Abstract
5-HTTLPR, episodic stressors, depressive and anxious symptoms were assessed prospectively (child and parent report) every 3 months over 1 year (5 waves of data) among community youth ages 9–15 (n = 220). Lagged HLM analyses showed 5-HTTLPR interacted with idiographic stressors (increases relative to the child’s own average level over time), but not nomothetic stressors (higher stress exposure relative to the sample), to predict prospective elevations in depressive, but not anxious, symptoms. Youth with copies of the S or LG alleles of 5-HTTLPR, who experienced more stressors relative to their typical level, exhibited prospective increases in depressive symptoms over time. These findings suggest that 5-HTTLPR confers susceptibility to depression via stress reactivity.
Keywords: Youth, depression, gene-environment interaction, serotonin, multi-wave, stress
Most individuals experience their first depressive episode in adolescence (Costello Mustillo, Erkanli, Keeler, & Angold, 2003), and adolescent-onset depression substantially increases risk for continuity into adulthood (Rutter, Kim-Cohen, & Maughan, 2006). Investigating gene-environment interactions offers a promising window to study putative pathophysiological and psychosocial risk mechanisms (Moffitt, Caspi, & Rutter, 2006). Caspi and colleagues (2003) demonstrated that a measured genotype (5-HTTLPR, the serotonin transporter-linked polymorphic region) by environment (major negative events) interaction (GxE) predicted increases in depression in adults. However, since this seminal paper, findings testing GxE with 5-HTTLPR and various environmental risks among adults have been mixed (Monroe & Reid, 2008; Risch et al., 2009).
Focus on 5-HTTLPR as a candidate gene of interest in the pathophysiology of depression arose after publications implicated the polymorphism in several biological phenotypes of the disorder including neuroticism (Lesch et al., 1996) and amygdala reactivity to threatening stimuli (Hariri et al., 2002). Despite initial support for 5-HTTLPR interacting with negative life events to predict depression in adults (Caspi et al., 2003), subsequent findings have been mixed with some studies demonstrating a clear GxE (e.g. Caspi et al., 2003; Taylor et al., 2006; Wilhelm et al., 2006; Zalsman et al., 2006), and others only partially replicating or failing to replicate these results (e.g. Grabe et al., 2005; Kendler et al., 2005; Gillespie, Whitfield, Heath, & Martin, 2005).
Recently, Risch and colleagues (2009) published a meta-analysis that sought to include exact replications of the original Caspi and colleagues (2003) 5-HTTLPR by stress interaction predicting depression. Results showed no overall significant GxE for depression but did reveal a significant main effect for stress. However, as several subsequent publications have noted, this meta-analysis is limited in numerous ways (Caspi, Hariri, Holmes, Uher, & Moffitt, 2010; Rutter, Thapar, & Pickles, 2009). The primary aim of the current study was to examine GxE effects on depressive symptoms, specifically among youth, using a powerful multi-wave design. In addition, we sought to address some of the limitations of prior studies included in the Risch et al. meta-analysis, including measurement precision for genetic risk and stressor exposure as well as approaches to testing GxE (i.e., idiographic and nomothetic approaches to stress reactivity; see below) in order to create a study design with more power to detect important etiological factors in the pathophysiology of depression.
The vast majority of the GxE literature in depression has focused predominantly on adult samples. Studying gene-environment interactions among youth is a relatively understudied area of importance given the strong risk for recurrence of adolescent onset depression. Of the extensive research literature, based primarily on adult samples, there are a few studies that have focused on youth samples and found an interaction between 5-HTTLPR and environment, including maltreatment (Cicchetti, Rogosch, & Sturge-Apple, 2007, mean age of 17; Kaufman et al., 2006, mean age of 9), general stressors (Eley et al., 2004, 12–19 years old), family structure (Nobile et al., 2009; ages 10–14), and chronic family stress (Hammen, Brennan, Kennan-Miller, Hazel, & Najman, 2010; ages 15 and 20). Moreover, most of the studies with youth samples are limited by cross-sectional and mono-informant designs (see Hammen et al., 2010 as an exception).
Given mixed GxE evidence among adults and few, limited studies among youth, the current study examined GxE effects on depressive symptoms specifically in a multi-wave study of youth. We examined the hypothesis that 5-HTTLPR allelic variation would moderate the longitudinal association between idiographic stress exposure (exposure relative to one’s own average) and elevations in depressive symptoms specifically, compared to anxious symptoms, over time and explored whether this longitudinal GxE effect would be stronger among girls or vary by youths’ age (as indexed by grade level).
Polymorphisms of the 5HT system are candidates of interest and biologically plausible for testing GxE effects on depression given 5HT’s involvement in emotion and cognition (Canli & Lesch, 2007). The serotonin transporter regulates serotonin function by terminating serotonin action in the synapse via reuptake. The short (S) allele is associated with decreased transcriptional efficiency compared with the long (L) allele (Canli & Lesch, 2007). The decreased transcriptional efficiency associated with the S allele results in less serotonin being recaptured in the pre-synaptic neuron when compared to the L allele. Although the exact mechanism by which this polymorphism gives rise to psychiatric outcomes has not been fully elucidated, there have been many studies investigating its role in depression (e.g. Brown & Harris, 2008; Gibb, Benas, Grassia, & McGeary, 2009; Gibb, Uhrlass, Grassia, Benas, & McGeary, 2009; Gotlib, Joormann, Minor, & Hallmayer, 2008; Hammen et al., 2010; Levinson, 2006; see recent review by Caspi et al., 2010). Findings suggest that although variations in 5-HTTLPR are not directly correlated with depression (Lesch, 2003), 5-HTTLPR may interact with other factors (e.g. attentional biases, negative cognitive styles, early adversity, negative life stress) to place individuals at higher risk for experiencing symptoms of depression. More recently, a common single nucleotide polymorphism (SNP) was found to occur (adenine to guanine; A to G) in the L allele (rs25531). Only the LA allele is high functioning (referred to as L), whereas the LG allele is more functionally equivalent to the S allele (collectively referred to as S) (Hu et al., 2005, 2006). Referred to as the triallelic method, this SNP is considered a better reflection of the transcriptional efficiency of 5-HTTLPR (Hu et al., 2006). The biallelic S and L approach has been most reported in the literature; however, this approach does not separate the LG from the LA alleles, which could result in individuals being incorrectly classified as low risk. It has been recommended that both triallelic and biallelic genotyping approaches be reported (Martin, Cleak, Willis-Owen, Flint, & Shifman, 2007) and we used both approaches to genotyping 5-HTTLPR in the current study.
In addition to genotyping, a reliable and valid assessment of stressors is essential for testing and interpreting GxE. Yet, most of the stress measures used in the adult GxE studies have unknown psychometric properties (Monroe & Reid, 2008). Importantly, comprehensive reviews of the GxE literature have found that methodological differences in stressor measurement affected whether studies replicated the original Caspi and colleagues finding (Caspi et al., 2010; Uher & McGuffin, 2010). Studies using more specific and clearly defined negative events and stress measures that are psychometrically strong were significantly more likely to find GxE effects in depression. Moreover, prior studies used assessments of stressors with variable time frames (e.g., 6 months in Eley et al., 2004 to 5 years in Caspi et al., 2003). Such imprecise timing of stressor occurrence can be problematic for GxE in depression because most of the GxE studies, especially those included in the Risch et al. (2009) meta-analysis, used broad, distal measures of environmental risk, as opposed to more specific, proximal episodic stressors (e.g., within past 3 months) that have been shown temporally to maximally predict increases in depression (Monroe & Reid, 2008). In particular, it is unclear how measures of distal stress would affect serotenergic function, and in turn, risk to depression. Indeed, Strickland and colleagues (2002) suggest that stressors should affect serotonergic function within a short time frame following the stressor. As such, using a shorter time frame for stressor assessment may provide a stronger and more conceptually meaningful test of GxE in depression. In sum, to test GxE predicting depressive symptoms, it is as important to assess the environment (i.e., proximal stressors) in a reliable and developmentally valid manner as it is to ascertain genetic risk (Monroe & Reid, 2008).
Accurately assessing genetic and environmental risks to depression is essential. But equally vital is conceptually considering how genes interact with the environment to predict increases in depressive symptoms and incorporating a design that enables a rigorous test of hypothesized GxE processes. The current hypothesis is that 5-HTTLPR may affect individuals’ sensitivity or reactivity to the environment (Caspi et al., 2010). Various lines of evidence, including human observational, neuroimaging, and animal research, is consistent with a stress reactivity conceptualization (see Caspi et al., 2010, for review). For example, Gotlib and colleagues (2008) showed that girls with the S allele of 5-HTTLPR reacted with greater cortisol release to a laboratory stressor. Experimental neuroscience research illustrates that S carriers exhibit exaggerated amygdala activity to threat-related stimuli (e.g., Hariri et al., 2002). Finally, animal research supports 5-HTTLPR as a gene that may affect stress reactivity (e.g., Li et al., 1999). To test the hypothesis that 5-HTTLPR may increase susceptibility to depression via stress reactivity, a multi-wave design, in which stressors and symptoms are assessed repeatedly, can increase precision and power.
