Abstract
Background
Treatment with histone deacetylases inhibitors (HDACi) such as valproic acid (VPA) and suberoylanilide hydroxamic acid (SAHA) has been shown to improve survival following lethal insults through mechanisms that are incompletely understood. Cell survival under adverse conditions requires a healthy network of capillaries to ensure adequate oxygen delivery. Angiogenetic activation of endothelial cells to migrate and to form sprouts is associated with characteristic changes in gene expression profiles. As HDACI can modulate expression of various genes involved in angiogenic activity, we investigated the effect of these agents on capillary-like sprout-formation in this study.
Methods
Human umbilical vein endothelial cells (HUVECs) were cultured as multicellular spheroids within a type I collagen matrix, which promotes formation of sprouts resembling angiogenesis in vitro. Human umbilical vein endothelial cells (HUVECs) were cultured as multicellular spheroids within a type I collagen matrix, which promotes formation of sprouts (in-vitro angiogenesis). Cells were cultured under following conditions: Control (no growth factors); VPA (1mM); VEGF (Vascular endothelial growth factor, 10 ng/ml); VPA+ VEGF; SAHA (5 mM) and SAHA+VEGF. After 24 hours of treatment the length of spheroid sprouting and cell migration was assessed quantitatively. The levels of acetylated histone H3, phosphor-ERK1/2 and β-Catenin in HUVECs were measured by Western blotting at 6 hours after treatment.
Results
High levels of acetylated histone H3 were detected in VPA and SAHA treated-groups. Compared to VEGF alone treated group (2379 ± 147.1 μm), the spheroid sprouting was 1.7 times increased with VPA and VEGF combined treatment (3996 ± 192.5 μm) (p < 0.01). Cell migrations did not show significant difference after addition of VPA, whereas SAHA suppressed migration. Expression of β-Catenin was significantly increased by VPA and SAHA treatments. Addition of VPA greatly enhanced expression of phosphor-ERK1/2.
Conclusions
Exposure of HUVECs to VPA and SAHA increased the expression of β-catenin and enhanced spheroid sprout formation in vitro. Modulation of HDAC dependent pathways may offer a novel approach to alter angionegenic processes and provide a useful therapeutic target.
Keywords: Endothelial cells, angiogenesis, Histone deacetylase inhibitor
INTRODUCTION
The growth of the vascular system during development, tissue repair or in disease conditions involves the sprouting, migration and proliferation of endothelial cells in a process termed angiogenesis. Angiogenic activation of endothelial cells (EC) to migrate and to form sprouts is associated with characteristic changes in gene expression profiles (1). A balance between pro-angiogenic and anti-angiogenic growth factors and cytokines tightly controls angiogenesis (2).
The cellular actions of vascular endothelial growth factor (VEGF) need to be coordinated to guide vascular patterning during sprouting angiogenesis (3). A number of signal transduction molecules are activated or modified in response to VEGF stimulation, such as extracellular signal-regulated kinase (ERK), and p38 mitogen-activated protein kinase (4). In endothelial cells, ERK phosphorylation is known to promote cell survival and angiogenesis (5, 6). Wnt/β-catenin pathway is involved in various aspects of EC biology and in angiogenesis, such as proliferation and vessel assembly (7). In a previous study we have reported that valproic acid, a histone deacetylase inhibitor, upregulates β –Catenin expression in vivo (8). β-Catenin activates the expression of vascular endothelial growth factor (VEGF)-A and VEGF-C in endothelial cells, and promotes capillary formation and endothelial cell differentiation into network structures (9).
Histone deacetylases (HDACs) constitute a family of enzymes that regulate gene transcription by modifying the acetylation level of histones and nonhistone proteins (10, 11). In particular, protein kinase D–dependent phosphorylation of HDAC5 and HDAC7 play an important role in vascular endothelial growth factor (VEGF)–induced angiogenesis in vitro (12, 13). Inhibition of HDAC causes histone hyperacetylation and modulates angiogenic genes expression. However, the precise mechanisms through which HDAC inhibitors (HDACi) regulate angiogenic sprouting processes is still unclear. In this study, therefore, we examined the effect of two different HDACi valproic acid (VPA) and suberoylanilide hydroxamic acid (SAHA) on EC spheroid sprouting in relation to modulation of β-catenin expression.
MATERIALS AND METHODS
Cell culture
Pooled human umbilical vein endothelial cells (HUVECs) were purchased from Lonza Verviers (Verviers) and cultured in endothelial basal medium (EGM-2; Lonza) with completed supplement kit or in M199 medium supplemented with 10 ng/mL VEGF (Sigma), 200 mM Glutamin, 5000 U/mL heparin, 1.25 μg/mL thymidine, 5% FBS (Sigma), 22 and 100 μg/mL penicillin and streptomycin.
