Abstract
Background
Neoadjuvant treatment has proven beneficial for many GI malignancies, but no phase III trials have been completed examining this approach in pancreatic cancer. This meta-analysis examines the best available phase II trials using neoadjuvant treatment for resectable and borderline/unresectable pancreatic adenocarcinoma.
Methods
Phase II trials were identified using a MEDLINE search, and the Cochrane Central Register of Controlled Trials from 1960 to July 2010. Patients were divided into two groups: patients with initially resectable tumors (Group A), and patients with borderline/unresectable tumors (Group B). Primary outcome measures were rate of resection and survival. Pooled proportions and 95% confidence intervals (CIs) were calculated using random-effects or fixed-effects models based on the heterogeneity of included studies.
Results
A total of 14 phase II clinical trials including 536 patients were analyzed. Following treatment, resectability was 65.8% (95% CI 55.4%–75.6%) compared with 31.6% in Group B (95% CI 14.0%–52.5%). A significant partial response was observed in patients with borderline/unresectable tumors; 31.8 (95% CI 24.2%–39.8%) in Group B, and 9.5% (95% CI 2.9%–19.4%) in Group A (p=0.003). Progressive disease was seen in 17.0% (95% CI 11.9%–22.7) of patients in Group A versus 21.8% (95% CI 10.1%–36.5%) in Group B (p=0.006). Median survival in resected patients was 23 months for Group A, and 22.3 months for Group B.
Conclusion
Neoadjuvant treatment appears to have some activity in patients with borderline/unresectable pancreatic adenocarcinoma. Nearly one-third of tumors initially deemed marginal for operative intervention were ultimately able to be resected following treatment. Until more effective targeted chemotherapeutics are developed, the only group of patients with pancreatic cancer that may benefit from neoadjuvant treatment are those with locally advanced disease.
Introduction
In 2010, 43,140 individuals were diagnosed with pancreatic cancer in the United States, with an estimated 36,800 deaths. 1 Of those diagnosed, only 15 to 20 percent are expected to have resectable disease.2 Approximately 40 percent will have metastatic disease at the time of diagnosis, and another 30 to 40 percent will have locally advanced tumors. The large majority of masses found in the pancreas are adenocarcinomas that arise from ductal epithelium, and have an overall survival of less than 5 percent.
Surgical resection with negative margins (R0) continues to be the only opportunity for cure.2 In patients with advanced disease receiving chemotherapy alone, median survival is 8–12 months. In contrast, patients undergoing pancreaticoduodenectomy have a 5-year survival between 25–35 percent. 3,4 In the subset of patients receiving postoperative chemotherapy, both local and distant recurrence has been shown to occur within 13 months from surgery. 5 Early tumor dissemination is most likely explained by micro-metastasis or minimal residual disease left behind during surgery. 6,7 As such, adjuvant therapy has become standard following surgical resection, especially in those patients with positive nodes or surgical margins. 8,9
In patients with locally advanced pancreatic adenocarcinoma, neoadjuvant treatment has been proposed as a way to decrease tumor burden and downstage tumors.10 Locally advanced disease has been defined as the presence of tumor abutment to the celiac trunk or superior mesenteric artery (SMA), or the involvement/thrombosis of the superior mesenteric vein (SMV)/portal vein (PV) axis greater than 180 degrees. 11 However, the absence of standardized criteria for locally advanced pancreatic cancer has given way to other categories, such as “borderline resectable” tumors. The NCCN considers these tumors to include severe unilateral or bilateral SMV or portal infringement; less than half the circumference tumor abutment on the SMA; abutment or encasement of the hepatic artery; and short segment SMV occlusion. 12
Neoadjuvant treatment has been shown to downstage tumors and decrease local recurrence rates in several GI malignancies. 13,14,15 No phase III trials have been completed that analyze this approach in pancreatic cancer. Here, we have analyzed data from the best available phase II studies examining the use of neoadjuvant therapy in patients with pancreatic cancer, with emphasis on tumor resectability, survival, tumor response rates, toxicities, and recurrence.
Methods
Study Design and Data Collection
Studies considered for our meta-analysis included prospective phase II trials investigating the effects of neoadjuvant chemotherapy and/or neoadjuvant radiation on patients with locally advanced and unresectable pancreatic cancer. Retrospective studies, phase I trials, cohort studies, case series, and case reports were excluded.
Studies were identified using MEDLINE, and the Cochrane Central Register of Controlled Trials from 1960 to July 2010. The following key words and phrases were used to search the database: (“neoadjuvant” or “preoperative”), and (“pancreas” or “pancreatic”), and (“cancer” or “adenocarcinoma” or “carcinoma” or “neoplasm”), and (“chemotherapy” or “chemoradiation” or “radiation” or “radiotherapy”), and (“clinical trial” or “phase II trial” or “randomized controlled trial”) without language restriction. The search results were then reviewed and hand selected based on our inclusion criteria.
