Abstract
The presence and function of cannabinoid CB2 receptors in the brain have been subject to debate. We report here that systemic, intranasal or intra-accumbens local administration of JWH133, a selective CB2 receptor agonist, dose-dependently inhibits intravenous cocaine self-administration, cocaine-enhanced locomotion, and cocaine-enhanced accumbens dopamine (DA) in wild-type (WT) and CB1 receptor-knockout (CB1−/−), but not CB2−/−, mice. This inhibition is mimicked by GW405833, another CB2 receptor agonist with a different chemical structure, and is blocked by AM630, a selective CB2 receptor antagonist. Intra-accumbens JWH133 alone dose-dependently decreases, while intra-accumbens AM630 elevates, extracellular DA and locomotion in WT and CB1−/− mice, but not in CB2−/− mice. Intra-accumbens AM630 also blocks the reduction in cocaine self-administration and extracellular DA produced by systemic administration of JWH133. These findings, for the first time, suggest that brain CB2 receptors modulate cocaine’s rewarding and locomotor-stimulating effects, likely by a DA-dependent mechanism.
Keywords: Cannabinoid, CB2 receptors, JWH133, cocaine, dopamine, self-administration
Behavioral and psychoactive effects of cannabinoids are mediated by activation of brain cannabinoid receptors1, 2. Two major cannabinoid receptors (CB1 and CB2) have been identified. Since CB1 receptors are highly expressed in the brain2, 3 and CB2 receptors are found primarily in the periphery4, 5, it has been heretofore generally believed that the behavioral and psychotropic effects of cannabinoids are CB1-mediated1, 2 and that CB2 receptor ligands have no psychoactive effects6. However, the purported lack of brain CB2 receptors has been challenged by recent reports of low densities of CB2 receptors on microglia7 and neuronal8–11 cells in several brain regions - including the anterior olfactory nucleus, cerebral cortex, cerebellum, hippocampus, striatum and brainstem. Further, activation of CB2 receptors by 2-arachidonoylglycerol, JWH015 or JWH133 inhibits locomotion10, 11, morphine-6-glucuronide-induced emesis11 and neuropathic pain12, 13, while stimulating neural progenitor proliferation14 and producing neuroprotective effects15, 16. More recent studies suggest that CB2 receptor activation inhibits neuronal firing in dorsal-root ganglia and spinal cord17, 18 and GABAergic transmission in rat cerebral cortex19. These data suggest that functional CB2 receptors may be expressed on central nervous system neuronal cells, prompting us to re-examine the role of CB2 receptors in drug reward and addiction. To this end, we here used highly selective CB2 receptor agonists and antagonists, combined with specific CB1 receptor-knockout (CB1−/−) and CB2 receptor-knockout (CB2−/−) mice, to investigate possible involvement of brain CB2 receptors in cocaine’s behavioral and neurochemical effects.
RESULTS
JWH133 inhibits intravenous cocaine self-administration
To determine whether CB2 receptor activation alters intravenous cocaine self-administration, we used JWH133, a highly selective CB2 receptor agonist (200-fold selectivity for CB2 versus CB1)20, 21, and AM630, a highly selective CB2 receptor antagonist (160-fold selectivity for CB2 versus CB1)20, 21, as pharmacological tools. We found that over 50% of wild-type (WT) (20 of 34) and CB2−/− (22 of 36) mice, while only about 30% of CB1−/− (10 of 36) mice acquired stable intravenous cocaine self-administration, defined as 20 or more infusions per 3-h session, with a regular pattern of self-administration achieved after 10 days of training (Supplementary Fig. 1). Strikingly, CB1−/− mice displayed a significant reduction in both total number and rate (infusions per h) of cocaine infusions on days 1–5, compared to WT or CB2−/− mice (Supplementary Fig. 1a, b). In addition, the majority of CB1−/− mice (7 of 10) displayed a distinct “burst-like” drug-taking pattern with long inter-burst intervals, while WT and CB2−/− mice displayed evenly-paced drug-taking without significant difference between the two strains (Supplementary Fig. 1c). These findings suggest that deletion of CB1 receptors may lower cocaine’s rewarding efficacy, leading to a compensatory increase in drug intake during each individual drug-taking episode. This is further supported by the finding that CB1−/− mice displayed a significant reduction in break-point level for cocaine self-administration under progressive-ratio (PR) reinforcement, compared to WT mice (Supplementary Fig. 1d). Since PR break-point, defined as maximal work performed by the animal to get a cocaine infusion, is cocaine dose-dependent and positively correlated to reward strength22, the reduction in PR break-point observed in CB1−/− mice suggests a reduction in cocaine’s reward strength and/or motivation for cocaine-taking behavior. This is consistent with previous findings that CB1 receptor deletion impairs cocaine’s rewarding, locomotor-stimulating, and DA-elevating effects23, 24.
Intraperitoneal (i.p.) administration of JWH133 (10, 20 mg/kg) produced a significant and dose-dependent reduction in cocaine self-administration and cocaine intake in both WT and CB1−/− mice, but not in CB2−/− mice (Fig. 1a). This inhibition lasted for no longer than 24 hrs after 20 mg/kg JWH133 (Fig. 1b, c). Pretreatment with AM630, a selective CB2 receptor antagonist, but not with AM251, a selective CB1 receptor antagonist25, significantly attenuated JWH133-induced inhibition of cocaine self-administration (Fig. 1d). This suggests that JWH133’s attenuating effect is mediated by activation of CB2, not CB1, receptors. This conclusion is further supported by the additional finding that systemic administration of GW405833 (3, 10 mg/kg, i.p.), another highly selective but structurally distinct CB2 receptor agonist26, also inhibited cocaine self-administration in WT mice (Fig. 2a).
Figure 1.
