993; Mitchell et al., 2010). This particular hybrid is termed ‘Sp-couchianus’ because melanomas originate from the X. maculatus melanin pattern ‘spotted-side’ (abbreviated ‘Sp’; Fig. 1). The administration of five consecutive daily doses of narrow band UVB to neonatal fish results in a melanoma incidence that is 26% above background levels, whereas, neonatal UVA irradiation has no affect on melanoma incidence (Mitchell et al., 2010). To date, however, unlike the HGF-SF mice, no one has investigated whether chronic UV or the acute irradiation of adult fish is a factor in the initiation and progression of cutaneous malignant melanomas (CMM). The purpose of this study was to expand on our current knowledge of UV-induced melanomagenesis in the Sp-couchianus model, and thereby, allow for comparisons of UV-induced carcinogenesis between two divergent lineages: ray-finned fishes and mammals. In the first experiment, we irradiated Sp-couchianus BC1 adults with the identical UVB exposure protocol used for neonates in order to determine if adult fish are susceptible to UV-induced melanomas in the absence of any early life UV exposures. In the second experiment, we chronically exposed Sp-couchianus F1 adults to solar (UVB/UVA) irradiation for a period of nine months in order to determine if prolonged exposures to solar UV has any impact on melanomagenesis.
Figure 1.
The Sp-couchianus backcross hybrid breeding scheme. An F1 hybrid is produced by mating a non-spotted male X. couchianus (+/+) with a female X. maculatus that has the X-linked spot-sided pattern (Sp/Sp; termed Jp 163B strain). The female F1 hybrids (Sp/+) are subsequently back-crossed to the parental X. couchianus male (+/+). This breeding scheme produces first generation backcross progeny that are either Sp (Sp/+) pigmented or wild-type (+/+). These wildtype fish are discarded because they lack the Xmrk oncogene.
2. Materials and Methods
2.1. Animals and breeding
Parental strains were originally obtained from the Xiphophorus Genetic Stock Center located at Texas State University in San Marcos, TX and have been maintained in our facility since 2000. The X. couchianus stock was collected in 1961 from the Huasteca canyon (Nuevo Leon, Mexico). Progenitors of X. maculatus strain Jp 163 B were obtained from a single pregnant female collected in 1939 from the Rio Jamapa (Veracruz, Mexico). Therefore, both stocks are highly inbred although they are not true isogenic strains. The mating scheme to generate Sp-couchianus F1 individuals that were used in the chronic exposure experiment and to produce BC1 individuals was X. couchianus males bred to X. maculatus 163B females (Fig. 1). The reason for this breeding scheme is that the Sp pattern composed of melanocytes, from which melanomas develop, and the Xmrk oncogene are X-linked in the X. maculatus Jp 163 B strain. Therefore, only X. maculatus Jp 163 B females can be homozygous for Xmrk in this strain. In order to generate Sp-couchianus BC1 individuals used in the acute adult exposures, F1 hybrid females were bred to X. couchianus males. Therefore, the genetic background of BC1 individuals is primarily that of X. couchianus and not X. maculatus 163B (i.e., ~75% X. couchianus and 25% X. maculatus). Because a critical component for melanoma formation in Xiphophorus sp. is the presence of melanocytes and the Xmrk oncogene (Schartl et al., 1999), non-melanized BC1 individuals (i.e., fish that did not inherit the Sp pattern or Xmrk) were discarded in accordance with standard operating procedure for studies within the Xiphophorus melanoma model (Mitchell et al., 2010).
2.2. Initial Animal housing
F1 and BC1 juvenile fish were initially housed with their broodmates in 208 L communal tanks before the start of experimental treatments began or prior to setting up the breeding tanks for generating BC1 individuals. In the case of the F1 animals, tanks were monitored weekly for maturation. As males matured, they were removed from the larger communal tanks placed into single sex 19 L tanks. However, all BC1 individuals were allowed to mature in their communal environments until the acute UVB irradiations began at 5–6 months of age. We have very rarely observed broods being dropped in these BC1 communal tanks (likely due to males being sterile) and, therefore, do not segregate these individuals as they mature. By 5–6 months of age all BC1 individuals were mature. Unless otherwise mentioned, all fish used in these experiments were housed under standard laboratory conditions which included a 12:12 light-dark cycle and fed a daily diet of Tetramin© flakes and live brine shrimp (Artemia sp.).
