Abstract
Background
Laparotomy wound load forces are reduced when dehiscence and incisional hernia formation occur. The purpose of this study is to determine the effects of strain loss on abdominal fascial fibroblast proliferation, orientation and collagen compaction function.
Methods
Cultured rat linea alba fibroblasts were subjected to continuous cyclic strain (CS), cyclic strain interrupted at 24 or 48 hours followed by culture at rest (IS-24 and IS-48) or were cultured without mechanical strain (NS). Cell number was measured and images analyzed for cell orientation. Fibroblasts from these groups were seeded onto the surface of (FPCL-S) or mixed into (FPCL-M) a collagen gel matrix and gel area was measured over time.
Results
Continuous strain stimulated proliferation when compared to the non-strained cells. The loss of strain (IS) delayed proliferation compared to CS throughout (P<0.05). CS fibroblasts aligned perpendicular to the direction of strain within 12 hours. Within 12 hours of strain loss, IS-48 fibroblasts became significantly less aligned (P<.0001), and appeared similar to the randomly organized NS fibroblasts 48 hours after strain removal. The CS and IS-24 groups demonstrated faster and greater overall FPCL-M compaction than both the IS-48 and NS groups (P<.0002). The CS group contracted the gel faster than the NS group in FPCL-S (P=.029).
Conclusions
Mechanical strain rapidly induces a proliferative, morphological and functional response in abdominal wall fibroblasts that is dependent on the continued presence of the strain signal and quickly lost when the load force is removed. The loss of wound edge tension that occurs during laparotomy wound separation and hernia formation may contribute to impaired wound healing through loss of a key stimulatory mechanical signal with important implications for abdominal wall reconstruction.
Introduction
Laparotomy wound failure progressing to incisional hernia formation is the most frequent complication of abdominal surgery, with an overall estimated incidence of 11 to 16% following abdominal operations.1–5 The true incidence is probably underestimated, as 8–29% of incisional hernias are asymptomatic and may not be detected without careful physical examination.4, 6, 7 Despite many advances in surgical technique and patient care, the rate of primary incisional hernia formation has not changed appreciably in 75 years.8 Although the use of mesh has improved hernia repair, recurrence rates remain unacceptably high,9–11 worsening with each subsequent reoperation12.
Incisional abdominal hernias most frequently develop from early laparotomy wound separation and failure within the first postoperative month, often clinically occult1, 13 The cause of wound failure may be multifactorial – related to closure technique, wound ischemia, infection, excessive straining from coughing or weight lifting, or patient comorbidities such as older age, obesity, malnutrition, diabetes or chronic steroid therapy.3, 14–21 These same factors may prevent reestablishment of fascial healing once separation has occurred, and contribute to the increased frequency of recurrence following hernia repair, however the exact mechanism of progression from wound separation to hernia formation is unknown.22
Mechanotransduction, the perception of cellular signaling occurring in response to mechanical force, may also play a role in laparotomy wound healing. Many different cells throughout the body experience mechanical forces on a frequent basis such as vascular endothelial and smooth muscle cells,23–25 cardiac myocytes and fibroblasts,26, 27 pulmonary alveolar cells,28 bone osteoblasts,29 tendon and ligament fibroblasts,30, 31 and fibroblasts and myofibroblasts during the contraction phase of cutaneous wound healing.32, 33 Mechanical strain is known to signal repair or regenerative responses of cells in many of these same tissues. The abdominal wall is subjected to intermittent and constant strain caused by breathing, coughing, weight bearing, etc.34 Models of tendon injury suggest that load-bearing tissues like the abdominal wall are dependent on mechanical strain to signal repair.35, 36 This is believed to occur through mechanotransduction pathways that activate wound repair fibroblasts.30, 37, 38In vitro, strain induces uniform alignment in many cell types,23, 25, 39–41 and the parallel arrangement of fibroblasts and collagen fibers in normal abdominal fascia contributes to its strength and is required for optimal fascial wound healing.42 Fibroblasts play an essential role in wound repair, migrating into the wound bed, proliferating, synthesizing growth factors, producing and remodeling new extracellular matrix, and contracting the wound.33, 43 Our previous work found that a fibroblast wound healing defect is induced when laparotomy wounds are mechanically disrupted and form incisional hernias.44, 45 It may be that the ideal abdominal wall reconstruction reestablishes physiological load much like a tendon following repair.
The aims of this study are to confirm the stimulatory proliferative and cell alignment effects of strain on abdominal wall fibroblasts, and then measure the fibroblast response when the mechanical signal is removed in terms of proliferation, change in cell orientation and fibroblast populated collagen lattice (FPCL) contraction function.
Materials and Methods
Materials
Low glucose Dulbecco’s modified Eagle’s medium (DMEM) and penicillin/streptomycin antibiotics were purchased from Invitrogen (Carlsbad, CA). Fetal bovine serum (FBS) was purchased from Hyclone Laboratories, Inc. (Logan, UT). Rat tail collagen was purchased from BD Biosciences (San Jose, CA).
