Abstract
The juvenile onset form of neuronal ceroid lipofuscinoses (JNCL) is a recessively inherited lysosomal storage disorder characterized by progressive neurodegeneration. JNCL results from mutations in the CLN3 gene that encodes a lysosomal membrane protein with unknown function.
Utilizing a Cln3-knock-out mouse model of JNCL that was created on the 129S6/SvEv genetic background, we have previously demonstrated that CLN3-deficient cerebellar granule cells (CGCs) have a selectively increased sensitivity to AMPA-type glutamate receptor-mediated toxicity. Our recent findings that CGCs from 129S6/SvEv and C57BL/6J wild type (WT) mice have significant differences in glutamate receptor expression and in excitotoxic vulnerability indicated that the genetic background possibly have a strong influence on how glutamate receptor function is dysregulated in CLN3-deficient neurons. Indeed, here we show that in the Cln3Δex7/8-knock-in mouse model, that is on the C57BL/6J genetic background, mimics the most frequent mutation observed in JNCL patients and considered a null mutant, the sensitivity of CGCs to both AMPA- and NMDA-type glutamate receptor overactivations is altered. Cultured wild type and Cln3Δex7/8 CGCs were equally sensitive to AMPA toxicity after 2 or 3 weeks in vitro, whereas the subunit-selective AMPA receptor agonist, CPW-399, induced significantly more cell death in mature, 3-week-old Cln3Δex7/8 cultures. NMDA receptor-mediated toxicity changed during in vitro development: Cln3Δex7/8 CGCs were less sensitive to high concentration of NMDA after 2 weeks in culture but became more vulnerable than their WT counterparts after 3 weeks in vitro.
Abnormally altered glutamate receptor function in the cerebellum may result in motor deficits, and we confirmed that 7-week-old Cln3Δex7/8 mice, similarly to Cln3-knock-out mice, have a motor coordination deficit as measured by an accelerating rotarod.
Our results demonstrate altered glutamate receptor function in Cln3Δex7/8 neurons and suggest that both AMPA and NMDA receptors are potential therapeutic targets in JNCL.
Keywords: juvenile neuronal ceroid lipofuscinoses, Batten disease, Cln3, cerebellar granule cells, AMPA receptor, NMDA receptor
1. Introduction
Neuronal ceroid lipofuscinoses (NCLs, also known as Batten disease), are the most frequent childhood neurodegenerative diseases caused by mutations in one of ten genes, CLN1-10 (Jalanko and Braulke, 2009). These recessively inherited, fatal lysosomal storage disorders were originally categorized based on the onset and clinical course of the disease (Cooper, 2003). The juvenile onset form of NCL (JNCL) results from mutations in the CLN3 gene (Consortium, 1995). CLN3 encodes a lysosomal membrane protein with unknown function (Phillips et al., 2005) and therefore, the mechanism of how CLN3 mutations result in selective neurodegeneration is completely unclear. The clinical symptoms of JNCL include progressive vision impairment leading to blindness, frequent occurrence of seizures and progressive motor and cognitive decline. Since there is no cure for the disease, JNCL patients inevitably die in their late teens or early 20s (Goebel and Wisniewski, 2004).
The Cln3-knock-out (Cln3Δex1-6) mouse model of JNCL exhibits many characteristic features of the human disease (Kovacs et al., 2006; Mitchison et al., 1999; Weimer et al., 2009) and provides an invaluable tool to elucidate the molecular and cellular pathomechanism of JNCL. A recent study found elevated glutamate levels in the cerebellum and cortex of Cln3-knock-out mice (Pears et al., 2005), indicating that glutamate neurotransmission may be altered in the absence of the Cln3 gene. Glutamate, besides its normal physiological function as the main excitatory neurotransmitter in the central nervous system, has been implicated in the pathophysiology of a number of neurodegenerative disorders. Abnormal extracellular glutamate levels and/or dysregulation of glutamate receptor function can result in neurological deficits and/or neuronal death (Bowie, 2008; Planells-Cases et al., 2006). Glutamate activates three classes of fast-acting, ion channel-coupled receptors [NMDA (N-methyl-D-aspartate), AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate) and kainate receptors, originally named after their specific agonists] as well as G-protein-coupled, metabotropic receptors that modulates fast synaptic transmission (Kew and Kemp, 2005).
Utilizing the Cln3-knock-out mouse model of JNCL, we have previously demonstrated that CLN3-deficient cerebellar granule cells (CGCs) have a selectively increased sensitivity to AMPA-type glutamate receptor-mediated toxicity (Kovacs et al., 2006). Attenuating AMPA receptor activity via a specific AMPA antagonist in one-month-old Cln3-knock-out mice resulted in an immediate improvement of their motor skills (Kovacs and Pearce, 2008), confirming that an abnormally increased AMPA receptor activity contributes to their motor coordination deficit.
Here we show that in the Cln3Δex7/8-knock-in mouse model (Cotman et al., 2002), that mimics the most frequent mutation observed in JNCL patients, the sensitivity of CGCs to glutamate receptor overactivation is also altered. Our results suggest that both AMPA and NMDA receptors are potential therapeutic targets in JNCL.
2. Materials and methods
2.1. Chemicals
Neurobasal medium, B-27 neuronal serum replacement, glutamine, and penicillin-streptomycin solution are products of Gibco BRL, Invitrogen Corporation (Grand Island, NY). All glutamate receptor ligands used here [NMDA, MK-801, (RS)-AMPA, Cyclothiazide, and CPW-399] were purchased from Tocris Cookson (Bristol, UK). The clear, polystyrene 48- and 96-well plates used for cell culture, viability assay readout, and determination of protein concentration were procured from Corning (Corning, NY). Isopropanol used in this study was obtained from JT Baker/Mallinckrodt Baker (Phillipsburg, NJ). The surface cross-linker, BS3, is produced by Pierce (Rockford, IL). All other chemicals unless stated otherwise were acquired from Sigma Aldrich (St. Louis, MO).
2.2. Animals
Mice used in this study were wild type (WT) C57BL/6J and Cln3 Δex7/8 maintained on the same genetic background and obtained from our in house breeding colony. All experiments were carried out according to the Animal Welfare Act, NIH policies, and the guidelines developed by the University of Rochester Institutional Animal Care and Use Committee.