2.3. Ultraviolet light exposures
a. Adult acute exposures
The experimental procedures for UVB exposure of adult fish followed closely the established protocol developed in our laboratory for exposing neonatal Xiphophorus fry. This is because we wanted to make comparisons between melanoma frequencies after adult and neonatal exposure regimes. Detailed information about the UVB exposure protocol can be found in (Mitchell et al., 2010). In summary, 5–6 month old adult BC1 fish (N = 158) were individually placed into small UV-transparent Plexiglas© irradiation chambers and exposed for five consecutive days to a specified amount of UVB light (see below). All irradiations occurred in a large irradiation box that houses the UV-transparent chambers, which are suspended in the middle of the box such that un-anesthetized, free-swimming fish can be simultaneously exposed to UVB from both sides. In order to reduce ‘edge’ effects (i.e., decreased or uneven exposure rates towards the ends of the box), we only placed 3 chambers into the center of the box at one time. To prevent unwanted white light effects like light-inducible photoenzymatic repair (Mitani et al., 1991; Yasuhira et al., 1992; Mitchell et al., 1993), adult animals were kept in the dark for 24 h prior to the first exposure until 24 h after the last exposure. All BC1 individuals irradiated were exposed to a total daily dose of 6.4 kJ/m2 at a fluence rate of 12.2 J/m2/sec from 2 unfiltered Philips TL01 bulbs mounted on either side of the irradiation chamber (4 bulbs total). Thus, the total UVB exposure time was 8 min 45 sec each day. The peak emission of the UVB bulbs is 311 nm. Dose rates were measured using a Model IL 1400A radiometer/photometer coupled to a UVB detector (International Light Technologies, Peabody, MA, USA). Dose rates along the entire length of the irradiation boxes were verified using DNA damage dosimeters suspended in the filled UV-transparent chambers. Negligible attenuation by the plastic chamber or the small amount of water (~70 mL) in the chamber water was observed.
b. Adult chronic exposures
Pairs of Sp-couchianus F1 individuals were placed in 9.4 L aquaria in two adjacent rooms at our indoor fish facility. One room housed individuals that were to undergo the chronic solar UV treatment whereas the other room housed control pairs that did not receive exposure to solar UV. There were 32 male pairs and 32 female pairs in the UV condition and 36 pairs of males and 28 pairs of females in the control condition. Thus, there were a total of 128 individuals in both conditions however, due to limitations in our F1 Sp-couchianus stocks the amount of males and females in each condition were slightly different. Fish were paired in single sex dyads so that animals would not breed during the 9-month experiment. In order to minimize inter-tank variations as much as possible (e.g., attenuation of UV in the water column), all tanks were filled with 6 L of water and refilled weekly to a line on the side of each tank delineating a water volume of 6 L. Due to the numbers of animals (total N = 256), it was not feasible to control for the age of the animals used in this experiment. However, care was taken to ensure that all animals were sexually mature and that mean age of the animals was not different between the conditions (UV: mean age = 214.1 days old; Control: mean age = 213.4 days old).
All tanks that were exposed to chronic solar UV were placed upon two identical 2 ft × 16 ft flat bench top tables that were arranged parallel to one another and spaced ~ 3 ft apart. Each table held 32 tanks that were arranged in two adjacent rows of 16 tanks each. In each row, tanks were placed end to end (lengthwise) and opaque dividers were placed between each tank and each row. Solar UV exposure was administered from a suspended overhead bank of lights that were on a 12:12 light-dark cycle (6:00 am to 6:00 pm). A set of four-4 ft light fixtures were joined end to end (lengthwise) and aligned directly over the center of each 16 ft bench top table. Each 4 ft bank of lights housed 4 bulbs: 2 40-Watt FS-40 broad-spectrum UV sunlamps (Westinghouse Lighting Corporation, Philadelphia, PA, USA) and 2 T-8 Vita-Lite® fluorescent lamps (Duro-Test Lighting, Philadelphia, PA, USA). Because we wanted to eliminate UVC and ensure UV light would enter the water column, the glass lid on each tank of the 64 tanks was removed and replaced with fitted piece of Kodacel® film (Eastman Kodak Company, Rochester, NY, USA) that blocks wavelengths below 290 nm (UVC). Aside from the UV irradiation, all fish in this treatment received standard care and husbandry throughout the duration of the experiment. The overhead light banks were briefly turned off twice during the daytime light cycle, once during the morning feeding and again during the afternoon feeding (~15 min. total).
Because our laboratory had not attempted prolonged solar UV exposure previously, we wanted to ensure the exposure rate was similar across tanks and that sufficient solar UV was penetrating the 30 cm deep water column. Therefore, at the beginning and end of the experiment we strategically placed DNA damage dosimeters (i.e., quartz ampules containing purified commercial DNA) along the length of the bench top table. Half of the dosimeters were placed at the surface of the tank water and the remaining dosimeters were suspended freely in the exact middle of the water column (15 cm depth). Dosimeters were placed under the lights for a period of exactly two h with the Kodacel® film covering the top of the tanks. Radioimmunoassays (RIAs) designed to detect the amount of cyclobutane pyrimidine dimers (CPDs) per million nucleotide bases were conducted. Detailed protocols and procedures for the RIA can be found at (Mitchell, 2006). The results of these RIAs indicated that there was sufficient UVB to induce DNA damage at the water’s surface as well as in the middle of the water column. We found that in a two h period under this experimental light regime an average of 51.9 CPDs/mb were induced at the surface and within the water column an average of 34.7 CPDs/mb were induced. The lower induction rate in the water column would be expected due to UVB attenuation. Furthermore, the individual dosimeters readings confirmed that there were negligible edge effects along the 16 ft length of the benches and that there was a relatively uniform distribution of solar UV exposure. From the dosimeter data, we estimated that the incident dose at the center of the experimental aquaria was equivalent to 2.63 kJ/m2/day; hence, our total dose administered to the fish over the 9-month exposure period was ~721 kJ/m2.