Abdominal wall musculotendinous fibroblast (abMTF) cell culture
Biopsies of normal SD rat linea albae were treated in 0.0125% trypsin at 37 °C for 30 min. The peritoneal side of the biopsies was scraped to remove mesothelial cells. Biopsies were minced in culture medium, spread on the bottom of T-75 cm2 flasks and allowed to stand undisturbed for two hours at 37 °C in 5% CO2 until the tissue pieces adhered to the flask bottom. The medium was then gently drained. DMEM culture medium containing 15% FBS, 1,000 μ/mL penicillin G and 1,000 μg/mL streptomycin sulfate solution was then slowly added to the culture flask and the tissue samples were incubated at 37 °C in 5% CO2. The medium was changed every two days and the abMTF primary cell cultures were grown to confluence for passage. Experimental abMTFs were used at passage three for Flexcell experiments and passage four for FPCL assays.
Application of mechanical strain
2.5 × 105 fibroblasts were plated in the third passage on BioFlex six-well plates coated with type I collagen (Flexcell International Corp., Hillsborough, NC). The cells were cultured in DMEM culture medium containing 15% FBS, 1,000 μ/mL penicillin Gand 1,000 μg/m L streptomycin sulfate for 48 h followed by 24 h FBS starving in serum free medium. The culture medium was then replaced with low glucose DMEM containing 2.5% FBS, 1,000 μ/mL penicillin G and 1,000 μg/mL streptomycin sulfate. In order to minimize the effects of factors contained in serum, we used the lowest FBS concentration, 2.5%, that we previously identified as allowing significant strain response compared to unstrained controls. Strained cells were exposed to continuous cycles of strain/relaxation generated by a cyclic vacuum produced by a computer driven system (Flexcell 4000; Flexcell International Corp., Hillsborough, NC). The strain is transmitted to adherent cells cultured on the upper surface of the membrane. The membranes were deformed by a sine-wave vacuum force from below to a peak of 10% extension at 12 cycles/minute (cpm) for up to 192 hours (8 days). These extension and rate settings were determined to produce optimal fibroblast growth responses in our preliminary investigations. For cell proliferation experiments, cells in the Cyclic Strain (CS) group were subjected to this strain regimen for the entire 192 hours, cells in the Interrupted Strain (IS-48) group were strained for 48 hours followed by culture at rest for 144 hours until the 192 h time point and cells in the No Strain (NS) group were cultured at rest for the entire 192 hour period. The 48 hour time point for interrupting strain allowed for a significant and established response to strain while remaining in log phase growth prior to strain removal. For FPCL experiments, the same CS, IS-48 and NS groups were used and collected at 96 h, with an additional group, IS-24, subjected to strain for 48 hours followed by culture at rest for 24 hours and collected at 72 h. All six-well plates were incubated at 37°C with 5% CO2. The medium was changed after the first 48 hours and then every 24 hours.
Cell proliferation
Cell counts were obtained for the CS, IS-48 and NS groups at 48, 96, 144 and 192 h. The cell monolayer was washed with PBS. The cells were detached with 950 μl 0.05% Trypsin at 37 °C for 5 min. 50 μl FBS was added to each well to stop the trypsin reaction. Fibroblasts were washed from the well bottom, transferred to 1.5 ml Eppendorf tubes and saved on ice. Cells numbers were counted using a hemocytometer.
Cell orientation
To assess cell orientation in relation to strain, a Nikon TMS inverted phase contrast microscope with a Nikon Coolpix 5000 high resolution digital camera (Nikon Corp., Japan) was used to obtain digital microphotographs at 160× magnification. Images of individual wells were taken at 24, 48, 60, 72, 84 and 96 h. Images were centered at 1.0 cm from the center of the well, the approximate location of maximal radial strain.46 Images were analyzed using ImageJ software (NIH, USA). Each image was divided into four quadrants and 8 cells in each quadrant were identified. The angle of the fibroblast cell body axis in relation to the direction of radial strain was measured from 0 to 90 degrees. A total of 6 wells across two separate experiments totaling 192 cells for each group at each time point were analyzed.
Fibroblast-populated collagen lattice (FPCL)
Collagen lattices were prepared from type I rat tail collagen per the manufacturer’s directions (BD Biosciences, San Jose, CA). Trypsinized abMTFs obtained from the CS, IS-24, IS-48 and NS groups at the previously described time points were suspended in cold DMEM. 60-well tissue culture plates were precoated with 4% BSA in PBS for 24 h. For FPCL with fibroblasts cultured on the surface of the collagen gels (FPCL-S), 1.3 mL DMEM plus 0.2 mL FBS plus 0.5 mL type I collagen stock solution were mixed to yield a final concentration of 1.25 mg/mL of collagen. This collagen working solution (2 ml) was added to each well. After polymerization at 37°C, 105 abMTFs were poured on each collagen gel in 2 ml DMEM containing 10% FBS. The plates were incubated in a cell culture incubator for 2 hours until cells adhered to the gel surface. The gels were then detached from the well sidewalls by gently scoring around the circumference with a fine pipette tip. For mixed fibroblast collagen gels (FPCL-M), 105 fibroblasts in 1.3 mL DMEM suspension plus 0.2 mL FBS plus 0.5 mL type I collagen stock solution were mixed to yield a final concentration of 1.25 mg/mL of collagen. This collagen/abMTF mixture (2 ml) was then added to each well. After polymerization at 37°C, 2 mL DMEM containing 10% FBS was added to each well. The plates were incubated for 2 hours and then the gels were detached as above. After detachment, gel diameter was measured with a ruler and the gel area was calculated at designated time points.