2.3. Cell Cultures
Primary cerebellar granule cell (CGC) cultures were prepared from seven-day-old WT and Cln3 Δex7/8 mouse pups as previously described (Finn et al., 2010). Briefly, meninges were removed from cerebella and the tissue was minced with a tissue chopper (McIlwain Tissue Chopper, Brinkmann). Minced tissue was then subjected to trypsinization and mechanical dissociation. Cultures were plated at a density of 1.5 × 105 cells per well into 48 well plates previously coated with poly-L-lysine. Cells were cultured in Neurobasal medium (supplemented to include 2% B-27 neuronal serum replacement, 25 mM KCl, 0.5 mM glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin) and maintained in a humidified environment with an atmosphere of 5% CO2/95% air kept at 37 °C. The culture medium was replaced completely approximately 24 hours after plating, and half of the culture medium was removed and replaced every three days for the duration of time in culture.
2.4. Agonist treatment
After 21 days of in vitro development, cells were incubated for 30 min in culture medium lacking B27 (Neurobasal medium containing 25 mM KCl, 0.5 mM glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin) then exposed to AMPA, CPW-399 or NMDA for two hours in fresh culture medium lacking B27. Cyclothiazide was added to AMPA treatments to prevent desensitization of AMPA receptors. MK-801, a specific NMDA receptor blocker, was added to all AMPA receptor agonist treatments (AMPA and CPW-399) at a 50 μM concentration to prevent transactivation of NMDA receptors.
Upon termination of the two-hour treatments, the agonist-containing culture medium was removed and replaced with Neurobasal medium containing 2% B-27, 25 mM KCl, 0.5 mM glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. Twenty-four hours later, cell viability was determined using the MTT viability assay.
2.5. MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) viability assay
Microscopic inspection of cultures was performed to visualize shrinkage due to apoptosis and disintegration due to osmotic lysis and thus determine approximate levels of cell death before viability was quantified using the MTT assay. The results of the MTT viability assay correlate well with what was seen in the preliminary visual inspection. The assay was performed in 48 well culture plates as previously described (Kovacs et al., 2006). Briefly, culture medium was removed and replaced with a 0.3 mg/ml solution of MTT dissolved in Neurobasal medium containing 25 mM KCl, 0.5 mM glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. Cells were incubated at 37 °C for one hour before the medium was aspirated and plates dried. Isopropanol was added to the wells to lyse cells and dissolve the formazan crystals. Aliquots of the isopropanol/formazan mix were then transferred to a 96 well plate, and absorbance was read at 562 nm with background subtraction at 690 nm using a SpectraMax M5 multimode microplate reader (Molecular Devices). Viability as presented here is expressed as a percentage of untreated controls.
2.6. Surface cross-linking in acute cerebellar slices
Cell surface proteins were covalently cross-linked using a cell impermeable cross-linking agent, BS3, resulting in the formation of high molecular weight complexes. As there is no modification of intracellular proteins (Boudreau and Wolf, 2005; Conrad et al., 2008; Finn et al., 2010), surface and intracellular proteins can be separated based on molecular weight. The reaction was performed as previously described (Finn et al., 2010); briefly, one-month-old male WT and Cln3 Δex7/8 mice were euthanized with CO2 and decapitated. The brains were rapidly extracted and a 2-mm-thick coronal slice was cut through the middle of each cerebellum. Slices were immediately immersed in ice-cold artificial cerebrospinal fluid (ACSF; 20 mM HEPES, 147 mM NaCl, 2.7 mM KCl, 1.2 mM CaCl2, 1 mM MgCl2 and 10 mM dextrose) and kept on ice while subsequent brains were dissected. After being isolated from the slices, cerebellar tissue was cut into 400-μm-thick slices using a tissue chopper. Slices were transferred to 1.5-ml microtubes containing ice-cold ACSF. A 4 mM solution of BS3 was added to the tubes to start the cross-linking reaction, and the tubes were gently shaken at 4 °C for 30 min. Glycine was added to a final concentration of 100 mM to quench the cross-linking reaction and the tissue pieces were pelleted by centrifugation. Ice-cold lysis buffer with protease and phosphatase inhibitors [25 mM HEPES, pH 7.4, 500 mM NaCl, 2 mM EDTA 20 mM NaF, 1 mM sodium orthovanadate, 0.1% NP-40 substitute, 1X protease inhibitor cocktail, and 1X phosphatase inhibitor cocktail (Sigma, St. Louis, MO)] was used to resuspend the pellet. Samples were completely homogenized by sonication before protein concentration was determined by the Pierce 660 nm protein assay (Pierce, Rockford, IL). Samples were aliquotted and stored at −80°C until analyzed by Western blotting.
2.7. Western Blotting
Surface cross-linked cerebellar samples containing thirty or sixty μg of proteins were separated on 5% (NMDA receptor subunits) or 6% (AMPA receptor subunits) Tris-HCl-SDS gels. After the electrophoretic separation, proteins were transferred to nitrocellulose membranes. Membranes were rinsed twice with ultrapure water before being blocked in 5% non-fat dry milk in Tris-buffered saline (TBS) with 0.1% Tween-20 (TBS-T) for two hours at room temperature. After blocking, membranes were incubated with antibodies to either AMPA receptor subunits [rabbit anti-GluR1 (Cat. No. Ab1504), 1:2000, overnight; rabbit anti-GluR2 (Cat. No. Ab1768), 1:1000, 60 hrs; rabbit anti-GluR4 (Cat. No. 06-308), 1:1000, 72 hours; all from Millipore (Temecula, CA)] or NMDA receptor subunits [goat anti-NR1 (cat no SC-1467) 1:250, overnight, goat anti-NR2A (Cat. No. sc-1468), 1:1000, 60 hrs; goat anti-NR2B (Cat. No. sc-1469), 1:400, goat anti-NR2C (Cat. No. sc-1470), 1:200, 65-68 hrs; all from Santa Cruz Biotech. (Santa Cruz, CA)] in blocking buffer at 4 °C. Membranes were rinsed twice with ultrapure water and then washed in TBS-T, once for ten minutes followed by three five-minute washes. The HRP-conjugated secondary antibodies were also diluted in blocking buffer [anti-rabbit IgG (1:5000; GE Healthcare, Piscataway, NY) or anti-goat IgG (1:5000; Santa Cruz Biotech, Santa Cruz, CA)] and membranes were incubated in those solutions for 1.5 hours at room temperature. Membranes were then rinsed twice with ultrapure water and washed in TBS-T: once for ten minutes followed by four five-minute washes. Membranes were incubated in Amersham’s ECL Plus Chemiluminescence Detection Reagent (GE Healthcare, Piscataway, NY) for five minutes and imaged using a Biospectrum 500 Imaging System (UVP, Upland, CA). Image J (NIH) was used to quantify band densities. Care was taken to avoid measuring the density of saturated bands; many figures were made using longer exposure times to enhance visibility. Total protein load was determined by Ponceau S staining and used to normalize all band intensities as described previously (Boudreau and Wolf, 2005; Conrad et al., 2008; Finn et al., 2010).