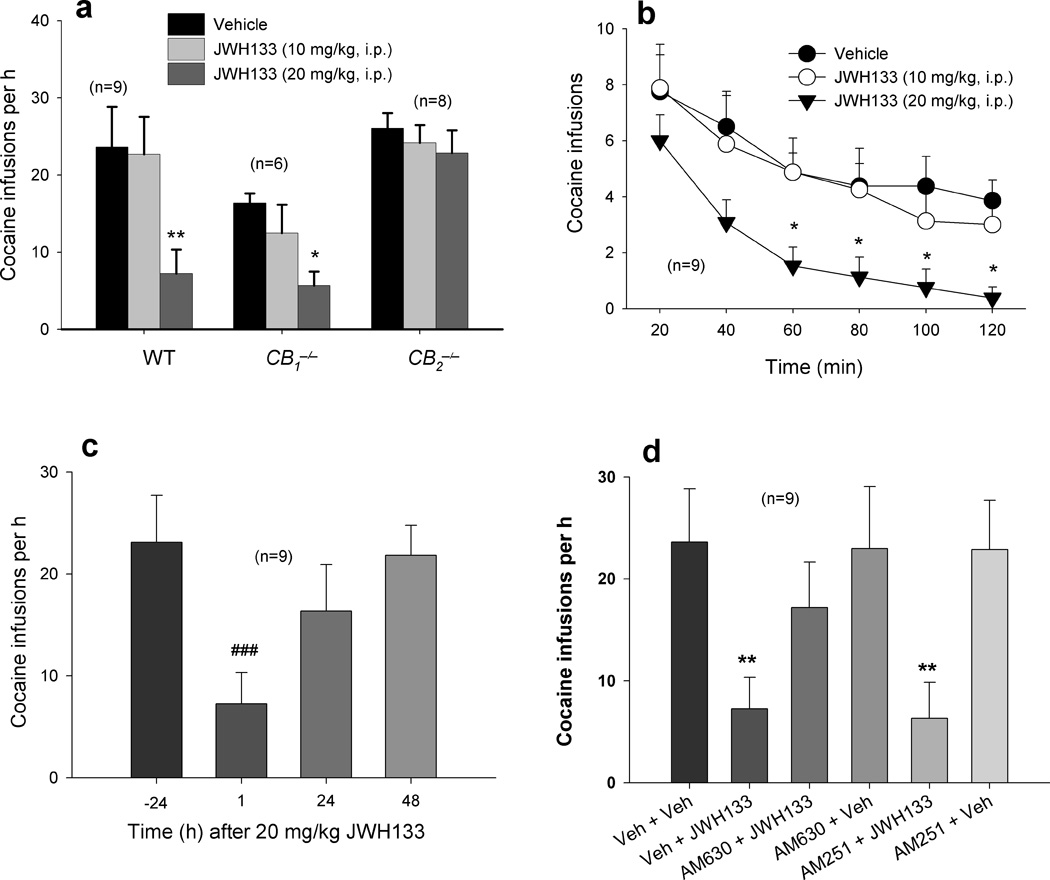
Effects of JWH133 on cocaine self-administration. (a) Systemic administration of JWH133 (10, 20 mg/kg, i.p., 30 min prior to testing) inhibits cocaine self-administration under FR1 reinforcement in WT (one-way ANOVA, F2,16 = 13.09, P < 0.001) and CB1−/−(F2,10 = 5.01, P < 0.05), but not CB2−/− (F2,14=0.56, P = 0.58), mice. (b) Time course of JWH133’s attenuation of cocaine self-administration in WT mice on the test day. (c) Time course of recovery of cocaine self-administration in WT mice after JWH133 administration. (d) In WT mice, JWH133-induced attenuation of cocaine self-administration is prevented by pretreatment with the CB2 receptor antagonist AM630 (10 mg/kg, i.p., 30 min prior to JWH133), but not by pretreatment with the CB1 receptor antagonist AM251 (3 mg/kg, i.p.) (F5,40 = 6.31, P < 0.001). Neither AM630 nor AM251 altered cocaine self-administration in WT mice. Data are means ± s.e.m. * P < 0.05, ** P < 0.01, compared to vehicle (Veh) control groups. ### P < 0.001, compared to pre-JWH133 (−24 h) condition.
Figure 2.
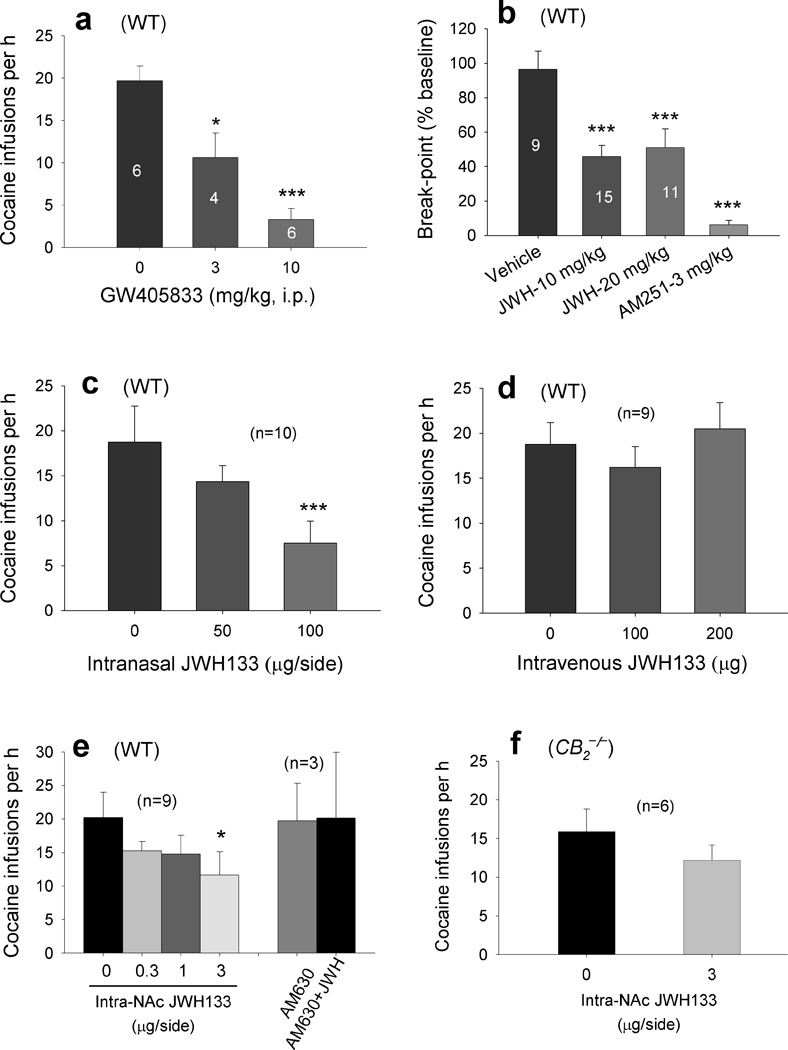
Effects of GW405833 or JWH133 on cocaine self-administration. (a) GW405833 (3, 10 mg/kg, i.p.) dose-dependently inhibited cocaine self-administration under FR1 reinforcement in WT mice (one-way ANOVA, F2,6 = 20.03, P < 0.01). (b) JWH133 (10, 20 mg/kg) or AM251 (3 mg/kg, i.p.) significantly lowered the cocaine self-administration break-point under PR reinforcement in WT mice (F3,37 = 13.83, P < 0.001). (c) Intranasal microinjections of JWH133 (50, 100 µg/nostril) dose-dependently inhibited cocaine self-administration under FR1 reinforcement (F2,18 = 14.34, P < 0.001). (d) Intravenous injection of the same micro-quantity (100, 200 µg) of JWH133 as used intranasally had no effect on cocaine self-administration (F2,16 = 1.59, P = 0.23). (e) Intra-NAc microinjections of JWH133 (0.3, 1, 3 µg/side) dose-dependently inhibited cocaine self-administration under FR1 reinforcement in WT mice. This inhibition was blocked by intra-NAc co-administration of AM630 (3 µg/side) (F3,24 = 4.49, P < 0.05). (f) Intra-NAc administration of JWH133 (3 µg/side) had no effect on cocaine self-administration in CB2−/− mice (F1,10 = 2.37, P = 0.15). Data are means ± s.e.m. * P < 0.05, *** P < 0.001, compared to vehicle control group.