Statistical analysis
Statistical analysis was performed using GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, CA, USA), R version 2.10.1 (R Foundation, www.r-project.org) and SPSS (IBM, Chicago, IL, USA). One-way ANOVA with Tukey post hoc test was used to analyze cell proliferation and FPCL data. The Kolmogorov-Smirnov test for two populations was used to compare abMTF orientation distributions in relation to the strain direction. A one population Kolmogorov-Smirnov test was used to compare the NS group to a uniform distribution at each time point. Significance level was set at P < 0.05 for all statistical analyses.
Results
Cell proliferative response to strain
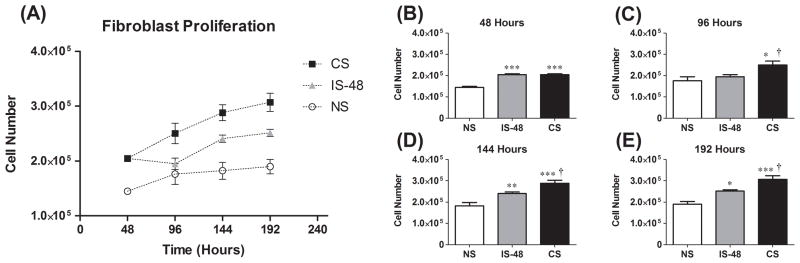
Cell counts are shown in Figure 1A–E for each group measured after 48, 96, 144 and 192 hours. Continuous strain and strain interrupted after 48 hours significantly stimulated fibroblast proliferation (P < .05, one-way ANOVA) over time. Further between groups comparison confirmed the proliferative response to strain, with cell number in the CS group higher than in the NS group at all time points (P < .0001 at 48 h, P = 0.022 at 96 h, P = .0002 at 144 h and P < .0001 at 192 h, post hoc Tukey test; Fig. 1A–E). This difference increased with time, with CS cell count 2.05×105 compared to 1.45×105 in the NS group at 48 h and 3.07×105 compared to 1.90×105 at 192 h. Removing strain resulted in decreased cell proliferation. IS-48 cell number was significantly lower than the CS group at 96 h, 48 hours after strain removal (1.95×105 vs. 2.50×105, P = .041; Fig. 1C) and this difference persisted through 192 h (P = .030 at 144 h and P = .019 at 192 h; Fig. 1D–E). There was no significant difference in cell number between the IS-48 and NS groups at 96 h (P = .64; Fig. 1C), however the IS-48 group was higher than the NS group at 144 h (P = .009; Fig. 1D) and 192 h (P = .010; Fig. 1E). Interestingly, at 96 h the IS-48 group cell number appeared to have decreased compared to at 48 h (1.95×105 vs. 2.05×105; Fig. 1A), however this change was not significant (P = .37, t-test).
Figure 1.
(A) Fibroblast cell number for No Strain (NS), 48 hour Interrupted Strain (IS-48) and continuous Cyclic Strain (CS) groups from 48 – 192 hours. (B)-(E) Cell number at 48 hours (B), 96 hours (C), 144 hours (D) and 192 hours (E). *: P < .05, **: P < .01, and ***: P <.001 compared to NS group. †: P <.05 compared to IS-48 group.
Cell orientation response to strain
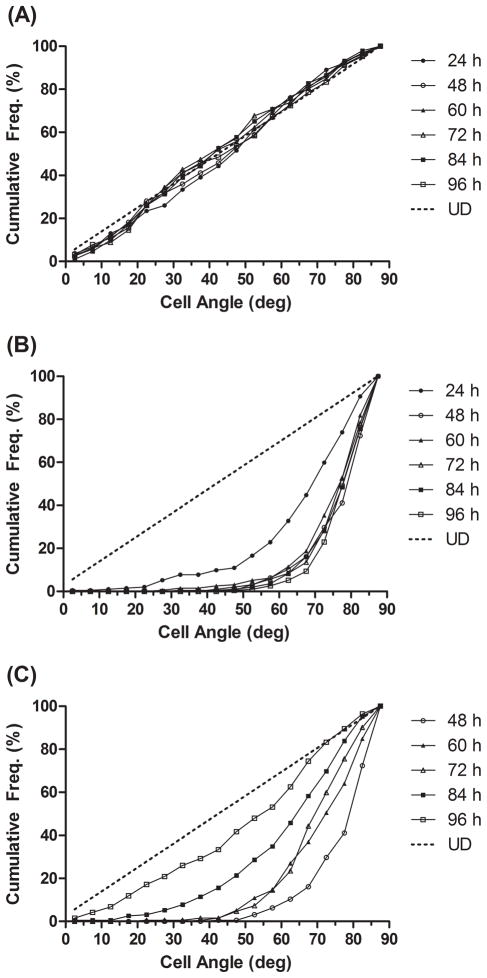
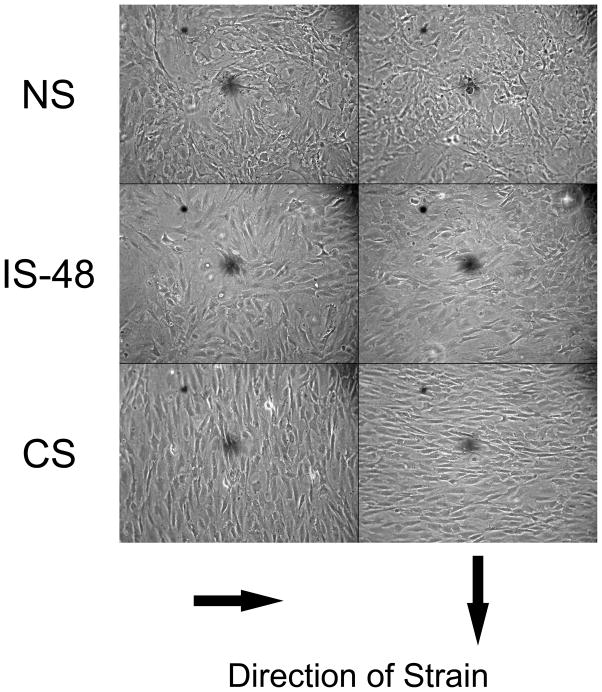