Spheroid sprouting assay
The assay was performed as described previously (14) with following modifications. HUVEC spheroids were generated overnight in hanging-drop culture consisting of 500 cells in EBM-2 medium, 10 % FCS and 20% methylcellulose (Sigma Biochemicals). Spheroids were embedded in collagen type I gels. The spheroid containing gel was rapidly transferred into prewarmed 24-well plates and allowed to polymerize (30 minutes), then 100 μl endothelial basal medium with or without VEGF (10 ng/ml) was added on top of the gel. After 24 hours, the pictures were taken using microscope, and In vitro capillary sprouting was quantified by measuring the cumulative sprout length per spheroid using ImagJ software (National Institutes of Health, bethesda, MD). To obtain a measure of the cumulative sprout length per spheroid, every sprout from 10 spheroids was assessed and from these data the mean cumulative sprout length per spheroid was calculated.
Cell migration assay: Scratch assay
Cells were seeded into 6 cm dishes previously labeled with a traced line. Once at confluence, cells were serum-starved in medium containing 0.5% FCS over night, and then scratch injury was applied using a cell scraper (width approximately10 mm). After injury, the monolayer was gently washed with PBS. Cell migration from the edge of the injured monolayer was examined and photographed 24 hours after scratching. The numbers of endothelial cells that moved across the injury line were counted as a standard marker of migration. Data were analyzed as a percentage of migration in untreated endothelial cells.
Western blot analysis
HUVECs were harvested after 6 hours incubation with following conditions, 1) no VEGF, 2) 10 ng/ml VEGF, 3) 10 ng/ml + 1 mM VPA, 4) 10 ng/ml + 5 mM SAHA. Cell lysates were prepared using a whole lysis kit (BD), according to manufacturer’s instructions. Protein concentration was determined by bicinchoninic acid (BCA) method, and equal amounts (15 μg) of samples were loaded per lane. Proteins were detected using specific antibodies against acetylated histone H3 (Lys9) (07–352, Millepore), p-ERK1/2 (Thr202/Tyr204) (#4377, Cell Signaling), and β-Catenin (13–8400, Invitrogen) and were visualized by enhanced chemiluminescence Western blotting detection system kit (Amersham). The results were scanned and quantified using ImageJ software (NIH).
Immunocytochemistry
HUVECs were prepared in 12 well dishes one day prior to experiment. After 6 hours incubation with following conditions, 1) no VEGF, 2) 10 ng/ml VEGF, 3) 10 ng/ml + 1 mM VPA, 4) 10 ng/ml + 5 mM SAHA, the cells were fixed in 4% paraformaldehyde for 20 min, and washed tree times in PBS. Cultures were incubated together with polyclonal anti-pospho-ERK antibody (Cell Signaling, #4376, 1:200) and monoclonal annti-β-catenin antibody (Invitrogen, #138400, 1:400) in bloking solution (1% BSA in PBS) at 4°C overnight. Cells were washed three times in PBS and incubated with the bloking solution including FITC-conjugated donkey anti-mouse IgG (Sigma) and TRITC-conjugated donkey anti-rabbit IgG (Sigma) at room temperature for 2 hours. Signals were examined using an Olympus microscope (BX71; Olympus) equipped with FITC and rhodamine filter set. All images were collected from microscope image system under the same conditions.
Statistical analyses
Data are presented as mean plus or minus SEM. As appropriate, one-way analysis of variance (ANOVA) and independent samples T test were performed using GraphPad Prism (version 5.00 for Windows, GraphPad Software, San Diego California). A p value of ≤ 0.05 was considered to be significant.
RESULTS
HDAC inhibitor upragulated acetylated histone 3 level
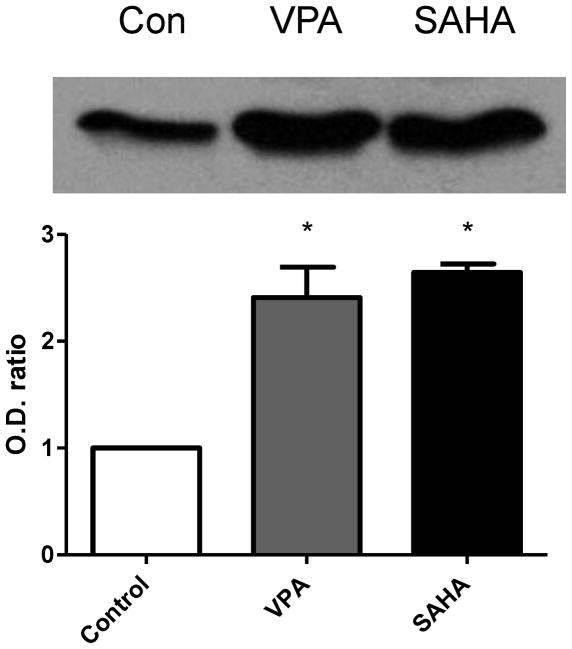
We first examined the protein acetylation level in brain tissue after treatment with VPA or SAHA. The significantly increased protein level of acetylated histone 3 at lysine 9 (AcH3K9) was detected by western blot in both VPA and SAHA treated groups (Fig. 1).