2.8. Rotarod test
An accelerating rotarod (AccuScan Instruments, Inc., Columbus, OH) was used to measure the motor skills of 7-week-old WT and Cln3 Δex7/8 male mice. During the training period, mice were placed on the rotarod starting at zero rpm and accelerating to 37 rpm in 120 seconds. Mice were trained in three sessions each consisting of two consecutive runs, with 15 min of rest between the sessions. Following training, mice rested for 3 h and then were tested in three test trials each consisting of two consecutive runs, with 15 min of rest between the trials. The average latency to fall from the rotating rod during the testing period was calculated for each mouse.
2.9. Statistical analysis
Two-way ANOVA with Bonferroni’s post test for multiple pairwise comparison and unpaired t-test were performed using GraphPad Prism version 4.03 (GraphPad Software, San Diego, CA).
3. Results
To investigate glutamate receptor function in the Cln3Δex7/8-knock-in mouse model of JNCL, we exposed primary cultures of cerebellar granule cells (CGCs) to varied concentrations of receptor agonists and then quantified the extent of glutamate receptor-mediated cell death using the MTT viability assay. The extent of glutamate receptor-mediated cell death can relay a wealth of information regarding receptor function because it depends on the expression level, subunit composition, and channel kinetics of glutamate receptors.
In primary neuronal cultures, glutamate receptor expression increases and subunit composition changes during in vitro development. To detect possible developmental differences, glutamate receptor-mediated cell death in WT and Cln3 Δex7-8 CGC cultures was compared at two different in vitro ages, on the 14th and 21st days in vitro. Twenty-one-day-old CGC cultures, as compared to 14-day-old cultures, were significantly more sensitive to AMPA and NMDA receptor overactivation; much lower agonist concentrations were necessary to induce cell death in 3-week-old than in 2-week-old cultures (Fig. 1–3).
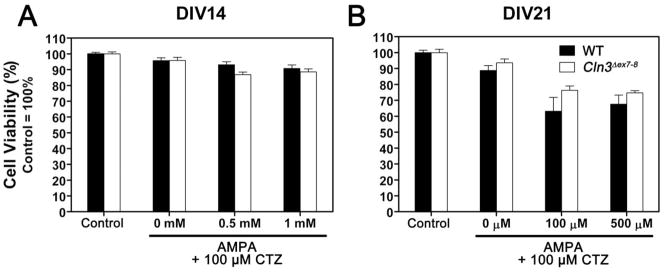
Fig. 1. Cln3 Δex7-8 and WT cerebellar granule cells have similar sensitivity to AMPA-induced cell death.
AMPA-induced cell death was investigated in primary cultures of CGCs prepared from seven-day-old WT (solid bars) and Cln3 Δex7-8 (open bars) mice. Cultures were maintained in Neurobasal medium with B27 serum replacement. On the 14th (A) or 21st (B) day in vitro (DIV14 or DIV21), cultures were exposed to the indicated concentrations of AMPA for two hours in Neurobasal medium without B27. Cyclothiazide (CTZ) was added at a concentration of 100 μM to prevent desensitization of AMPA receptors. Treatments were done in the presence of 50 μM MK-801 to prevent transactivation of NMDA receptors. Upon completion of the two-hour treatment, medium was replaced with complete, B27-containing Neurobasal medium. Cell viability was determined using the MTT viability assay twenty-four hours later. Results presented here represent mean ± S.E.M. of four separate culture preparations (n = 9–22). No statistically significant differences between WT and Cln3 Δex7-8 cultures were found by two-way ANOVA with Bonferroni’s post-test for multiple comparisons.
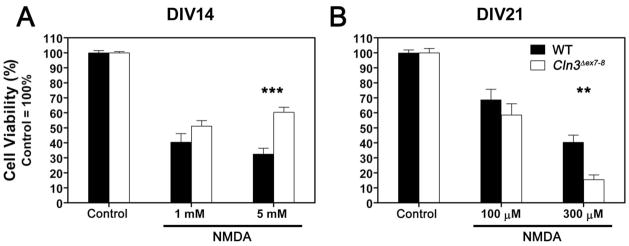
Fig. 3. Relative sensitivity of Cln3 Δex7-8 cerebellar granule cells to NMDA receptor-mediated toxicity changes during in vitro development.
NMDA receptor-mediated cell death was investigated in primary cultures of CGC prepared from seven-day-old WT (solid bars) and Cln3 Δex7-8 (open bars) mice. Cultures were maintained in Neurobasal medium with B27 serum replacement. On the 14th (A) or 21st (B) day in vitro (DIV14 or DIV21), cultures were exposed to the indicated concentrations of NMDA for two hours in Neurobasal medium without B27. Upon completion of the two-hour treatment, medium was replaced with complete, B27-containing Neurobasal medium. Cell viability was determined using the MTT viability assay twenty-four hours later. Results presented here represent mean ± S.E.M. of four separate culture preparations (n = 11–19). Statistical significance was determined by two-way ANOVA with Bonferroni’s post-test for multiple comparisons: ** p<0.01, *** p<0.001.
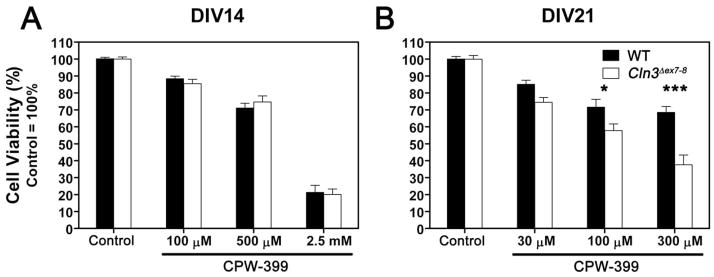
3.1. Cln3 Δex7-8 cerebellar granule cells are significantly more sensitive to the subtype-selective AMPA receptor agonist, CPW-399, in mature, three-week-old cultures
Cerebellar granule cell (CGC) cultures were isolated from seven-day-old WT and Cln3 Δex7-8 mouse pups. Cultures were maintained for two or three weeks in vitro before being treated. To induce AMPA receptor-mediated cell death, cultures were treated with AMPA or with the subtype-selective AMPA receptor agonist, CPW-399. Both AMPA and CPW-399 treatments were done in the presence of 50 μM MK-801 to prevent transactivation of NMDA receptors. In the case of AMPA exposure, 100 μM cyclothiazide was also added to the cells to prevent desensitization of AMPA receptors. AMPA exposure on the 14th or 21st day in vitro induced a similar amount of cell death in WT and Cln3 Δex7-8cultures (Fig. 1).