A multi-wave design with repeated assessments of symptoms and stressors enables a more precise investigation of how stress exposure over time is related to elevations in depressive symptoms as a function of 5-HTTLPR allelic variation. When testing this stress reactivity hypothesis using multi-wave data, one can distinguish between nomothetic versus idiographic stress conceptualizations and approaches to analysis (Abela & Hankin, 2008). The nomothetic approach states that individuals who experience high stress levels, compared to the sample as a whole, regardless of their individual fluctuations in stress over time, are the most likely to become depressed, especially when vulnerable. In contrast, the idiographic approach suggests that individuals who experience higher stress exposure, compared to their own average over time and regardless of their rank-order in the sample, are most likely to become depressed, especially when vulnerable. To our knowledge, no prior GxE study has investigated stress reactivity as a function of genotype to predict depressive symptoms from nomothetic and idiographic perspectives. Importantly, however, research testing cognitive vulnerability interacting with stress, which is a stress reactivity hypothesis grounded in cognitive theories of depression (e.g., Abramson, Metalsky, & Alloy, 1989; Beck, 1987), has found stronger and more consistent support when using an idiographic as opposed to nomothetic approach when examining the impact of stress on depressive symptoms as a function of cognitive risk in adults (Abela, Webb, Ho, Wagner, & Adams, 2006; Gibb, Beevers, Andover, & Holleran, 2006; Stone, Gibb, & Coles, 2010) and youth (Abela, Skitch, Adams & Hankin, 2006; Abela, Zuroff, Ho, Adams, & Hankin, 2006; see Abela & Hankin, 2008 for review). Testing an idiographic approach to stress requires multi-wave data to ascertain individuals’ average stress exposure and deviations from their own average (i.e., increases or decreases from typical stress exposure). However, there are very few multi-wave GxE studies of depression (Gibb, Benas, et al., 2009, 8–12 years old; Gibb, Uhrlass, et al., 2009, 8–12 years old). To our knowledge, no study has examined how 5-HTTLPR interacts with stressors, conceptualized nomothetically as well as idiographically, to predict prospective changes in symptoms. All of the studies included in the Risch et al. (2009) meta-analysis conceptualized stress reactivity in a nomothetic manner.
Research has demonstrated that anxiety and depression are highly comorbid in youth (Chavira, Stein, Bailey & Stein, 2004; Costello et al., 2003; Lewinsohn, Zinbarg, Seeley, Lewinsohn, & Sack, 1997). Additionally, prospective studies have shown anxiety symptoms and disorders tend to precede depression in youth (Beesdo et al., 2007, Pine, Cohen, Gurley, Brook, & Ma, 1998). Given the degree of comorbidity, as well as the temporal ordering of these two internalizing syndromes, it is important to investigate whether GxE is a shared etiology or specific to predicting depressive symptoms. The few studies that have examined the role of 5-HTTLPR interacting with stressful life events to predict anxiety in youth (Cicchetti et al., 2007; Gunthert et al., 2007; Olsson et al., 2005) and adults (Laucht et al., 2009; Stein, Schork, & Gelernter, 2008; Xie et al., 2009) have yielded mixed findings. Additionally, both the type of anxiety examined (e.g. anxiety sensitivity, post-traumatic stress disorder, and state anxiety) as well as the nature of the environmental risk (e.g. childhood adversity, mild daily stressors, and insecure attachment style) were highly variable among the studies. Concurrently examining both anxious and depressive symptoms among youth provides a stringent test of the specificity of GxE in their etiology.
In summary, this study examined gene-environment interactions, specifically the interaction of the 5-HTTLPR polymorphism with proximal episodic stressors over time, to predict prospective elevations in depressive symptoms among youth. Moreover, we investigated the specificity of this GxE to predict depressive versus anxious symptoms, which commonly co-occur with depression (Costello et al., 2003). Last, we explored the potential moderation of this GxE interaction by sex and age given prior research showing that the 5-HTTLPR x stress interaction was associated with depression among adolescent girls only (e.g., Eley et al., 2004; Hammen et al., 2010), behavioral genetic research finding GxE among adolescent girls (Silberg et al., 1999), and the sex difference in depression emerging in early adolescence and diverging strongly through middle adolescence (Hankin & Abramson, 2001).
To provide a more exacting test of GxE, we followed Rutter’s (2005) recommendations for methodologically rigorous developmental psychopathology research and incorporated (1) a multi-wave longitudinal design allowing for more precise temporal ordering of events before depression, (2) a community sample of youth, and (3) multiple informants of key constructs such as depressive symptoms and stressors which reduces common source information bias. In particular, we assessed developmentally salient episodic stressors and anxious and depressive symptoms every 3 months over 1 year (5 waves of data) with both youth and parent report in a cohort design including samples of 3rd, 6th, and 9th grade youth. This powerful multi-wave design enabled a more exacting examination of processes over time, that is not possible with cross-sectional or even 2-time point studies (Curran & Willoughby, 2003), and provided more statistical power (Snijders & Bosker, 1999) for testing GxE predictions.
We hypothesized that youth carrying at least one copy of the 5-HTTLPR S variant with less transcriptional efficiency, who encountered more idiographic stressors over time, would report greater prospective elevations specifically in depressive and not anxious symptoms over time. We did not expect to find a significant GxE predicting depression when stressors were analyzed in a nomothetic manner, consistent with the nonsignificant GxE effect observed in the Risch et al. (2009) meta-analysis using these methods, as an idiographic approach to stress reactivity has proved to be more consistent and strongly predictive of future depressive symptoms (Abela & Hankin, 2008). Finally, given previous findings of a GxE predicting depression among adolescent girls only (Eley et al., 2004, Hammen et al., 2010) and the paucity of research investigating children, we examined whether sex and/or age moderates the GxE interaction in depressive symptoms.
Method
Participants
Children and adolescents were recruited by brief information letters sent home directly by the participating school districts to families with a child in 3rd, 6th, or 9th grades of public schools. Approximately 2,000 families had a child in 3rd, 6th, or 9th grade in a participating school district, and therefore were eligible to receive letters. The short letter stated that we were conducting a study on social and emotional development in children and adolescents and requested that interested participants call the laboratory to receive more detailed information on the study. Of the brief introductory letters sent by the school districts, 305 families were counted as contacted as a parent responded to the letter and called the laboratory for more information. Briefly, the 305 contacted parents called the laboratory and responded to a brief phone screen that established that both the parent and child were fluent in English, and the child did not carry an autism spectrum or psychotic disorder and had an IQ > 70. Of the 305 initially contacted families, 220 (72%) qualified as a study participant as they met criteria and arrived at the laboratory for the first assessment. The remaining 85 (28%) are considered non-participants for the following reasons: 3 (1%) were excluded due to child with autism spectrum disorder or non-English speaking family; 12 (4%) declined after learning about the study’s requirements, 49 (16%) were scheduled but did not arrive for assessment, and 21 (7%) were never reached to schedule an assessment. In sum, the present study has a participation rate of 72%. Our participation rate is above the 70% rate recommended by epidemiologists for having a representative sample of the target population (Tolonen, 2005; Tolonen, Dobson, & Kulathinal, 2005) and is comparable to that found in previous community-based, general samples of youth depression (e.g., 61% in Lewinsohn, Hops, Roberts, Seeley, & Andrews, 1993).
Participants were 220 youth ranging in age from 9–15 (M=11.4, SD=2.27). The sample was approximately evenly divided by sex (boys: 43%, girls: 57%) and grade (31% 3rd grade, 41% 6th grade, 28% 9th grade). The present sample, drawn from the general community of youth attending public schools, was representative of both the broader population of the particular geographical area and school districts from which the sample was drawn, including socioeconomic status, ethnicity and race. Ethnicity was as follows: Caucasian: 68%, African American: 22%, Latino: 5%, Asian/Pacific Islander: 3%, Other/Mixed Ethnicity and Race: 2%, which is comparable to that of the community and school districts from which the sample was recruited (Caucasian: 65%, African American: 11%, Latino: 19%, Asian/Pacific Islander: 4%, American-Indian: 1%); 25% of youth from the entire school districts received free/reduced lunch. Sex and ethnicity did not differ and was approximately evenly distributed across grades (e.g., 44% boys in 3rd, 46% in 6th, and 40% in 9th grades, respectively). Parents of the youth were predominantly mothers (87%), and 73% were married, 11% single, 15% divorced or separated, and 1% widowed. Median annual parental income was $70,000 (range $10,000 to $200,000), and 24% of the youth received free/reduced lunch at school.
Procedures
The parent and youth visited the laboratory for the baseline assessment. Parents provided informed written consent for their participation and for their child; youth provided written assent. The initial baseline assessment consisted of a battery of questionnaires completed by youth and parents about the child and collection of youth DNA via saliva. Regular follow up assessments over the phone with parent and youth occurred every 3 months over a 1-year period (5 waves of data) to assess stressors and symptoms. The Institutional Review Board approved all procedures. Youth and parents were reimbursed for participation at baseline and each follow-up.