To determine whether JWH133-induced attenuation of cocaine self-administration was due to a reduction in cocaine’s rewarding efficacy, we studied JWH133’s effect on i.v. cocaine self-administration under PR reinforcement. We found that systemic administration of JWH133 (10, 20 mg/kg, i.p.) significantly lowered the PR break-point for cocaine self-administration in WT mice (Fig. 2b), suggesting a reduction in cocaine’s reward strength and/or motivation for drug-taking behavior after JWH133 administration. We previously showed that CB1 receptor blockade by AM251 significantly lowered the PR break-point for cocaine self-administration in rats27. We therefore also tested AM251 in the present study, and found that AM251 (3 mg/kg) lowered the PR break-point for cocaine self-administration in WT mice (Fig. 2b). These data suggest that the JWH133-induced reduction in cocaine self-administration resulted from a reduction in cocaine’s rewarding efficacy.
JWH133 inhibits cocaine self-administration by activation of brain CB2 receptors
To further determine whether JWH133’s action was mediated by activation of brain or peripheral CB2 receptors, we first studied the effects of intranasal microinjections of JWH133 on i.v. cocaine self-administration. Extensive studies have shown that a wide variety of compounds that cannot penetrate the blood-brain barrier can be delivered directly from nose into brain28. We found that intranasal microinjections of JWH133 (50, 100 µg/10 µl/side) dose-dependently inhibited i.v. cocaine self-administration (Fig. 2c). To explore the possibility that effects of intranasal JWH133 might be mediated by drug absorption into the nasal vasculature with subsequent venous delivery of drug to pharmacological site(s) of action, we observed the effects of i.v. injection of the same micro-quantities of JWH133 as used intranasally on cocaine self-administration. We found that i.v. microinjections of JWH133 (100, 200 µg) had no effect on cocaine self-administration (Fig. 2d). These data suggest that intranasal JWH133-induced pharmacological effects are mediated by activating brain rather than peripheral CB2 receptors. To further explore this issue, we observed the effects of local administration of JWH133 into the nucleus accumbens (NAc) on cocaine self-administration. We found that intra-NAc microinjections of JWH133 (0.3, 1, 3 µg/side) significantly and dose-dependently inhibited cocaine self-administration in WT mice (Fig. 2e), but not in CB2−/− mice (Fig. 2f). This inhibition was blocked by intra-NAc co-administration of AM630 (3 µg/side).
JWH133 itself has no reinforcing or aversive effects
We further examined whether JWH133 itself has cocaine-like rewarding effects. To address this issue, we first trained mice to acquire stable cocaine self-administration, and then cocaine was replaced by JWH133 (1 mg/kg/infusion) or vehicle. We found that neither JWH133 nor vehicle sustained stable self-administration in mice previously trained for cocaine self-administration (Supplementary Fig. 2a). In fact, the self-administration behavior underwent gradual extinction over the 5 days of substitution testing. This extinction pattern was essentially identical to that seen when vehicle was substituted for cocaine. However, when JWH133 or vehicle was replaced by cocaine, self-administration behavior returned to levels previously observed during stable cocaine self-administration. In addition, we also found that cocaine (10, 20 mg/kg, i.p.) produced a significant conditioned place preference, while JWH133, at the same doses, produced neither conditioned place preference nor place aversion in WT mice (Supplementary Fig. 2b). These findings suggest that JWH133 has no cocaine-like reinforcing nor aversive effects in mice.
JWH133 inhibits cocaine-enhanced locomotion
To determine whether JWH133’s effect on cocaine self-administration generalizes to other cocaine actions, we investigated the effects of JWH133 on cocaine-enhanced locomotion. Systemic administration of 10 mg/kg cocaine produced a significant increase in locomotion in all 3 mouse strains (Fig. 3). Pretreatment with JWH133 (10, 20 mg/kg, 30 min prior to cocaine) dose-dependently attenuated cocaine-enhanced locomotion in WT (Fig. 3a) and CB1−/− (Fig. 3b) mice, but not in CB2−/− (Fig. 3c) mice. Systemic administration of the same doses of JWH133 alone also significantly inhibited locomotion in a dose-dependent manner in WT and CB1−/− mice, but not in CB2−/− mice (Fig. 4a), suggesting an effect mediated by activation of CB2 receptors. Since the same doses of JWH133 alone failed to alter locomotor performance on a fast-running rotarod device in all 3 mouse strains (Supplementary Fig. 3), we infer that JWH133’s inhibition of cocaine self-administration or locomotion is not produced by nonspecific impairment of locomotor capacity.
Figure 3.
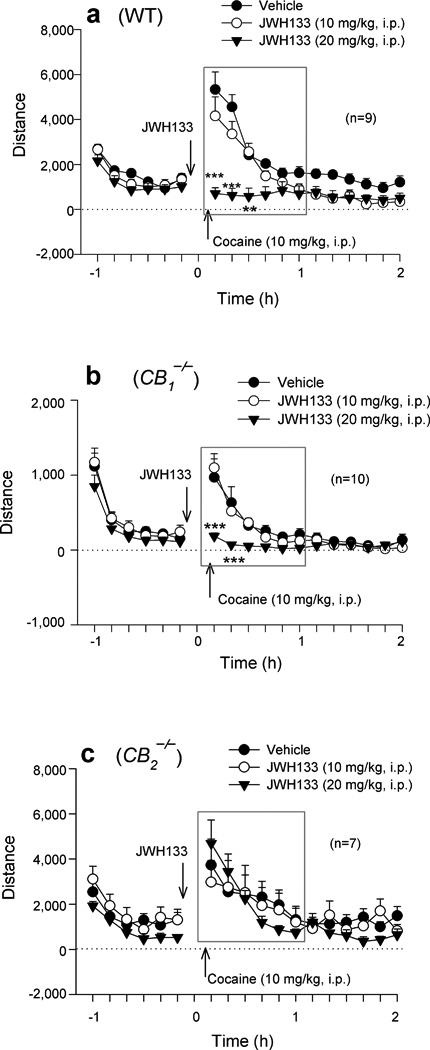
Systemic administration of JWH133 (10, 20 mg/kg, i.p., 30 min prior to cocaine) dose-dependently inhibited cocaine-enhanced locomotion in WT (a, two-way ANOVA for repeated measures over time, F2,16 = 14.45, P < 0.001) and CB1−/− (b, F2,18=12.57, p<0.001), but not in CB2−/− (c, F2,12 = 0.17, P = 0.85), mice. Data are means ± s.e.m. ** P < 0.01, *** P < 0.001, compared to vehicle treatment group.
Figure 4.