The following data was collected from each included study: publication details, including journal and year of publication; study details, including study period, and number and age of subjects; trial interventions, including chemotherapy type and dose, and radiation dose (if administered); and outcome measures. The patients were then subdivided into two groups. Group A included those patients with initially resectable tumors, and Group B included patients with borderline or unresectable disease. The trials were analyzed and tumor resectability was grouped according to the National Comprehensive Cancer Network (NCCN) criteria for resectability. 16 In those trials with poorly defined tumor resectability guidelines, tumors were grouped according to the stated resectability criteria.
Primary outcome measures were resectability and survival. Resectability was defined as the percentage of patients resected divided by the total number of patients that received neoadjuvant treatment and surgical treatment. Secondary outcome measures included tumor response as graded according to the RECIST criteria 17, toxicity as defined by the RTOG/EORTC criteria,18 and recurrence. Tumor response was defined as 1) complete response: disappearance of all target lesions (radiographic) or no vital tumor cells (histopathologic); 2) partial response: 30% decrease of the target lesion (radiographic) or marked signs of tumor regression (histopathologic); 3) progressive disease: 20% increase of the target lesion (radiographic), or distant metastases (radiographic or histopathologic); 4) stable disease: no change or small changes that did not meet the other criteria.
Statistical Analysis
All statistical analyses were performed using the software package R (Version 2.10.1; R Foundation for Statistical Computing, Vienna, Austria). The R function metaprop in package meta (Schwarzer 2008) was used for meta-analysis of proportion outcomes. Both the fixed-effects model and the random-effects model were considered. Pooled proportion estimates with corresponding 95% confidence intervals were calculated using the Freeman-Tukey double arcsine transformation. 19,20 For each meta-analysis, the Cochran Q statistic and I2 statistic were calculated to investigate the between-study heterogeneity. For Cochran Q tests with a p-value less than .10, the assumption of homogeneity was considered invalid 21 and results from a random-effects model were reported. Otherwise, results from a fixed-effects model were reported. Pooled median survival time was estimated by assuming an exponential distribution for the survival time, following the method described in Gillen et al. 22 Since confidence intervals for median estimates were not calculable, range of median survival times was provided instead. Funnel plots were created to assess potential publication bias. The Wilson score interval was used for the proportion outcomes. Plots were created using a Web-based tool (Eastern Region Public Health Observatory (erpho);tools.erpho.org.uk/binomial.aspx).
A general linear model was performed to explore the causes of heterogeneity, using year of study, resectability (yes, borderline, no, both), study design (multi-arm, single-arm), tumor response criteria (RECIST, well-defined, not well-defined), resectability criteria (NCCN, well-defined, not well-defined), chemotherapy (monotherapy, combination therapy), or median age (<60 yrs, ≥60 yrs) as the covariate. The outcome variable was the proportion with an arcsine transformation. The study population was used as residual weight within the regression model. Covariates with a p-value less than .05 were considered significant and the fraction of explained variance was reported.
Results
Out of 397 initially retrieved studies, 14 phase II trials from 1993 to 2010 were chosen to include in this meta-analysis (Table S1). The 14 phase II trials included 536 patients. The University of Texas M.D. Anderson Cancer Center (Houston, TX) and the Fox Chase Cancer Center (Philadelphia, PA) each published two studies (Table S1). All other studies were published by independent groups.
All fourteen trials were prospective studies. Twelve (86%) were single arm studies, while two trials randomized patients to receive different types of neoadjuvant treatment. Data from all 14 studies included pancreatic ductal adenocarcinoma distributed in the head, body, and tail. No separate analysis was done regarding tumor location. Two out of 14 studies also reported data on periampullary tumors (duodenal adenocarcinoma, biliary tract carcinoma), without separate analysis regarding different tumor types. In 12 trials (86%) tumor diagnosis was histologically or cytologically proven prior to the start of treatment.
Patients were classified according to tumor resectability and put into two groups. Group A included 402 (75%) patients and had tumors that were considered resectable before treatment. Group B included 134 (25%) patients with borderline or unresectable tumors (Table 1). Primary outcome measures were rate of resection and survival.
Table 1.
Summary of included studies and group assignments.
| Group | Total number of studies | Total number of patients |
|---|---|---|
| All patients | 14 | 536 |
| Group A (resectable before treatment) | 9 | 402 |
| Group B (borderline/unresectable before treatment) | 5 | 134 |
In all 14 trials, chemotherapy was applied as neoadjuvant treatment. Different combinations of chemotherapeutic agents were used, and doses varied between trials. Eight (57%) trials used gemcitabine based chemotherapy regimens. The remaining 6 trials used 5-FU based regimens, the majority of which ended prior to 2004. Three trials used gemcitabine monotherapy, two trials used gemcitabine in combination with cisplatin, and one trial used gemcitabine with docetaxel. The three oldest studies were performed using 5-FU and mitomycin C (MMC), and one study utilized 5-FU and cisplatin (Table S1). Overall, there were 11 studies using combination therapy and 3 studies using single agents.
