Abstract
Objective
To examine the Institute of Medicine (IOM) guidelines for gestational weight gain in adolescents.
Study Design
Retrospective cohort using the Missouri Birth Certificate Registry. Included subjects were primiparous, singleton gestations, <20 years, delivered 24–44 weeks gestation. The exposure was defined as weight gain less than, within, or greater than IOM recommendations. Outcomes examined were small-for-gestational-age infants (SGA), large-for-gestational age infants (LGA), preterm delivery, infant death, preeclampsia, cesarean delivery, and operative vaginal delivery. The analysis was stratified by body mass index (BMI) category.
Results
In any BMI category, inadequate weight gain was associated with increased odds of SGA, preterm delivery and infant death. When subjects gained more than IOM recommendations, SGA decreased with slight increases in LGA, preeclampsia, and cesarean delivery.
Conclusions
Adolescents should be counseled regarding adequate weight gain in pregnancy. Further research is necessary to determine if the IOM recommendations recommend enough weight gain in adolescents to optimize pregnancy outcomes.
Keywords: Adolescent pregnancy, Gestational Weight Gain, body mass index
Introduction
Adolescent pregnancy is complicated not only by its unique social aspects, but also by the increased risk of complications, including preeclampsia, preterm birth, and low infant birthweight.1,2 In 2006, the rate of low birth weight (less than 2500 g) in teenagers was as high as 13.4%, compared to 8.3% for the entire population.3 Although the reason for the increased rate of low birth weight infants among adolescent mothers is unclear, it is well known that maternal weight gain is correlated to infant birth weight,4 either because gestational weight gain is causally associated with fetal growth or because the same determinants of gestational weight gain (diet and exercise) also impact fetal growth.5 Whether the effect of gestational weight gain is causal or merely associative, this association may provide a pathway to decrease low birth weight infants in this population.
On the other hand, excessive gestational weight gain leads to post-partum weight retention and obesity,6,7 a fact particularly important in adolescents who are likely to have subsequent pregnancies. Additionally, excessive weight gain is linked with a higher rate of large-for-gestational-age infants, fetal distress, gestational diabetes and preeclampsia.1,4,8
The Institute of Medicine (IOM) recommendations on gestational weight gain focus on adults without specific recommendations for adolescents.5 In fact, the use of adult body mass index (BMI) categories misclassifies adolescents into a lighter BMI group,5,9,10 resulting in higher weight gain recommendations than if using adolescent-specific BMI guidelines. For example, a 16-year-old with a BMI of 18 would be underweight according to IOM classifications and the recommendations for her weight gain would be 28–45-lbs. However, according to age-specific BMI categories, she would be normal weight, resulting in weight gain recommendations of 25–35-lbs. The IOM justifies this misclassification because “…young teens often need to gain more to improve birth weight outcomes.”5 However, whether misclassifying adolescents results in recommendations for enough gestational weight gain to maximize pregnancy outcome or whether it results in excessive gestational weight gain remains unclear.
Therefore, we sought to examine the IOM recommendations for gestational weight gain in adolescents by investigating the relationship of weight gain less than, within, or greater than the IOM recommendations with pregnancy outcomes and infant birthweight.
Materials and Methods
This was a population-based, retrospective cohort study based on Missouri linked birth, fetal, and infant death certificate data from 1989 to 2005. The database includes parental demographic information, medical and obstetrical characteristics and complications, and neonatal status at birth for each birth that occurred in the state. The study was exempt from institutional board review as all information was de-identified.
Our study sample consisted of primiparous women less than 20 years old who delivered singleton pregnancies between 24 and 44 weeks of gestation in Missouri between January 1, 1989, and December 31, 2005. We elected to include only primiparous patients to remove confounding factors of the prior pregnancy, such as prior cesarean. Gestational age from the present study was based on the birth certificate variable “clinical estimate of gestation” because it more accurately reflects gestational age at delivery than length of pregnancy calculated using the last menstrual period.11 Pregnancies complicated by major fetal anomalies or breech presentation were excluded as this would impact the outcomes of interest. Multifetal gestations were excluded as the IOM recommendations are specifically for singleton gestations and multifetal are anticipated to require higher weight gains.
The exposure of interest for this study was gestational weight gain, which is a self-reported measure obtained from the birth certificate. The study sample was divided into three exposure groups based on the IOM recommendations for gestational weight gain according to each BMI weight category. The three groups included those who gained below recommendations, within recommendations, and above recommendations for gestational weight gain. BMI, calculated as weight per height (kg/m2), was categorized according to the IOM recommendations: underweight (BMI, < 18.5 kg/m2), normal weight (BMI, 18.5 – 24.9 kg/m2), overweight (BMI, 25 – 29.9 kg/m2), and obese (BMI, ≥ 30 kg/m2). As gestational weight gain is in part dependent on how long a subject remains pregnant, expected gestational weight gain for preterm patients was calculated based on IOM recommendations by using the recommended first trimester weight gain (1.1–4.4 kg) and the estimated weight gain per week based on BMI category.