2.4 Experimental housing and tumor monitoring
After the adult acute exposures, 3 to 4 Sp-couchianus BC1 individuals were placed in mixed sex 19 L tanks in order to facilitate tumor observation in swimming fish. BC1 animals remained in these tanks for 10 months at which time the experimented was terminated and a final tumor count was conducted. The Sp-couchianus F1 individuals, both control (no UV) and chronic solar UV treatment groups, were continually housed in the aforementioned tanks for a period of 9 months. Weekly tumor inventories were conducted on all fish. The identification of tumors was primarily based on the presence of exophytic melanized growth, which is easily detected in free-swimming fish of the Sp-couchianus model. In the rare cases of enhanced pigmentation without nodular growth, animals were fixed in 10% neutral buffered formalin (NBF) and sent for histopathological tumor analyses. Individual fish with severe tumor burden were euthanized prior to the termination of the experiment in accordance with the guidelines of Institutional Animal Care and Use Committee (IACUC).
2.5 Statistics
A Fisher’s exact test was conducted to determine if there was a difference in the frequency of melanomas between F1 animals chronically exposed to solar UV and the F1 control (no UV) animals. In the case of the BC1 animals exposed as 5–6 month adults to UVB, chi-squared tests were conducted to compare the frequency of melanomas between adult acute UVB irradiations, neonatal acute UVB irradiation and no UVB exposure. Melanoma frequencies for Sp-couchianus BC1 animals exposed to neonatal acute UVB irradiation and no UVB were taken from a recent publication out of our laboratory (Mitchell et al., 2010). All statistical analyses were performed using Graphpad Prism® Ver. 5.0a (Graphpad Software, La Jolla, CA, USA).
3. Results
3.1 Melanomagenesis and adult acute exposures
At the age of 5 months old (i.e., the beginning of the UVB irradiations), we had already observed a considerable number of spontaneous melanomas in the non-treated BC1 animals (tumor bearing fish, TBF). In all, 25 out of the 183 animals developed exophytic lesions before beginning the adult acute irradiations. We believe that these animals represent the background melanoma frequency for the Sp-couchianus BC model that is due to the genetic predisposition of BC Xiphophorus fishes (for review see Patton et al. 2010). In support of this, the observed frequency was not significantly different from that of non-irradiated Sp-couchianus BC animals (25 TBF out of 183 individuals, melanoma frequency = 13.7%; published background frequency: 40 TBF out of 216 individuals, melanoma frequency = 18.5%; χ2=1.71, P=0.19). Because we had no experience irradiating adult fish, the question arose about how to handle the fish that developed melanoma before the UVB irradiations began. We believed the proper thing to do was to include these animals with those animals that develop melanoma after being irradiated with UVB as adults. The reason for this is simple. When we irradiate 5 day old neonates for five consecutive days and calculate ‘induced’ melanoma frequencies in adult animals, we do not differentiate the melanomas that formed due to the UVB irradiation (induced melanomas) from spontaneous melanomas. Therefore, the published frequency of UVB ‘induced’ melanomas after neonatal irradiation, against which we want to compare the adult UVB irradiated melanoma frequency, included both spontaneous and induced melanomas (Mitchell et al., 2010).
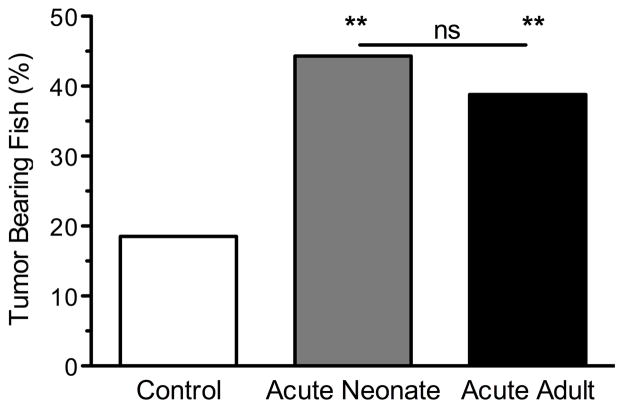
Out of the 158 fish that were irradiated as adults (i.e., 183 minus the 25 TBF that developed melanomas before we began irradiations), 46 fish developed melanomas by the conclusion of the experiment. Thus, acute UVB adult irradiation significantly induced melanoma formation above the background frequency (adult acute irradiation: 71 total TBF out of 183 individuals, melanoma frequency = 38.8%; no irradiation: 40 TBF out of 216 individuals, melanoma frequency = 18.5%; χ2=20.29, P<0.0001). It should be noted that even if we do not include the 25 animals that developed melanomas before the treatment, adult acute UVB exposure still resulted in the induction of melanomas above background levels (χ2=5.79, P<0.01). Importantly, and rather remarkably, the incidence of melanomas was not significantly different between the fish that were irradiated as neonates compared to mature adults (adult acute irradiation: 71 total TBF out of 183 individuals, melanoma frequency = 38.8%; neonate UVB irradiation: 86 TBF out of 194 individuals, melanoma frequency = 44.3%; χ2 =1.19, P=0.28; Fig. 2).
Figure 2.