In 85% of the studies patients received neoadjuvant radiotherapy (Table SI). Radiation doses ranged between 30 and 50.4 Gy. Most patients received 1.8 Gy/fraction (8/14) or 3 Gy/fraction (2/14). None of the trials incorporated intraoperative radiotherapy.
Toxicity
Treatment toxicity data was available for nine studies only. Severe toxicity (grade 3/4) data from those studies using RTOG/EORTC criteria (1995) 17 were included for subsequent analysis. Grade 3/4 toxicity from neoadjuvant treatment for all patients was 39.9% (95% CI 21.3, 60.3). In those patients with resectable tumors, 37.0% (95% CI 12.1, 66.3) were estimated to have grade 3/4 treatment toxicity, compared to 46.2% (95% CI 36.1, 56.4) in patients with borderline/unresectable tumors (Table 2).
Table 2.
Grade 3/4 toxicity for neoadjuvant treatment, including 95% confidence interval from the random effect model and number of studies in each group.
| Group | Grade 3/4 toxicity |
|---|---|
| All patients |
39.9% [21.3%, 60.3%] I2 = 93.4% [89.6%, 95.8%] (n=346) |
| Group A (resectable before treatment) |
37.0% [12.1%, 66.3%] I2 = 95.8% [93.1%, 97.5%] (n=256) |
| Group B (borderline/unresectable before treatment) |
46.2% [36.1%, 56.4%] I2 = 0.0% [0.0%, 89.1%] (n=90) |
Recurrence
Eight out of fourteen trials reported complete data regarding local recurrence, and were subsequently included in this analysis. Two studies did not report any data on tumor recurrence and four studies reported incomplete data. The eight trials used in this analysis did not clearly state criteria for local versus distant recurrence. Peritoneal disease was classified as distant recurrence. Local recurrence was estimated as 11.1% (95% CI 5.6, 18.0) in all patients (Table 3). There was no statistically significant difference between the proportion of recurrence between patients who were initially resectable prior to treatment (13.9%, 95% CI 7.4, 22.0) and those who were not (4.4%, 95% CI 2.3, 13.4, p = 0.41).
Table 3.
Local and Distant Recurrence, including 95% confidence interval from the random effect model and number of studies in each group.
| Group | Local Recurrence | Distant Recurrence |
|---|---|---|
| All patients |
11.1% [5.6%, 18.0%] I2 = 45.3% [0.0%, 75.8%] (n=345) |
43.9% [34.5%, 53.6%] I2 = 46.9% [0.0%, 75.3%] (n=364) |
| Group A (resectable before treatment) |
14.5% [9.6%, 20.0%] I2 = 44.5% [0.0%, 79.7%] (n=252) |
44.6% [37.6%, 51.7%] I2 = 4.6% [0.0%, 75.8%] (n=271) |
| Group B (borderline/unresectable before treatment) |
4.4% [0.2%, 13.4%] I2 = 0.0% [0.0%, 88.3%] (n=93) |
46.5% [13.3%, 81.6%] I2 = 79.6% [35.4%, 93.6%] (n=93) |
Distant recurrence was analyzed in nine out of fourteen studies. Two studies did not include any data on distant recurrence and three studies provided incomplete data. The proportion of distant recurrence found in all patients was 43.9% (95% CI 34.5, 53.6). There was no significant difference between patients in Group A and B, 44.6% (95% CI 37.6, 51.7) and 46.5% (95% CI 13.3, 18.6), respectively (Table 3).
Tumor Response
Tumor response to neoadjuvant therapy was evaluated in all trials according to clinical or radiographic evidence. In five out of fourteen studies (36%) the RECIST criteria 17 were used to grade tumor response. Another five trials did not use RECIST criteria but the criteria to assess tumor response were clearly stated and well-defined. In contrast, four studies did not state well-defined criteria to grade tumor response.
For all patients, only 1.8% (95% CI 0.6, 3.6) demonstrated a complete response, whereas 18.8% (95% CI 9.7, 29.9) of all patients had a partial response (Table 4, Figure 1). Patients with more advanced tumors were found to have a significantly higher partial response (31.8%, 95% CI 24.2, 39.8), compared to those patients with resectable tumors (9.5%, 95% CI 2.9, 19.4). In contrast, 73.9% (95% CI 63.2, 83.3) of patients with resectable tumors had stable disease (Figure 2) compared to 40.9% (95% CI 32.8, 49.3) of patients with borderline/unresectable tumors (Table 4). Progressive disease was demonstrated in 18.9% (95% CI 13.1, 25.4) of all patients, with minimal variation between Group A and B (Table 4).
Table 4.