Outcomes of interest for the present study included small-for-gestational-age infant (SGA), large-for-gestational-age infant (LGA), preterm delivery, infant death, preeclampsia, and cesarean delivery. SGA and LGA were measured as birth weight below the 10th percentile and above the 90th percentile, respectively, for gestational age and race/ethnicity, with the United States population serving as the reference for fetal growth.12 Preterm delivery was defined as delivery less than 37 weeks gestation. Infant deaths were those that occurred within the first year of life. Because of the small number of subjects with eclampsia, all adolescents who had the condition “pregnancy-induced hypertension (preeclampsia)” or “eclampsia” checked on the Missouri birth certificate were considered as having preeclampsia. For the cesarean delivery outcome, two binary indicators were constructed: one for all cesarean deliveries referring to primary elective and emergency cesarean as indicated on the birth certificate and unplanned cesarean for primary emergency cesarean.
The 1990 IOM recommendations for gestational weight gain suggest that adolescents should gain in the upper half of the recommendations for each BMI category, although the 2009 recommendations do not make this suggestion.5 As a secondary analysis, we examined the incidence of outcomes in those gaining in the bottom half of IOM recommendations versus the top half of IOM recommendations.
Differences in sample characteristics by BMI categories were assessed by using the Pearson Chi-square test (χ2) for categorical variables and t-test for continuous variables. For each BMI weight category, we examined the relationship of weight gain less than, within, or greater than the IOM recommendations with pregnancy outcomes and infant birthweight. Odds ratios (OR) and the 95% confidence intervals (95% CI) were estimated using binary logistic regression.
Factors that may be associated with gestational weight gain and outcomes of interest were evaluated as potential confounders. Data for the following maternal demographic and lifestyle variables were obtained from the birth certificate: maternal age, race (White American or African American), smoking or alcohol use during pregnancy (yes or no), Medicaid use (yes or no), pre-pregnancy body mass index (BMI), Kotelchuck prenatal care index (a measure of the adequacy of prenatal care as determined by the month in which prenatal care began and the percentage of visits attended),13 and a binary composite maternal medical risk factor (includes chronic hypertension, diabetes, and renal disease). For easier parameter estimate interpretation, maternal age was mean centered.14 All tests were 2 tailed and p < .05 was considered significant. All statistical analyses were performed with STATA (version 10.0, STATA Corp, College Station, TX).
Results
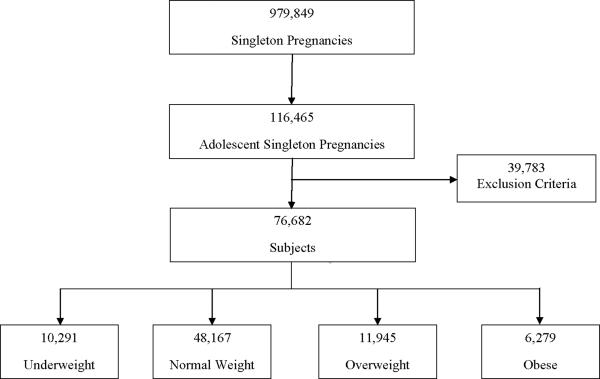
Of 979,849 singleton pregnancies in the Missouri birth certificate registry during the study period, 116,465 were to adolescents (Figure 1). After exclusion of multiparous subjects, congenital fetal anomalies, and breech presentation, the cohort consisted of 76,682 subjects. In our study sample, 10,291 (13.42%) were underweight, 48,167 (62.81%) were normal weight, 11,945 (15.58%) were overweight, and 6,279 (8.19%) were obese (Table 1). The majority of adolescents in our study were age 17–19 with an average age of 17. Overweight and obese adolescents were more likely to be black, Medicaid recipients, and have chronic hypertension and diabetes. Underweight and normal weight adolescents were more likely to be white and smoke during pregnancy.
Figure 1.
Subjects Included
Table 1.
Characteristics of Population by BMI Category
| Underweight BMI < 18.5 | Normal Weight BMI 18.5–24.9 | Overweight BMI 25.0–29.9 | Obese BMI ≥30 | p | |
|---|---|---|---|---|---|
| (n=10291) | (n=48167) | (n=11945) | (n=6279) | ||
| Mean Age | 17.49 ± 1.30 | 17.55 ± 1.31 | 17.73 ± 1.28 | 17.96 ± 1.16 | <0.01 |
| White (%) | 8565 (83.23%) | 36012 (74.76%) | 8254 (69.10%) | 4209 (67.03%) | <0.01 |
| Black (%) | 1726 (16.77%) | 12155 (25.24%) | 3691 (30.90%) | 2070 (32.97%) | |
| Medicaid (%) | 7756 (75.51%) | 35556 (73.95%) | 9227 (77.38%) | 5042 (80.43%) | <0.01 |
| Smoking (%) | 3263 (31.74%) | 11701 (24.32%) | 2697 (22.62%) | 1460 (23.30%) | <0.01 |
| Alcohol Use (%) | 361 (0.75%) | 73 (0.71%) | 77 (0.65%) | 46 (0.73%) | 0.68 |
| Chronic Hypertension (%) | 84 (0.17%) | 8 (0.08%) | 49 (0.41%) | 67 (1.07%) | <0.01 |
| Diabetes (%) | 51 (0.50%) | 325 (0.68%) | 145 (1.21%) | 153 (2.44%) | <0.01 |
| Weight Gain | <0.01 | ||||
| Less than IOM | 2808 (27.2%) | 10249 (21.3%) | 1050 (8.8%) | 688 (11.0%) | |
| Within IOM | 4404 (42.8%) | 16296 (33.8%) | 2411 (20.2%) | 1090 (17.4%) | |
| More than IOM | 3079 (29.9%) | 21621 (44.9%) | 8478 (71.0%) | 4501 (71.7%) | |
Tables 2–5 detail the association between gestational weight gain and pregnancy outcomes among adolescents by their BMI category. As prepregnancy BMI increased, so did the number of subjects gaining more than the IOM recommendations. Consequently, the vast majority overweight and obese adolescents gained more than the recommended amount.