Figure 1.
Western blot for acetylated histone 3 at lysine 9 (AcH3K9) in ECs at 6 hours after treatment with VPA or SAHA. AcH3K9 level clearly increased after VPA or SAHA treatment. N=3.
Sprouting of EC spheroids is enhanced by HDAC inhibitor
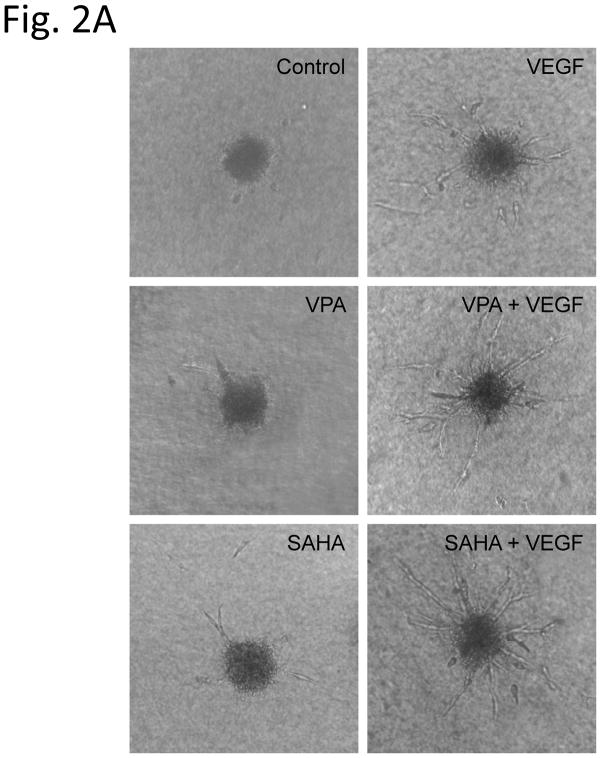
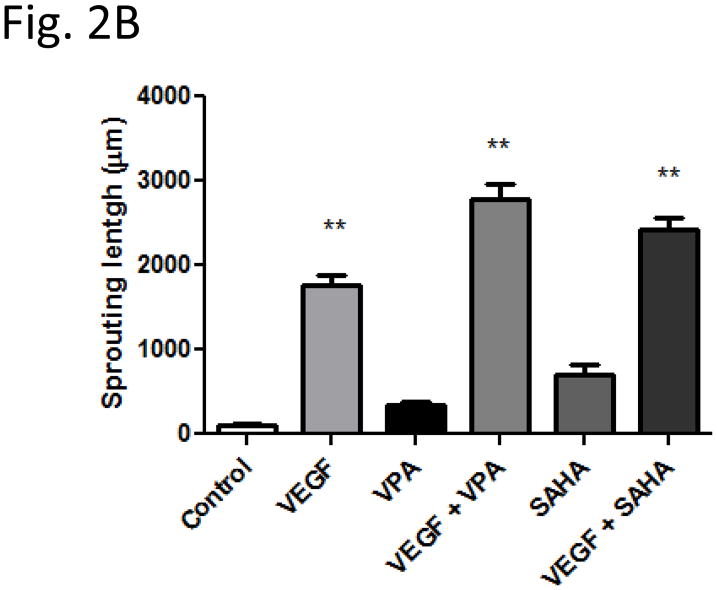
Spheroids generated from HUVECs with a defined cell number of 500 cells per spheroid were embedded into type I collagen gels and incubated for 24 hours in the absence or presence of HDACi with VEGF. There was almost complete absence of spheroid sprouting in group without VEGF (cumulative sprout length 101 ± 30 μm) (Fig. 2A, Control). A slightly increased sprouting was showed in VPA (337 ± 52 μm) (Fig. 2A, VPA) or SAHA (706 ± 122 μm) (Fig. 2A, SAHA) alone treatment groups, but the increase did not reach statistical difference in VPA group. As expected, addition of VEGF significantly increased spheroid length to 1755 ± 122 μl (Fig. 2A, VEGF). Compared to VEGF alone treated group, the treatment of VEGF combined VPA (2777 ± 189 μm) (Fig. 2A, VEGF + VPA) or SAHA (2417 ± 150 μm) (Fig. 2A, VEGF + SAHA) greatly enhanced the HUVECs sprouting further; the increase was highly significant (Fig. 2B).
Figure 2.
Sprouting of HUVEC spheroid in collagen gels. After 24 h of treatment with HDAC inhibitor, spheroid sprouting was analyzed. (Control) Low baseline sprouting was observed. (VEGF) in the presence of VEGF, the sprouting increased significantly (2B). Increasing of HUVEC sprouting in VPA (VPA) or SAHA (SAHA) alone groups were not significant (2B). Treatment of VEGF combined VPA (VEGF + VPA) or SAHA (VEGF + SAHA) greatly enhanced the HUVECs sprouting (2B). ** p < 0.01.