Melanoma frequencies in unirradiated and UVB-irradiated BC1 fish. The Y-axis indicates the percentage of fish that developed melanomas in the three experimental groups: fish not exposed to UV (‘Control’), fish exposed to acute UVB as neonates (‘Acute Neonate’), and fish exposed to acute UVB as adults (‘Acute Adult’). The neonatal and adult administration of narrow band UVB resulted in a significant induction of melanoma compared to the unirradiated control BC1 fish. However, there was no difference in the frequency of melanomas between the acute neonate and acute adult groups. Data for the control and acute neonate groups are taken from Mitchell et al., 2010. ** indicates P < 0.0001 and ns indicates not significant.
3.2 Melanomagenesis and adult chronic exposures
We observed very few melanomas in either the adult F1 hybrids chronically exposed to solar UV or the F1 hybrids not exposed to UV. Four fish developed melanomas out of the 128 fish exposed to solar UV (~3.1%) and 3 fish developed melanomas out of the 128 fish in the control group (~2.3%). Furthermore, we did not observe any noticeable cases of enhanced pigmentation (melanosis) or any other gross effects from the chronic solar UV exposures. The lack of melanoma induction after chronic solar UV in Xiphophorus fishes is consistent with similar studies conducted on other melanoma models including HGF/SF mice (Table 2).
4. Discussion
Although there is a clear consensus that sunlight is a risk factor for melanoma, there is plenty of debate about the type, dose and duration of UV exposure that is necessary to initiate CMM (Walker, 2008). Obvious evidence for the involvement of UV light in melanomagenesis comes from the genetic disorder Xeroderma pigmentosum (XP). Individuals with XP have deficient nucleotide excision repair and, therefore, cannot repair bulky adducts in DNA which are produced via DNA’s direct absorption of UVB light. As a result, XP patients are 1000 times more likely to develop melanoma than individuals with normal DNA repair capacity (Kraemer et al., 1994). In light of this, it seems reasonable that CMM would be readily induced in animal models given prolonged UVR exposures. However, the results presented here demonstrate that the Sp-couchianus hybrids are not susceptible to melanoma formation after prolonged chronic exposure to solar UV. Furthermore, to our knowledge, the administration of chronic UVB or solar UV has resulted in CMM induction in only a single study (2 out of 10 Tyr-Hras mice developed CMM after 38 weeks of high intensity solar UV exposure; Powell et al., 1999), whereas all other studies conducted on melanoma models show no effect of chronic UVR exposure on CMM induction (Monodelphis: Robinson et al., 1995; Ley, 1997; HGF/SF mice: Noonan et al., 2000; SKN-2 hairless mice: van Schanke et al., 2005). Clearly, the role of chronic UVR irradiation is very limited in the initiation of melanomas in animal models despite the recent evidence of UV signature mutations in human melanoma (Pleasance et al., 2010; Wang et al., 2010).
An extraordinary finding of this study is the initiation of CMM after acute adult UVB exposures in the absence of any early adult (neonatal) exposure of Sp-couchianus BC1 fish. This is because previous research conducted on M. domestica and HGF/SF mice documented CMM formation in animals exposed as adults to UVR only if it was preceded by acute neonatal UVR exposures (Robinson et al., 1994; Noonan et al., 2001). Therefore, this study is the first to document the induction of CMM in animals exposed to UV only as adults and not as neonates.
The equivalent rates of CMM induction in Sp-couchianus BC1 adults exposed to narrow band UVB as either adults (this study) or neonates (Mitchell et al., 2010) are surprising and somewhat bewildering (Fig. 2). This is especially true given the tremendous differences in the anatomy and physiology of 5 day old fry and 5 to 6 month old mature adult fish (e.g., neuroendocrine pathways, reproductive tissues, and gross morphology). These differences raise several interesting aspects about CMM induction in Xiphophorus sp. fishes. The first point is that the epidermal skin of neonates lacks any visible melanocytic pigmentation, whereas the adult BC1 fish are heavily pigmented (Fig. 1). As with mammals, the skin of neonates contains immature differentiating melanocytes (melanoblasts; Schartl et al., 1982) that may handle insults from UVR less effectively than mature melanocytes (Erickson, 1993). Another issue related to melanin pigmentation is the greater potential for the production of detrimental reactive oxygen species (ROS) in heavily melanized skin compared to non-melanized skin (Hill et al., 1997). Although ROS has been implicated as a causative agent in Xiphophorus melanomas (Wood et al., 2006), we have recently proposed a hypothesis that sets forth early transitory events due to UVB induced DNA damage [specifically the (6-4) pyrimidine-pyrimidone dimer] in initiating melanomas (Mitchell et al., 2011). Although this precludes a role for ROS in initiation, it does not suggest that ROS (and UVA) do not play significant roles in tumor progression. With this in mind, repeating the acute adult exposures with narrow band UVA would be an interesting follow-up to this study because; (1) unlike UVB, UVA does not directly produce DNA damage and (2) we have previously documented that irradiating neonates with UVA does not induce melanomas in Sp-couchianus BC1 fish (Mitchell et al., 2010). In addition to generating ROS, melanin produced by melanocytes also has a photoprotective effect that further complicates the significant melanoma induction of heavily melanized BC1 fish after receiving only adult acute UVB exposures.