Tumor Response, including 95% confidence interval from the random effect model and number of studies in each group.
| Group | Complete Response | Partial Response | Stable Disease | Progressive Disease |
|---|---|---|---|---|
| All patients |
1.8% [0.6%, 3.6%] I2 = 35.1% [0.0%, 68.1%] (n=330) |
18.8% [9.7%, 29.9%] I2 = 81.8% [68.6%, 89.5%] (n=330) |
59.2% [46.1%, 71.6%] I2 = 81.9% [68.8%, 89.5%] (n=330) |
18.9% [13.1%, 25.4%] I2 = 49.9% [0.1%, 74.9%] (n=330) |
| Group A (resectable before treatment) |
0.8% [0.0%, 2.6%] I2 = 0.0% [0.0%, 0.0%] (n=196) |
9.5% [2.9%, 19.4%] I2 = 72.8% [37.3%, 88.2%] (n=196) |
73.9% [63.2%, 83.3%] I2 = 58.2% [0.0%, 83.1%] (n=196) |
17.0% [11.9%, 22.7%] I2 = 0.0% [0.0%, 69.6%] (n=196) |
| Group B (borderline/unresectable before treatment) |
4.0% [0.3%, 11.6%] I2 = 65.1% [8.6%, 86.7%] (n=134) |
31.8% [24.2%, 39.8%] I2 = 45.0% [0.0%, 79.8%] (n=134) |
40.9% [32.8%, 49.3%] I2 = 27.1% [0.0%, 71.2%] (n=134) |
21.8% [10.1%, 36.5%] I2 = 72.1% [29.6%, 88.9%] (n=134) |
Figure 1.
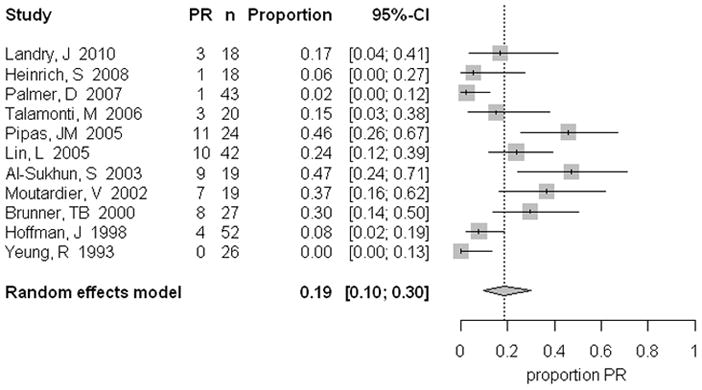
Partial response pooled percentages in patients following neoadjuvant treatment, including the 95% confidence interval from the random effects model and number of patients in each group.
Figure 2.
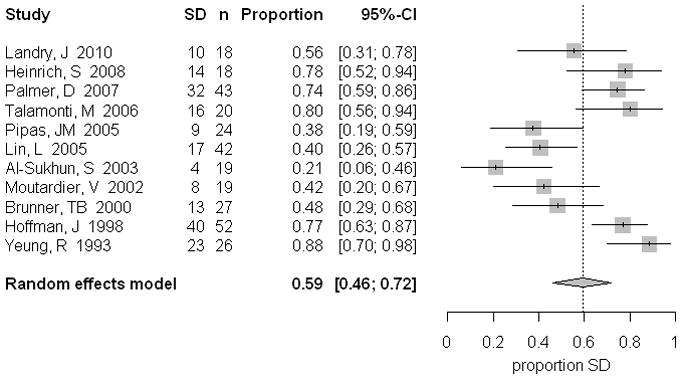
Stable disease pooled percentages in patients following neoadjuvant treatment, including the 95% confidence interval from the random effects model and number of patients in each group.
Rate of Resection
Operations in this analysis included curative resections, such as pancreaticoduodenectomy, distal pancreatectomy, and total pancreatectomy. Palliative and staging procedures were excluded. All fourteen trials reported resection data and were included in this analysis. One trial utilized NCCN guidelines for resection, 12 eight trials (57%) alternatively used other clearly stated well-defined criteria, and five did not have well-defined criteria for resection.
Rate of resection was 54.2% (95% CI 41.5, 66.6) in all patients (Table 5, Figure 3). Of those patients who were staged with resectable tumors prior to treatment, 65.8% (95% CI 55.4, 75.6) had significantly higher rates of resection, compared with 31.6% (95% CI 14.0, 52.5) of patients with borderline/unresectable disease. Margin-negative resections (R0) were achieved in 80.6% (95% CI 71.0, 88.7) in all patients. As expected, a higher proportion of patients with resectable tumors underwent R0 resections (85.1%, 95% CI 76.8, 91.9). To assess for publication bias, a funnel plot was generated regarding resection rate that demonstrated no considerable imbalance. Thus, there was no strong evidence for publication bias in either group of patients.
Table 5.