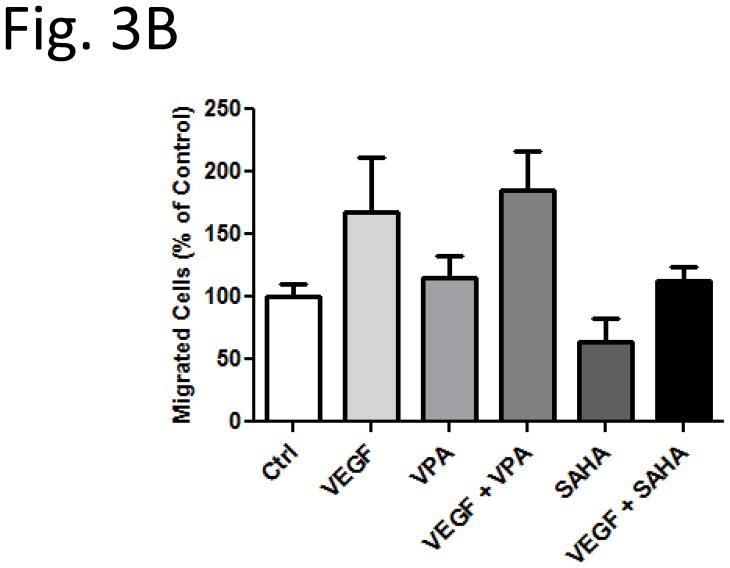
EC migration enhanced by VPA but not by SAHA
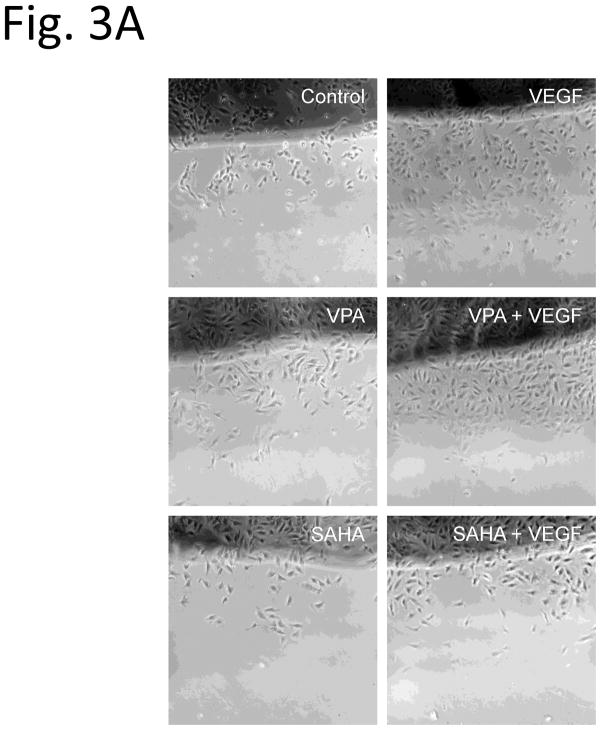
To examine the functional effects of HDACi on HUVECs, we tested the in vitro scratch (wound) assay. Confluent monolayers HUVECs were subjected to scratch wounds, and then incubated with the HDACi for 24 hr. Compared to VEGF absence control group, EC migration increased to 168 % (Fig. 3A, Control and VEGF, Fig. 3B) after VEGF treatment. Compared with control and VEGF alone treated groups, the EC migrations did not show significant difference after addition of VPA (Fig. 3A, VPA and VEGF + VPA, Fig. 3B). Interestingly, with SAHA treatment, the cell migration was reduced in both groups with or without VEGF (Fig. 3A, SAHA and VEGF + SAHA, Fig. 3B).
Figure 3.
Migration of HUVECs in an in vitro scratch assay. Compared with control and VEGF alone treated groups, the EC migrations did not show significant difference after addition of VPA (VPA and VEGF + VPA, 3B). With SAHA treatment, the cell migration was reduced in both groups with or without VEGF (SAHA and VEGF + SAHA, 3B). * p < 0.05.