We also treated CGC cultures with CPW-399, an AMPA receptor agonist that elicits slow or non-desensitizing responses (Campiani et al., 2001). CPW-399 exhibits strong subunit preferences; the compound has a significantly higher affinity for GluR1 and GluR2 that manifests as a 20 fold preference for receptors containing these subunits as opposed to GluR3 and GluR4 (Campiani et al., 2001). No difference in sensitivity to CPW-399 was found in two-week-old cultures (Fig. 2A). When cells were treated after three weeks in culture, we observed a concentration-dependent difference between WT and Cln3 Δex7-8 cultures (Fig. 2B). At the lowest concentration of CPW-399 (30 μM), Cln3 Δex7-8 neurons seemed to be slightly more sensitive to the agonist treatment but the difference was not statistically significant. Increasing the CPW-399 concentration to 100 μM increased the difference between the two populations to the point of statistical significance (p<0.05). The highest concentration of CPW-399 (300 μM) induced twice as much cell death in Cln3 Δex7-8 cultures than in WT cultures (p<0.001).
Fig. 2. Cln3 Δex7-8 cerebellar granule cells are significantly more sensitive to the subtype-selective AMPA receptor agonist, CPW-399, in mature, three-week-old cultures.
CPW-399-induced cell death was investigated in primary cultures of CGC prepared from seven-day-old WT (solid bars) and Cln3 Δex7-8 (open bars) mice. Cultures were maintained in Neurobasal medium with B27 serum replacement. On the 14th (A) or 21st (B) day in vitro (DIV14 or DIV21), cultures were exposed to the indicated concentrations of CPW-399 for two hours in Neurobasal medium without B27. CPW-399 is a non-desensitizing, subtype-selective AMPA receptor agonist. Treatments were done in the presence of 50 μM MK-801 to prevent transactivation of NMDA receptors. Upon completion of the two-hour treatment, medium was replaced with complete, B27-containing Neurobasal medium. Cell viability was determined using the MTT viability assay twenty-four hours later. Results presented here represent mean ± S.E.M. of four separate culture preparations (n = 8–22). Statistical significance was determined by two-way ANOVA with Bonferroni’s post-test for multiple comparisons: * p<0.05, *** p<0.001.
3.2. Relative sensitivity of Cln3 Δex7-8 cerebellar granule cells to NMDA receptor-mediated toxicity changes during in vitro development
To investigate the properties of NMDA receptors in Cln3 Δex7-8 and WT neurons, we treated CGC cultures with various concentrations of NMDA after two or three weeks of in vitro development. After two weeks in culture, we saw no difference in response to 1 mM NMDA between Cln3 Δex7-8 and WT cells. When cultures were exposed to 5 mM NMDA, Cln3 Δex7-8 CGCs were significantly less sensitive than their WT counterparts (Fig. 3A, p<0.001). Cultures treated after three weeks of in vitro development have similar sensitivity to the lowest NMDA concentration tested (100 μM). Increasing the NMDA concentration to 300 μM resulted in a significant difference in viability with the Cln3 Δex7-8 neurons being significantly more vulnerable to the treatment (Fig. 3B, p<0.01).
3.3. Surface and intracellular expression levels of AMPA and NMDA receptor subunits in the cerebellum of one-month-old Cln3 Δex7-8 mice
After demonstrating altered glutamate receptor function in Cln3Δex7/8 cerebellar granule cell cultures in vitro, we wanted to examine if glutamate receptor function of Cln3 Δex7-8 neurons is also altered in the cerebellum in vivo. Since AMPA and NMDA receptor function is regulated in part by surface expression, we measured the surface and intracellular expression levels of AMPA and NMDA receptor subunits in acutely isolated cerebellar slices from one-month-old Cln3 Δex7-8 and WT male mice. (We chose the age of one month because it roughly corresponds to the absolute age of the 3-week-old cerebellar granule cell cultures, prepared from seven-day-old pups, in which the alterations in AMPA and NMDA receptor function were observed.) Using the membrane-impermeable cross-linking agent, BS3, the cell surface receptors (the receptor subunits forming the receptor and adjacent proteins) are covalently cross-linked resulting in high-molecular-weight aggregates, whereas intracellular receptors are not modified (Boudreau and Wolf, 2005; Conrad et al., 2008; Finn et al., 2010). Accordingly, the surface and intracellular receptors can be separated by SDS-PAGE and quantified by Western blotting (Fig. 4A). This method has several advantages as compared to surface biotinylation, an alternative method for monitoring receptor surface expression. A drawback of the surface biotinylation method is that biotin-treated samples must be purified by avidin/streptavidin-coupled beads into biotinylated and non-biotinylated fractions before analysis. Then, biotinylated and non-biotinylated fractions must be measured in separate SDS-PAGE lanes and normalized to total protein for comparison. Potential errors are introduced at purification and normalization steps. In contrast, BS3-cross-linked samples can be analyzed directly by SDS-PAGE without purification, and surface and intracellular bands are measured in the same lane, avoiding the need for normalization. Due to these advantages, the surface cross-linking method has increasingly been applied to measure glutamate receptor surface expression in different brain regions (see e.g., Conrad et al., 2008; Davies et al., 2008; Ferrario et al., 2010; Finn et al., 2010; Gould et al., 2008; Mao et al., 2009; Mickiewicz and Napier, 2011; Morice et al., 2008).