Cell orientation relative to the direction of strain from 0–90 degrees was measured at 24, 48, 60, 72, 84 and 96 hours. A summary of cell orientation distributions are shown in Figure 2. As expected, the NS group cells maintained a uniform distribution at all time points (P > 0.05 for null hypothesis; Fig. 2A). Within 24 hours of strain application, the CS group cells had shifted significantly toward 90 degree alignment in relation to the strain force (P < 0.0001, Fig. 2B). Maximal alignment was achieved by 48 h. Following strain removal, IS-48 cells became increasingly less aligned, significant within 12 hours (P < 0.0001; Fig. 2C). At 96 h, 48 hours after strain removal, the IS-48 cell group orientation appeared similar to the NS group, however the difference in distributions remained significant (P = 0.010). Representative images of the fibroblasts at the 96 h time point are shown in Figure 3. The IS-48 cell orientation, 48 hours after strain removal, appears similar to the randomly oriented NS cells, while the CS cells are oriented perpendicular to the direction of strain.
Figure 2.
Cumulative distributions of cell orientation in relation to the direction of strain (0 to 90 degrees) at each experimental time point from 24 to 96 hours. Each point represents a 5 degree interval. UD (Uniform Distribution): Hypothetical uniform distribution of cell angles. Results from all three groups are demonstrated: (A) NS; (B) CS; (C) IS-48. Strain was removed at the 48 hour mark in the IS-48 group (C).
Figure 3.
Representative images demonstrating cell orientation in relation to the direction of strain (arrows) at 96 hours in the NS, IS-48 and CS groups. This time point is 48 hours after strain removal in the IS-48 group. Images are taken at 160× magnification.
FPCL
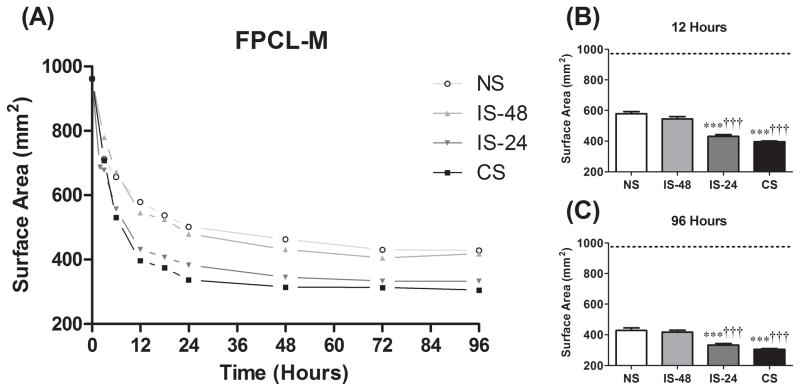
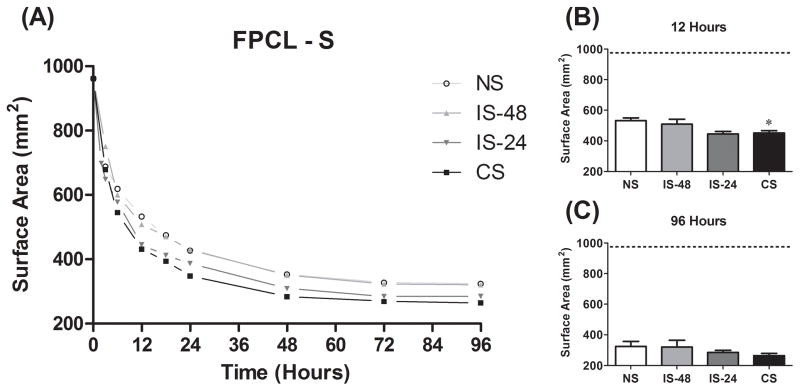
FPCL is an accepted model of granulation tissue and wound contraction.47 To determine if loss of cyclic strain impairs fibroblast function, the intrinsic kinetic activity of abMTFs in terms of ability to bind to and remodel a collagen matrix was measured and compared among all four groups. FPCL contraction was measured in both mixed (FPCL-M; Fig. 4) and surface (FPCL-S; Fig. 5) models. The majority of contraction in both models occurred within the first 24 hours, reaching maximal contraction by approximately 72 hours (Fig. 4A and 5A). In FPCL-M, the CS and IS-24 groups demonstrated faster (gel area at 12 hours, P < .0001; Fig. 4B) and greater overall (gel area at 96 hours, P < .0001; Fig. 4C) contraction than both the IS-48 and NS groups. FPCL-S contraction was faster in the CS compared to the NS group (P = .029; Fig. 5B), but no difference was seen in overall contraction (P = .44, one-way ANOVA; Fig. 5C). There was no difference between the CS and IS-24 groups (P > 0.05) or the IS-48 and NS groups (P > 0.05) for these measures in either FPCL-M or FPCL-S.