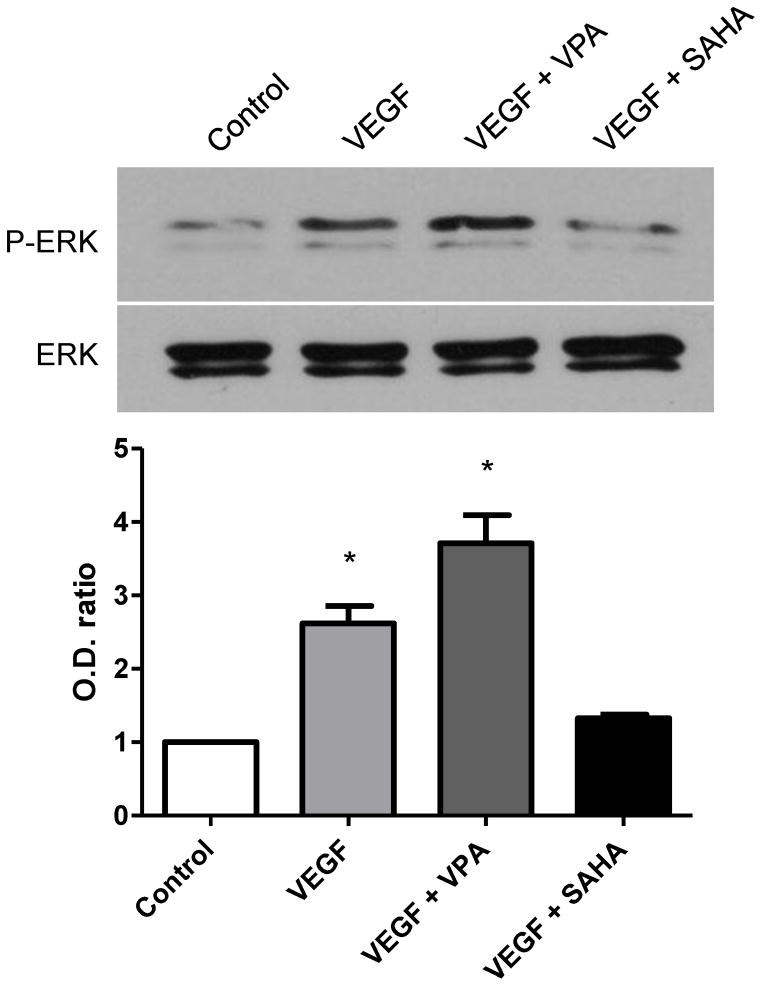
Expressions of phosphor-ERK and β-catenin enhanced by HDAC inhibitor
With Western blot analysis for phosphor-ERK, only a weak band was detectable in VEGF absence control group, while that the signal increased to 1.6 folds with incubation of 10 ng/ml VEGF after 6 hours (Fig. 4). Compared with VEGF only treatment, phosphor-ERK expression was greatly enhanced (3.7 folds) with the addition of VPA (Fig. 4). In contrast, expression of phosphor-ERK was decreased to almost control level after addition of SAHA. Consistently the enhancement of pospho-ERK signals were detected in the immunocytochemistry (Fig. 6, red). No significant changes were detected in total ERK expressions.
Figure 4.
Western blot for p-ERK and ERK at 6 hours after treatment with VPA or SAHA. Uper panel and bar graph: Presence of VEGF significantly induced p-ERK expression, and the expression of p-ERK greatly increased by addition of VPA. However, the expression of p-ERK was decreased to control level by addition of SAHA. Lower panel: No significant changes were detected in total ERK expressions. * p < 0.05.
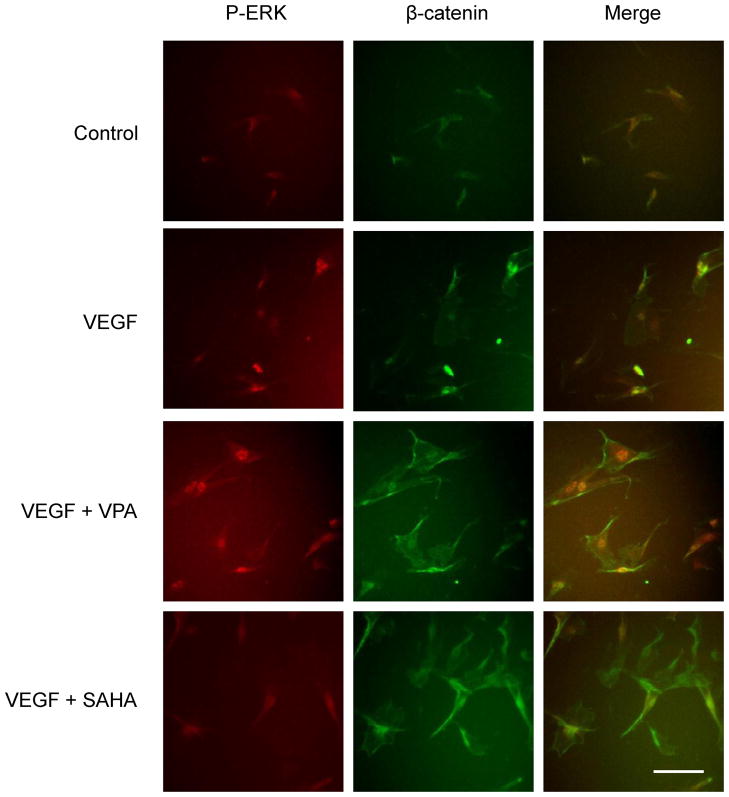
Figure 6.