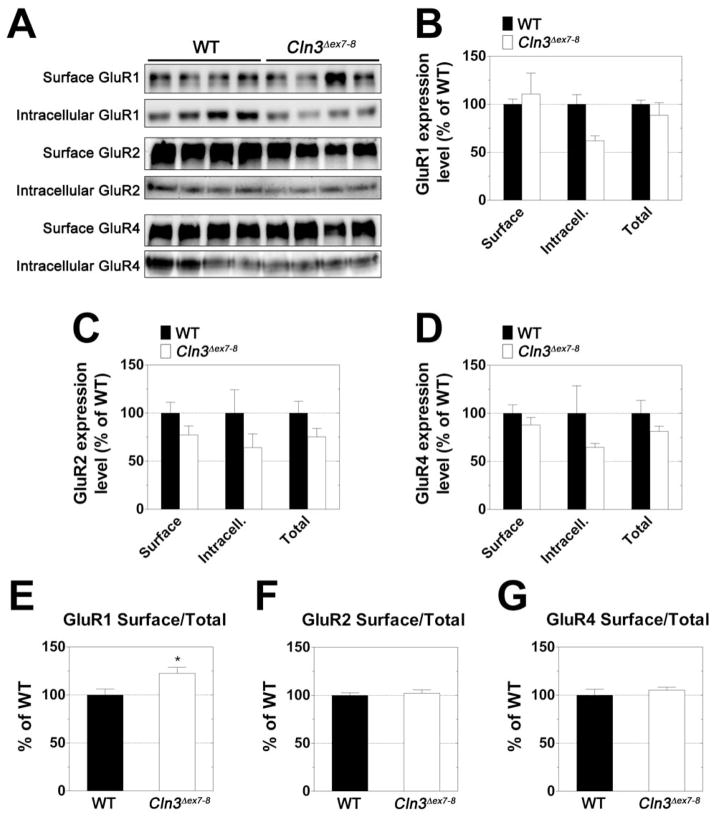
Fig. 4. Surface/Total ratio of the GluR1 AMPA receptor subunit is significantly higher in the cerebellum of one-month-old Cln3 Δex7-8 mice.
Surface and intracellular expression levels of the GluR1, GluR2, and GluR4 AMPA receptor subunits were investigated using acutely isolated slices from the cerebella of one-month-old WT (solid bars) and Cln3 Δex7-8 (open bars) mice. Slices were subjected to surface cross-linking and then analyzed by Western blot for GluR1, GluR2, and GluR4. A: Western blots showing separation of surface (cross-linked) and intracellular receptor subunits. B-D: Quantification of surface, intracellular, and total (surface + intracellular) subunit expression levels. E-G: Comparison of relative surface/total subunit expression ratios. For graphs B-G, Subunit expression levels were normalized to total protein as determined by Ponceau S staining. All measurements for Cln3 Δex7-8 samples are expressed as a percentage of WT. Four mice were analyzed for each genotype; histogram bars represent mean ± S.E.M. Statistical significance was determined by two-way ANOVA with Bonferroni’s post-test for multiple comparisons (B-D) or unpaired t-test (E-G), * p<0.05.
Our experiments revealed that surface expression levels of the AMPA receptor subunits GluR1 and GluR4 were very similar in the cerebellum of Cln3 Δex7-8 and WT mice (Fig. 4A, B and D). Surface expression of the GluR2 AMPA receptor subunit and intracellular expression of GluR1, GluR2 and GluR4 were lower in the Cln3 Δex7-8 cerebellum (Fig. 4A-D); the differences, however, were not statistically significant. The surface to total ratio of each of the receptor subunits was also determined. Cln3 Δex7-8 mice were found to have an increased ratio of surface to total GluR1 in their cerebellum as compared to their WT counterparts (Fig. 4E). There were no differences in this ratio for GluR2 or GluR4 (Fig. 4F-G).
We also investigated the expression levels of the NMDA receptor subunits NR1, NR2A, NR2B and NR2C. The surface expression of NR2A and NR2B, the intracellular level of NR1 and the surface to total ratio of NR2A were all higher in the cerebellum of Cln3 Δex7-8 mice but the differences were not statistically significant (Fig. 5). Surface and intracellular levels of NR2C were very similar in Cln3 Δex7-8 and WT cerebellar samples (data not shown).
Fig. 5. Expression levels of the NR1, NR2A and NR2B NMDA receptor subunits in the cerebellum of one-month-old Cln3 Δex7-8 and WT mice.
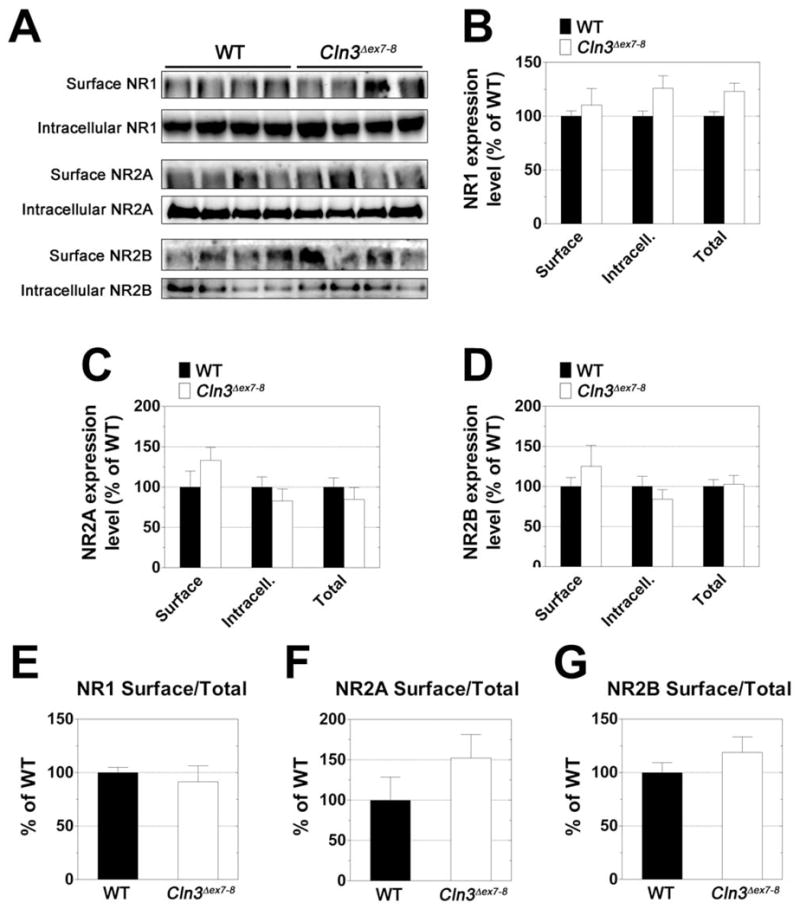
Surface and intracellular expression levels of the NR1, NR2A, and NR2B NMDA receptor subunits were investigated using acutely isolated slices from the cerebella of one-month-old WT (solid bars) and Cln3 Δex7-8 (open bars) mice. Slices were subjected to surface cross-linking and then analyzed by Western blot for NR1, NR2A, and NR2B. A: Western blots showing separation of surface (cross-linked) and intracellular receptor subunits. B-D: Quantification of surface, intracellular, and total (surface + intracellular) subunit expression levels. E-G: Comparison of relative surface/total subunit expression ratios. For graphs B-G, Subunit expression levels were normalized to total protein as determined by Ponceau S staining. All measurements for Cln3 Δex7-8 samples are expressed as a percentage of WT. Four mice were analyzed for each genotype; histogram bars represent mean ± S.E.M. Statistical significance was determined by two-way ANOVA with Bonferroni’s post-test for multiple comparisons (B-D) or unpaired t-test (E-G).