Rate of Resection, including 95% confidence interval from the random effect model and number of studies in each group.
| Group | Resected Patients/All | RO/Resected Patients |
|---|---|---|
| All patients |
54.2% [41.5%, 66.6%] I2 = 88.7% [82.9%, 92.6%] (n=536) |
80.6% [71.0%, 88.7%] I2 = 72.4% [52.9%, 83.9%] (n=536) |
| Group A (resectable before treatment) |
65.8% [55.4%, 75.6%] I2 = 78.2% [58.7%, 88.4%] (n=402) |
85.1% [76.8%, 91.9%] I2 = 65.6% [30.1%, 83.1%] (n=402) |
| Group B (borderline/unresectable before treatment) |
31.6% [14.0%, 52.5%] I2 = 84.4% [65.1%, 93.0%] (n=134) |
62.2% [29.9%, 89.4%] I2 = 78.8% [49.3%, 91.1%] (n=134) |
Figure 3.
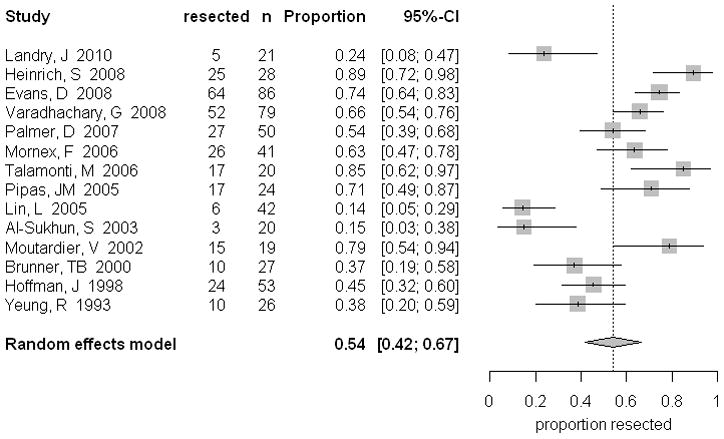
Rate of resection pooled percentages in patients following neoadjuvant treatment, including the 95% confidence interval from the random effects model and number of patients in each group.
Survival
Estimated median survival times were calculated and provided with ranges appropriately. Data was reported in twelve of fourteen studies, and survival times were calculated from diagnosis or start of neoadjuvant treatment. The overall median survival was 13.8 mo (9–26.5) in all patients, with no significant difference between the two groups (Table 6). As expected, surgically resected patients had a longer median survival times. In those patients with operable tumors prior to treatment median survival was 23.0 mo (range 11.7–34), and 22.3 mo (range 18–26.3) in patients with more advanced tumors (Table 6).
Table 6.
Overall median survival (mo) and survival in resected patients (mo) for each group.
| Group | Overall median survival (range) | Median survival in resected patients (range) |
|---|---|---|
| All patients | 13.8 (9–26.5) (n=490) |
22.9 (11.7–34) (n=447) |
| Group A (resectable before treatment) | 15.1 (9.4–26.5) (n=356) |
23.0 (11.7–34) (n=357) |
| Group B (borderline/unresectable before treatment) | 11.2 (9–19.4) (n=134) |
22.3 (18–26.3) (n=90) |
Discussion
The aim of this meta-analysis and review of the literature was to investigate the potential role for neoadjuvant treatment in patients with pancreatic adenocarcinoma. Neoadjuvant therapy has been shown to have significant impact in several GI malignancies, and has many theoretical advantages over adjuvant treatment in patients with pancreatic adenocarcinoma. Preoperative treatment has been proposed to have greater benefits on well-oxygenated, non-devascularized tissue, with improved delivery of chemotherapeutic agents. 9 Secondly, preoperative treatment may be better tolerated, allowing for higher completion rates. Neoadjuvant treatment may also reduce any delay in therapy, and could potentially downstage unresectable tumors. 23
Of those patients with resectable pancreatic adenocarcinoma, 65.8% underwent curative resection. This finding is similar to the rate of resection reported in patients with resectable disease who did not receive neoadjuvant treatment. 24 Not surprisingly, 85.1% of the patients in this group had RO resections (Table 5). Patients with resectable tumors were also found to have significantly more stable disease than those patients with more advanced disease, 73.9% versus 40.9%, respectively (Table 4). The longest estimated median survival (23.0 months, range 11.7–34 months) was observed in Group A patients. Therefore, patients with resectable disease have similar outcomes, regardless of neoadjuvant treatment.
Nearly one-third of tumors initially deemed borderline/unresectable were ultimately suitable for surgical resection after treatment. Group B patients also demonstrated a significantly higher partial response (31.8%) when compared to those patients with resectable disease. This finding is likely explained by the larger overall size of tumors found in Group B patients. This patient population, by definition, had larger, more advanced tumors that were considered borderline or unresectable masses. Those patients with increased tumor burden were able to demonstrate a higher partial response when compared to patients with smaller tumors. The overall median survival for patients with locally advanced disease was 11.2 months (range 9–19.4 months). In this same group of patients, the estimated survival time increased to 22.3 months after resection. From this data we conclude that patients with borderline/unresectable disease have increased partial response rates and a survival time similar to patients with resectable disease after receiving neoadjuvant treatment plus resection.