Table 2.
Pregnancy Outcomes by Gestational Weight Gain Category for Underweight Adolescents (BMI <18.5)
| Gain Less than IOM <28 lbs | AOR* (95% CI) | Within IOM†28–40 lbs | Gain More than IOM >40 lbs | AOR* (95% CI) | |
|---|---|---|---|---|---|
| (n=2808) | (n = 4404) | (n=3079) | |||
| Neonatal Outcomes | |||||
| SGA (%) | 836 (29.78%) | 2.00 (1.77–2.24) | 815 (18.51%) | 355 (11.53%) | 0.54 (0.47–0.62) |
| LGA (%) | 143 (3.25%) | 0.82 (0.62–1.1) | 79 (2.81%) | 236 (7.66%) | 2.53 (2.03–3.14) |
| Preterm Delivery (%) | 408 (14.53%) | 2.82 (2.37–3.36) | 239 (5.43%) | 94 (3.05%) | 0.58 (0.45–0.74) |
| Infant Death (%) | 44 (1.57%) | 3.99 (2.25–7.05) | 19 (0.43%) | 13 (0.42%) | 0.96 (0.46–2.02) |
| Maternal Outcomes | |||||
| Preeclampsia (%) | 86 (3.07%) | 0.85 (0.65–1.13) | 154 (3.50%) | 162 (5.26%) | 1.62 (1.29–2.04) |
| Cesarean-All (%) | 292 (10.40%) | 1.02 (0.87–1.20) | 459 (10.42%) | 365 (11.85%) | 1.15 (0.99–1.34) |
| Unplanned Cesarean (%) | 198 (7.58%) | 0.94 (0.78–1.14) | 337 (8.18%) | 246 (8.70%) | 1.08 (0.91–1.29) |
| Operative Vaginal Delivery (%) | 398 (14.17%) | 0.83 (0.73–0.95) | 753 (17.10%) | 573 (18.61%) | 1.11 (0.99–1.26) |
Adjusted for maternal age, maternal race, tobacco use, alcohol use, a composite maternal medical risk factor (includes chronic hypertension, diabetes, and renal disease), Medicaid, and the Kotel chuck prenatal care index
Reference group
Table 5.
Pregnancy Outcomes by Gestational Weight Gain Category for Obese Adolescents (BMI >30.0)
| Gain Less than IOM <11 lbs | AOR* (95% CI) | Within IOM†11–20 lbs | Gain More than IOM >20 lbs | AOR* (95% CI) | |
|---|---|---|---|---|---|
| (n=688) | (n=1090) | (n=4501) | |||
| Neonatal Outcomes | |||||
| SGA (%) | 97 (14.10%) | 1.00 (0.75–1.33) | 155 (14.23%) | 389 (8.64%) | 0.55 (0.45–0.68) |
| LGA (%) | 36 (5.23%) | 0.91 (0.59–1.40) | 67 (6.15%) | 533 (11.84%) | 2.15 (1.63–2.83) |
| Preterm Delivery (%) | 76 (11.05%) | 1.52 (1.07–2.16) | 80 (7.34%) | 158 (3.51%) | 0.43 (0.32–0.58) |
| Infant Death (%) | 17 (2.47%) | 1.70 (0.83–3.48) | 16 (1.47%) | 22 (0.49%) | 0.36 (0.19–0.71) |
|
| |||||
| Maternal Outcomes | |||||
| Preeclampsia (%) | 52 (7.58%) | 0.80 (0.56–1.14) | 107 (9.82%) | 637 (14.16%) | 1.46 (1.17–1.83) |
| Cesarean-All (%) | 132 (19.19%) | 0.69 (0.54–0.88) | 288 (26.42%) | 1306 (29.02%) | 1.12 (0.96–1.31) |
| Unplanned Cesarean (%) | 87 (13.90%) | 0.69 (0.52–0.91) | 197 (20.08%) | 878 (22.07%) | 1.12 (0.94–1.34) |
| Operative Vaginal Delivery (%) | 64 (9.30%) | 0.91 (0.65–1.26) | 116 (10.64%) | 508 (11.29%) | 1.06 (0.86–1.32) |
Adjusted for maternal age, maternal race, tobacco use, alcohol use, a composite maternal medical risk factor (includes chronic hypertension, diabetes, and renal disease), Medicaid, and the Kotel chuck prenatal care index
Reference group
For every BMI category, the odds of having a SGA baby decreased as gestational weight gain increased. Significantly, the incidence of SGA infants ranged from 14.23% (obese subjects) to 18.51% (underweight subjects) in adolescents who gained within the IOM recommendations. The incidence of SGA in adolescents gaining more than the IOM recommendations was between 8.64–11.53%. Subjects who gained more than the IOM recommendations had decreased odds of having an SGA infant compared to adolescents gaining within the IOM recommendations, depending on their BMI category (AOR 0.54–0.62). Although the rate of LGA increased 2–2.5 fold in adolescents gaining more than the IOM recommendations compared to those gaining within, the absolute rate of LGA infants did not exceed 10% except for obese adolescents who gained more than the IOM recommendations.