Double immunostaining for p-ERK (red) and β-catenin (green) at 6 hours after the treatment with VPA or SAHA. VEGF induced p-ERK signal was greatly enhanced by the addition of VPA. Enhanced β-catenin immunoreactivity was detected in the cells treated with VPA or SAHA. Scale bar: 20 μm.
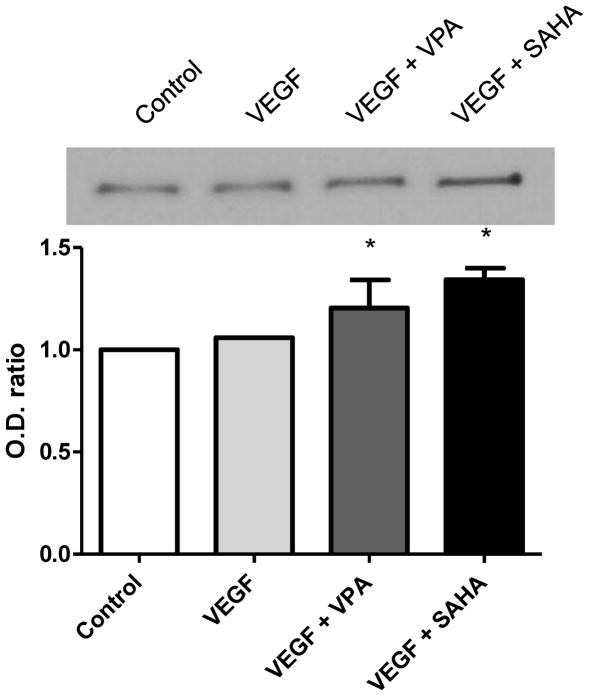
At 6 hours after VEGF treatment, no significant difference was observed in the expression of β-catenin compared with the control. However, the expression of β-catenin increased significantly after treatment with VPA or SAHA (Fig. 5, 6, green). In the immunostaining, the increased β-catenin expressions were showed in the relatively large endothelial cells. No statistical difference was shown between VPA and SAHA treatments.
Figure 5.
Western blot for β-catenin at 6 hours after treatment with VPA or SAHA. Compared with the control, no significant difference was observed after VEGF treatment. The expression of β-Catenin became increased significantly after treatment with VPA or SAHA. * p < 0.05.
DISCUSSION
The current study suggests that HDACi may play an important role in the endothelial cell sprouting process. We observed that: (i) acetylation of histone 3 was clearly increased 6 hours after VPA or SAHA treatment, (ii) endothelial cell spheroid sprouting was greatly enhanced after treatment with VPA and SAHA, when combined with VEGF, (iii) VPA treatment did not alter endothelial cell migration, whereas SAHA treatment slightly suppressed the migration, (iv) The expression of p-ERK was increased by VPA treatment, but not by SAHA, (v) the expression of β-catenin was increased after VPA and SAHA treatment.
The cellular actions of VEGF need to be coordinated to guide vascular patterning during sprouting angiogenesis (3). Our results show that VEGF promotes EC sprouting and migration (Fig. 2 and 3). Acetylation of chromatin proteins and transcription factors is part of a complex signaling system that is involved in the control of gene expression (15, 16). Ha and colleagues have reported that the nuclear export of HDAC5 regulates VEGF-induced MEF2 transcriptional activation in ECs (12). Another recent study reported that over expression of HDAC5 decreases sprout formation, indicating that HDAC5 is a negative regulator of angiogenesis (17). Consistently, we have shown in current study that spheroid sprouting was greatly promoted by addition of VPA or SAHA (Fig. 2). These observations indicate that HDAC inhibitor modulates angiogenic gene expression in the endothelial cells (18), which contributes to capillary-like sprouting of endothelial cells (17). We also discovered in this study that VPA and SAHA treatments upregulated the β-Catenin signal (Fig. 5). This is consistent with our previous studies that have demonstrated that VPA administration increases transcription of β-catenin gene as well the protein level (8). The canonical Wnt/β-catenin pathway has been reported to be crucial for angiogenesis (19). This has also been confirmed in human umbilical vein ECs (HUVECs) infected with a kinase-mutant form of GSK3β, leading to increased β-catenin signaling and downstream VEGF expression (9). In our study, we found that VPA or SAHA amplified the VEGF agiogenic response (Fig. 2), suggesting that this mechanism might be involved.
VPA is considered a first-generation HDAC inhibitor, it inhibits HDACs 1–5 and HDAC 7 in a concentration of 1 mM (20), which is within the range of patient’s plasma levels (21). Whereas, the second-generation HDAC inhibitor SAHA inhibits HDACs 4–7 and HDACs 9–10 (22). A recent study implicates HDAC7 as a key modulator of endothelial cell migration, and HDAC7 silencing caused an inhibition of endothelial cell migration by increasing PDGF-B expression (23). In our study, SAHA treatment was associated with significantly suppressed VEGF induced cell migration, but not with VPA treatment (Fig. 3). On the other hand, only VPA treatment increased the expression of phosphor-ERK signal (Fig.5). This is similar to previous studies that have reported that VPA increases phosphor-ERK expression (5, 20), which is likely induced by a mechanism independently of HDAC inhibition (5). The phosphorylation of ERK in endothelial cells is generally thought to be a proangiogenic event (24). Therefore, VPA induced phosphor-ERK may partially contributes to EC migration, which is consistent with our observation that VPA had higher migration level than SAHA group.