Figure 4.
(A) Mixed FPCL (FPCL-M) results for change in gel surface area over time from 0 to 96 hours. The 12 hour time point (B) is used as a measure of rate of gel contraction. The 96 hour time point (C) is used as a measure of overall gel compaction. ***: P <.001 compared to NS group. †††:P <.001 compared to IS-48 group.
Figure 5.
(A) Surface FPCL (FPCL-S) results for change in gel surface area over time from 0 to 96 hours. The 12 hour time point (B) is used as a measure of rate of gel contraction. The 96 hour time point (C) is used as a measure of overall gel compaction. *: P <.05 compared to NS group.
Discussion
We have demonstrated that cyclic strain stimulates abdominal wall fascial fibroblast proliferation and induces uniform alignment perpendicular to the direction of strain. Differences in proliferation were seen within 48 hours of strain application and fibroblast alignment began within 24 hours, reaching a maximum by 48 hours. This is consistent with previous studies finding that increased fibroblast and osteoblast proliferation occurs within 48 hours and fibroblasts, osteoblasts and endothelial cells align perpendicular to the strain force within 12 to 24 hours.23, 39, 40, 48 Other studies have demonstrated that initial proliferative and mechanical cellular responses begin even earlier, with anti-apoptotic signal and MAP kinase activation occurring within minutes,49 upregulation of β actin mRNA within one hour,31 integrin and collagen upregulation within 2 hours following 3 hours of cyclic strain,31 cell alignment starting within one to three hours31, 50 and DNA synthesis and PCNA expression increasing within 24 hours of strain application.49, 51
There are, however, fewer studies of the effects of strain removal on cellular responses. We believe that there may be a fundamental biological mechanism activated or deactivated during laparotomy wound failure, with implications toward the techniques of abdominal wall reconstruction. We observe here that strain-induced fibroblast proliferation is lost within 48 hours of strain removal and loss of cell alignment begins within 12 hours. Neidlinger-Wilke et al.50 found a significant shift toward perpendicular alignment of dermal fibroblasts after three hours of cyclic strain, which was lost within four hours following strain removal. Most other studies are limited to examining the effects of releasing intrinsically-developed static cellular tension within collagen gels. Fibroblasts seeded on the surface of or mixed into attached collagen gels will quickly reorganize and contract the collagen, developing an isometric tension. DNA synthesis, collagen production and cell proliferation is stimulated and cells develop a bipolar morphology and align uniformly.52 Beginning within one to several hours after gel release, however, DNA and protein synthesis is inhibited, collagen production declines, apoptosis is induced and cell population recedes, response to growth factors diminishes and cells develop a stellate morphology with loss of cell alignment.53–56 Similar results are seen in epithelial cells in released collagen gels.57 Although static tensions are certainly present in the abdominal wall, it is a dynamic structure subject to significant cyclic and variable strains, and the forces to which fibroblasts in a healing laparotomy wound are subjected are likely better modeled through application of cyclic strain to cell substrate rather than intrinsic, static strain. In terms of cell proliferation and morphology, however, our results do appear consistent with those of attached static collagen gels.
We have also shown that strain-stimulated fibroblasts are able to contract a collagen matrix faster and to a greater extent than unstrained controls, and that a significant impairment of this ability occurs between 24 and 48 hours following strain removal. These findings were most prominent in the mixed FPCL (FPCL-M) model, in which no difference in the rate of or overall gel contraction was found between cells 24 hours removed from strain (IS-24) and continuously strained cells (CS), whereas 48 hours after strain removal (IS-48) this increased contraction ability was lost and there was no difference from unstrained cells (NS). In surface FPCL (FPCL-S), faster gel contraction was only observed in the cyclic strain (CS) group and no differences in overall contraction were seen. FPCL-M more closely models in vivo laparotomy wound healing conditions, allowing three-dimensional fibroblast-matrix interactions, which provides a more tissue-like environment.58
To our knowledge, this is the first study to examine the impact of cyclic strain removal on fibroblast contraction function in FPCL. We have previously identified deficits in the kinetic properties of fibroblasts cultured from laparotomy wound and hernia biopsies obtained from a rat model of incisional hernias. It was observed that fibroblasts cultured from normally healing laparotomy wounds caused 80% FPCL contraction over five days, while incisional hernia fibroblasts caused only 50% lattice contraction (unpublished data). Together with our observations of proliferation and orientation loss following strain removal, this suggests that a certain degree of wound tension may be necessary for optimal abMTF function.
Laparotomy wound healing is a complex process involving interplay between many different types of cells and failure with progression to hernia formation is multifactorial. Caution must be exercised in relating in vitro studies of wound healing to the dynamic processes of the abdominal wall in which load forces are variable and technical and patient factors play a role. However, the fibroblast is the dominant cell type during the proliferative and remodeling phases, in which new extracellular matrix including collagen is synthesized and ultimate wound strength is developed. Our findings suggest that early fascial separation and diminished wound tension may lead to loss of a key stimulatory mechanical signal for fibroblast proliferation, alignment and contraction function, resulting in the inability to heal the initial wound failure with subsequent progression to hernia formation. Thus, preventing the initial fascial separation by optimizing patient factors for wound healing and rigorous adherence to technical principles of wound closure are critical to minimizing the risk of hernia formation. Additionally, tension, to a certain degree, may be necessary to stimulate appropriate wound healing, and a truly “tension free” hernia repair may be suboptimal. Where the balance lies is a key question that has yet to be answered.