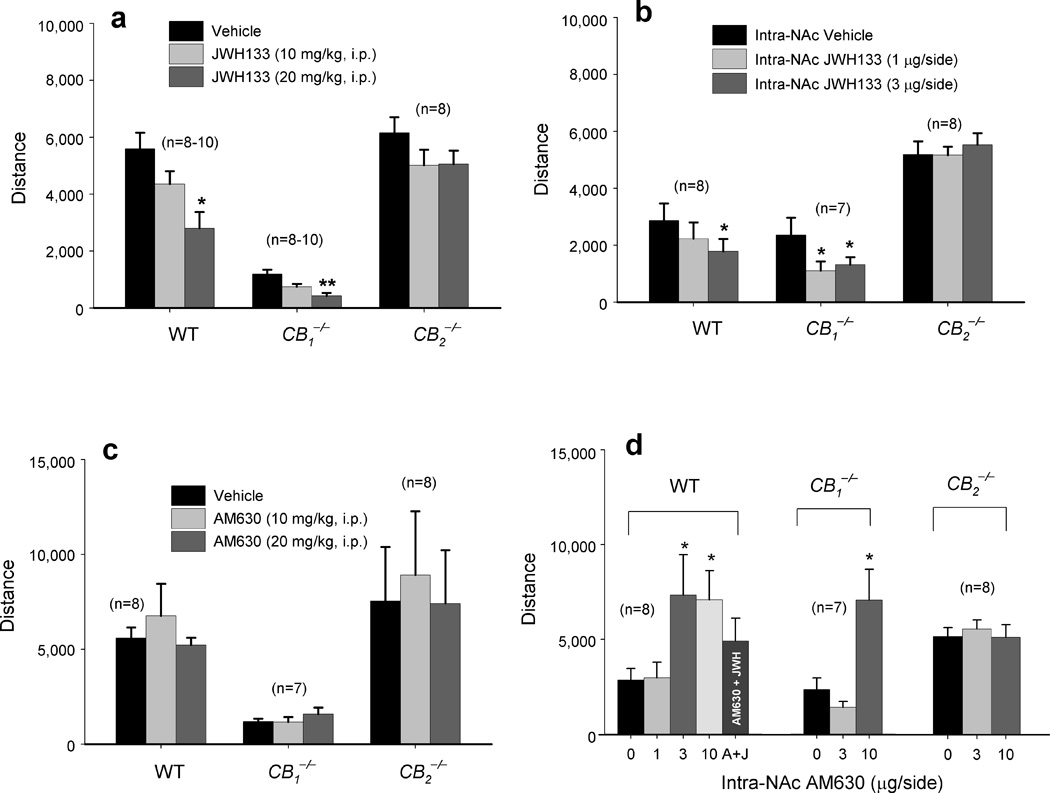
Effects of systemic or local intra-NAc administration of JWH133 or AM630 on locomotor activity. (a) Systemic administration of JWH133 (10, 20 mg/kg, i.p.) dose-dependently inhibited locomotion in WT (one-way ANOVA, F2,24 = 8.03, P = 0.002) and CB1−/− (F2,25 = 13.44, P < 0.001) mice, but not in CB2−/− (F2,14 = 3.36, P > 0.05) mice. (b) Intra-NAc microinjections of JWH133 (1, 3 µg/side) significantly inhibited locomotion in WT (F2,14=4.17, p<0.05) and CB1−/− (F2,12 = 4.91, P < 0.05), but not in CB2−/− (F2,14 = 0.04, P > 0.05), mice (c) Systemic administration of AM630 failed to alter locomotion in any strain of mice. (d) Intra-NAc administration of AM630 (1, 3, 10 µg/side) significantly augmented locomotion in WT (F3,21 = 4.62, P < 0.05) and CB1−/− (F2,12 = 10.57, P < 0.01), but not in CB2−/−(F2,14 = 0.05, P > 0.05), mice. Data are means ± s.e.m. * P < 0.05, ** P < 0.01, compared to vehicle control group.
To further determine whether such locomotor inhibition is mediated by activation of brain CB2 receptors, we observed the effects of intra-NAc JWH133 and/or AM630 on locomotion. We found that intra-NAc microinjections of JWH133 (1, 3 µg/side) significantly inhibited locomotion in WT or CB1−/− mice, but not in CB2−/− mice (Fig. 4b), in a dose-dependent manner similar to systemic administration (Fig. 4a). We note that systemic administration of AM630 failed to alter locomotion in any mouse strain tested (Fig. 4c). However, when locally administered into the NAc, AM630 (1, 3, 10 µg/side) significantly increased locomotor activity (Fig. 4d) in WT and CB1−/− mice, but not in CB2−/− mice. These data suggest that CB2 receptors may tonically modulate locomotion. A higher brain AM630 level, achieved by local rather than by systemic administration, appears to be required to block endocannabinoid action on brain CB2 receptors.
JWH133 inhibits cocaine-enhanced extracellular DA in the nucleus accumbens
Given the crucial role of the mesolimbic DA system in cocaine self-administration and modulation of locomotion29, we further investigated the effects of JWH133 on basal and cocaine-enhanced DA in the NAc by in vivo microdialysis. We did not see significant differences in basal levels of extracellular NAc DA between WT and CB2−/− mice (Supplementary Fig. 4). However, CB1−/− mice displayed a significant basal reduction, compared to WT mice (Supplementary Fig. 4). Consistent with the findings in cocaine self-administration and locomotion, systemic administration of JWH133 (3, 10, 20 mg/kg, i.p.) also significantly and dose-dependently lowered extracellular NAc DA in WT (Fig. 5a) and CB1−/− (Fig. 5b) mice, but not in CB2−/− (Fig. 5c) mice. This reduction in NAc DA was blocked by AM630 (10 mg/kg, i.p.) in CB1−/− mice (Fig. 5b), suggesting that JWH133’s DA-inhibiting effect is mediated by activation of CB2 receptors. Moreover, pretreatment with the same doses of JWH133 also significantly attenuated cocaine-enhanced NAc DA in WT (Fig. 5d), CB1−/− (Fig. 5e), but not in CB2−/− (Fig. 5f) mice.
Figure 5.