Although we showed that HDACi altered various parameters of angiogenesis in vitro, we cannot be entirely sure what mechanisms are specifically involved. Global inhibition of HDAC activity inhibits angiogenesis by modifying expression of proangiogenic and antiangiogenic factors (23, 25, 26). It is likely that accumulated multiple effects of HDAC inhibitor lead to different specific angiogenic response in different biological conditions and status. But how individual subtype of HDAC signals responses to different angiogenic processes? What is the long-term effect? Different inhibitors block very different classes of HDAC (27). We tested pan-HDACi in the current study. It is possible that using more specific inhibitors or a combination treatment would generate a more robust response. These questions are the focus of future studies. Our current study only provides proof of concept focused on EC sprouting.
In conclusion, we have shown that exposure to VPA and SAHA in vitro increase the expression of β-catenin, thereby enhancing HUVECs spheroid sprout formation. Additionally, as a proangiogenic signal, phosphor-ERK1/2 induced by VPA may contribute to an enhanced angiogenic response. Modulation of HDAC pathway may therefore be a useful therapeutic approach to alter angionegenic processes.
Acknowledgments
Supported by the NIH grant RO1 GM084127 (to HBA).
Footnotes
Data presented at the 6th Annual Academic Surgical Congress, Huntington Beach, CA (February, 2011).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Glesne DA, Zhang W, Mandava S, Ursos L, Buell ME, Makowski L, Rodi DJ. Subtractive transcriptomics: establishing polarity drives in vitro human endothelial morphogenesis. Cancer Res. 2006;66:4030–4040. doi: 10.1158/0008-5472.CAN-05-3294. [DOI] [PubMed] [Google Scholar]
- 2.Pandya NM, Dhalla NS, Santani DD. Angiogenesis--a new target for future therapy. Vascul Pharmacol. 2006;44:265–274. doi: 10.1016/j.vph.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Gerhardt H. VEGF and endothelial guidance in angiogenic sprouting. Organogenesis. 2008;4:241–246. doi: 10.4161/org.4.4.7414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsumoto T, Claesson-Welsh L. VEGF receptor signal transduction. Sci STKE. 2001;2001:re21. doi: 10.1126/stke.2001.112.re21. [DOI] [PubMed] [Google Scholar]
- 5.Michaelis M, Suhan T, Michaelis UR, Beek K, Rothweiler F, Tausch L, Werz O, Eikel D, Zornig M, Nau H, Fleming I, Doerr HW, Cinatl J., Jr Valproic acid induces extracellular signal-regulated kinase 1/2 activation and inhibits apoptosis in endothelial cells. Cell Death Differ. 2006;13:446–453. doi: 10.1038/sj.cdd.4401759. [DOI] [PubMed] [Google Scholar]
- 6.Liu W, Ahmad SA, Reinmuth N, Shaheen RM, Jung YD, Fan F, Ellis LM. Endothelial cell survival and apoptosis in the tumor vasculature. Apoptosis. 2000;5:323–328. doi: 10.1023/a:1009679307513. [DOI] [PubMed] [Google Scholar]
- 7.Liebner S, Plate KH. Differentiation of the brain vasculature: the answer came blowing by the Wnt. J Angiogenes Res. 2010;2:1. doi: 10.1186/2040-2384-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alam HB, Shuja F, Butt MU, Duggan M, Li Y, Zacharias N, Fukudome EY, Liu B, Demoya M, Velmahos GC. Surviving blood loss without blood transfusion in a swine poly-trauma model. Surgery. 2009;146:325–333. doi: 10.1016/j.surg.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 9.Skurk C, Maatz H, Rocnik E, Bialik A, Force T, Walsh K. Glycogen-Synthase Kinase3beta/beta-catenin axis promotes angiogenesis through activation of vascular endothelial growth factor signaling in endothelial cells. Circ Res. 2005;96:308–318. doi: 10.1161/01.RES.0000156273.30274.f7. [DOI] [PubMed] [Google Scholar]
- 10.Gray SG, Ekstrom TJ. The human histone deacetylase family. Exp Cell Res. 2001;262:75–83. doi: 10.1006/excr.2000.5080. [DOI] [PubMed] [Google Scholar]