There were inherent limitations to our meta-analysis. The power of our study was low and the insignificant differences in subgroup comparisons are probably due to lack of statistical power, especially when compared to others that included all study types in their analysis. Given that there is no data from phase III trials, we limited our scope to prospective, phase II trials to obtain the best data available. Despite this, our findings are consistent with similar, larger studies.21
Although trials included in this analysis were from 1993 to 2010, actual treatment dates spanned from 1986 to 2006 (Table S1). Most trials were 2–4 years in length, with three trials lasting 5–6 years. During this 20 year period, there have been major changes in the treatment of pancreatic cancer, including chemotherapy, radiotherapy, and surgical management. This aspect introduces great variability in patient treatment between trials. The definition for resectability varied widely between studies. Studies included in this analysis spanned seven years and had a wide range of resection criteria, some of which were not clearly stated altogether. This finding is most evident in patients with locally advanced disease, especially those with “borderline tumors”. As a result of this variation, our analysis grouped patients with borderline disease and those patients with unresectable disease together into one group. Despite this limitation, our analysis reflects the best data available on this particular topic.
Another limitation of our study is the deficiency of margin status data. It is widely accepted that margin status in pancreatic resection is a crucial factor when determining patient outcomes. However, margins were not clearly defined in these trials, and no analysis could be performed to relate margin status and patient outcomes after receiving neoadjuvant treatment.
Publication bias is one of the major concerns of meta-analysis. The estimates could be inflated when the results are mainly based on published studies. We used funnel plots as a visual aid to investigate potential publication bias. However, due to the limited number of studies in our meta-analyses, it might be difficult to identify all publication bias from the funnel plot, since a funnel plot with a small number of studies may appear asymmetric simply by chance. It is well known that the techniques we have for correcting for publication bias are sometimes poor. Thus, we might not able to remove the potential publication bias completely. The likelihood of potential publication bias could be further reduced if unpublished studies that meet the inclusion criteria were available.
The problem of heterogeneity found in all meta-analysis studies was partially handled using the random effects model. The Cochran Q test was used to detect heterogeneity of the meta-analysis. We used a p-value of less than 0.10 to indicate heterogeneity rather than the conventional cutpoint of 0.05 because the Cochran Q test has low statistical power at detecting heterogeneity when there are few studies. Approximately 2/3 of our meta-analyses show significant evidence of heterogeneity. Random-effects models were used to take into account the between-study variation. Causes of heterogeneity were investigated by using a general linear model. However, no significant source of heterogeneity was detected.
In conclusion, the data compiled from this meta-analysis highlights the importance of preoperative staging. Despite the limitations of this study, the data suggest that patients with borderline or unresectable disease may see the most benefit from neoadjuvant treatment. Like other GI malignancies, treating patients with borderline resectable pancreatic cancer before surgery may ultimately allow for resection in a significant number of these patients, and provides a period of time to select those patients with better tumor biology that will benefit from resection. In contrast, preoperative therapy in those patients with resectable tumors has minimal benefit and may delay a potentially curative operation. With the advent of more effective chemotherapeutic or targeted treatments, a neoadjuvant approach may evolve into a more effective option. However, there is a great need to establish and adhere to a set of standardized resection criteria for patient stratification to enable the pursuit of larger, more comprehensive trials in the future.