An intriguing possibility for explaining melanoma formation after only adult UVB irradiation presents itself when one focuses on what is the same between the fish at the time of exposure rather than what is different, mainly the presence of cells with pluripotent capacities. The melanoma literature over the last decade is filled with conceptual and empirical evidence of possible melanoma stem cells (MSC; reviewed by Schatton et al., 2008), and more recently with the theory of melanoma-initiating cells (MIC; Refaeli et al., 2009). Unfortunately, stem cell markers have not been characterized in Xiphophorus and in vivo work with melanoma models to isolate melanoma stem cells and melanoma-initiating cells is still in its infancy (Refaeli et al., 2009). Future research is needed that utilizes the high melanoma incidence of these inducible animal models to facilitate the isolation of pure MSC and MIC, which is extremely difficult in cell cultures and xenograft assays given current technology.
An important aspect of the adult chronic exposures is the use of Sp-couchianus F1 hybrids rather than BC1 individuals. Although the large sample sizes of the two experiments made this inevitable, F1 hybrids (unlike BC1) are heterozygous for the Xmrk oncogene and more importantly possess both parental alleles for the autosomal tumor suppressor Cdkn2x (P16 homolog). The elevated rates of spontaneous and induced melanoma formation in BC1 fish is thought to be limited to those fish possessing Xmrk in the absence of the X. maculatus allele for Cdkn2x (for details see Patton et al., 2010). However, theoretically, there is an increased likelihood of UV-induced lesions and subsequent mutations occurring in the remaining copy of X. maculatus Cdkn2x of the F1 hybrids that could impair the function of this tumor suppressor and thereby lead to melanoma formation. Yet, we found no difference in the occurrence of melanomas in F1 hybrids in the chronically UV treated population when compared to the control group (no UV; see below). This result does corroborate work on rodents which shows that Cdk4 deficient mice (Ink4a−/−:Arf−/− and Cdk4R24C/R24C) are not susceptible to UVR induced melanomas (Serrano et al., 1996; Sotillo et al., 2001). An explanation for this is provided Walker and Hayward (Walker et al., 2002) who suggest that the dysregulation of upstream proteins (e.g., Braf and nRAS in humans; Xmrk in BC1 hybrids) is more successful at ‘priming’ melanocytes for transformation and melanoma formation than knocking out downstream regulators such as Cdkn2 and Cdk4.
The lack of melanoma formation in the chronically exposed Sp-couchinaus F1 hybrids is also not surprising, given two recent studies on the underlying photobiology in this system. First, we recently observed that sequential irradiations of F1 fish results in a photoadaptive effect that reduces DNA damage induction as a consequence of repeated irradiation (Mitchell et al., 2009). In these fish, DNA damage did not accumulate during the five consecutive days of UVB treatment. Remarkably, the amount of DNA damage present after the fifth day of irradiation similar to or even less than the amount of DNA damage induced after the first day of irradiation. Therefore, although the total 9-month exposure of F1 hybrids to solar UV was estimated at ~82 kJ/m2 based on the dosimeter data, the combined attenuation of DNA damage due to this photoadaptation and DNA repair probably resulted in a much smaller frequency of induced DNA damage than expected from the incident dose. Second, UVB-induced melanomas are completely abrogated by photoenzymatic repair (PER) if the animals are exposed to white light immediately after UVB exposure (Mitchell et al., 2011). Due to the extended time frame of this experiment is was not feasible to remove white light exposure, therefore, throughout the experiment these animals could ameliorate the negative effects of UV exposure by employing PER. The result of this experiment further highlights the importance of DNA damage and repair in the initiation of melanomas.
Unlike breast and prostate cancers, the nature and sequence of critical genetic and epigenetic events involved in the initiation and progression of melanoma is not well understood (Meierjohann and Schartl, 2006). We believe the continued development and use of appropriate animal models will be essential to elucidate the elusive mechanisms underlying melanomagenesis. Despite the divergent evolutionary paths of the three primary UV induced melanoma models, in vivo experimentation has revealed several key elements of melanoma susceptibility that is shared across these distantly related organisms. First, studies of UVR irradiation in these models demonstrate that the initiation of melanomas is not related to chronic UV exposures and the concomitant accumulation and persistence of DNA lesions and mutations. Second, the action spectrum for melanoma formation is conserved. UVB but not UVA induces CMM in all models tested (Robinson et al., 1994; Ley, 2001; De Fabo et al., 2004; van Schanke et al., 2005; Mitchell et al., 2010). Furthermore, the importance of early life acute exposures to UVB is a general phenomenon although Xiphophorus BC1 hybrids are also sensitive to acute UVB exposure as mature adults in the absence of prior neonatal exposures. The ability of adult exposures to induce melanomas in humans is, thus, an open question. Lastly, as with human melanoma formation (Haluska et al., 2007), RTKs, Ras-Raf-Mapk and PI3-kinase-Akt signaling pathways are quintessential to melanomagenesis in in vivo animal models (Walker et al., 2002; Meierjohann and Schartl, 2006).