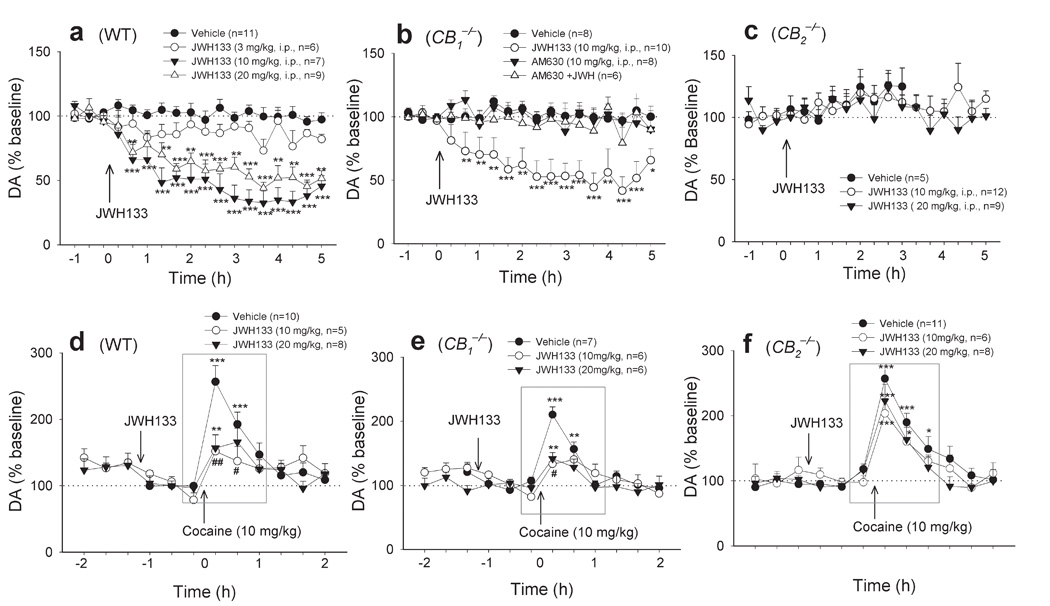
Systemic administration of JWH133 (3, 10, 20 mg/kg, i.p.) dose-dependently inhibited basal (a, b, c) or cocaine-enhanced (d, e, f) extracellular NAc DA in WT (a, two-way ANOVA for repeated measures over time, F3,29 = 25.97, P < 0.001; d, F2,19 = 4.47, P < 0.05) and CB1−/− (b, F3,28 = 10.07, P < 0.001; e, F2,16 = 4.78, P < 0.05) mice, but not in CB2−/− (c, F2,23 = 0.10, P > 0.05; f, F2,22 = 1.53, P > 0.05) mice. AM630 alone (10 mg/kg, i.p.) failed to alter NAc DA in CB1−/− mice, while AM630 pretreatment (10 mg/kg, i.p.) prevented JWH133-induced inhibition of NAc DA in CB1−/− mice (b). Data are means ± s.e.m. * P < 0.05, ** P < 0.01, *** P < 0.001, compared to pre-drug baseline.# P < 0.05,## P < 0.01, compared to vehicle treatment group.
To determine whether this inhibition is mediated by activation of brain or peripheral CB2 receptors, we also observed the effects of intranasal or intra-NAc local administration of JWH133 on extracellular DA. We found that intranasal administration of JWH133 (100 µg/nostril) produced a significant reduction in extracellular NAc DA in WT and CB1−/− mice, but not in CB2−/− mice (Fig. 6a). Similarly, intra-NAc local perfusion of JWH133 (1–1000 µM) significantly lowered extracellular DA in both WT and CB1−/− mice, but not in CB2−/− mice (Fig. 6b). In fact, an unexpected increase in extracellular DA was observed in CB2−/− mice after local administration of JWH133. The underlying mechanisms are unclear. One possibility is that JWH133 may bind to other (non-CB2) receptors in CB2−/− mice, producing an increase in extracellular DA. Congruent with this finding, intra-NAc local perfusion of AM630 (1, 10, 100 µM) elevated extracellular DA in a concentration-dependent manner in both WT and CB1−/− mice, but not in CB2−/− mice (Fig. 6c), suggesting that endocannabinoids tonically modulate NAc DA release by activation of brain CB2 receptors. Further, AM630-enhanced extracellular DA appears more robust in CB1−/− mice than in WT mice (Fig. 6c), suggesting higher endocannabinoid tone on brain CB2 receptors in CB1−/− mice. Moreover, intra-NAc local perfusion of AM630 also blocked the reduction in extracellular NAc DA produced by systemic administration of JWH133 seen in WT and CB1−/− mice (Fig. 6c, d), suggesting that JWH133-induced inhibition of DA release is mediated by activation of NAc CB2 receptors. The locations of microdialysis probes or microinjection cannulae were within the NAc (Supplementary Fig. 5).
Figure 6.
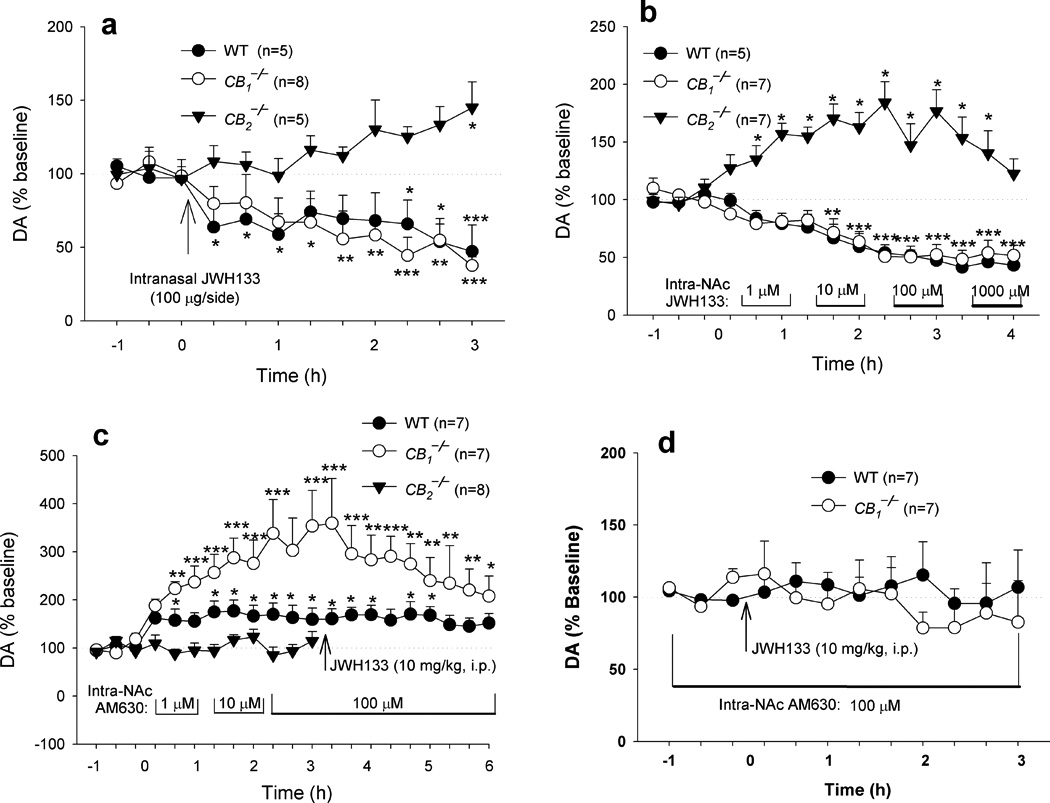
Effects of intranasal or intra-NAc local perfusion of JWH133 or AM630 on extracellular NAc DA. (a) Intranasal administration of JWH133 (50 µg/nostril) significantly lowered extracellular DA in WT and CB1−/− mice, but not in CB2−/− mice (two-way ANOVA for repeated measures over time, F2,15 = 10.81, P = 0.001). (b) Intra-NAc local perfusion of JWH133 lowered extracellular DA in WT and CB1−/− mice in a dose-dependent manner, while elevating extracellular DA in CB2−/− mice (F2,18=47.00, P < 0.001). (c) Intra-NAc local perfusion of AM630 elevated extracellular DA in WT and CB1−/− mice, but not in CB2−/− mice (F2,18 = 12.13, P < 0.001). Further, AM630-enhanced extracellular DA appears more robust in CB1−/− mice than in WT mice (F1,12 = 7.50, P < 0.05). (d) Renormalized data over new baselines 1 h before JWH133 administration from the data in Panel c, illustrating that intra-NAc local perfusion of AM630 blocked JWH133’s action on extracellular DA in WT and CB1−/− mice. Data are means ± s.e.m. * P < 0.05, ** P < 0.01, *** P < 0.001, compared to pre-drug baseline.