Supplementary Material
Acknowledgments
Financial Support: This study was supported by the National Institutes of Health (CA016042 and P01AT003960)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer Statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Bradley EL. Long-term survival after pancreatoduodenectomy for ductal adenocarcinoma: the emperor has no clothes? Pancreas. 2008;37:349–351. doi: 10.1097/MPA.0b013e31818e9100. [DOI] [PubMed] [Google Scholar]
- 3.Yeo CJ, Cameron JL, Lillemoe KD, Sitzmann JV, Hruban RH, Goodman SN, et al. Pancreaticoduodenectomy for cancer of the head of the pancreas: 201 patients. Ann Surg. 1995;221:721–733. doi: 10.1097/00000658-199506000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kazanjian KK, Hines OJ, Duffy JP, Yoon DY, Cortina G, Reber HA. Improved survival following pancreaticoduodenectomy to treat adenocarcinoma of the pancreas: the influence of operative blood loss. Arch Surg. 2008;143:116–71. doi: 10.1001/archsurg.143.12.1166. [DOI] [PubMed] [Google Scholar]
- 5.Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, et al. Adjuvant chemotherapy with gemictabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297:267–277. doi: 10.1001/jama.297.3.267. [DOI] [PubMed] [Google Scholar]
- 6.Esposito I, Kleeff J, Bergmann F, Reiser C, Herpel E, Friess H, et al. Most pancreatic cancer resections are R1 resections. Ann Surg Oncol. 2008;15:1651–1660. doi: 10.1245/s10434-008-9839-8. [DOI] [PubMed] [Google Scholar]
- 7.Kleeff J, Reiser C, Hinz U, Bachmann J, Debus J, Jaeger D, et al. Surgery for recurrent pancreatic ductal adenocarcinoma. Ann Surg. 2007;245:566–572. doi: 10.1097/01.sla.0000245845.06772.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolff RA, Varadhachary CR, Evans DB. Adjuvant therapy for adenocarcinoma of the pancreas: analysis of reported trials and recommendations for the future. Ann Surg Oncol. 2008;15:2773–2786. doi: 10.1245/s10434-008-0002-3. [DOI] [PubMed] [Google Scholar]
- 9.Thomas A, Dajani K, Neoptolemos J, Ghaneh P. Adjuvant therapy in pancreatic cancer. Dig Dis. 2010;28:684–692. doi: 10.1159/000320099. [DOI] [PubMed] [Google Scholar]
- 10.Quiros RM, Brown KM, Hoffman JP. Neoadjuvant therapy in pancreatic cancer. Cancer Invest. 2007;25:267–273. doi: 10.1080/07357900701206356. [DOI] [PubMed] [Google Scholar]
- 11.Katz MH, Wang H, Fleming JB, Sun CC, Hwang RF, Wolff RA, et al. Long-term survival after multidisciplinary management of resected pancreatic adenocarcinoma. Ann Surg Oncol. 2009;16:836–847. doi: 10.1245/s10434-008-0295-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Comprehensive Cancer Network (NCCN) guidelines. 2010 August; doi: 10.6004/jnccn.2010.0125. www.nccn.org. [DOI] [PubMed]
- 13.Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Rietkau R, et al. German Rectal Cancer Study Group. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 14.Mezhirr JJ, Tang LH, Coit DG. Neoadjuvant therapy for locally advanced gastric cancer. J Surg Oncol. 2010;101:305–314. doi: 10.1002/jso.21483. [DOI] [PubMed] [Google Scholar]
- 15.Campbell NP, Villaflor VM. Neoadjuvant treatment of esophageal cancer. World J Gastroenterol. 2010;16:3793–3803. doi: 10.3748/wjg.v16.i30.3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tempero M, Arnoletti JP, Ben-Josef E, Bhargava P, Casper ES, Kim P, et al. Pancreatic adenocarcinoma. Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2007;10:998–1033. doi: 10.6004/jnccn.2007.0085. [DOI] [PubMed] [Google Scholar]
- 17.Therasse P, Arbuck SG, Eisenhauer EA, Wander J, Kaplan RS, Rubinstein L, Verwe, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 18.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment. Int J Radiation Oncology Biol Phys. 1995;31:1341–1346. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 19.Mills EJ, Nachega JB, Buchan I, Orbinski J, Attaran A, Singh S, et al. Adherence to antiretroviral therapy in sub-saharan Africa and North America- a meta-analysis. Journal of the American Medical Association. 2006;296:679–690. doi: 10.1001/jama.296.6.679. [DOI] [PubMed] [Google Scholar]
- 20.Freeman MF, Tukey JW. Transformations related to the angular and the square root. Annals of Mathematical Statistics. 1950;21:607–611. [Google Scholar]
- 21.Lau J, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127:820–826. doi: 10.7326/0003-4819-127-9-199711010-00008. [DOI] [PubMed] [Google Scholar]