For every BMI category, the odds of preterm delivery was significantly increased in those gaining less than the IOM recommendations compared to subjects gaining within the recommendations (aOR 1.52, 95% CI 1.07–2.16 for obese subjects, aOR 2.96, 95% CI 2.51–3.50 for underweight subjects). Subjects who gained more than the recommended weight gain had significantly decreased odds of delivering preterm compared to those gaining within the guidelines (aOR 0.43, 95% CI 0.32–0.58 for obese subjects, aOR 0.58, 95% CI 0.45–0.74 for underweight subjects). Subjects gaining less than recommended also had significantly increased odds of infant death (2.64–3.99) in every BMI category except obese patients. In obese subjects, those that gained less than recommended did not experience a statistically significant increase in odds of infant death compared to those gaining within the IOM recommendations (aOR 1.70, 95% CI 0.83–3.48). However, in obese subjects those who gained more than IOM recommendations had significantly decreased odds of infant death compared to those gaining within recommendations (aOR 0.36, 95% CI 0.19–0.71). This decrease was not seen in other BMI categories.
In examining maternal outcomes, the rate of preeclampsia and cesarean delivery increased as gestational weight gain increased. For adolescents gaining more than the recommendations, the odds of preeclampsia was increased (AOR 1.46–1.89) compared to those gaining within the recommended amount, depending on their prepregnancy BMI category. However, the absolute rate of preeclampsia was approximately 7%, except in overweight and obese subjects where it reached 10–14%. The odds of cesarean delivery were increased (AOR 1.12–1.35, depending on BMI category) in adolescents gaining more than the recommended amount compared to adolescents gaining within the recommendations, except in obese subjects where the odds were the same between the two groups. Of note, the cesarean delivery rates were below the national rate of about 30% for all BMI and weight gain categories. For normal weight adolescents, the rate of operative vaginal delivery increased as gestational weight gain increased, although the differences between those gaining within and greater than IOM recommendations were not significant. The rate of operative vaginal delivery was not altered by gestational weight gain in other BMI categories.
In examining outcomes with weight gain in the bottom and top half of the IOM recommendations, few differences are seen (Table 6). The incidence of SGA remained greater than 10% in each BMI category, although it decreased in underweight and normal weight patients (from 21.56% to 15.99% and from 16.34% to 13.50% respectively). For other outcomes, the incidence was similar between those gaining in the bottom and top half of the IOM recommendations.
Table 6.
Neonatal and Maternal Outcomes in Bottom and Top Half of IOM Recommendations
| Underweight | Normal Weight | Overweight | Obese | |||||
|---|---|---|---|---|---|---|---|---|
| In Bottom Half of IOM (28–34 lbs) | In Top Half of IOM (34.1–40 lbs) | In Bottom Half of IOM (25–30 lbs) | In Top Half of IOM (30.1–35 lbs) | In Bottom Half of IOM (15–20 lbs) | In Top Half of IOM (20.1–25 lbs) | In Bottom Half of IOM (11–15.5 lbs) | In Top Half of IOM (15.6–20 lbs) | |
| (n=2208) | (n=2246) | (n=8729) | (n=7211) | (n=1098) | (n=1196) | (n=416) | (n=630) | |
| Neonatal Outcomes | ||||||||
| SGA | 21.56 | 15.99 | 16.34 | 13.50 | 15.76 | 14.13 | 14.18 | 14.92 |
| LGA | 2.22 | 3.65 | 3.44 | 4.60 | 3.37 | 4.60 | 4.57 | 6.67 |
| Preterm Delivery | 7.20 | 7.52 | 4.12 | 7.29 | 5.37 | 5.43 | 6.01 | 5.24 |
| Infant Death | 0.59 | 0.62 | 0.50 | 0.68 | 0.46 | 1.00 | 0.24 | 1.59 |
| Maternal Outcomes | ||||||||
| Preeclampsia | 3.08 | 3.83 | 3.70 | 4.72 | 5.46 | 7.27 | 10.82 | 8.57 |
| Cesarean-All | 10.14 | 10.69 | 11.58 | 12.9 | 15.94 | 16.22 | 25.72 | 26.03 |
| Unplanned Cesarean | 8.05 | 8.46 | 8.80 | 9.88 | 11.30 | 11.70 | 18.96 | 20.28 |
| Operative Vaginal Delivery | 15.99 | 17.68 | 14.60 | 15.41 | 9.22 | 12.46 | 11.54 | 10.95 |
Data presented as percentage (%)
Comment
In this large retrospective cohort study, we found that, in every BMI category, adolescents gaining less than the recommended amount had significantly increased odds of SGA, preterm delivery and infant death. The increase in the risk of infant death is likely due in part to the increases in preterm delivery, as this increase in infant death is not seen when only term patients were analyzed (data not shown). It is therefore extremely important to counsel teenagers regarding the importance of adequate weight gain during pregnancy. Further, using adult BMI classifications in adolescents to determine gestational weight gain recommendations seems appropriate based on this information and does not appear to recommend too much weight gain.