Acknowledgments
Funding for this work was provided by NCI grant CA113671 and NIEHS Center grant ES07784. We would like to thank David Trono for helping to care for the animals used in these experiments and his assistance in the experimental design of the chronic adult solar UV irradiations.
Footnotes
This paper is based on a presentation given at the 5th Aquatic Annual Models of Human Disease conference: hosted by Oregon State University and Texas State University-San Marcos, and convened at Corvallis, OR, USA September 20–22, 2010.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Chang CC, Ferrone S. HLA-G in melanoma: can the current controversies be solved? Semin Cancer Biol. 2003;13:361–369. doi: 10.1016/s1044-579x(03)00027-0. [DOI] [PubMed] [Google Scholar]
- Curtin JA, Fridlyand J, Kageshita T, Patel HN, Busam KJ, Kutzner H, Cho KH, Aiba S, Brocker EB, LeBoit PE, Pinkel D, Bastian BC. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135–2147. doi: 10.1056/NEJMoa050092. [DOI] [PubMed] [Google Scholar]
- De Fabo EC, Noonan FP, Fears T, Merlino G. Ultraviolet B but not ultraviolet A radiation initiates melanoma. Cancer Res. 2004;64:6372–6376. doi: 10.1158/0008-5472.CAN-04-1454. [DOI] [PubMed] [Google Scholar]
- de Wit PE, Moretti S, Koenders PG, Weterman MA, van Muijen GN, Gianotti B, Ruiter DJ. Increasing epidermal growth factor receptor expression in human melanocytic tumor progression. J Invest Dermatol. 1992;99:168–173. doi: 10.1111/1523-1747.ep12616793. [DOI] [PubMed] [Google Scholar]
- Elwood JM, Jopson J. Melanoma and sun exposure: An overview of published studies. Int J Cancer. 1997;73:198–203. doi: 10.1002/(sici)1097-0215(19971009)73:2<198::aid-ijc6>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Erickson CA. From the crest to the periphery: control of pigment cell migration and lineage segregation. Pigment Cell Res. 1993;6:336–347. doi: 10.1111/j.1600-0749.1993.tb00611.x. [DOI] [PubMed] [Google Scholar]
- Fleury C, Bérard F, Balme B, Thomas L. The study of cutaneous melanomas in camargue-type gray-skinned horses (1): Clinical–pathological characterization. Pigment Cell Res. 2000;13:39–46. doi: 10.1034/j.1600-0749.2000.130108.x. [DOI] [PubMed] [Google Scholar]
- Hacker E, Muller K, Whiteman DC, Pavey S, Hayward N, Walker G. Reduced expression of IL-18 is a marker of ultraviolet radiation-induced melanomas. Int J Cancer. 2008;123:227–231. doi: 10.1002/ijc.23389. [DOI] [PubMed] [Google Scholar]
- Haluska F, Pemberton T, Ibrahim N, Kalinsky K. The RTK/RAS/BRAF/PI3K pathways in melanoma: biology, small molecule inhibitors, and potential applications. Semin Oncol. 2007;34:546–554. doi: 10.1053/j.seminoncol.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Hill HZ, Li W, Xin P, Mitchell DL. Melanin: a two edged sword? Pigment Cell Res. 1997;10:158–161. doi: 10.1111/j.1600-0749.1997.tb00478.x. [DOI] [PubMed] [Google Scholar]
- Homburger F. Chemical carcinogenesis in the Syrian golden hamster: A review. Cancer. 1969;23:313–338. doi: 10.1002/1097-0142(196902)23:2<313::aid-cncr2820230209>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Huang TS, Rauth S, Das Gupta TK. Overexpression of EGF receptor is associated with spontaneous metastases of a human melanoma cell line in nude mice. Anticancer Res. 1996;16:3557–3563. [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer Statistics, 2007. CA: A Cancer Journal for Clinicians. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- Kraemer KH, Levy DD, Parris CN, Gozukara EM, Moriwaki S, Adelberg S, Seidman MM. Xeroderma pigmentosum and related disorders: examining the linkage between defective DNA repair and cancer. J Invest Dermatol. 1994;103:96S–101S. doi: 10.1111/1523-1747.ep12399329. [DOI] [PubMed] [Google Scholar]
- Kusewitt DF, Applegate LA, Ley RD. Ultraviolet radiation-induced skin tumors in a South american opossum (Monodelphis domestica) Vet Pathol. 1991;28:55–65. doi: 10.1177/030098589102800108. [DOI] [PubMed] [Google Scholar]
- Larue L, Beermann F. Cutaneous melanoma in genetically modified animals. Pigment Cell Res. 2007;20:485–497. doi: 10.1111/j.1600-0749.2007.00411.x. [DOI] [PubMed] [Google Scholar]
- Ley RD. Ultraviolet radiation A-induced precursors of cutaneous melanoma in Monodelphis domestica. Cancer Res. 1997;57:3682–3684. [PubMed] [Google Scholar]