- 22.Gillen S, Schuster T, Meyerzum Buschenfelde C, Friess H, Kleeff J. Preoperative/neoadjuvant therapy in pancreatic cancer: A systematic review and meta-analysis of response and resection percentages. PLoS Med. 2010;7:e10000267. doi: 10.1371/journal.pmed.10000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reni M. Neoadjuvant treatment for resectable pancreatic cancer: Time for phase III testing? World J Gastroenterol. 2010;16:4883–488. doi: 10.3748/wjg.v16.i39.4883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bilimoria JY, Bentrem DJ, Ko CY, Stewart AK, Winchester DP, Talamonti MS. National failure to operate on early stage pancreatic cancer. Ann Surg. 2007;246:173–180. doi: 10.1097/SLA.0b013e3180691579. [DOI] [PMC free article] [PubMed] [Google Scholar]
References
- 1.Landry J, Catalano PJ, Staley C, Harris W, Hoffman J, Talamonti M, et al. Randomized phase II study of gemcitabine plus radiotherapy versus gemcitabine plus radiotherapy versus gemcitabine, 5-fluorouracil for patients with locally advanced potentially resectable pancreatic adenocarcinoma. J Surg Oncol. 2010;101:587–592. doi: 10.1002/jso.21527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heinrich S, Pestalozzi BC, Schafer M, Weber A, Bauerfeind P, Knuth A, et al. Prospective phase II trial of neoadjuvant chemotherapy with gemcitabine and cisplatin for resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26:2526–2531. doi: 10.1200/JCO.2007.15.5556. [DOI] [PubMed] [Google Scholar]
- 3.Evans DB, Varadhachary GR, Crane CH, Sun CC, Lee JE, Pisters PWT, et al. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26:3496–3502. doi: 10.1200/JCO.2007.15.8634. [DOI] [PubMed] [Google Scholar]
- 4.Varadhachary GR, Wolff RA, Crane CH, Sun CC, Lee JE, Pisters PWT, et al. Preoperative gemcitabine and cisplatin followed by gemcitabine-based chemoradiation for resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26:3487–3495. doi: 10.1200/JCO.2007.15.8642. [DOI] [PubMed] [Google Scholar]
- 5.Palmer DH, Stocken DD, Hewitt H, Markam CE, Hassan AB, Johnson PJ, et al. A randomized phase 2 trial of neoadjuvant chemotherapy in resectable pancreatic cancer: gemcitabine alone versus gemcitabine combined with cisplatin. Ann Surg Oncol. 2007;14:2088–2096. doi: 10.1245/s10434-007-9384-x. [DOI] [PubMed] [Google Scholar]
- 6.Mornex F, Girard N, Scoazec JY, Bossard N, Ychou M, Smith D, et al. Feasibility of p reoperative combined radiation therapy and chemotherapy with 5-fluorouracil and cisplatin in potentially resectable pancreatic adenocarcinoma: The French SFRO-FFCD 97-04 Phase II Trial. Int J Rad Oncol Biol Phys. 2006;65:1471–1478. doi: 10.1016/j.ijrobp.2006.02.054. [DOI] [PubMed] [Google Scholar]
- 7.Talamonti MS, Small W, Jr, Mulcahy MF, Wayne JF, Attaluri V, Colletti LM, et al. A multi-institutional phase II trial of preoperative full-dose gemcitabine and concurrent radiation for patients with potentially resectable pancreatic carcinoma. Ann Surg Oncol. 2006;13:150–158. doi: 10.1245/ASO.2006.03.039. [DOI] [PubMed] [Google Scholar]
- 8.Pipas JM, Barth RJ, Zaki B, Tsapakos MJ, Suriawinata AA, Bettmann MA, et al. Docetaxel/gemcitabine gollowed by gemcitabine and external beam radiotherapy in patients with pancreatic adenocarcinoma. Ann Surg Oncol. 2005;12:995–1004. doi: 10.1245/ASO.2005.04.503. [DOI] [PubMed] [Google Scholar]
- 9.Lin LL, Picus J, Drebin JA, Linehan DC, Solis J, Strasberg SM, et al. A phase II study of alternating cycles of split course radiation therapy and gemcitabine chemotherapy for inoperable pancreatic or biliary tract carcinoma. Am J Clin Oncol. 2005;28:234–241. doi: 10.1097/01.coc.0000156920.11091.12. [DOI] [PubMed] [Google Scholar]
- 10.Al-Sukhun S, Zalupski MM, Ben-Josef E, Vaitkevicius VK, Philip PA, Soulen R, et al. Chemoradiotherapy in the treatment of regional pancreatic carcinoma; A phase II study. Am J Clin Oncol. 2003;26:543–549. doi: 10.1097/01.coc.0000037143.60502.54. [DOI] [PubMed] [Google Scholar]
- 11.Moutardier V, Giovannini M, Lelong B, Monges G, Vardou VJ, Magnin V, et al. A phase II single institutional experience with preoperative readiochemotherapy in pancreatic adenocarcinoma. EJSO. 2002;28:531–539. doi: 10.1053/ejso.2002.1293. [DOI] [PubMed] [Google Scholar]
- 12.Brunner TB, Grabenbauer GG, Kastl S, Herrmann O, Baum U, Fietkau R, et al. Preoperative chemoradiation in locally advanced pancreatic carcinoma: A phase II study. Onkologie. 2000;23:436–442. doi: 10.1159/000027214. [DOI] [PubMed] [Google Scholar]
- 13.Hoffman JP, Lipsitz S, Pisansky T, Weese JL, Solin L, Benson AB., III Phase II trial of preoperative radiation therapy and chemotherapy for patients with localized, resectable adenocarcinoma of the pancreas: An Eastern Cooperative Oncology Group Study. J Clin Oncol. 1998;16:317–323. doi: 10.1200/JCO.1998.16.1.317. [DOI] [PubMed] [Google Scholar]
- 14.Yeung RS, Weese JL, Hoffman JP, Solin LJ, Paul AR, Engstrom PF, et al. Neoadjuvant chemoradiation in pancreatic and duodenal carcinoma: A phase II study. Cancer. 1993;72:2124–2133. doi: 10.1002/1097-0142(19931001)72:7<2124::aid-cncr2820720711>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