3.4. Seven-week-old Cln3 Δex7/8 mice have a motor coordination deficit
Dysregulated glutamate receptor function in the cerebellum may cause motor deficits. Osorio et al. (2009) recently reported an impaired motor performance of 8-week-old Cln3 Δex7/8 mice in a constant speed rotarod test. Using a more challenging, accelerating rotarod test (0-37 rpm in 120 s), we found that Cln3 Δex7/8 mice already have a motor coordination deficit at the age of 7 weeks (Fig. 6). Cln3 Δex7/8 mice fell from the rotating rod 10 s sooner than WT mice.
Fig. 6. Cln3 Δex7/8 mice have a motor coordination deficit.
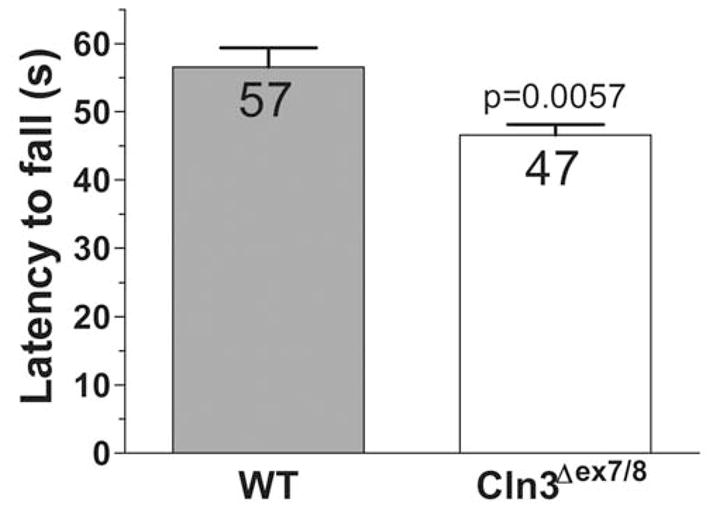
An accelerating rotarod (from 0 to 37 rpm in 120 s) was used to measure the motor skills of 7-week-old WT and Cln3 Δex7-8 male mice. Data are plotted as the time at which animals fell from the rotarod. Cln3 Δex7/8 mice had a reduced ability to remain on the rod as it accelerated. Columns and bars represent mean ± S.E.M. (n = 12). The data sets passed the normality test (α level 0.05), and therefore, statistical significance was determined by unpaired t-test.
4. Discussion
The Cln3-knock-out (Cln3Δex1-6) mouse model of JNCL has been characterized in a number of studies (see e.g., Kovacs et al., 2006; Mitchison et al., 1999; Weimer et al., 2009), giving important clues to the patho-mechanism of the disease and also to the potential function of the CLN3 protein. In the Cln3Δex7/8-knock-in mouse model of JNCL (Cotman et al., 2002), both copies of the normal Cln3 gene were replaced by a mutant Cln3Δex7/8 gene that mimics the most common, disease-causing human mutation, a 1.02 kb deletion that removes exons 7 and 8, theoretically resulting in a truncated protein that contains the first 153 amino acids of CLN3 and 28 novel amino acids at the C-terminus. Although, a recent study (Kitzmuller et al., 2008), solely based on vacuolar and lysosomal size analysis in yeast and cultured human cells, suggested that the 1 kb deletion-associated truncated protein may have residual function, Chan et al. (2008) provided convincing evidence that the truncated CLN3 protein is unlikely to be expressed since cellular quality control mechanisms at the RNA level are likely to degrade the mutant transcript. The lack of clinical phenotype differences in JNCL patients who were homozygous for CLN3Δex7/8 or compound heterozygous with one copy of CLN3Δex7/8 (Adams et al., 2010) also supports the view that Cln3Δex7/8 is a null mutation.
Cln3-knock-out and Cln3Δex7/8-knock-in mice, however, are on different genetic backgrounds, the former being on the 129S6/SvEv while the latter on the C57BL/6J background. The genetic background, as several studies have shown, can modify (even suppress) the effect of a gene deletion (Bilovocky et al., 2003; Duysen and Lockridge, 2006; Mahajan et al., 2004; Tang et al., 2003; Yang et al., 2005). We have recently demonstrated that cerebellar granule cells from 129S6/SvEv and C57BL/6J mice have significant differences in glutamate receptor expression and in excitotoxic vulnerability, and these intrinsic differences possibly have a strong influence on how glutamate receptor function is dysregulated in CLN3-deficient neurons. Indeed, in the present study, comparing the vulnerability of cultured C57BL/6J WT and Cln3Δex7/8-knock-in CGCs to AMPA and NMDA receptor-mediated toxicity, our findings were different from the results obtained previously in 129S6/SvEv WT and Cln3-knock-out CGC cultures (Kovacs et al., 2006). In the present study, Cln3Δex7/8-knock-in CGCs were cultured in serum-free medium (Neurobasal medium with 2% B27 supplement) for up to 3 weeks to study in vitro maturation and developmental changes. In fact, we found remarkable developmental differences. Although, cultured WT and Cln3Δex7/8-knock-in CGCs were equally sensitive to AMPA toxicity after 2 or 3 weeks in vitro, the GluR1/GluR2 subunit-selective AMPA receptor agonist, CPW-399 (Campiani et al., 2001), induced significantly more cell death in mature, 3-week-old Cln3Δex7/8 cultures. NMDA receptor-mediated toxicity changed during in vitro development: Cln3Δex7/8 CGCs were less sensitive to high concentration of NMDA after 2 weeks in culture but became more vulnerable than their WT counterparts after 3 weeks in vitro.
After demonstrating altered glutamate receptor function in Cln3Δex7/8 cerebellar granule cell cultures in vitro, we examined if glutamate receptor function of Cln3 Δex7-8 neurons is also altered in the cerebellum in vivo. Since AMPA and NMDA receptor function is regulated in part by surface expression, we measured the surface and intracellular expression levels of AMPA and NMDA receptor subunits in acutely isolated cerebellar slices from one-month-old Cln3 Δex7-8 and WT male mice.