- Ley RD. Dose response for ultraviolet radiation A–induced focal melanocytic hyperplasia and nonmelanoma skin tumors in Monodelphis domestica. Photochem Photobiol. 2001;73:20–23. doi: 10.1562/0031-8655(2001)073<0020:drfura>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Ley RD. Animal models of ultraviolet radiation (UVR)-induced cutaneous melanoma. Front Biosci. 2002;7:d1531–1534. doi: 10.2741/A857. [DOI] [PubMed] [Google Scholar]
- Meierjohann S, Schartl M. From Mendelian to molecular genetics: the Xiphophorus melanoma model. Trends Genet. 2006;22:654–661. doi: 10.1016/j.tig.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Misfeldt ML, Grimm DR. Sinclair miniature swine: an animal model of human melanoma. Vet Immunol Immunopathol. 1994;43:167–175. doi: 10.1016/0165-2427(94)90133-3. [DOI] [PubMed] [Google Scholar]
- Mitani H, Yasuhira S, Komura JI, Shima A. Enhancement of repair of UV-irradiated plasmids in cultured fish cells by fluorescent light preillumination and growth arrest. Mutat Res. 1991;255:273–280. doi: 10.1016/0921-8777(91)90031-j. [DOI] [PubMed] [Google Scholar]
- Mitchell DL. Quantification of photoproducts in mammalian cell DNA using radioimmunoassay. In: Henderson DS, editor. DNA Repair Protocols. Humana Press; Totowa: 2006. pp. 239–249. [DOI] [PubMed] [Google Scholar]
- Mitchell DL, Fernandez AA. Different types of DNA damage play different roles in the etiology of sunlight-induced melanoma. Pigment Cell Melanoma Res. 2011;24:119–124. doi: 10.1111/j.1755-148X.2010.00789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DL, Fernandez AA, Nairn RS, Garcia R, Paniker L, Trono D, Thames HD, Gimenez-Conti I. Ultraviolet A does not induce melanomas in a Xiphophorus hybrid fish model. Proc Natl Acad Sci USA. 2010;107:9329–9334. doi: 10.1073/pnas.1000324107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DL, Paniker L, Douki T. DNA damage, repair and photoadaptation in a Xiphophorus fish hybrid. Photochem Photobiol. 2009;85:1384–1390. doi: 10.1111/j.1751-1097.2009.00591.x. [DOI] [PubMed] [Google Scholar]
- Mitchell DL, Scoggins JT, Morizot DC. DNA repair in the variable platyfish (Xiphophorus variatus) irradiated in vivo with ultraviolet B light. Photochem Photobiol. 1993;58:455–459. doi: 10.1111/j.1751-1097.1993.tb09590.x. [DOI] [PubMed] [Google Scholar]
- Noonan FP, Otsuka T, Bang S, Anver MR, Merlino G. Accelerated ultraviolet radiation-induced carcinogenesis in hepatocyte growth factor/scatter factor transgenic mice. Cancer Res. 2000;60:3738–3743. [PubMed] [Google Scholar]
- Noonan FP, Recio JA, Takayama H, Duray P, Anver MR, Rush WL, De Fabo EC, Merlino G. Neonatal sunburn and melanoma in mice. Nature. 2001;413:271–272. doi: 10.1038/35095108. [DOI] [PubMed] [Google Scholar]
- Patton EE, Mitchell DL, Nairn RS. Genetic and environmental melanoma models in fish. Pigment Cell Melanoma Res. 2010;23:314–337. doi: 10.1111/j.1755-148X.2010.00693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleasance ED, Cheetham RK, Stephens PJ, McBride DJ, Humphray SJ, Greenman CD, Varela I, Lin ML, Ordonez GR, Bignell GR, Ye K, Alipaz J, Bauer MJ, Beare D, Butler A, Carter RJ, Chen L, Cox AJ, Edkins S, Kokko-Gonzales PI, Gormley NA, Grocock RJ, Haudenschild CD, Hims MM, James T, Jia M, Kingsbury Z, Leroy C, Marshall J, Menzies A, Mudie LJ, Ning Z, Royce T, Schulz-Trieglaff OB, Spiridou A, Stebbings LA, Szajkowski L, Teague J, Williamson D, Chin L, Ross MT, Campbell PJ, Bentley DR, Futreal PA, Stratton MR. A comprehensive catalogue of somatic mutations from a human cancer genome. Nature. 2010;463:191–196. doi: 10.1038/nature08658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell MB, Gause PR, Hyman P, Gregus J, Lluria-Prevatt M, Nagle R, Bowden GT. Induction of melanoma in TPras transgenic mice. Carcinogenesis. 1999;20:1747–1753. doi: 10.1093/carcin/20.9.1747. [DOI] [PubMed] [Google Scholar]
- Rahn JJ, Trono D, Gimenez-Conti I, Butler AP, Nairn RS. Etiology of MNU-induced melanomas in Xiphophorus hybrids. Comp Biochem Physiol C Toxicol Pharmacol. 2009;149:129–133. doi: 10.1016/j.cbpc.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refaeli Y, Bhoumik A, Roop DR, Ronai ZA. Melanoma-initiating cells: a compass needed. EMBO Rep. 2009;10:965–972. doi: 10.1038/embor.2009.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivers JK. Is there more than one road to melanoma? Lancet. 2004;363:728–730. doi: 10.1016/S0140-6736(04)15649-3. [DOI] [PubMed] [Google Scholar]