Measures
Depressive symptoms
The Children’s Depression Inventory (CDI; Kovacs, 1985) assessed youths’ depressive symptoms. Both the child (CDI-C) and parent (CDI-P) reported on the child’s level of depressive symptoms to enable multiple informants of depression. CDI-C and CDI-P were given at all 5 assessments. CDI-C and CDI-P scores were moderately correlated (r’s ranged from .34 to .44, p’s < .001), so they were standardized and averaged together to form an overall depressive symptoms score at each wave. There extensive evidence to support the reliability and validity of the CDI as a measure of depressive symptoms in children (Klein, Dougherty, & Olino, 2005). In the current sample, internal consistency (α) was above .80 at all waves. At baseline, the range of CDI scores (child report: mean CDI was 6.87; SD = 5.0, range 0–35; parent report: mean CDI was 5.69; SD = 5.6, range 0–28) was comparable to published norms (Kovacs, 1985) and prior research with general community samples (Cole et al., 1998; Petersen et al., 1993). Using recommended clinical cutoffs for the CDI (i.e., CDI scores > 13; Kazdin, 1989; Smucker, Craighead, Craighead, & Green, 1986) revealed that 15.9% of youth-report and 13.8% of parent report were above cut-scores for the CDI at baseline.
Anxious symptoms
The Multidimensional Anxiety Scale for Children (MASC; March, Sullivan, & Parker, 1999) assessed youths’ overall anxious symptoms and was completed by both the child (MASC-C) and parent about the child (MASC-P). MASC-C and MASC-P were given at all 5 assessments. MASC-C and MASC-P scores were moderately correlated (r’s ranged from .18 to .31, p’s < .001) consistent with prior research (Baldwin & Dadds, 2007), so they were standardized and averaged together to form an overall anxious symptoms score at each wave. There is evidence to support the reliability and validity of the MASC as an measure of anxiety in children (Silverman & Ollendick, 2005). In the current sample, internal consistency (α) was above .80 at all waves. At baseline, the range of MASC scores (child report: mean MASC was 43.26; SD = 22.2, range 5–96; parent report: mean MASC was 43.99; SD = 19.96, range 15–91) was comparable to published norms (March et al., 1999). Using recommended clinical cutoffs for the MASC (March et al., 1999) revealed that 12% of youth-report and 7% of parent report were above cut-scores (T scores > 65) for the MASC at baseline.
Stressors
The Adolescent Life Events Questionnaire (ALEQ; Hankin & Abramson, 2002) consists of 37 items that assess the number of stressors occurring within the past 3 months. The ALEQ assesses a broad range of negative life events that typically occur among adolescents, including school, friendship, romantic, and family events. Respondents indicated whether the event occurred within the past 3 months, which resulted in a count of stressors over the last 3 months. Based on methodological recommendations on assessing stress and adversity (e.g., Brewin, Andrews, & Gotlib, 1993), the ALEQ includes concrete, specific anchors and descriptions of events, rather than overly general subjective event descriptions commonly seen in other self-report stress checklists. The use of concrete anchors is intended to help reduce over-reporting of trivial events or misinterpretation of events due to personality, mood, or memory bias. For example, instead of the event, “did poorly on a test,” which could be prone to misinterpretation (e.g., a straight A student receives a B+ and endorses the event), the ALEQ specifically defines and anchors the event: “Did poorly on – i.e., C or worse, or failed, a test or class project.” Both the child (ALEQ-C) and parent (ALEQ-P) reported on the child’s exposure to stressors by indicating whether or not a stressor occurred within the last 3 months. ALEQ-C and ALEQ-P were given at all 5 assessments. ALEQ-C and ALEQ-P scores were moderately correlated (r’s ranged from .37 to .41, p’s < .001), so they were standardized and averaged together to form an overall score at each wave. Scores for ALEQ-C ranged from 0 to 35 (M = 16.5, SD = 7.5), and ALEQ-P scores ranged from 2 to 37 (M = 17.2, SD = 7.3). The ALEQ demonstrated good validity in past research (a, Hankin, 2008b; Hankin, Stone, & Wright, 2010), including significant correlations with ratings of episodic stressors (r = .44, p < .001) from a contextual stress interview (Rudolph & Flynn, 2007). In sum, the ALEQ possesses strong psychometric properties and provides a reasonably reliable, valid, and developmentally appropriate assessment of stressors among youth.
Genotyping
Children provided buccal cells for DNA collection via Oragene® kits from DNA Genotek (Ottawa, Ontario, Canada) and genomic DNA was collected and isolated using standard salting out and solvent precipitation methods. The 5-HTTLPR alleles were assayed (Anchordoquy, McGeary, Liu, Krauter, & Smolen, 2003) and modified by using primers reported by Hu et al. (2005). The rs25531 SNP genotypes (LA vs. LG) were obtained by incubating the PCR products with MspI (Wendland, Martin, Kruse, Lesch, & Murphy, 2006). Samples were analyzed on an ABI PRISMR® 3130xl Sequencer. For the triallelic analyses, three groups of participants were formed based on their genotyping: children homozygous for the lower expressing S or LG alleles, those heterozygous, and those homozygous for the higher expressing LA allele. For biallelic analyses, trichotomous groups of SS, SL, and LL genotypes were formed.
Results
Preliminary Analyses
Table 1 shows descriptive statistics, as an average across time and across informants, for the whole sample and by grade and sex. Sex differences were observed for MASC. Specifically, girls on average across time reported more anxious symptoms compared with boys. Grade differences were noted for CDI and ALEQ. Youth in the 9th grade reported more depressive symptoms and stressors on average over time. Depressive and anxious symptoms were moderately correlated across time points (r’s = .26 at baseline, .36 at Time 2, .25 at Time 3, .13 at Time 4, and .27 at Time 5). 5-HTTLPR polymorphisms were in Hardy-Weinberg equilibrium. Genotype frequencies for biallelic 5-HTTLPR were 33% L/L, 46% S/L, and 21% S/S. Genotype frequencies for triallelic 5-HTTLPR were 21% LA homozygotes, 46% heterozygotes, and 33% homozygotes for S/LG. Neither genotype nor allelic frequencies varied significantly by race(White vs. African-American; χ2 < 1.37).
Table 1.
Descriptive statistics overall and by sex and grade group
| Full sample | Girls | Boys | 3rd grade | 6th grade | 9th grade | |
|---|---|---|---|---|---|---|
| Variable | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) |
| CDI | 6.47 (5.20) | 7.17 (5.28) | 5.98 (5.64) | 6.19 (5.28) | 6.17 (4.77) | 7.28 (5.72) * |
| MASC | 43.01 (19.89) | 46.22 (21.26) | 38.58 (18.35)*** | 43.43 (19.04) | 41.26 (13.84) | 40.69 (15.36) |
| ALEQ | 16.02 (7.85) | 16.41 (7.66) | 15.48 (8.12) | 12.96 (7.26) | 15.12 (7.72) | 21.02 (6.72)*** |
Note. CDI=Children’s Depression Inventory; MASC =Multidimensional Anxiety Scale for Children; ALEQ=Adolescent Life Events Questionnaires. All measures are composites of parent and child report and are averaged across the 5 time points.
p < .05,
p < .001
Statistical Approach
Hierarchical linear modeling (HLM 5.04; Raudenbush, Bryk, Cheong, & Congdon, 2001) was used to investigate the main study questions. We used lagged analyses to test for 5-HTTLPR interacting with stressors assessed over time to predict prospective elevations in symptoms specifically. Symptoms (depressive or anxious) at time T served as the dependent variable in the HLM analysis and time T-1 symptoms were included in level 1 so that prospective increases in symptoms from one wave to the next were predicted after controlling for prior wave’s symptoms. To test whether 5-HTTLPR interacts with idiographic stressors, we centered ALEQ scores around each youth’s mean level over time (group-mean centering). This analysis tests the stress reactivity hypothesis that 5-HTTLPR moderates the longitudinal association between increases in stressors, relative to the individual’s average level, and prospective elevations in symptoms over time. In contrast, we tested the nomothetic approach by leaving stress scores uncentered. We controlled for age, race and sex in all analyses.
5-HTTLPR Interacts with Idiographic Stressors to Predict Prospective Elevations in Depressive Symptoms Specifically
We first examined potential gene-environment correlations (rGE). There was no significant association between 5-HTTLPR and stressors at any time point (r’s < .08, ns). However, high-risk 5-HTTLPR alleles, when analyzed in the biallelic manner with three groups (S/S, S/L, and L/L), interacted with greater idiographic stress exposure to predict prospective elevations in depressive symptoms over 1 year (top portion of Table 2). Following up this significant interaction to examine the effect of stressors on depressive for each genotype group showed that youth with two S copies exhibited the largest association between stressors and depressive symptoms (b = .15, SE = .03, t = 3.98, p < .001; r effect size = .43), followed by youth with S/L (b = .09, SE = .02, t = 3.34, p < .001; r effect size = .37), and last by homozygous L carriers (b = .05, SE = .03, t = 1.37, p =.17; r effect size = .16).