DISCUSSION
Here we report that systemic administration of the CB2 receptor agonist JWH133 significantly and dose-dependently inhibits intravenous cocaine self-administration under both FR1 and PR reinforcement and inhibits cocaine-enhanced locomotion and extracellular NAc DA in WT and CB1−/− mice, but not in CB2−/− mice. This effect was mimicked by GW405833 (another selective CB2 receptor agonist), and blocked by AM630, a selective CB2 receptor antagonist, but not by AM251, a selective CB1 receptor antagonist, suggesting an effect mediated by activation of CB2 receptors. Further, intranasal microinjections of JWH133, but not intravenous injections of the same micro-quantities of JWH133 as injected intranasally, also significantly and dose-dependently inhibited intravenous cocaine self-administration, suggesting an effect mediated by activation of brain, not peripheral, CB2 receptors. This is further supported by the finding that local intra-NAc administration of JWH133 also significantly inhibited cocaine self-administration in a dose-dependent manner, an effect that was blocked by intra-NAc co-administration of AM630. In addition, intra-NAc local administration of JWH133 dose-dependently lowered, while AM630 elevated, basal levels of locomotion and extracellular NAc DA. Intra-NAc local perfusion of AM630 blocked the reduction in cocaine self-administration and NAc DA produced by systemic administration of JWH133. These data suggest that both the behavioral and neurochemical effects of JWH133 are mediated by activation of brain CB2 receptors.
We note that systemic administration of AM630 failed to alter, while intra-NAc local administration of AM630 elevated, extracellular DA and locomotion, suggesting that local AM630 administration is more effective than systemic administration. This may be related to AM630’s relatively poor pharmacokinetic properties and/or blood-brain barrier passage. In addition, we also note that intra-NAc AM630 significantly elevated extracellular DA and locomotion, but failed to alter cocaine self-administration. This may be related to previous findings that locomotion is largely DA-dependent30, while cocaine self-administration is dependent on both DA and non-DA mechanisms31. We also note that pharmacological blockade of NAc CB2 receptors elevated, while genetic deletion of CB2 receptors did not alter, basal extracellular DA in the NAc. The reasons are unclear. One possibility is that CB2 receptor deletion-induced disinhibition of NAc DA release may be compromised by actions in other brain loci that modulate the mesolimbic DA system. Another possibility is that neuroadaptative processes may antagonize CB2 receptor inactivation-induced DA neuronal disinhibition after CB2 receptor deletion. Whatever the exact mechanism(s), the present data strongly suggest that brain CB2 receptors functionally modulate the mesolimbic DA system and DA-related functions. Activation of brain CB2 receptors by JWH133 inhibits both the behavioral and neurochemical effects of cocaine. Since JWH133 neither alters locomotor performance as assessed by the rotarod test, nor produces drug rewarding or aversive effects as assessed by i.v. self-administration and conditioned place preference, JWH133-induced inhibition of cocaine self-administration is most likely mediated by attenuation of cocaine’s rewarding efficacy secondary to the reduction in cocaine-enhanced NAc DA rather than by nonspecific locomotor impairment or malaise.
We fully recognize that these findings challenge the currently accepted opinion that selective CB2 receptor agonists have no CNS effects. This opinion is largely based upon previous reports that the selective CB2 receptor agonist AM1241 neither inhibits locomotion or rotarod performance, nor produces catalepsy or hypothermia in rats or mice32. In addition, AM1241 also failed to alter brain functional activity as assessed by pharmacological MRI33. The ineffectiveness of AM1241 may be related to the relatively lower doses (30 µg-3.3 mg/kg) used in those studies, relatively poor selectivity, and species differences in CB2 receptor response to AM124134–36. For example, AM1241 is reported to act as a full or partial agonist at human CB2 receptors35, while acting as an inverse agonist at rodent CB2 receptors36. Further, the analgesic effects produced by AM1241 are reported to be blocked by the opioid receptor antagonist naloxone37, suggesting that AM1241 may interact with other, non-cannabinoid, receptors. However, the CB2 receptor agonist GW405833, at high doses (30–100 mg/kg), produces significant CNS effects such as analgesia, sedation and catalepsy26, consistent with our finding that GW405833 (3–10 mg/kg) significantly inhibits cocaine self-administration in mice.
The presence of CB2 receptors in the CNS, in particular on neurons, has been subject to debate10, 38. Previous studies using in situ hybridization and Northern blot assays failed to detect CB2 receptor mRNA in brain5, 39, 40. However, recent studies with more sensitive RT-PCR and immunolabeling techniques have claimed to find significant CB2 receptor expression in microglia and subpopulations of neuronal cells in brain7–11. By using highly sensitive and specific Taqman probes, we have recently identified two CB2 receptor isoforms (CB2A, CB2B) in both brain and peripheral tissues, which display significant species differences in both structure and expression between humans, rats and mice41. It is now well accepted that CB2 receptors are expressed on microglia and a subset of neurons with levels increasing under certain pathological conditions such as neuroinflammation and brain injury38. There are two possibilities to explain the present findings. First, a low density of CB2 receptors may be expressed on mesolimbic DA neurons. Since CB2 receptors are Gi/o coupled42, activation of CB2 receptors on DA neurons in the midbrain ventral tegmental area (VTA) may directly inhibit VTA DA neurons and decrease NAc DA release, and therefore inhibit intravenous cocaine self-administration and cocaine-enhanced locomotion as observed in the present study. Although direct evidence of CB2 receptor expression in the mesolimbic DA neurons is lacking at present, functional CB2 receptors are found in other neurons. For example, CB2 receptor mRNA is expressed on striatal GABAergic neurons in non-human primates43, and activation of CB2 receptors inhibits GABAergic neurotransmission in the medial entorhinal cortex of the rat19. In addition, CB2 receptors are also found on neurons in the dorsal-root ganglion (DRG) and spinal cord (SC)44, 45, and activation of CB2 receptors on DRG-SC neurons inhibits neuronal response to noxious stimuli45, 46, thereby contributing to the antinociceptive effects of CB2 receptor agonists47. The second possibility is that activation of CB2 receptors located on microglial cells or astrocytes in the VTA and/or NAc may indirectly inhibit NAc DA release by releasing cytokines and inflammatory factors48, thereby inhibiting cocaine self-administration and cocaine-enhanced locomotion as observed in the present study.