Adolescents who attained a gestational weight gain within the IOM recommendations had an incidence of small-for-gestational age infants (defined as a birth weight <10th percentile) between 14–19%, while those who gained more than the IOM recommendations for their BMI category had fewer SGA infants (8–12%). Adolescents who gained more than IOM recommendations also had fewer preterm deliveries than those gaining within the recommendations. Increasing gestational weight gain did have drawbacks, namely increased odds of LGA infants and cesarean delivery; though the absolute increase in LGA and cesarean delivery is small, about 1–5%, depending on prepregnancy BMI. An increased odds of preeclampsia with increasing gestational weight gain was also noted; however, as edema is also reflected as increased weight, determining if the weight gain caused preeclampsia or was a reflection of preeclampsia is impossible to determine in a retrospective study.
Minimizing SGA and preterm infants is crucial because these infants are at increased risk of stillbirth, seizures, sepsis, intubation, and neonatal death.15 Although it may be suggested that adolescents benefit from delivering smaller infants due to their immature pelvic structure, no data has suggested that SGA or preterm infants born to adolescents fare better than SGA or preterm infants born to adult women. Further investigation into whether adolescents need to gain even more weight than currently recommended is warranted based on these findings.
Prior studies have also suggested that inadequate weight gain in adolescent pregnancy contributes substantially to low birth weight and small-for-gestational-age infants. In a prospective study, Stevens-Simon and McAnarney demonstrated that adolescents with a slow weight gain had smaller infants compared to those with average and rapid weight gain, but those with a rapid weight gain had high post-partum weight retention.16 No differences were seen in the rate of pregnancy-induced hypertension, glucose intolerance, and cesarean delivery based on gestational weight gain. Scholl et al reported a secondary analysis of the impact of maternal growth and weight gain on infant birth weight in a cohort of adolescent pregnancies from the Camden Study.17 Despite larger gestational weight gains, growing teenagers had smaller infant birth weights than non-growing adolescents or mature controls, suggesting that maternal growth represents a competition for nutrients and that growing adolescents have higher energy and weight gain requirements for a healthy pregnancy.
In contrast, a study by Nielsen et al suggests that increased gestational weight gain in African American adolescents does not improve infant birth weights.18 In this retrospective chart review, adolescents who gained in the upper half of the recommended range or more than the IOM recommendations had a similar risk of SGA infants compared to adolescents who gained in the lower half. It should be noted that this study uses the IOM guidelines released in 1990, which used a different BMI classification. Also, the size of the study was small, especially once patients were stratified by both BMI and weight gain category, thereby limiting the statistical power to detect differences in SGA among study groups.
Recent retrospective studies have suggested that obese women do not need to gain any weight during pregnancy.19,20 However, these studies were restricted to adult women, and adolescents should be examined separately due to their unique metabolic needs.
To our knowledge, ours is the first study to specifically evaluate the IOM recommendations for gestational weight gain released in May 2009 specifically in adolescent pregnancies. The large sample size of the cohort allowed us to stratify patients by prepregnancy BMI while retaining an adequate sample size to examine both neonatal and maternal outcomes. Also, the inclusion of maternal outcomes is itself unique, as the majority of prior studies have focused solely on infant outcomes. Finally, retaining the use of adult BMI categories makes this information more accessible to the obstetrician, who typically does not use age-specific BMI charts.
One limitation of this study that deserves comment is the use of birth certificate data. Missouri state birth certificate data report the mother's height, prepregnancy weight, and amount of gestational weight loss or gain, in addition to pregnancy complications. Prepregnancy weight is typically a self-reported weight, but it has been demonstrated that adolescents can accurately report their prepregnancy weight.21 In comparisons of birth certificate data to medical records, the reported birth weight, gestational weight gain, gestational age, and mode of delivery on the birth certificate agrees with medical record with good accuracy.22–25 Also, the use of check-box formats rather than open-ended questions improves the accuracy of data contained in birth certificates.25,26 Birth certificate data has reduced sensitivity for reporting medical complications of pregnancy such as hypertension and diabetes;27–29 under-reporting has been associated in particular with minority status and non-English speakers. However, under-reporting is likely randomly distributed across BMI categories and unlikely to introduce bias into the analysis. Where inaccuracies in birth certificate data exist, they would be random and result in a non-differential misclassification that would bias our results toward the null. Therefore, we feel that reliable information can be obtained using these birth certificate data.