Table 2.
5-HTTLPR X stress interactions predicting prospective elevations of depressive symptoms over 1 year
| Predictor | b | SE | t | df | ES(r) | |
|---|---|---|---|---|---|---|
| IDIOGRAPHIC STRESS REACTIVITY
| ||||||
| Fixed Effects | ||||||
| Level 1 | ||||||
| CDI T-1 | .65 | .02 | 25.69*** | 1,219 | .87 | |
| Idiographic stress | .10 | .02 | 4.15*** | 1, 218 | .27 | |
| Level 2 | ||||||
| Race | −.66 | .85 | −.78 | 1,216 | .05 | |
| Sex | .38 | .23 | 1.62 | 1,216 | .11 | |
| SERT | .83 | .57 | 1.44 | 1,216 | .10 | |
| SERT x Idiographic stress | .08 | .02 | 3.79*** | 1,218 | .25 | |
| Variance components | ||||||
| Level 1 | Within person | 3.36 | ||||
| Level 2 | Initial Status | 8.67*** | ||||
| Stress slope | .004*** | |||||
| CDI T-1 | .068*** | |||||
|
| ||||||
| NOMOTHETIC STRESS REACTIVITY
| ||||||
| Fixed Effects | ||||||
| Level 1 | ||||||
| CDI T-1 | .61 | .03 | 22.68*** | 1,219 | .83 | |
| Nomothetic stress | .09 | .02 | 3.95*** | 1, 218 | .26 | |
| Level 2 | ||||||
| Race | −.34 | .35 | .98 | 1,216 | .06 | |
| Sex | .47 | .51 | .92 | 1,216 | .06 | |
| SERT | .30 | .36 | .82 | 1, 216 | .05 | |
| SERT x Nomothetic stress | .01 | .02 | .88 | 1, 218 | .05 | |
| Variance components | ||||||
| Level 1 | Within person | 3.33 | ||||
| Level 2 | Initial Status | 13.75*** | ||||
| Stress slope | .004*** | |||||
| CDI T-1 | .068*** | |||||
Note. CDI=Children’s Depression Inventory. CDI T-1= Prior wave CDI score. SERT=Serotonin transporter promoter polymorphism. ES (r) = effect size r (Rosnow & Rosenthal, 1991). Generally, ES (r) .10– .23 is viewed as small, moderate is .24–.36, and > .37 is large, respectively (Cohen 1988).
p < .05;
p < .01;
p < .001
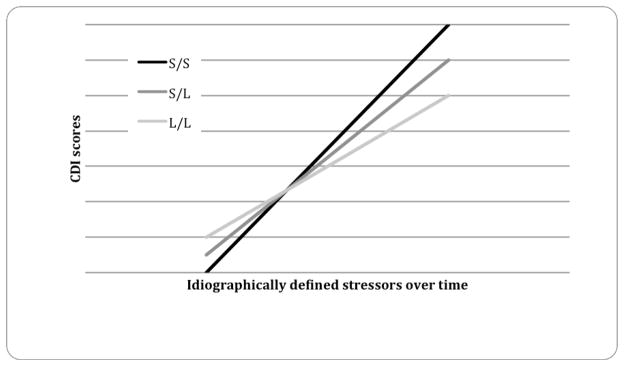
This GxE effect is shown in Figure 1 with stressors depicted at 1 SD above and below the mean for idiographic ALEQ scores. Youth with two short copies of 5-HTTLPR (S/S) who experienced more stressors over time, relative to their typical exposure to stressors, reported the greatest prospective increases in depressive symptoms. In contrast, when stressors were analyzed in a nomothetic manner, the GxE effect was not significant (bottom portion of Table 2). The main effect of nomothetic stressors predicted prospective elevations in depressive symptoms, consistent with Risch and colleagues’ meta-analysis (2009). Moreover, when 5-HTTLPR was genotyped using the triallelic genotyping with trichotomous grouping, similar results were observed as the GxE (b = .06, SE = .02, t = 2.86, p < .01, r effect size = .19) was significant in predicting prospective elevations in depressive symptoms. Finally, neither sex (b = .005, SE = .004, t = 1.36, p = .17, r effect size = .09) nor grade (b = .023, SE = .015, t = 1.54, p = .09, r effect size = .10) moderated the GxE effect.
Figure 1.
Interaction between 5-HTTLPR and idiographic stressors (combined parent and youth report) predicts prospective elevations in depressive symptoms (combined parent and youth report) over time.
Specificity of Effects
This specificity analysis examines the unique prediction of GxE for depressive versus anxious symptoms given their known co-occurrence. Similar lagged HLM analyses were conducted as above except that composite MASC anxiety scores served as the dependent variable. We entered MASC scores at T-1 and idiographic stressors at level 1, 5-HTTLPR at level 2, and the cross-level interaction of 5-HTTLPR x idiographic stressors. Results showed main effects for biallelic genotyping of 5-HTTLPR (b = 2.86, SE = 1.12, t = 2.38, p < .05, r effect size = .19), prior anxiety symptoms (b = 1.98, SE = .42, t = 4.75, p < .001, r effect size = .30), and idiographic stressors (b = .88, SE = .09, t = 9.96, p < .001, r effect size = .56), yet no significant interaction (b = .14, SE = .12, t = 1.17, p= .24, r effect size = .08). Likewise, no significant interaction was observed for the nomothetic stress approach (b = .14, SE = .18, t = .67, p = .51, r effect size = .04). Similar results were observed when triallelic genotyping trichotomous groups were analyzed. Such non-significant GxE for anxiety symptoms demonstrates specificity to depression.
Finally, a more stringent test of specificity and sensitivity is to examine whether 5-HTTLPR x idiographic stressors continues to predict prospective elevations in depressive symptoms over time after controlling for the concurrent co-occurrence of anxious symptoms. In this final analysis, we used the same HLM equation for 5-HTTLPR x idiographic stress predicting prospective elevations in depressive symptoms except that MASC scores at Time T were also included at level 1 to control for the overlap between anxious and depressive symptoms. The biallelic genotyped 5-HTTLPR x idiographic stress interaction still significantly predicted prospective elevations in depressive symptoms (b = .087, SE = .02, t = 4.14, p < .001, r effect size = .26), and the within person main effect of MASC was likewise significant (b = .045, SE = .008, t = 5.69, p < .001, r effect size = .36). The same pattern was seen with triallelic genotyping. In sum, youth carrying high-risk susceptibility 5-HTTLPR alleles who experienced greater idiographic stress exposure experienced increases in prospective elevations in depressive symptoms over 1 year even after accounting for the co-occurring influence of anxiety symptoms.
Discussion
Theoretical and empirical interest in GxE has surged since Caspi and colleagues’ seminal findings, yet there has been doubt concerning the replicability of GxE in depression since Risch et al.’s meta-analysis (2009). Also, the extent to which measured genes interact with stressors among youth to predict prospective elevations in depressive symptoms specifically has been unclear. Results from this study indicate that youth who carry at least one S allele of the variant of 5-HTTLPR exhibited higher prospective elevations in depressive symptoms specifically over a 1-year follow-up when they experienced more stressors relative to their own typical exposure to stress. This overall significant GxE effect represented a moderate effect size. This 5-HTTLPR x idiographic stressor interaction is consistent with a stress reactivity conceptualization of how 5-HTTLPR may enhance susceptibility to depression in concert with environmental risk (cf., Caspi et al., 2010). No GxE effect was observed when stressors were analyzed in a nomothetic manner. These findings were observed for both boys and girls as well as youth of all ages as neither sex nor grade moderated the GxE effect. Last, this GxE effect specifically predicted depressive, not anxious, symptoms. In sum, this study provides evidence for GxE effects in youth depression using a multi-wave longitudinal design with reliable, developmentally valid assessments of proximal stressors (Monroe & Reid, 2008).
An exciting and unique contribution of the present study is the use of a multi-wave design to enable an idiographic as well as a nomothetic approach (Abela & Hankin, 2008) to analyze stress reactivity and environmental risk in GxE. The significant GxE effect predicting prospective elevations in depressive symptoms was observed only when analyzed from an idiographic conceptualization, in which individuals’ increases or decreases in stressors over time were compared relative to their own average exposure to stress. We based our a priori prediction that GxE effects would hold when conceptualized and analyzed from an idiographic perspective due to consistent and robust results for stress reactivity demonstrated in cognitive vulnerability-stress research using an idiographic versus nomothetic approach (Abela & Hankin, 2008). This study’s findings suggest that 5-HTTLPR allelic variation may underlie individuals’ stress reactivity to increases in one’s typical exposure to stressors, as indicated by the significant genetic interaction with idiographic stressors. The present study used a multi-wave design in GxE depression research which provides a strong test of whether 5-HTTLPR conveys susceptibility to depression, in concert with idiographic stress exposure, via stress reactivity processes. This differentiation of environmental stress conceptualization and analysis (i.e., idiographic and nomothetic) offers another potential explanation for the lack of an overall significant GxE effect in the Risch et al. (2009) meta-analysis (see Caspi et al., 2010; Rutter et al., 2009; Uher & McGuffin, 2010, for other limitations), as stress reactivity has been conceptualized and analyzed in a purely nomothetical manner in all prior GxE research. Taking the present evidence that genetically susceptible (at least 5-HTTLPR) youth who are exposed to idiographic stressors exhibited the greatest elevations in depressive symptoms in concert with prior human (e.g., Gotlib et al., 2008; Hariri et al., 2002) and animal (e.g., Li et al., 1999) research, an increasing and coherent literature is building suggesting that 5-HTTLPR may confer risk to depression via stress reactivity processes (cf., Caspi et al., 2010).