- Robinson ES, Hubbard GB, VandeBerg JL. Ultraviolet radiation-induced skin lesions in laboratory opossums (Monodelphis domestica) exposed from the weanling stage. Arch Dermatol Res. 1995;287:333–337. doi: 10.1007/BF01105088. [DOI] [PubMed] [Google Scholar]
- Robinson ES, VandeBerg JL, Hubbard GB, Dooley TP. Malignant melanoma in ultraviolet irradiated laboratory opossums: initiation in suckling young, metastasis in adults, and xenograft behavior in nude mice. Cancer Res. 1994;54:5986–5991. [PubMed] [Google Scholar]
- Schartl A, Schartl M, Anders F. Promotion and regression of neoplasia by testosterone-promoted cell differentiation in Xiphophorus and Girardinus. Carcinog Compr Surv. 1982;7:427–434. [PubMed] [Google Scholar]
- Schartl M, Hornung U, Gutbrod H, Volff JN, Wittbrodt J. Melanoma loss-of-function mutants in Xiphophorus caused by Xmrk-oncogene deletion and gene disruption by a transposable element. Genetics. 1999;153:1385–1394. doi: 10.1093/genetics/153.3.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatton T, Frank MH. Cancer stem cells and human malignant melanoma. Pigment Cell Melanoma Res. 2008;21:39–55. doi: 10.1111/j.1755-148X.2007.00427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuchter LM. Adjuvant interferon therapy for melanoma: high-dose, low-dose, no dose, which dose? J Clin Oncol. 2004;22:7–10. doi: 10.1200/JCO.2004.10.907. [DOI] [PubMed] [Google Scholar]
- Schwab M, Scholl E. Neoplastic pigment cells induced by N-Methyl-N-nitrosourea (MNU) in Xiphophorus and genetic control of their terminal differentiation. Differentiation. 1981;19:77–83. [Google Scholar]
- Serrano M, Lee HW, Chin L, Cordon-Cardo C, Beach D, DePinho RA. Role of the INK4a locus in tumor suppression and cell mortality. Cell. 1996;85:27–37. doi: 10.1016/s0092-8674(00)81079-x. [DOI] [PubMed] [Google Scholar]
- Setlow RB, Grist E, Thompson K, Woodhead AD. Wavelengths effective in induction of malignant melanoma. Proc Natl Acad Sci USA. 1993;90:6666–6670. doi: 10.1073/pnas.90.14.6666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotillo R, García JF, Ortega S, Martín J, Dubus P, Barbacid M, Malumbres M. Invasive melanoma in Cdk4-targeted mice. Proc Natl Acad Sci USA. 2001;98:13312–13317. doi: 10.1073/pnas.241338598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama H, LaRochelle WJ, Sharp R, Otsuka T, Kriebel P, Anver M, Aaronson SA, Merlino G. Diverse tumorigenesis associated with aberrant development in mice overexpressing hepatocyte growth factor/scatter factor. Proc Natl Acad Sci USA. 1997;94:701–706. doi: 10.1073/pnas.94.2.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker MAG, Alisa M. Melanoma etiology: where are we? Oncogene. 2003;22:3042–3052. doi: 10.1038/sj.onc.1206444. [DOI] [PubMed] [Google Scholar]
- van Schanke A, Jongsma MJ, Bisschop R, van Venrooij GM, Rebel H, de Gruijl FR. Single UVB overexposure stimulates melanocyte proliferation in murine skin, in contrast to fractionated or UVA-1 exposure. J Invest Dermatol. 2005;124:241–247. doi: 10.1111/j.0022-202X.2004.23551.x. [DOI] [PubMed] [Google Scholar]
- Volff JN, Schartl M. Evolution of signal transduction by gene and genome duplication in fish. J Struct Funct Genomics. 2003;3:139–150. [PubMed] [Google Scholar]
- Walker G. Cutaneous melanoma: how does ultraviolet light contribute to melanocyte transformation? Future Oncol. 2008;4:841–856. doi: 10.2217/14796694.4.6.841. [DOI] [PubMed] [Google Scholar]
- Walker GJ, Hayward NK. Pathways to melanoma development: lessons from the mouse. J Invest Dermatol. 2002;119:783–792. doi: 10.1046/j.1523-1747.2002.00217.x. [DOI] [PubMed] [Google Scholar]
- Wang H-T, Choi B, Tang M-S. Melanocytes are deficient in repair of oxidative DNA damage and UV-induced photoproducts. Proc Natl Acad Sci USA. 2010;107:12180–12185. doi: 10.1073/pnas.1005244107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SR, Berwick M, Ley RD, Walter RB, Setlow RB, Timmins GS. UV causation of melanoma in Xiphophorus is dominated by melanin photosensitized oxidant production. Proc Natl Acad Sci USA. 2006;103:4111–4115. doi: 10.1073/pnas.0511248103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuhira S, Mitani H, Shima A. Enhancement of photorepair of ultraviolet-induced pyrimidine dimers by preillumination with fluorescent light in the goldfish cell line. The relationship between survival and yield of pyrimidine dimers. Photochem Photobiol. 1992;55:97–101. doi: 10.1111/j.1751-1097.1992.tb04214.x. [DOI] [PubMed] [Google Scholar]