Acknowledgments
Supported by NIH Institute of General Medical Sciences Grant Numbers 5R01GM0782880 (Michael G. Franz, MD) and 5F32GM08887302 (Eric J. Culbertson, MD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pollock AV, Evans M. Early prediction of late incisional hernias. Br J Surg. 1989;76(9):953–4. doi: 10.1002/bjs.1800760926. [DOI] [PubMed] [Google Scholar]
- 2.Seiler CM, Bruckner T, Diener MK, et al. Interrupted or continuous slowly absorbable sutures for closure of primary elective midline abdominal incisions: a multicenter randomized trial (INSECT: ISRCTN24023541) Ann Surg. 2009;249(4):576–82. doi: 10.1097/SLA.0b013e31819ec6c8. [DOI] [PubMed] [Google Scholar]
- 3.Hoer J, Lawong G, Klinge U, et al. Factors influencing the development of incisional hernia. A retrospective study of 2,983 laparotomy patients over a period of 10 years. Chirurg. 2002;73(5):474–80. doi: 10.1007/s00104-002-0425-5. [DOI] [PubMed] [Google Scholar]
- 4.Mudge M, Hughes LE. Incisional hernia: a 10 year prospective study of incidence and attitudes. Br J Surg. 1985;72(1):70–1. doi: 10.1002/bjs.1800720127. [DOI] [PubMed] [Google Scholar]
- 5.Israelsson LA, Jonsson T. Incisional hernia after midline laparotomy: a prospective study. Eur J Surg. 1996;162(2):125–9. [PubMed] [Google Scholar]
- 6.Ellis H, Gajraj H, George CD. Incisional hernias: when do they occur? Br J Surg. 1983;70(5):290–1. doi: 10.1002/bjs.1800700514. [DOI] [PubMed] [Google Scholar]
- 7.Read RC, Yoder G. Recent trends in the management of incisional herniation. Arch Surg. 1989;124(4):485–8. doi: 10.1001/archsurg.1989.01410040095022. [DOI] [PubMed] [Google Scholar]
- 8.Carlson MA. Acute wound failure. Surg Clin North Am. 1997;77(3):607–36. doi: 10.1016/s0039-6109(05)70571-5. [DOI] [PubMed] [Google Scholar]
- 9.Luijendijk RW, Hop WC, van den Tol MP, et al. A comparison of suture repair with mesh repair for incisional hernia. N Engl J Med. 2000;343(6):392–8. doi: 10.1056/NEJM200008103430603. [DOI] [PubMed] [Google Scholar]
- 10.Burger JW, Luijendijk RW, Hop WC, et al. Long-term follow-up of a randomized controlled trial of suture versus mesh repair of incisional hernia. Ann Surg. 2004;240(4):578–83. doi: 10.1097/01.sla.0000141193.08524.e7. discussion 583–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vidovic D, Jurisic D, Franjic BD, et al. Factors affecting recurrence after incisional hernia repair. Hernia. 2006;10(4):322–5. doi: 10.1007/s10029-006-0097-z. [DOI] [PubMed] [Google Scholar]
- 12.Flum DR, Horvath K, Koepsell T. Have outcomes of incisional hernia repair improved with time? A population-based analysis. Ann Surg. 2003;237(1):129–35. doi: 10.1097/00000658-200301000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burger JW, Lange JF, Halm JA, et al. Incisional hernia: early complication of abdominal surgery. World J Surg. 2005;29(12):1608–13. doi: 10.1007/s00268-005-7929-3. [DOI] [PubMed] [Google Scholar]
- 14.McNeil PM, Sugerman HJ. Continuous absorbable vs interrupted nonabsorbable fascial closure. A prospective, randomized comparison. Arch Surg. 1986;121(7):821–3. doi: 10.1001/archsurg.1986.01400070091019. [DOI] [PubMed] [Google Scholar]
- 15.Wissing J, van Vroonhoven TJ, Schattenkerk ME, et al. Fascia closure after midline laparotomy: results of a randomized trial. Br J Surg. 1987;74(8):738–41. doi: 10.1002/bjs.1800740831. [DOI] [PubMed] [Google Scholar]
- 16.Kendall SW, Brennan TG, Guillou PJ. Suture length to wound length ratio and the integrity of midline and lateral paramedian incisions. Br J Surg. 1991;78(6):705–7. doi: 10.1002/bjs.1800780623. [DOI] [PubMed] [Google Scholar]
- 17.Regnard JF, Hay JM, Rea S, et al. Ventral incisional hernias: incidence, date of recurrence, localization and risk factors. Ital J Surg Sci. 1988;18(3):259–65. [PubMed] [Google Scholar]
- 18.da Silva AL, Petroianu A. Incisional hernias: factors influencing development. South Med J. 1991;84(12):1500, 1504. doi: 10.1097/00007611-199112000-00021. [DOI] [PubMed] [Google Scholar]
- 19.Bucknall TE, Cox PJ, Ellis H. Burst abdomen and incisional hernia: a prospective study of 1129 major laparotomies. Br Med J (Clin Res Ed) 1982;284(6320):931–3. doi: 10.1136/bmj.284.6320.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sugerman HJ, Kellum JM, Jr, Reines HD, et al. Greater risk of incisional hernia with morbidly obese than steroid-dependent patients and low recurrence with prefascial polypropylene mesh. Am J Surg. 1996;171(1):80–4. doi: 10.1016/S0002-9610(99)80078-6. [DOI] [PubMed] [Google Scholar]