The retrospective nature of the study limited us to using total gestational weight gain for the entire pregnancy, although the trimester of the weight gain and the rate of weight gain may also be important factors in both maternal and infant outcomes.30 It also forced us to assume a linear weight gain throughout the second and third trimester of pregnancy; if this weight gain is not linear then preterm subjects may have been consistently misclassified into an erroneous weight gain category. However, similar results, with the exception of infant death, were seen when the analysis was limited to term births only (data available upon request), suggesting that the formula used to determine weight gain recommendations in preterm births was a reasonable estimate. Also, nutritional intake and the quality of diet may play a role in determining infant birth weight and maternal outcomes. Although these may be reflected in weight gain, we cannot analyze the impact of nutritional status on outcomes with this study.
Despite these limitations, we feel that clinically important information is contained in this study. Teenagers who gain less than the IOM recommendations have increased odds of SGA infants, preterm delivery, and infant death compared to those who gain within the recommendations. Adolescents who gain within the IOM recommendations for their BMI category have 14–19% incidence of small-for-gestational-age infants. The incidence of SGA and preterm deliveries decreases with weight gain above the IOM recommendations. Based on these findings, we feel that the current IOM weight gain recommendations for pregnancy should be reexamined with regard to adolescent pregnancies.
Clinical Implications.
Teenagers who gain less than the IOM recommendations have an increased risk for SGA, preterm delivery and infant death.
Using adult BMI classifications in adolescents to determine gestational weight gain recommendations seems appropriate.
Further investigation into whether adolescents need to gain even more weight than currently recommended is warranted based on these findings.
Table 3.
Pregnancy Outcomes by Gestational Weight Gain Category for Normal Weight Adolescents (BMI 18.5–24.9)
| Gain Less than IOM <25 lbs | AOR* (95% CI) | Within IOM†25–35 lbs | Gain More than IOM >35 lbs | AOR* (95% CI) | |
|---|---|---|---|---|---|
| (n=10249) | (n=16296) | (n=21621) | |||
| Neonatal Outcomes | |||||
| SGA (%) | 2117 (20.67%) | 1.55 (1.45–1.66) | 2399 (14.72%) | 2019 (9.32%) | 0.57 (0.54–0.61) |
| LGA (%) | 371 (3.62%) | 0.72 (0.63–0.82) | 772 (4.74%) | 1986 (9.19%) | 2.10 (1.92–2.29) |
| Preterm Delivery (%) | 1399 (13.65%) | 2.48 (2.27–2.72) | 901 (5.53%) | 649 (3.00%) | 0.54 (0.48–0.60) |
| Infant Death (%) | 157 (1.53%) | 3.26 (2.45–4.35) | 75 (0.46%) | 84 (0.39%) | 0.82 (0.59–1.13) |
| Maternal Outcomes | |||||
| Preeclampsia (%) | 440 (4.30%) | 0.92 (0.81–1.04) | 736 (4.52%) | 1710 (7.91%) | 1.89 (1.72–2.07) |
| Cesarean-All (%) | 1204 (11.75%) | 1.00 (0.92–1.08) | 1978 (12.14) | 3456 (15.98%) | 1.35 (1.27–1.44) |
| Unplanned Cesarean (%) | 881 (9.14%) | 1.02 (0.93–1.12) | 1406 (9.27%) | 2381 (12.05%) | 1.32 (1.23–1.42) |
| Operative Vaginal Delivery (%) | 13.01 (12.69%) | 0.84 (0.78–0.91) | 2468 (15.14%) | 3517 (16.27%) | 1.06 (1.00–1.13) |
Adjusted for maternal age, maternal race, tobacco use, alcohol use, a composite maternal medical risk factor (includes chronic hypertension, diabetes, and renal disease), Medicaid, and the Kotel chuck prenatal care index
Reference group
Table 4.