Whatever the mechanisms, the present findings, for the first time, suggest that activation of brain CB2 receptors inhibits cocaine’s rewarding and psychomotor-stimulating effects, which is congruent with a rapidly expanding corpus of published reports implicating brain CB2 receptors in modulating a variety of CNS functions such as locomotion10, pain13, 47, emesis11, neurogenesis14, and neuroprotection15. This finding not only challenges current views that CB2 receptors are absent from the CNS and that CB2 receptor ligands lack CNS effects, but also suggests that brain CB2 receptors may be a novel target for the pharmacotherapy of drug abuse and addiction.
Supplementary Material
ACKNOWLEDGETS
This research was supported by the Intramural Research Program (IRP) of the National Institute on Drug Abuse (NIDA), National Institutes of Health (NIH). We thank Drs. Yavin Shaham and Elliot A. Stein of NIDA/IRP, and Dr. Ken Mackie of Indiana University for their helpful comments on this manuscript.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
AUTHOR CONTRIBUTIONS
Z.-X.X. developed the original research proposal, designed and supervised all experiments, analyzed all data and wrote the manuscript. X.-Q.P., X.L. and R.S. conducted the cocaine self-administration experiments. X.L., G.-H.B and H.-Y.Z. conducted the in vivo microdialysis experiments. X.L., H.-J.Y, R.S. and J.L. conducted the locomotor behavioral experiments. X.-Q.P, R.S. and H.-J.Y conducted the conditioned place preference/aversion experiments. Q-R.L. contributed to the original research proposal. E.L.G. contributed to the original conceptualization of this work and was responsible for overall supervision of the research and for revisions and modifications to the manuscript.
References
- 1.Parolaro D, Rubino T. The role of the endogenous cannabinoid system in drug addiction. Drug News Perspect. 2008;21:149–157. [PubMed] [Google Scholar]
- 2.Mackie K. Cannabinoid receptors: where they are and what they do. J Neuroendocrinol. 2008;20(Suppl 1):10–14. doi: 10.1111/j.1365-2826.2008.01671.x. [DOI] [PubMed] [Google Scholar]
- 3.Glass M, Dragunow M, Faull RL. Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience. 1997;77:299–318. doi: 10.1016/s0306-4522(96)00428-9. [DOI] [PubMed] [Google Scholar]
- 4.Griffin G, Tao Q, Abood ME. Cloning and pharmacological characterization of the rat CB2 cannabinoid receptor. J Pharmacol Exp Ther. 2000;292:886–894. [PubMed] [Google Scholar]
- 5.Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 6.Malan TP, Jr, et al. CB2 cannabinoid receptor agonists: pain relief without psychoactive effects? Curr Opin Pharmacol. 2003;3:62–67. doi: 10.1016/s1471-4892(02)00004-8. [DOI] [PubMed] [Google Scholar]
- 7.Stella N. Cannabinoid and cannabinoid-like receptors in microglia, astrocytes, and astrocytomas. Glia. 2010;58:1017–1030. doi: 10.1002/glia.20983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baek JH, Zheng Y, Darlington CL, Smith PF. Cannabinoid CB2 receptor expression in the rat brainstem cochlear and vestibular nuclei. Acta Otolaryngol. 2008:1–7. doi: 10.1080/00016480701796944. [DOI] [PubMed] [Google Scholar]
- 9.Gong J-P, et al. Cannabinoid CB2 receptors: immunohistochemical localization in rat brain. Brain Res. 2006;1071:10–23. doi: 10.1016/j.brainres.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 10.Onaivi ES, et al. Discovery of the presence and functional expression of cannabinoid CB2 receptors in brain. Ann N Y Acad Sci. 2006;1074:514–536. doi: 10.1196/annals.1369.052. [DOI] [PubMed] [Google Scholar]
- 11.Van Sickle MD, et al. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310:329–332. doi: 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- 12.Guindon J, Hohmann AG. Cannabinoid CB2 receptors: a therapeutic target for the treatment of inflammatory and neuropathic pain. Br J Pharmacol. 2008;153:319–334. doi: 10.1038/sj.bjp.0707531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jhaveri MD, et al. Evidence for a novel functional role of cannabinoid CB2 receptors in the thalamus of neuropathic rats. Eur J Neurosci. 2008;27:1722–1730. doi: 10.1111/j.1460-9568.2008.06162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goncalves MB, et al. A diacylglycerol lipase-CB2 cannabinoid pathway regulates adult subventricular zone neurogenesis in an age-dependent manner. Mol Cell Neurosci. 2008;38:526–536. doi: 10.1016/j.mcn.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Viscomi MT, et al. Selective CB2 receptor agonism protects central neurons from remote axotomy-induced apoptosis through the PI3K/Akt pathway. J Neurosci. 2009;29:4564–4570. doi: 10.1523/JNEUROSCI.0786-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sagredo O, et al. Cannabinoid CB2 receptor agonists protect the striatum against malonate toxicity: relevance for Huntington's disease. Glia. 2009;57:1154–1167. doi: 10.1002/glia.20838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elmes SJR, Jhaveri MD, Smart D, Kendall DA, Chapman V. Cannabinoid CB2 receptor activation inhibits mechanically evoked responses of wide dynamic range dorsal horn neurons in naive rats and in rat models of inflammatory and neuropathic pain. Eur J Neurosci. 2004;20:2311–2320. doi: 10.1111/j.1460-9568.2004.03690.x. [DOI] [PubMed] [Google Scholar]
- 18.Sagar DR, et al. Inhibitory effects of CB1 and CB2 receptor agonists on responses of DRG neurons and dorsal horn neurons in neuropathic rats. Eur J Neurosci. 2005;22:371–379. doi: 10.1111/j.1460-9568.2005.04206.x. [DOI] [PubMed] [Google Scholar]
- 19.Morgan NH, Stanford IM, Woodhall GL. Functional CB2 type cannabinoid receptors at CNS synapses. Neuropharmacology. 2009;57:356–368. doi: 10.1016/j.neuropharm.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 20.Ashton JC, Wright JL, McPartland JM, Tyndall JDA. Cannabinoid CB1 and CB2 receptor ligand specificity and the development of CB2-selective agonists. Curr Med Chem. 2008;15:1428–1443. doi: 10.2174/092986708784567716. [DOI] [PubMed] [Google Scholar]
- 21.Huffman JW. CB2 receptor ligands. Mini Rev Med Chem. 2005;5:641–649. doi: 10.2174/1389557054368844. [DOI] [PubMed] [Google Scholar]
- 22.Richardson NR, Roberts DCS. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- 23.Soria G, et al. Lack of CB1 cannabinoid receptor impairs cocaine self-administration. Neuropsychopharmacology. 2005;30:1670–1680. doi: 10.1038/sj.npp.1300707. [DOI] [PubMed] [Google Scholar]