- 21.Sauerland S, Korenkov M, Kleinen T, et al. Obesity is a risk factor for recurrence after incisional hernia repair. Hernia. 2004;8(1):42–6. doi: 10.1007/s10029-003-0161-x. [DOI] [PubMed] [Google Scholar]
- 22.Franz MG. The biology of hernias and the abdominal wall. Hernia. 2006;10(6):462–71. doi: 10.1007/s10029-006-0144-9. [DOI] [PubMed] [Google Scholar]
- 23.Iba T, Sumpio BE. Morphological response of human endothelial cells subjected to cyclic strain in vitro. Microvasc Res. 1991;42(3):245–54. doi: 10.1016/0026-2862(91)90059-k. [DOI] [PubMed] [Google Scholar]
- 24.Kona S, Chellamuthu P, Xu H, et al. Effects of cyclic strain and growth factors on vascular smooth muscle cell responses. Open Biomed Eng J. 2009;3:28–38. doi: 10.2174/1874120700903010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dartsch PC, Hammerle H, Betz E. Orientation of cultured arterial smooth muscle cells growing on cyclically stretched substrates. Acta Anat (Basel) 1986;125(2):108–13. doi: 10.1159/000146146. [DOI] [PubMed] [Google Scholar]
- 26.Butt RP, Laurent GJ, Bishop JE. Mechanical load and polypeptide growth factors stimulate cardiac fibroblast activity. Ann N Y Acad Sci. 1995;752:387–93. doi: 10.1111/j.1749-6632.1995.tb17446.x. [DOI] [PubMed] [Google Scholar]
- 27.Komuro I, Katoh Y, Kaida T, et al. Mechanical loading stimulates cell hypertrophy and specific gene expression in cultured rat cardiac myocytes. Possible role of protein kinase C activation. J Biol Chem. 1991;266(2):1265–8. [PubMed] [Google Scholar]
- 28.Wirtz HR, Dobbs LG. Calcium mobilization and exocytosis after one mechanical stretch of lung epithelial cells. Science. 1990;250(4985):1266–9. doi: 10.1126/science.2173861. [DOI] [PubMed] [Google Scholar]
- 29.Buckley MJ, Banes AJ, Jordan RD. The effects of mechanical strain on osteoblasts in vitro. J Oral Maxillofac Surg. 1990;48(3):276–82. doi: 10.1016/0278-2391(90)90393-g. discussion 282–3. [DOI] [PubMed] [Google Scholar]
- 30.Yang G, Crawford RC, Wang JH. Proliferation and collagen production of human patellar tendon fibroblasts in response to cyclic uniaxial stretching in serum-free conditions. J Biomech. 2004;37(10):1543–50. doi: 10.1016/j.jbiomech.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 31.Kaneko D, Sasazaki Y, Kikuchi T, et al. Temporal effects of cyclic stretching on distribution and gene expression of integrin and cytoskeleton by ligament fibroblasts in vitro. Connect Tissue Res. 2009;50(4):263–9. doi: 10.1080/03008200902846270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Snowden JM, Cliff WJ. Wound contraction. Correlations between the tension generated by granulation tissue, cellular content and rate of contraction. Q J Exp Physiol. 1985;70(4):539–48. doi: 10.1113/expphysiol.1985.sp002940. [DOI] [PubMed] [Google Scholar]
- 33.Grinnell F. Fibroblasts, myofibroblasts, and wound contraction. J Cell Biol. 1994;124(4):401–4. doi: 10.1083/jcb.124.4.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cobb WS, Burns JM, Kercher KW, et al. Normal intraabdominal pressure in healthy adults. J Surg Res. 2005;129(2):231–5. doi: 10.1016/j.jss.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 35.Enwemeka CS. Functional loading augments the initial tensile strength and energy absorption capacity of regenerating rabbit Achilles tendons. Am J Phys Med Rehabil. 1992;71(1):31–8. doi: 10.1097/00002060-199202000-00008. [DOI] [PubMed] [Google Scholar]
- 36.Lin TW, Cardenas L, Soslowsky LJ. Biomechanics of tendon injury and repair. J Biomech. 2004;37(6):865–77. doi: 10.1016/j.jbiomech.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 37.Aspenberg P. Stimulation of tendon repair: mechanical loading, GDFs and platelets. A mini-review. Int Orthop. 2007;31(6):783–9. doi: 10.1007/s00264-007-0398-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ingber DE. Tensegrity: the architectural basis of cellular mechanotransduction. Annu Rev Physiol. 1997;59:575–99. doi: 10.1146/annurev.physiol.59.1.575. [DOI] [PubMed] [Google Scholar]
- 39.Buckley MJ, Banes AJ, Levin LG, et al. Osteoblasts increase their rate of division and align in response to cyclic, mechanical tension in vitro. Bone Miner. 1988;4(3):225–36. [PubMed] [Google Scholar]