Pregnancy Outcomes by Gestational Weight Gain Category for Overweight Adolescents (BMI 25.0–29.9)
| Gain Less than IOM <15 lbs | AOR* (95% CI) | Within IOM†15–25 lbs | Gain More than IOM >25 lbs | AOR* (95% CI) | |
|---|---|---|---|---|---|
| (n=1050) | (n=2411) | (n=8478) | |||
| Neonatal Outcomes | |||||
| SGA (%) | 226 (21.56%) | 1.58 (1.30–1.92) | 357 (14.81%) | 835 (9.85%) | 0.62 (0.54–0.71) |
| LGA (%) | 43 (4.39%)(0. | 0.94 (0.66–1.35) | 116 (4.81%) | 891 (10.51%) | 2.35 (1.91–2.88) |
| Preterm Delivery (%) | 157 (14.95%) | 2.14 (1.67–2.73) | 27.17 (7.01%) | 296 (3.49%) | 0.49 (0.40–0.60) |
| Infant Death (%) | 23 (2.19%) | 2.64 (1.41–4.94) | 20 (0.83%) | 42 (0.50%) | 0.64 (0.37–1.10) |
| Maternal Outcomes | |||||
| Preeclampsia (%) | 67 (6.38%) | 0.84 (0.62–1.15) | 166 (6.89%) | 905 (10.67%) | 1.57 (1.32–1.87) |
| Cesarean-All (%) | 170 (16.19%) | 0.94 (0.77–1.15) | 403 (16.72%) | 1755 (20.69%) | 1.27 (1.13–1.43) |
| Unplanned Cesarean (%) | 123 (12.56%) | 0.99 (0.78–1.25) | 270 (12.18%) | 1240 (16.03%) | 1.33 (1.16–1.54) |
| Operative Vaginal Delivery (%) | 125 (11.90%) | 0.96 (0.77–1.21) | 303 (12.57%) | 1188 (14.00%) | 1.10 (0.96–1.26) |
Adjusted for maternal age, maternal race, tobacco use, alcohol use, a composite maternal medical risk factor (includes chronic hypertension, diabetes, and renal disease), Medicaid, and the Kotel chuck prenatal care index
Reference group
Acknowledgments
Financial Support: Dr. Harper is supported by a grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (T32HD- PI: Macones) and by UL1RR024992 (PI: Evanoff). Dr. Chang is supported by a KL2 Multidisciplinary Clinical Research Career Development Program Scholar award (KL2RR024994) from the National Institute of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reprints Not Available
References
- 1.DeVader SR, Neeley HL, Myles TD, Leet TL. Evaluation of gestational weight gain guidelines for women with normal prepregnancy body mass index. Obstetrics & Gynecology. 2007;110(4):745–51. doi: 10.1097/01.AOG.0000284451.37882.85. [DOI] [PubMed] [Google Scholar]
- 2.American College of Obstetricians and G. ACOG Practice Bulletin. Assessment of risk factors for preterm birth. Clinical management guidelines for obstetrician-gynecologists. Number 31, October 2001. (Replaces Technical Bulletin number 206, June 1995; Committee Opinion number 172, May 1996; Committee Opinion number 187, September 1997; Committee Opinion number 198, February 1998; and Committee Opinion number 251, January 2001). Obstetrics & Gynecology. 2001;98(4):709–16.
- 3.Martin J, Hamilton B, Sutton P, Ventura S, Menacker F, Kirmeyer S, Mathews T. Births: Final data for 2006. National Vital Statistics Reports. 2009;57(7) [PubMed] [Google Scholar]
- 4.Edwards LE, Hellerstedt WL, Alton IR, Story M, Himes JH. Pregnancy complications and birth outcomes in obese and normal-weight women: effects of gestational weight change. Obstetrics & Gynecology. 1996;87(3):389–94. doi: 10.1016/0029-7844(95)00446-7. [DOI] [PubMed] [Google Scholar]
- 5.Institute of Medicine . Weight Gain During Pregnancy: Reexamining the Guidelines. The National Academies Press; Washington, DC: 2009. [PubMed] [Google Scholar]
- 6.Scholl TO, Hediger ML, Schall JI, Ances IG, Smith WK. Gestational weight gain, pregnancy outcome, and postpartum weight retention. Obstetrics & Gynecology. 1995;86(3):423–7. doi: 10.1016/0029-7844(95)00190-3. [DOI] [PubMed] [Google Scholar]
- 7.Butte NF, Ellis KJ, Wong WW, Hopkinson JM, Smith EO. Composition of gestational weight gain impacts maternal fat retention and infant birth weight. American Journal of Obstetrics & Gynecology. 2003;189(5):1423–32. doi: 10.1067/s0002-9378(03)00596-9. [DOI] [PubMed] [Google Scholar]
- 8.Cedergren M. Effects of gestational weight gain and body mass index on obstetric outcome in Sweden. International Journal of Gynaecology & Obstetrics. 2006;93(3):269–74. doi: 10.1016/j.ijgo.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Groth S. Are the Institute of Medicine recommendations for gestational weight gain appropriate for adolescents? JOGNN - Journal of Obstetric, Gynecologic, & Neonatal Nursing. 2007;36(1):21–7. doi: 10.1111/j.1552-6909.2006.00117.x. [DOI] [PubMed] [Google Scholar]
- 10.Fernandez ID, Olson CM, De Ver Dye T. Discordance in the assessment of prepregnancy weight status of adolescents: a comparison between the Centers for Disease Control and Prevention sex- and age-specific body mass index classification and the Institute of Medicine-based classification used for maternal weight gain guidelines. Journal of the American Dietetic Association. 2008;108(6):998–1002. doi: 10.1016/j.jada.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wingate MS, Alexander GR, Buekens P, Vahratian A. Comparison of gestational age classifications: date of last menstrual period vs. clinical estimate. Ann Epidemiol. 2007;17(6):425–30. doi: 10.1016/j.annepidem.2007.01.035. [DOI] [PubMed] [Google Scholar]
- 12.Alexander GR, Kogan MD, Himes JH. 1994–1996 U.S. singleton birth weight percentiles for gestational age by race, Hispanic origin, and gender. Matern Child Health J. 1999;3(4):225–31. doi: 10.1023/a:1022381506823. [DOI] [PubMed] [Google Scholar]
- 13.Bloch JR, Dawley K, Suplee PD. Application of the Kessner and Kotelchuck prenatal care adequacy indices in a preterm birth population. Public Health Nurs. 2009;26(5):449–59. doi: 10.1111/j.1525-1446.2009.00803.x. [DOI] [PubMed] [Google Scholar]
- 14.Singer JD, Willett JB. Applied longitudinal data analysis: modeling change and event occurrence. Oxford University Press; Oxford, NY: 2003. [Google Scholar]
- 15. ACOG Practice Bulletin No 12, January 2000. Intrauterine Growth Restriction.