A few prior studies have found a GxE predicting depressive symptoms specifically in females (e.g., Eley et al., 2004; Hammen et al., 2010), yet our data did not support sex moderation for the GxE effect. The precise reason for the inconsistencies across studies is unclear, although none of the previous studies finding sex moderation examined environmental stress from an idiographic conceptualization. In accordance with our findings, studies investigating the role of stress reactivity in relation to depression have found this effect across both males and females (Hankin, Badanes, Abela, & Watamura, 2010; Hariri et al., 2002, Li et al., 1999; Williams et al., 2009). Our evidence suggests a plausible psychobiological process for how the GxE effect in depression works in both males and females and moves beyond merely investigating GxE interactions with purely statistical techniques by examining GxE effects using psychological and biologically plausible approaches and conceptualizations (Rutter et al., 2009). Finally, grade cohort did not moderate the significant GxE effect in this sample. As the majority of youth GxE research has used adolescent samples and little research has investigated GxE in pre-pubertal children, the lack of moderation by grade cohort suggests that 5-HTTLPR may confer susceptibility to depressive symptoms when both children and adolescents experience idiographic stressors. Clearly, though, additional research with younger children is needed to replicate this finding.
Interestingly, when our data were analyzed in an idiographic manner, a slight cross-over interaction was observed (see Figure 1). These types of interactions have also been observed in previous GxE studies predicting depression (e.g. Caspi et al., 2003; Eley et al., 2004; Wilhelm et al., 2006). The implications of these findings have been extensively discussed in relation to Belsky’s (1997) differential-susceptibility hypothesis and Boyce and Ellis’s (2005) biological-sensitivity-to-context thesis (also see Belsky & Pluess, 2009a, 2009b; Ellis & Boyce, 2008). Both of these theoretical arguments emphasize the importance of examining how certain risk factors, such as the S allele of the 5-HTTLPR gene, could be a marker of environmental sensitivity in which those possessing one or both of these alleles would either thrive in an enriched environment or be especially vulnerable in an adverse environment (Belsky & Pluess, 2009a). As seen in Figure 1, our data demonstrate that the participants who carry either one or two copies of the S allele tend to have lower levels of depressive symptoms in the presence of low idiographic stress, as well as higher levels of depressive symptoms in the presence of high idiographic stress when compared to those homozygous for the long allele. Our findings lend support to both Belsky and Boyce and Ellis’s conceptualization of how S 5-HTTLPR alleles may be related to differential-susceptibility to environment as opposed to the conceptualization that possessing an S allele confers only risk or vulnerability.
Strengths and Limitations
Several features of the present study are unique and provide a more methodologically and conceptually rigorous test of hypotheses that enhance confidence in the results and advance knowledge of GxE in youth depression. The study included a multi-wave, multi-informant design with a community-based sample, which increased statistical power and enabled us to evaluate both idiographic and nomothetic approaches to measuring stress. Additionally, we combined child and parent reports of youths’ anxious and depressive symptoms and stressors to reduce reliance on a single source. We used a general community sample of youth, as opposed to a clinical sample with biases that are known to reduce generalizability and accurate statistical tests (Cohen & Cohen, 1984).
Next, we examined GxE specificity to depression versus anxiety given the strong co-occurrence of these internalizing symptoms and found unique prediction to depression. This finding provides some important information about specific etiological processes predicting depression versus anxiety. Of note, our findings supported a main effect of the 5-HTTLPR gene for anxiety symptoms. Meta-analyses have not found support for the 5-HTTLPR polymorphism predicting certain subtypes of anxiety in adults, such as harm avoidance, but have found a main effect predicting neuroticism (Munafo et al., 2008; Schinka, Busch, & Robichauz-Keene, 2004). Indeed, research examining main effects of 5-HTTLPR on anxiety has been relatively rare (Gonda et al., 2009), and finding this direct association, especially with youth, is novel.
Last, we used both biallelic and triallelic method for genotyping 5-HTTLPR (Hu et al., 2005, 2006). GxE effects were found for both approaches. We used relatively reliable, valid, and developmentally appropriate assessments of negative life events, as reported by both youth and parent, to capture stress exposure as environmental risk to test GxE more accurately.
Still, study limitations provide avenues for future research. First, the sample size was relatively modest, although significant GxE was observed when the appropriate characterization of environmental stress (idiographic) was used. Also mitigating the concern of sample size, reviewers of the GxE literature (e.g., Uher & McGuffin, 2008, 2010) suggest minimum sample sizes, based on power considerations (e.g., Luan, Wong, Day, & Wareham, 2001), and the present study exceeded minimum thresholds, at least those recommended for cross-sectional GxE research. Still, there is the possibility that the present findings may represent chance effects, and replication of these effects are needed with larger samples that assess stressors, preferably with reliable and valid methods, repeatedly over time. Alternatively, it may be that relatively modest instead of significantly more sizeable sample sizes are sufficient to detect GxE, especially when investigated with a multi-wave design, which increases statistical power (Snijders & Bosker, 1999); accurate measurement of genes and environment (Monroe & Reid, 2008), which reduce measurement error; and a construct-validity, theory-driven approach (Caspi et al., 2010). Indeed, prior work has demonstrated that inaccurate assessments decrease statistical power to such a substantial degree that smaller samples with accurate measurement may be preferable (Wong, Day, Luan, Chan, & Wareham, 2003).
Second, the significant idiographic GxE predicted prospective elevations in depressive symptoms, but we did not assess clinical depression. Most research suggests that depression can be represented and conceptualized best as a dimensional continuum, rather than discrete category (Hankin, Fraley, Lahey & Waldman, 2005). Also, subclinical depressive symptoms are important to study in their own right as they represent more than mere “moodiness” and predict diagnosable disorder (Klein, Shankman, Lewinsohn, & Seeley, 2009; Pine, Cohen, Cohen, & Brook, 1999) and other psychosocial impairments (Gotlib et al., 1995). The percentage of youth with depressive symptoms scores above normative cutoffs was within the range expected in unselected community samples of youth of these ages (Cole et al., 1998; Petersen et al., 1993). Thus, although the current sample reported relatively low levels of depressive symptoms, understanding GxE effects predicting elevations in subclinical depressive symptoms over time, especially among youth, is an important area of research. Nevertheless, diagnostic interviews to ascertain clinical depression across multiple assessments can address potential concerns about continuity of findings to depressive disorder. Additionally, the majority of our sample was comprised of younger youth (72% in the 3rd or 6th grade), and research has demonstrated that significant increases in depression generally occur after age 13 (Hankin et al., 1998). Although we controlled for age in our analyses and it can be considered a strength that our sample was mostly children and early adolescents, future research is still needed to investigate possible developmental influences in GxE processes over time and whether static genetic polymorphisms interact with increases in stressors during adolescence to predict the surge in depression in middle to late adolescence.
Third, some have criticized self-report of stressors and argued that questionnaire assessments may not be as rigorous or accurate compared with contextual stress interviews (Monroe & Reid, 2008). The stress measure used in this study has shown good reliability and validity in prior research (a,e.g., Hankin, 2008b; Hankin et al., 2010), including significant and moderate correlations with a contextual stress interview. Also, the stress measure uses very specific language with precise anchors and examples to define stressors as recommended to more accurately assess negative events (Brewin et al., 1993; Dohrenwend, 2006). We assessed stressor occurrence with multiple informants (parent and youth) to reduce subjectivity unreliability and overreporting of trivial events. These methodological features match those used by Caspi and colleagues (2003). Still, the use of a self-reported measure of negative life events is a limitation of the study. Future GxE research examining stress would benefit from use of contextual stress interviews, as seen in research by Hammen and colleagues (2010), and doing so in a multi-wave design.
Finally, we analyzed DNA sequence variation of one specific allelic variation in 5-HTTLPR. The environment has a significant impact on gene expression and outcomes through epigenetic processes (Tsankova, Renthal, Kumar, & Nestler, 2007). Incorporating epigenetics (Mill & Petronis, 2007) and static DNA sequence variation of other genes in addition to 5-HTTLPR is a clear priority for future research. For example, Meaney and colleagues’ research with mice (Weaver et al., 2004) and human post-mortem brain tissue (McGowan et al., 2009) reveals epigenetic influences of the glucocorticoid receptor in response to environmental influences. Similar epigenetic effects accounting for GxE is likely in the development of psychopathology (Mill & Petronis, 2007; Tsankova et al., 2007).