Although, we did not find any statistically significant differences in the surface expression of the examined receptor subunits (GluR1, GluR2, GluR4, NR1, NR2A, NR2B and NR2C), the possibility that CLN3 deficiency affects the expression levels of the GluR3 AMPA and NR3 NMDA receptor subunits cannot be excluded. Furthermore, if CLN3 deficiency selectively affects glutamate receptor surface expression in cerebellar granule cells, all the other neuronal and glial cell types present in the cerebellum could mask the observable differences. Another possibility is that CLN3 deficiency alters AMPA and NMDA receptor functions by changing their posttranslational modification (e.g., phosphorylation), affecting the electrophysiological properties of the receptors (Wang et al., 2006). Dysregulated glutamate receptor function in the cerebellum of Cln3-knock-out mice largely contributes to their motor coordination deficit (Kovacs and Pearce, 2008; Kovacs et al., 2006). This motor deficit, that can be detected as early as on postnatal day 14, has been characterized using an accelerating rotarod test (0–30 rpm in 240 s) (Kovacs and Pearce, 2008; Kovacs et al., 2006; Weimer et al., 2009). Osorio et al. (2009) recently reported an impaired motor performance of 8-week-old Cln3Δex7/8-knock-in mice in a constant speed rotarod test. Applying a more challenging, accelerating rotarod test (0-37 rpm in 120 s), we were able to detect the motor coordination deficit in 7-week-old Cln3Δex7/8-knock-in mice (see Fig. 6). In summary, our results demonstrate altered glutamate receptor function in Cln3Δex7/8 neurons and suggest that both AMPA and NMDA receptors are potential therapeutic targets in JNCL.
Research highlights.
The genetic background affects mouse models of juvenile Batten disease.
Sensitivity of Cln3ΔΔex7/8 neurons to glutamate receptor overactivation is altered.
AMPA and NMDA receptor expression is not changed in the Cln3ΔΔex7/8 cerebellum.
Seven-week-old Cln3ΔΔex7/8-knock-in mice have a motor coordination deficit.
Acknowledgments
This work was supported by the Luke and Rachel Batten Foundation and in part by National Institutes of Health (NIH) R01 NS044310 and R21 TW008433.
Abbreviations
- ACSF
artificial cerebrospinal fluid
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate
- CGC
cerebellar granule cell
- Cln3-KO
Cln3-knock-out
- CTZ
cyclothiazide
- DIV
day in vitro
- JNCL
juvenile neuronal ceroid lipofuscinoses
- NMDA
N-methyl-D-aspartate
- WT
wild type
Footnotes
Conflict of interest
None of the authors of this paper has any conflict of interest relating to the publication.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams HR, Beck CA, Levy E, Jordan R, Kwon JM, Marshall FJ, Vierhile A, Augustine EF, de Blieck EA, Pearce DA, Mink JW. Genotype does not predict severity of behavioural phenotype in juvenile neuronal ceroid lipofuscinosis (Batten disease) Dev Med Child Neurol. 2010;52:637–643. doi: 10.1111/j.1469-8749.2010.03628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilovocky NA, Romito-DiGiacomo RR, Murcia CL, Maricich SM, Herrup K. Factors in the genetic background suppress the engrailed-1 cerebellar phenotype. J Neurosci. 2003;23:5105–5112. doi: 10.1523/JNEUROSCI.23-12-05105.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau AC, Wolf ME. Behavioral sensitization to cocaine is associated with increased AMPA receptor surface expression in the nucleus accumbens. J Neurosci. 2005;25:9144–9151. doi: 10.1523/JNEUROSCI.2252-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie D. Ionotropic glutamate receptors & CNS disorders. CNS Neurol Disord Drug Targets. 2008;7:129–143. doi: 10.2174/187152708784083821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campiani G, Morelli E, Nacci V, Fattorusso C, Ramunno A, Novellino E, Greenwood J, Liljefors T, Griffiths R, Sinclair C, Reavy H, Kristensen AS, Pickering DS, Schousboe A, Cagnotto A, Fumagalli E, Mennini T. Characterization of the 1H-cyclopentapyrimidine-2,4(1H,3H)-dione derivative (S)-CPW399 as a novel, potent, and subtype-selective AMPA receptor full agonist with partial desensitization properties. J Med Chem. 2001;44:4501–4504. doi: 10.1021/jm015552m. [DOI] [PubMed] [Google Scholar]
- Chan CH, Mitchison HM, Pearce DA. Transcript and in silico analysis of CLN3 in juvenile neuronal ceroid lipofuscinosis and associated mouse models. Hum Mol Genet. 2008;17:3332–3339. doi: 10.1093/hmg/ddn228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M, Wolf ME. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consortium TIBD. Isolation of a novel gene underlying Batten disease, CLN3. Cell. 1995;82:949–957. doi: 10.1016/0092-8674(95)90274-0. [DOI] [PubMed] [Google Scholar]
- Cooper JD. Progress towards understanding the neurobiology of Batten disease or neuronal ceroid lipofuscinosis. Curr Opin Neurol. 2003;16:121–128. doi: 10.1097/01.wco.0000063762.15877.9b. [DOI] [PubMed] [Google Scholar]
- Cotman SL, Vrbanac V, Lebel LA, Lee RL, Johnson KA, Donahue LR, Teed AM, Antonellis K, Bronson RT, Lerner TJ, MacDonald ME. Cln3(Deltaex7/8) knock-in mice with the common JNCL mutation exhibit progressive neurologic disease that begins before birth. Hum Mol Genet. 2002;11:2709–2721. doi: 10.1093/hmg/11.22.2709. [DOI] [PubMed] [Google Scholar]
- Davies KD, Goebel-Goody SM, Coultrap SJ, Browning MD. Long term synaptic depression that is associated with GluR1 dephosphorylation but not alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor internalization. J Biol Chem. 2008;283:33138–33146. doi: 10.1074/jbc.M803431200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duysen EG, Lockridge O. Phenotype comparison of three acetylcholinesterase knockout strains. J Mol Neurosci. 2006;30:91–92. doi: 10.1385/JMN:30:1:91. [DOI] [PubMed] [Google Scholar]