- 24.Li X, et al. Attenuation of basal and cocaine-enhanced locomotion and nucleus accumbens dopamine in cannabinoid CB1-receptor-knockout mice. Psychopharmacology. 2009;204:1–11. doi: 10.1007/s00213-008-1432-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thakur GA, Nikas SP, Makriyannis A. CB1 cannabinoid receptor ligands. Mini Rev Med Chem. 2005;5:631–640. doi: 10.2174/1389557054368772. [DOI] [PubMed] [Google Scholar]
- 26.Valenzano KJ, et al. Pharmacological and pharmacokinetic characterization of the cannabinoid receptor 2 agonist, GW405833, utilizing rodent models of acute and chronic pain, anxiety, ataxia and catalepsy. Neuropharmacology. 2005;48:658–672. doi: 10.1016/j.neuropharm.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 27.Xi Z-X, et al. Cannabinoid CB1 receptor antagonists attenuate cocaine's rewarding effects: experiments with self-administration and brain-stimulation reward in rats. Neuropsychopharmacology. 2008;33:1735–1745. doi: 10.1038/sj.npp.1301552. [DOI] [PubMed] [Google Scholar]
- 28.Costantino HR, Illum L, Brandt G, Johnson PH, Quay SC. Intranasal delivery: physicochemical and therapeutic aspects. Int J Pharm. 2007;337:1–24. doi: 10.1016/j.ijpharm.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 29.Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- 30.Schwarting RKW, Huston JP. Behavioral and neurochemical dynamics of neurotoxic meso-striatal dopamine lesions. Neurotoxicology. 1997;18:689–708. [PubMed] [Google Scholar]
- 31.Bardo MT. Neuropharmacological mechanisms of drug reward: beyond dopamine in the nucleus accumbens. Crit Rev Neurobiol. 1998;12:37–67. doi: 10.1615/critrevneurobiol.v12.i1-2.30. [DOI] [PubMed] [Google Scholar]
- 32.Malan TP, Jr, et al. CB2 cannabinoid receptor-mediated peripheral antinociception. Pain. 2001;93:239–245. doi: 10.1016/S0304-3959(01)00321-9. [DOI] [PubMed] [Google Scholar]
- 33.Chin C-L, et al. Differential effects of cannabinoid receptor agonists on regional brain activity using pharmacological MRI. Br J Pharmacol. 2008;153:367–379. doi: 10.1038/sj.bjp.0707506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mukherjee S, et al. Species comparison and pharmacological characterization of rat and human CB2 cannabinoid receptors. Eur J Pharmacol. 2004;505:1–9. doi: 10.1016/j.ejphar.2004.09.058. [DOI] [PubMed] [Google Scholar]
- 35.Yao BB, et al. In vitro pharmacological characterization of AM1241: a protean agonist at the cannabinoid CB2 receptor? Br J Pharmacol. 2006;149:145–154. doi: 10.1038/sj.bjp.0706838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bingham B, et al. Species-specific in vitro pharmacological effects of the cannabinoid receptor 2 (CB2) selective ligand AM1241 and its resolved enantiomers. Br J Pharmacol. 2007;151:1061–1070. doi: 10.1038/sj.bjp.0707303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ibrahim MM, et al. CB2 cannabinoid receptor activation produces antinociception by stimulating peripheral release of endogenous opioids. Proc Natl Acad Sci U S A. 2005;102:3093–3098. doi: 10.1073/pnas.0409888102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Atwood BK, Mackie K. CB2: a cannabinoid receptor with an identity crisis. Br J Pharmacol. 2010;160:467–479. doi: 10.1111/j.1476-5381.2010.00729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown SM, Wager-Miller J, Mackie K. Cloning and molecular characterization of the rat CB2 cannabinoid receptor. Biochim Biophys Acta. 2002;1576:255–264. doi: 10.1016/s0167-4781(02)00341-x. [DOI] [PubMed] [Google Scholar]
- 40.Schatz AR, Lee M, Condie RB, Pulaski JT, Kaminski NE. Cannabinoid receptors CB1 and CB2: a characterization of expression and adenylate cyclase modulation within the immune system. Toxicol Appl Pharmacol. 1997;142:278–287. doi: 10.1006/taap.1996.8034. [DOI] [PubMed] [Google Scholar]
- 41.Liu Q-R, et al. Species differences in cannabinoid receptor 2 (CNR2 gene): identification of novel human and rodent CB2 isoforms, differential tissue expression and regulation by cannabinoid receptor ligands. Genes Brain Behav. 2009;8:519–530. doi: 10.1111/j.1601-183X.2009.00498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bayewitch M, et al. The peripheral cannabinoid receptor: adenylate cyclase inhibition and G protein coupling. FEBS Lett. 1995;375:143–147. doi: 10.1016/0014-5793(95)01207-u. [DOI] [PubMed] [Google Scholar]
- 43.Lanciego JL, et al. Expression of the mRNA coding the cannabinoid receptor 2 in the pallidal complex of Macaca fascicularis. J Psychopharmacol. 2010 doi: 10.1177/0269881110367732. [DOI] [PubMed] [Google Scholar]
- 44.Zhang J, et al. Induction of CB2 receptor expression in the rat spinal cord of neuropathic but not inflammatory chronic pain models. Eur J Neurosci. 2003;17:2750–2754. doi: 10.1046/j.1460-9568.2003.02704.x. [DOI] [PubMed] [Google Scholar]
- 45.Anand U, et al. Cannabinoid receptor CB2 localisation and agonist-mediated inhibition of capsaicin responses in human sensory neurons. Pain. 2008;138:667–680. doi: 10.1016/j.pain.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 46.Ross RA, et al. Actions of cannabinoid receptor ligands on rat cultured sensory neurones: implications for antinociception. Neuropharmacology. 2001;40:221–232. doi: 10.1016/s0028-3908(00)00135-0. [DOI] [PubMed] [Google Scholar]
- 47.Beltramo M, et al. CB2 receptor-mediated antihyperalgesia: possible direct involvement of neural mechanisms. Eur J Neurosci. 2006;23:1530–1538. doi: 10.1111/j.1460-9568.2006.04684.x. [DOI] [PubMed] [Google Scholar]
- 48.Haydon PG, Blendy J, Moss SJ, Rob Jackson F. Astrocytic control of synaptic transmission and plasticity: a target for drugs of abuse? Neuropharmacology. 2009;56(Suppl 1):83–90. doi: 10.1016/j.neuropharm.2008.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zimmer A, Zimmer AM, Hohmann AG, Herkenham M, Bonner TI. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc Natl Acad Sci U S A. 1999;96:5780–5785. doi: 10.1073/pnas.96.10.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Buckley NE, et al. Immunomodulation by cannabinoids is absent in mice deficient for the cannabinoid CB2 receptor. Eur J Pharmacol. 2000;396:141–149. doi: 10.1016/s0014-2999(00)00211-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