Implications for Research, Policy, and Practice
Understanding what risk factors and processes predict increases in depressive symptoms among youth is clearly important for policy and practice. Elevated depressive symptoms are among the most potent predictors of later onset of clinical depression (Klein et al., 2009). Youth with subthreshold depression represent important targets for selective interventions to forestall onset of major depression and subsequent interpersonal and academic difficulties associated with elevated depression (Gotlib et al., 1995). Most recently, Garber and colleagues (2009) demonstrated that onset of major depression could be significantly prevented and delayed among high-risk youth, as defined by elevated, subthreshold levels of depression and/or parents with current or past depression. As the present study showed that genetic risk, specifically 5-HTTLPR, interacts with idiographic increases in stressors to prospectively predict elevations in depressive symptoms, future preventive research may benefit by incorporating an integrated genetic perspective to further understand how and for whom selective interventions and prevention efforts work to reduce depression onset (cf., Uher, 2008).
In sum, this study showed that youth who experience more idiographic stressors over time are most likely to exhibit prospective increases in depressive symptoms specifically, especially when those youth are at measured genetic risk. These data suggest that 5-HTTLPR may confer susceptibility to depression through the process of stress reactivity in that youth carrying at least one copy of the 5-HTTLPR lower expressing variant may be more susceptible to react to their environment (Belsky, Bakermans-Kranenburg, & van IJzendoorn2007; Caspi et al., 2010). When that environment is characterized by relatively greater exposure to stressful negative life events, youth with 5-HTTLPR susceptibility are more likely to experience increases in depressive symptoms specifically over time.
Acknowledgments
This work was supported by NIMH grant 5R01 MH077195 (Hankin and Abela). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or National Institutes of Health.
Contributor Information
Benjamin L. Hankin, Department of Psychology, University of Denver
Jessica Jenness, Department of Psychology, University of Denver.
John R.Z. Abela, Department of Psychology, Rutgers University
Andrew Smolen, Institute for Behavioral Genetics, University of Colorado-Boulder.
References
- Abela JRZ, Hankin BL. Cognitive vulnerability to depression in children and adolescents: A developmental psychopathology perspective. In: Abela JRZ, Hankin BL, editors. Handbook of child and adolescent depression. New York: The Guilford Press; 2008. pp. 35–78. [Google Scholar]
- Abela JRZ, Skitch SA, Adams P, Hankin BL. The timing of parent and child depression: A hopelessness theory perspective. Journal of Clinical Child and Adolescent Psychology. 2006;35:253–263. doi: 10.1207/s15374424jccp3502_9. [DOI] [PubMed] [Google Scholar]
- Abela JRZ, Webb CA, Ho M, Wagner C, Adams P. The role of self-criticism, dependency, and hassles in the course of depressive illness: A multi-wave longitudinal study of vulnerability and resiliency. Personality and Social Psychology Bulletin. 2006;32:328–338. doi: 10.1177/0146167205280911. [DOI] [PubMed] [Google Scholar]
- Abela JRZ, Zuroff DC, Ho MR, Adams P, Hankin BL. Excessive reassurance seeking, hassles, and depressive symptoms in children of affectively-ill parents: A multi-wave longitudinal study. Journal of Abnormal Child Psychology. 2006;34:171–187. doi: 10.1007/s10802-005-9011-x. [DOI] [PubMed] [Google Scholar]
- Abramson L, Metalsky G, Alloy L. Hopelessness depression: A theory-based subtype of depression. Psychological Review. 1989;96(2):358–372. [Google Scholar]
- Anchordoquy HC, McGeary C, Liu L, Krauter KS, Smolen A. Genotyping of three candidate genes following whole genome preamplification of DNA collected from buccal cells. Behavior Genetics. 2003;33:73–78. doi: 10.1023/a:1021007701808. [DOI] [PubMed] [Google Scholar]
- Baldwin J, Dadds M. Reliability and Validity of Parent and Child Versions of the Multidimensional Anxiety Scale for Children in Community Samples. Journal of the American Academy of Child &Adolescent Psychiatry. 2007;46(2):252–260. doi: 10.1097/01.chi.0000246065.93200.a1. [DOI] [PubMed] [Google Scholar]
- Beck A. Cognitive models of depression. Journal of Cognitive Psychotherapy. 1987;1(1):5–37. [Google Scholar]
- Beesdo K, Bittner A, Pine D, Stein M, Hofler M, Lieb R, et al. Incidence of social anxiety disorder and the consistent risk for secondary depression in the first three decades of life. Archives of General Psychiatry. 2007;64(8):903–912. doi: 10.1001/archpsyc.64.8.903. [DOI] [PubMed] [Google Scholar]
- Belsky J. Variation in susceptibility to environmental influence: An evolutionary argument. Psychological Inquiry. 1997;8(3):182–186. [Google Scholar]
- Belsky J, Bakermans-Kranenburg M, van IJzendoorn M. For better and for worse: Differential susceptibility to environmental influences. Current Directions in Psychological Science. 2007;16(6):300–304. [Google Scholar]
- Belsky J, Pluess M. Beyond diathesis stress: Differential susceptibility to environmental influences. Psychological Bulletin. 2009a;135(6):885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Belsky J, Pluess M. The nature (and nurture?) of plasticity in early human development. Perspectives on Psychological Science. 2009b;4(4):345–351. doi: 10.1111/j.1745-6924.2009.01136.x. [DOI] [PubMed] [Google Scholar]
- Boyce W, Ellis B. Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Development and Psychopathology. 2005;17(2):271–301. doi: 10.1017/s0954579405050145. [DOI] [PubMed] [Google Scholar]
- Brewin CR, Andrews B, Gotlib IH. Psychopathology and early experience: A reappraisal of retrospective reports. Psychological Bulletin. 1993;113:82–98. doi: 10.1037/0033-2909.113.1.82. [DOI] [PubMed] [Google Scholar]
- Brown G, Harris T. Depression and the serotonin transporter 5-HTTLPR polymorphism: A review and a hypothesis concerning gene-environment interaction. Journal of Affective Disorders. 2008;111(1):1–12. doi: 10.1016/j.jad.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Canli T, Lesch KP. Long story short: The serotonin transporter in emotion regulation and social cognition. Nature Neuroscience Review. 2007;10:1103–1109. doi: 10.1038/nn1964. [DOI] [PubMed] [Google Scholar]
- Caspi A, Ahmad R, Holmes A, Uher R, Moffitt TE. Genetic Sensitivity to the Environment: The case of the serotonin transporter gene and its implications for studying complex diseases and traits. American Journal of Psychiatry. 2010 doi: 10.1176/appi.ajp.2010.09101452. published March 15, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTTLPR gene. Science. 2003;301(5631):386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Chavira DA, Stein MB, Bailey K, Stein MT. Comorbidity of generalized social anxiety disorder and depression in a pediatric primary care sample. Journal of Affective Disorders. 2004;80:163–171. doi: 10.1016/S0165-0327(03)00103-4. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA, Sturge-Apple ML. Interactions of child maltreatment and serotonin transporter and monoamine oxidase A polymorphisms: Depressive symptomatology among adolescents from low socioeconomic status backgrounds. Development and Psychopathology. 2007;19:1161–1180. doi: 10.1017/S0954579407000600. [DOI] [PubMed] [Google Scholar]
- Cohen P, Cohen J. The clinician’s illusion. Archives of General Psychiatry. 1984;41:1178–1182. doi: 10.1001/archpsyc.1984.01790230064010. [DOI] [PubMed] [Google Scholar]
- Cole DA, Peeke LG, Martin JM, Truglio R, Seroczynski AD. Longitudinal look at the relation between depression and anxiety in children and adolescents. Journal of Consulting and Clinical Psychology. 1998;66:41–460. doi: 10.1037//0022-006x.66.3.451. [DOI] [PubMed] [Google Scholar]
- Costello JE, Mustillo S, Erkanli A, Keeler G, Angold A. Prevalence and development of psychiatric disorders in childhood and adolescence. Archives of General Psychiatry. 2003;60(8):837–844. doi: 10.1001/archpsyc.60.8.837. [DOI] [PubMed] [Google Scholar]
- Curran PJ, Willoughby MT. Implications of latent trajectory models for the study of developmental psychopathology. Development and Psychopathology. 2003;15:581–612. doi: 10.1017/s0954579403000300. [DOI] [PubMed] [Google Scholar]