- 40.Buck RC. Reorientation response of cells to repeated stretch and recoil of the substratum. Exp Cell Res. 1980;127(2):470–4. doi: 10.1016/0014-4827(80)90456-5. [DOI] [PubMed] [Google Scholar]
- 41.Wang JH, Grood ES. The strain magnitude and contact guidance determine orientation response of fibroblasts to cyclic substrate strains. Connect Tissue Res. 2000;41(1):29–36. doi: 10.3109/03008200009005639. [DOI] [PubMed] [Google Scholar]
- 42.Dubay DA, Franz MG. Acute wound healing: the biology of acute wound failure. Surg Clin North Am. 2003;83(3):463–81. doi: 10.1016/S0039-6109(02)00196-2. [DOI] [PubMed] [Google Scholar]
- 43.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341(10):738–46. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 44.Franz MG, Kuhn MA, Nguyen K, et al. Transforming growth factor beta(2) lowers the incidence of incisional hernias. J Surg Res. 2001;97(2):109–16. doi: 10.1006/jsre.2001.6083. [DOI] [PubMed] [Google Scholar]
- 45.DuBay DA, Adamson B, Wang X, et al. Fascial wound failure and incisional hernia formation is associated with fibroblast cell-cycle arrest. Wound Repair Regen. 2002;10(2):1. [Google Scholar]
- 46.Gilbert JA, Weinhold PS, Banes AJ, et al. Strain profiles for circular cell culture plates containing flexible surfaces employed to mechanically deform cells in vitro. J Biomech. 1994;27(9):1169–77. doi: 10.1016/0021-9290(94)90057-4. [DOI] [PubMed] [Google Scholar]
- 47.Carlson MA, Longaker MT. The fibroblast-populated collagen matrix as a model of wound healing: a review of the evidence. Wound Repair Regen. 2004;12(2):134–47. doi: 10.1111/j.1067-1927.2004.012208.x. [DOI] [PubMed] [Google Scholar]
- 48.Neidlinger-Wilke C, Grood ES, Wang J-C, et al. Cell alignment is induced by cyclic changes in cell length: studies of cells grown in cyclically stretched substrates. J Orthop Res. 2001;19(2):286–93. doi: 10.1016/S0736-0266(00)00029-2. [DOI] [PubMed] [Google Scholar]
- 49.Danciu TE, Gagari E, Adam RM, et al. Mechanical strain delivers anti-apoptotic and proliferative signals to gingival fibroblasts. J Dent Res. 2004;83(8):596–601. doi: 10.1177/154405910408300803. [DOI] [PubMed] [Google Scholar]
- 50.Neidlinger-Wilke C, Grood E, Claes L, et al. Fibroblast orientation to stretch begins within three hours. J Orthop Res. 2002;20(5):953–6. doi: 10.1016/S0736-0266(02)00024-4. [DOI] [PubMed] [Google Scholar]
- 51.Adam RM, Roth JA, Cheng HL, et al. Signaling through PI3K/Akt mediates stretch and PDGF-BB-dependent DNA synthesis in bladder smooth muscle cells. Journal of Urology. 2003;169(6):2388–2393. doi: 10.1097/01.ju.0000063980.99368.35. [DOI] [PubMed] [Google Scholar]
- 52.Nakagawa S, Pawelek P, Grinnell F. Long-term culture of fibroblasts in contracted collagen gels: effects on cell growth and biosynthetic activity. J Invest Dermatol. 1989;93(6):792–8. doi: 10.1111/1523-1747.ep12284425. [DOI] [PubMed] [Google Scholar]
- 53.Mochitate K, Pawelek P, Grinnell F. Stress relaxation of contracted collagen gels: disruption of actin filament bundles, release of cell surface fibronectin, and down-regulation of DNA and protein synthesis. Exp Cell Res. 1991;193(1):198–207. doi: 10.1016/0014-4827(91)90556-a. [DOI] [PubMed] [Google Scholar]
- 54.Nakagawa S, Pawelek P, Grinnell F. Extracellular matrix organization modulates fibroblast growth and growth factor responsiveness. Exp Cell Res. 1989;182(2):572–82. doi: 10.1016/0014-4827(89)90260-7. [DOI] [PubMed] [Google Scholar]
- 55.Grinnell F, Zhu M, Carlson MA, et al. Release of mechanical tension triggers apoptosis of human fibroblasts in a model of regressing granulation tissue. Exp Cell Res. 1999;248(2):608–19. doi: 10.1006/excr.1999.4440. [DOI] [PubMed] [Google Scholar]
- 56.Fluck J, Querfeld C, Cremer A, et al. Normal human primary fibroblasts undergo apoptosis in three-dimensional contractile collagen gels. J Invest Dermatol. 1998;110(2):153–7. doi: 10.1046/j.1523-1747.1998.00095.x. [DOI] [PubMed] [Google Scholar]
- 57.Iwig M, Glaesser D, Bethge M. Cell shape-mediated growth control of lens epithelial cells grown in culture. Exp Cell Res. 1981;131(1):47–55. doi: 10.1016/0014-4827(81)90404-3. [DOI] [PubMed] [Google Scholar]
- 58.Rhee S. Fibroblasts in three dimensional matrices: cell migration and matrix remodeling. Exp Mol Med. 2009;41(12):858–65. doi: 10.3858/emm.2009.41.12.096. [DOI] [PMC free article] [PubMed] [Google Scholar]