- 16.Stevens-Simon C, McAnarney ER. Adolescent pregnancy. Gestational weight gain and maternal and infant outcomes. American Journal of Diseases of Children. 1992;146(11):1359–64. doi: 10.1001/archpedi.1992.02160230117031. [DOI] [PubMed] [Google Scholar]
- 17.Scholl TO, Hediger ML, Schall JI, Khoo CS, Fischer RL. Maternal growth during pregnancy and the competition for nutrients. American Journal of Clinical Nutrition. 1994;60(2):183–8. doi: 10.1093/ajcn/60.2.183. [DOI] [PubMed] [Google Scholar]
- 18.Nielsen JN, O'Brien KO, Witter FR, Chang SC, Mancini J, Nathanson MS, Caulfield LE. High gestational weight gain does not improve birth weight in a cohort of African American adolescents. American Journal of Clinical Nutrition. 2006;84(1):183–9. doi: 10.1093/ajcn/84.1.183. [DOI] [PubMed] [Google Scholar]
- 19.Bianco AT, Smilen SW, Davis Y, Lopez S, Lapinski R, Lockwood CJ. Pregnancy outcome and weight gain recommendations for the morbidly obese woman. Obstetrics & Gynecology. 1998;91(1):97–102. doi: 10.1016/s0029-7844(97)00578-4. [DOI] [PubMed] [Google Scholar]
- 20.Kiel DW, Dodson EA, Artal R, Boehmer TK, Leet TL. Gestational weight gain and pregnancy outcomes in obese women: how much is enough? Obstetrics & Gynecology. 2007;110(4):752–8. doi: 10.1097/01.AOG.0000278819.17190.87. see comment. [DOI] [PubMed] [Google Scholar]
- 21.Stevens-Simon C, McAnarney ER, Coulter MP. How accurately do pregnant adolescents estimate their weight prior to pregnancy? Journal of Adolescent Health Care. 1986;7(4):250–4. doi: 10.1016/s0197-0070(86)80017-1. [DOI] [PubMed] [Google Scholar]
- 22.Buescher PA, Taylor KP, Davis MH, Bowling JM. The quality of the new birth certificate data: a validation study in North Carolina. American Journal of Public Health. 1993;83(8):1163–5. doi: 10.2105/ajph.83.8.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reichman NE, Hade EM. Validation of birth certificate data. A study of women in New Jersey's HealthStart program. Annals of Epidemiology. 2001;11(3):186–93. doi: 10.1016/s1047-2797(00)00209-x. [DOI] [PubMed] [Google Scholar]
- 24.Piper JM, Mitchel EF, Jr., Snowden M, Hall C, Adams M, Taylor P. Validation of 1989 Tennessee birth certificates using maternal and newborn hospital records. American Journal of Epidemiology. 1993;137(7):758–68. doi: 10.1093/oxfordjournals.aje.a116736. [DOI] [PubMed] [Google Scholar]
- 25.Woolbright LA, Harshbarger DS. The revised standard certificate of live birth: analysis of medical risk factor data from birth certificates in Alabama, 1988–92. Public Health Rep. 1995;110(1):59–63. [PMC free article] [PubMed] [Google Scholar]
- 26.Frost F, Starzyk P, George S, McLaughlin JF. Birth complication reporting: the effect of birth certificate design. American Journal of Public Health. 1984;74(5):505–6. doi: 10.2105/ajph.74.5.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lydon-Rochelle MT, Holt VL, Cardenas V, Nelson JC, Easterling TR, Gardella C, Callaghan WM. The reporting of pre-existing maternal medical conditions and complications of pregnancy on birth certificates and in hospital discharge data. Am J Obstet Gynecol. 2005;193(1):125–34. doi: 10.1016/j.ajog.2005.02.096. [DOI] [PubMed] [Google Scholar]
- 28.Bailit JL. Rates of labor induction without medical indication are overestimated when derived from birth certificate data. Am J Obstet Gynecol. 203(3):269, e1–3. doi: 10.1016/j.ajog.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 29.Reichman NE, Schwartz-Soicher O. Accuracy of birth certificate data by risk factors and outcomes: analysis of data from New Jersey. Am J Obstet Gynecol. 2007;197(1):32, e1–8. doi: 10.1016/j.ajog.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 30.Scholl TO, Hediger ML, Ances IG, Belsky DH, Salmon RW. Weight gain during pregnancy in adolescence: predictive ability of early weight gain. Obstetrics & Gynecology. 1990;75(6):948–53. [PubMed] [Google Scholar]