- Dohrenwend BP. Inventorying stressful life events as risk factors for psychopathology: Toward resolution of the problem of intracategory variability. Psychological Bulletin. 2006;132:477–495. doi: 10.1037/0033-2909.132.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eley TC, Sugden K, Corsico A, Gregory AM, Sham P, McGuffin P, et al. Gene-environment interaction analysis of serotonin system markers with adolescent depression. Molecular Psychiatry. 2004;9:908–915. doi: 10.1038/sj.mp.4001546. [DOI] [PubMed] [Google Scholar]
- Ellis B, Boyce W. Biological sensitivity to context. Current Directions in Psychological Science. 2008;17(3):183–187. [Google Scholar]
- Garber J, Clarke GN, Weersing VR, Beardslee WR, Brent DA, Gladstone TRG, et al. Prevention of depression in at-risk adolescents: A randomized controlled trial. Journal of American Medical Association. 2009;301(21):2215–2224. doi: 10.1001/jama.2009.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb B, Beevers C, Andover M, Holleran K. The Hopelessness Theory of Depression: A Prospective Multi-Wave Test of the Vulnerability-Stress Hypothesis. Cognitive Therapy and Research. 2006;30(6):763–772. [Google Scholar]
- Gibb B, Benas J, Grassia M, McGeary J. Children’s attentional biases and 5-HTTLPR genotype: Potential mechanisms linking mother and child depression. Journal of Clinical Child and Adolescent Psychology. 2009;38(3):415–426. doi: 10.1080/15374410902851705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb B, Uhrlass D, Grassia M, Benas J, McGeary J. Children’s inferential styles, 5-HTTLPR genotype, and maternal expressed emotion-criticism: An integrated model for the intergenerational transmission of depression. 2009 doi: 10.1037/a0016765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie N, Whitfield J, Williams B, Heath A, Martin N. The relationship between stressful life events, the serotonin transporter (5-HTTLPR) genotype and major depression. Psychological Medicine: A Journal of Research in Psychiatry and the Allied Sciences. 2005;35(1):101–111. doi: 10.1017/s0033291704002727. [DOI] [PubMed] [Google Scholar]
- Gonda X, Fountoulakis KN, Juhasz G, Rihmer Z, Lazary J, Laszik A, et al. Association of the s allele of the 5-HTTLPR with neuroticism-related traits and temperaments in a psychiatrically healthy population. European Archives of Psychiatry and Clinical Neuroscience. 2009;259:106–113. doi: 10.1007/s00406-008-0842-7. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Joormann J, Minor KL, Hallmayer J. HPA axis reactivity: A mechanism underlying the associations among 5-HTTLPR, stress, and depression. Biological Psychiatry. 2008;63:847–851. doi: 10.1016/j.biopsych.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, Lewinsohn PM, Seeley JR. Symptoms versus a diagnosis of depression: Differences in psychosocial functioning. Journal of Consulting and Clinical Psychology. 1995;63:90–100. doi: 10.1037//0022-006x.63.1.90. [DOI] [PubMed] [Google Scholar]
- Grabe H, Lange M, Wolff B, Völzke H, Lucht M, Freyberger H, et al. Mental and physical distress is modulated by a polymorphism in the 5-HT transporter gene interacting with social stressors and chronic disease burden. Molecular Psychiatry. 2005;10(2):220–224. doi: 10.1038/sj.mp.4001555. [DOI] [PubMed] [Google Scholar]
- Gunthert K, Conner T, Armeli S, Tennen H, Covault J, Kranzler H. Serotonin transporter gene polymorphism (5-HTTLPR) and anxiety reactivity in daily life: A daily process approach to gene-environment interaction. Psychosomatic Medicine. 2007;69(8):762–768. doi: 10.1097/PSY.0b013e318157ad42. [DOI] [PubMed] [Google Scholar]
- Hammen C, Brennan P, Keenan-Miller D, Hazel N, Najman J. Chronic and acute stress, gender, and serotonin transporter gene environment interactions predicting depression symptoms in youth. Journal of Child Psychology and Psychiatry. 2010;51(2):180–187. doi: 10.1111/j.1469-7610.2009.02177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL. Cognitive vulnerability-stress model of depression during adolescence: Investigating symptom specificity in a multi-wave prospective study. Journal of Abnormal Child Psychology. 2008;36:999–1014. doi: 10.1007/s10802-008-9228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL. Cognitive vulnerability-stress model of depression during adolescence: Investigating symptom specificity in a multi-wave prospective study. Journal of Abnormal Child Psychology. 2008b;36:999–1014. doi: 10.1007/s10802-008-9228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, Abramson LY. Development of gender differences in depression: An elaborated cognitive vulnerability-transactional stress theory. Psychological Bulletin. 2001;127:773–796. doi: 10.1037/0033-2909.127.6.773. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Abramson LY. Measuring cognitive vulnerability to depression in adolescence: Reliability, validity and gender differences. Journal of Clinical Child and Adolescent Psychology. 2002;31:491–504. doi: 10.1207/S15374424JCCP3104_8. [DOI] [PubMed] [Google Scholar]
- Hankin B, Badanes L, Abela J, Watamura S. Hypothalamic pituitary adrenal axis dysregulation in dysphoric children and adolescents: Cortisol reactivity to psychosocial stress from preschool through middle adolescence. Biological Psychiatry. 2010;68(5):484–490. doi: 10.1016/j.biopsych.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, Fraley RC, Lahey BB, Waldman I. Is youth depressive disorder best viewed as a continuum or discrete category? A taxometric analysis of childhood and adolescent depression in a population-based sample. Journal of Abnormal Psychology. 2005;114:96–110. doi: 10.1037/0021-843X.114.1.96. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Stone LB, Wright PA. Co-rumination, interpersonal stress generation, and internalizing symptoms: Sex differences and transactional influences in a multi-wave study of adolescents. Development and Psychopathology. 2010;22:217–235. doi: 10.1017/S0954579409990368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri A, Mattay V, Tessitore A, Kolachana B, Fera F, Goldman D, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297(5580):400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Hu XY, Lipsky RH, Zhu G, Akhtar LA, Taubman J, Greenberg BD, et al. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. American Journal of Human Genetics. 2006;78:815–826. doi: 10.1086/503850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Oroszi G, Chun J, Smith TL, Goldman D, Schuckit MA. An expanded evaluation of the relationship of four alleles to the level of response to alcohol and the alcoholism risk. Alcohol: Clinical and Experimental Research. 2005;29:8–16. doi: 10.1097/01.alc.0000150008.68473.62. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Yang B, Douglas-Palumberi H, Grasso D, Lipshitz D, Houshyar S, et al. Brain-derived neurotrophic factor 5-HHTLPR gene interactions and environmental modifiers of depression in children. Biological Psychiatry. 2006;59:673–680. doi: 10.1016/j.biopsych.2005.10.026. [DOI] [PubMed] [Google Scholar]
- Kazdin AE. Identifying depression in children: A comparison of alternative selection criteria. Journal of Abnormal Child Psychology. 1989;17:437–454. doi: 10.1007/BF00915037. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Kuhn JW, Vittum J, Prescott CA, Riley B. The interaction of stressful life events and a serotonin transporter polymorphism in the prediction of episodes of major depression. Archives of General Psychiatry. 2005;62:529–535. doi: 10.1001/archpsyc.62.5.529. [DOI] [PubMed] [Google Scholar]
- Klein DN, Dougherty LR, Olino TM. Toward guidelines for evidence-based assessment of depression in children and adolescents. Journal of Clinical Child and Adolescent Psychology. 2005;34:412–432. doi: 10.1207/s15374424jccp3403_3. [DOI] [PubMed] [Google Scholar]
- Klein DN, Shankman SA, Lewinsohn PM, Seeley JR. Subthreshold depressive disorder in adolescents: Predictors of escalation to full-syndrome depressive disorders. J Am Acad Child Adolesc Psychiatry. 2009;48:703–710. doi: 10.1097/CHI.0b013e3181a56606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs M. Children’s depression inventory manual. New York: Multi-Health Systems, Inc; 1992. [Google Scholar]
- Kovacs M. The Children’s Depression Inventory (CDI) Psychomarmachology Bulletin. 1985;21:995–998. [PubMed] [Google Scholar]
- Laucht M, Treutlein J, Blomeyer D, Buchmann A, Schmid B, Becker K, et al. Interaction between the 5-HTTLPR serotonin transporter polymorphism and environmental adversity for mood and anxiety psychopathology: Evidence from a high-risk community sample of young adults. International Journal of Neuropsychopharmacology. 2009;12(6):737–747. doi: 10.1017/S1461145708009875. [DOI] [PubMed] [Google Scholar]