- 11.Glozak MA, Sengupta N, Zhang X, Seto E. Acetylation and deacetylation of non-histone proteins. Gene. 2005;363:15–23. doi: 10.1016/j.gene.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 12.Ha CH, Wang W, Jhun BS, Wong C, Hausser A, Pfizenmaier K, McKinsey TA, Olson EN, Jin ZG. Protein kinase D-dependent phosphorylation and nuclear export of histone deacetylase 5 mediates vascular endothelial growth factor-induced gene expression and angiogenesis. J Biol Chem. 2008;283:14590–14599. doi: 10.1074/jbc.M800264200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang S, Li X, Parra M, Verdin E, Bassel-Duby R, Olson EN. Control of endothelial cell proliferation and migration by VEGF signaling to histone deacetylase 7. Proc Natl Acad Sci U S A. 2008;105:7738–7743. doi: 10.1073/pnas.0802857105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Korff T, Augustin HG. Tensional forces in fibrillar extracellular matrices control directional capillary sprouting. J Cell Sci. 1999;112 ( Pt 19):3249–3258. doi: 10.1242/jcs.112.19.3249. [DOI] [PubMed] [Google Scholar]
- 15.McKinsey TA, Olson EN. Toward transcriptional therapies for the failing heart: chemical screens to modulate genes. J Clin Invest. 2005;115:538–546. doi: 10.1172/JCI24144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Backs J, Olson EN. Control of cardiac growth by histone acetylation/deacetylation. Circ Res. 2006;98:15–24. doi: 10.1161/01.RES.0000197782.21444.8f. [DOI] [PubMed] [Google Scholar]
- 17.Urbich C, Rossig L, Kaluza D, Potente M, Boeckel JN, Knau A, Diehl F, Geng JG, Hofmann WK, Zeiher AM, Dimmeler S. HDAC5 is a repressor of angiogenesis and determines the angiogenic gene expression pattern of endothelial cells. Blood. 2009;113:5669–5679. doi: 10.1182/blood-2009-01-196485. [DOI] [PubMed] [Google Scholar]
- 18.Mottet D, Castronovo V. Histone Deacetylases: Anti-Angiogenic Targets in Cancer Therapy. Curr Cancer Drug Targets. 2010 doi: 10.2174/156800910793358014. [DOI] [PubMed] [Google Scholar]
- 19.Liebner S, Cavallaro U, Dejana E. The multiple languages of endothelial cell-to-cell communication. Arterioscler Thromb Vasc Biol. 2006;26:1431–1438. doi: 10.1161/01.ATV.0000218510.04541.5e. [DOI] [PubMed] [Google Scholar]
- 20.Gurvich N, Tsygankova OM, Meinkoth JL, Klein PS. Histone deacetylase is a target of valproic acid-mediated cellular differentiation. Cancer Res. 2004;64:1079–1086. doi: 10.1158/0008-5472.can-03-0799. [DOI] [PubMed] [Google Scholar]
- 21.Thisted E, Ebbesen F. Malformations, withdrawal manifestations, and hypoglycaemia after exposure to valproate in utero. Arch Dis Child. 1993;69:288–291. doi: 10.1136/adc.69.3_spec_no.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benn CL, Butler R, Mariner L, Nixon J, Moffitt H, Mielcarek M, Woodman B, Bates GP. Genetic knock-down of HDAC7 does not ameliorate disease pathogenesis in the R6/2 mouse model of Huntington’s disease. PLoS One. 2009;4:e5747. doi: 10.1371/journal.pone.0005747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mottet D, Bellahcene A, Pirotte S, Waltregny D, Deroanne C, Lamour V, Lidereau R, Castronovo V. Histone deacetylase 7 silencing alters endothelial cell migration, a key step in angiogenesis. Circ Res. 2007;101:1237–1246. doi: 10.1161/CIRCRESAHA.107.149377. [DOI] [PubMed] [Google Scholar]
- 24.Sebolt-Leopold JS, Herrera R. Targeting the mitogen-activated protein kinase cascade to treat cancer. Nat Rev Cancer. 2004;4:937–947. doi: 10.1038/nrc1503. [DOI] [PubMed] [Google Scholar]
- 25.Michaelis M, Michaelis UR, Fleming I, Suhan T, Cinatl J, Blaheta RA, Hoffmann K, Kotchetkov R, Busse R, Nau H, Cinatl J., Jr Valproic acid inhibits angiogenesis in vitro and in vivo. Mol Pharmacol. 2004;65:520–527. doi: 10.1124/mol.65.3.520. [DOI] [PubMed] [Google Scholar]
- 26.Chou CW, Chen CC. HDAC inhibition upregulates the expression of angiostatic ADAMTS1. FEBS Lett. 2008;582:4059–4065. doi: 10.1016/j.febslet.2008.10.048. [DOI] [PubMed] [Google Scholar]
- 27.Li Y, Alam HB. Modulation of acetylation: creating a pro-survival and anti-inflammatory phenotype in lethal hemorrhagic and septic shock. J Biomed Biotechnol. 2011:523481. doi: 10.1155/2011/523481. Epub 2011 Feb 15. [DOI] [PMC free article] [PubMed] [Google Scholar]