- Ferrario CR, Li X, Wang X, Reimers JM, Uejima JL, Wolf ME. The role of glutamate receptor redistribution in locomotor sensitization to cocaine. Neuropsychopharmacology. 2010;35:818–833. doi: 10.1038/npp.2009.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn R, Kovacs AD, Pearce DA. Altered sensitivity to excitotoxic cell death and glutamate receptor expression between two commonly studied mouse strains. J Neurosci Res. 2010;88:2648–2660. doi: 10.1002/jnr.22433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel HH, Wisniewski KE. Current state of clinical and morphological features in human NCL. Brain Pathol. 2004;14:61–69. doi: 10.1111/j.1750-3639.2004.tb00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TD, O'Donnell KC, Dow ER, Du J, Chen G, Manji HK. Involvement of AMPA receptors in the antidepressant-like effects of lithium in the mouse tail suspension test and forced swim test. Neuropharmacology. 2008;54:577–587. doi: 10.1016/j.neuropharm.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalanko A, Braulke T. Neuronal ceroid lipofuscinoses. Biochim Biophys Acta. 2009;1793:697–709. doi: 10.1016/j.bbamcr.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Kew JN, Kemp JA. Ionotropic and metabotropic glutamate receptor structure and pharmacology. Psychopharmacology (Berl) 2005;179:4–29. doi: 10.1007/s00213-005-2200-z. [DOI] [PubMed] [Google Scholar]
- Kitzmuller C, Haines RL, Codlin S, Cutler DF, Mole SE. A function retained by the common mutant CLN3 protein is responsible for the late onset of juvenile neuronal ceroid lipofuscinosis. Hum Mol Genet. 2008;17:303–312. doi: 10.1093/hmg/ddm306. [DOI] [PubMed] [Google Scholar]
- Kovacs AD, Pearce DA. Attenuation of AMPA receptor activity improves motor skills in a mouse model of juvenile Batten disease. Exp Neurol. 2008;209:288–291. doi: 10.1016/j.expneurol.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs AD, Weimer JM, Pearce DA. Selectively increased sensitivity of cerebellar granule cells to AMPA receptor-mediated excitotoxicity in a mouse model of Batten disease. Neurobiol Dis. 2006;22:575–585. doi: 10.1016/j.nbd.2005.12.018. [DOI] [PubMed] [Google Scholar]
- Mahajan MA, Das S, Zhu H, Tomic-Canic M, Samuels HH. The nuclear hormone receptor coactivator NRC is a pleiotropic modulator affecting growth, development, apoptosis, reproduction, and wound repair. Mol Cell Biol. 2004;24:4994–5004. doi: 10.1128/MCB.24.11.4994-5004.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao LM, Wang W, Chu XP, Zhang GC, Liu XY, Yang YJ, Haines M, Papasian CJ, Fibuch EE, Buch S, Chen JG, Wang JQ. Stability of surface NMDA receptors controls synaptic and behavioral adaptations to amphetamine. Nat Neurosci. 2009;12:602–610. doi: 10.1038/nn.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickiewicz AL, Napier TC. Repeated exposure to morphine alters surface expression of AMPA receptors in the rat medial prefrontal cortex. Eur J Neurosci. 2011;33:259–265. doi: 10.1111/j.1460-9568.2010.07502.x. [DOI] [PubMed] [Google Scholar]
- Mitchison HM, Bernard DJ, Greene ND, Cooper JD, Junaid MA, Pullarkat RK, de Vos N, Breuning MH, Owens JW, Mobley WC, Gardiner RM, Lake BD, Taschner PE, Nussbaum RL. Targeted disruption of the Cln3 gene provides a mouse model for Batten disease. The Batten Mouse Model Consortium [corrected] Neurobiol Dis. 1999;6:321–334. doi: 10.1006/nbdi.1999.0267. [DOI] [PubMed] [Google Scholar]
- Morice E, Andreae LC, Cooke SF, Vanes L, Fisher EM, Tybulewicz VL, Bliss TV. Preservation of long-term memory and synaptic plasticity despite short-term impairments in the Tc1 mouse model of Down syndrome. Learn Mem. 2008;15:492–500. doi: 10.1101/lm.969608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio NS, Sampaio-Marques B, Chan CH, Oliveira P, Pearce DA, Sousa N, Rodrigues F. Neurodevelopmental delay in the Cln3Deltaex7/8 mouse model for Batten disease. Genes Brain Behav. 2009;8:337–345. doi: 10.1111/j.1601-183X.2009.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pears MR, Cooper JD, Mitchison HM, Mortishire-Smith RJ, Pearce DA, Griffin JL. High resolution 1H NMR-based metabolomics indicates a neurotransmitter cycling deficit in cerebral tissue from a mouse model of Batten disease. J Biol Chem. 2005;280:42508–42514. doi: 10.1074/jbc.M507380200. [DOI] [PubMed] [Google Scholar]
- Phillips SN, Benedict JW, Weimer JM, Pearce DA. CLN3, the protein associated with batten disease: structure, function and localization. J Neurosci Res. 2005;79:573–583. doi: 10.1002/jnr.20367. [DOI] [PubMed] [Google Scholar]
- Planells-Cases R, Lerma J, Ferrer-Montiel A. Pharmacological intervention at ionotropic glutamate receptor complexes. Curr Pharm Des. 2006;12:3583–3596. doi: 10.2174/138161206778522092. [DOI] [PubMed] [Google Scholar]
- Tang Y, McKinnon ML, Leong LM, Rusholme SA, Wang S, Akhurst RJ. Genetic modifiers interact with maternal determinants in vascular development of Tgfb1(−/−) mice. Hum Mol Genet. 2003;12:1579–1589. doi: 10.1093/hmg/ddg164. [DOI] [PubMed] [Google Scholar]
- Wang JQ, Liu X, Zhang G, Parelkar NK, Arora A, Haines M, Fibuch EE, Mao L. Phosphorylation of glutamate receptors: a potential mechanism for the regulation of receptor function and psychostimulant action. J Neurosci Res. 2006;84:1621–1629. doi: 10.1002/jnr.21050. [DOI] [PubMed] [Google Scholar]
- Weimer JM, Benedict JW, Getty AL, Pontikis CC, Lim MJ, Cooper JD, Pearce DA. Cerebellar defects in a mouse model of juvenile neuronal ceroid lipofuscinosis. Brain Res. 2009;1266:93–107. doi: 10.1016/j.brainres.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Huang YG, Ye W, Hansen P, Schnermann JB, Briggs JP. Influence of genetic background and gender on hypertension and renal failure in COX-2-deficient mice. Am J Physiol Renal Physiol. 2005;288:F1125–1132. doi: 10.1152/ajprenal.00219.2004. [DOI] [PubMed] [Google Scholar]