- Lesch K. Neuroticism and serotonin: A developmental genetic perspective. In: Plomin R, DeFries JC, Craig IW, McGuffin P, Plomin R, DeFries JC, McGuffin P, editors. Behavioral genetics in the postgenomic era. 2003. pp. 389–423. [Google Scholar]
- Lesch K, Bengel D, Heils A, Sabol S, Greenberg BD, Petri S, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274(5292):1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Levinson D. The Genetics of Depression: A Review. Biological Psychiatry. 2006;60(2):84–92. doi: 10.1016/j.biopsych.2005.08.024. [DOI] [PubMed] [Google Scholar]
- Lewinsohn P, Hops H, Roberts R, Seeley J, Andrews J. Adolescent psychopathology: I. Prevalence and incidence of depression and other DSM-III R disorders in high school students. Journal of Abnormal Psychology. 1993;102(1):133–144. doi: 10.1037//0021-843x.102.1.133. [DOI] [PubMed] [Google Scholar]
- Lewinsohn P, Zinbarg R, Seeley J, Lewinsohn M, Sack W. Lifetime comorbidity among anxiety disorders and between anxiety disorders and other mental disorders in adolescents. Journal of Anxiety Disorders. 1997;11(4):377–394. doi: 10.1016/s0887-6185(97)00017-0. [DOI] [PubMed] [Google Scholar]
- Li Q, Wichems C, Heils A, Van D Dar LD, Lesch KP, Murphy DL. Reduction of 5-HT1a binding sites in 5-HT transporter knockout mice. Journal of Pharmacology and Experimental Therapeutics. 1999;291:999–1007. [PubMed] [Google Scholar]
- Luan JA, Wong MY, Day NE, Wareham NJ. Sample size determination of gene-environment interaction. International Journal of Epidemiology. 2001;30:1035–1040. doi: 10.1093/ije/30.5.1035. [DOI] [PubMed] [Google Scholar]
- March JS, Sullivan K, Parker J. Test-retest reliability of the Multidimensional Anxiety Scale for Children. Journal of Anxiety Disorders. 1999;13:349–358. doi: 10.1016/s0887-6185(99)00009-2. [DOI] [PubMed] [Google Scholar]
- Martin J, Cleak J, Willis-Owen SAG, Flint J, Shifman S. Mapping regulatory variants for the serotonin transporter gene based on allelic expression imbalance. Molecular Psychiatry. 2007;12:421–422. doi: 10.1038/sj.mp.4001952. [DOI] [PubMed] [Google Scholar]
- McGowan PO, Sasaki A, D’Alessio AC, Dymov S, Labonté B, et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nature Neuroscience. 2009;12:342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mill J, Petronis A. Molecular studies of major depressive disorder: The epigenetic perspective. Molecular Psychiatry. 2007;12:799–814. doi: 10.1038/sj.mp.4001992. [DOI] [PubMed] [Google Scholar]
- Moffitt TE, Caspi A, Rutter M. Measured gene-environment interactions in psychopathology. Perspective on Psychological Science. 2006;1:5–27. doi: 10.1111/j.1745-6916.2006.00002.x. [DOI] [PubMed] [Google Scholar]
- Monroe SM, Reid MW. Gene-environment interactions in depression research: Genetic polymorphisms and life-stress polyprocedures. Psychological Science. 2008;19:947–956. doi: 10.1111/j.1467-9280.2008.02181.x. [DOI] [PubMed] [Google Scholar]
- Munafo MR, Freimer NB, Ng W, Ophoff R, Veijola J, Miettunen J, et al. 5-HTTLPR genotype and anxiety-related personality traits: a meta-analysis and new data. American Journal of Medical Genetics Part B, Neuropsychiatric Genetics. 2008 doi: 10.1002/ajmg.b.30808. Published online 10 June 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobile M, Rusconi M, Bellina M, Marino C, Giorda R, Carlet O, Vanzin L, Molteni M, Battaglia M. The influence of family structure, the TPH2 G-703T and the 5-HTTLPR serotonergic genes upon affective problems in children aged 10–14 years. Journal of Child Psychology and Psychiatry. 2009;50:317–325. doi: 10.1111/j.1469-7610.2008.01958.x. [DOI] [PubMed] [Google Scholar]
- Olsson CA, Byrnes GB, Lotfi-Miri M, Collins V, Williamson R, Patton C, Anney RJ. Association between 5-HTTLPR genotypes and persisting patterns of anxiety and alcohol use: results from a 10-year longitudinal study of adolescent mental health. Molecular Psychiatry. 2005;10:868–876. doi: 10.1038/sj.mp.4001677. [DOI] [PubMed] [Google Scholar]
- Petersen A, Compas B, Brooks-Gunn J, Stemmler M, Ey S, Grant K. Depression in adolescence. American Psychologist. 1993;48(2):155–168. doi: 10.1037//0003-066x.48.2.155. [DOI] [PubMed] [Google Scholar]
- Pine D, Cohen E, Cohen P, Brook J. Adolescent depressive symptoms as predictors of adult depression: Moodiness or mood disorder? The American Journal of Psychiatry. 1999;156(1):133–135. doi: 10.1176/ajp.156.1.133. [DOI] [PubMed] [Google Scholar]
- Pine DS, Cohen P, Gurley D, Brook J, Ma Y. The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Archives of General Psychiatry. 1998;55:56–64. doi: 10.1001/archpsyc.55.1.56. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk A, Cheong YF, Congdon R. Hierarchical linear and nonlinear modeling 5.04. Lincolnwood, IL: Scientific Software International; 2001. [Google Scholar]
- Risch N, Herrell R, Lehner T, Liang KY, Eaves L, Hoh J, et al. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: A meta-analysis. Journal of the American Medical Association. 2009;301:2462–2471. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph KD, Flynn M. Childhood adversity and youth depression: The role of gender and pubertal status. Development and Psychopathology. 2007;19:497–521. doi: 10.1017/S0954579407070241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M. Environmentally mediated risks for psychopathology: Research strategies and findings. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44:3–18. doi: 10.1097/01.chi.0000145374.45992.c9. [DOI] [PubMed] [Google Scholar]
- Rutter M, Moffitt TE, Caspi A. Gene-environment interplay and psychopathology: Multiple varieties but real effects. Journal of Child Psychology and Psychiatry. 2006;47:226–261. doi: 10.1111/j.1469-7610.2005.01557.x. [DOI] [PubMed] [Google Scholar]
- Rutter M, Thapar A, Pickles A. Gene-environment interactions: Biologically valid pathway or artifact? Archives of General Psychiatry. 2009;66:1287–1289. doi: 10.1001/archgenpsychiatry.2009.167. [DOI] [PubMed] [Google Scholar]
- Schinka J, Busch R, Robichaux-Keene N. A meta-analysis of the association between the serotonin transporter gene polymorphism (5-HTTLPR) and trait anxiety. Molecular Psychiatry. 2004;9(2):197–202. doi: 10.1038/sj.mp.4001405. [DOI] [PubMed] [Google Scholar]
- Silberg J, Pickles A, Rutter M, Hewitt J, Simonoff E, Maes H, et al. The influence of genetic factors and life stress on depression among adolescent girls. Archives of General Psychiatry. 1999;56:225–232. doi: 10.1001/archpsyc.56.3.225. [DOI] [PubMed] [Google Scholar]
- Silverman WK, Ollendick TH. Evidence-Based Assessment of Anxiety and Its Disorders in Children and Adolescents. Journal of Clinical Child and Adolescent Psychology. 2005;34:380–411. doi: 10.1207/s15374424jccp3403_2. [DOI] [PubMed] [Google Scholar]
- Smucker MR, Craighead WE, Craighead LW, Green BJ. Normative and reliability data for the Children’s Depression Inventory. Journal of Abnormal Child Psychology. 1986;14:25–40. doi: 10.1007/BF00917219. [DOI] [PubMed] [Google Scholar]
- Snijders TAB, Bosker RJ. Multilevel analysis: An introduction to basic and advanced multilevel modeling. London: Sage; 1999. [Google Scholar]
- Stein M, Schork N, Gelernter J. Gene-by-environment (serotonin transporter and childhood maltreatment) interaction for anxiety sensitivity, an intermediate phenotype for anxiety disorders. Neuropsychopharmacology. 2008;33(2):312–319. doi: 10.1038/sj.npp.1301422. [DOI] [PubMed] [Google Scholar]
- Stone L, Gibb B, Coles M. Does the hopelessness theory account for sex differences in depressive symptoms among young adults? Cognitive Therapy and Research. 2010;34(2):177–187. doi: 10.1007/s10608-009-9241-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland PL, Deakin JFW, Percival C, Dixon J, Gater RA, Goldberg DP. Bio-social origins of depression in the community: Interactions between social adversity, cortisol and serotonin neurotransmission. British Journal of Psychiatry. 2002;180:168–173. doi: 10.1192/bjp.180.2.168. [DOI] [PubMed] [Google Scholar]
- Taylor S, Way B, Welch W, Hilmert C, Lehman B, Eisenberger N. Early Family Environment, Current Adversity, the Serotonin Transporter Promoter Polymorphism, and Depressive Symptomatology. Biological Psychiatry. 2006;60(7):671–676. doi: 10.1016/j.biopsych.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Tolonen H. Standardization and quality Control. National Public Health Institute; Helsinki: 2005. Towards the high quality of population health surveys; p. A27/2005. Available from: http://www.ktl.fi/attachments/suomi/julkaisut/julkaisusarja_a/2005/2005a27.pdf. [Google Scholar]
- Tolonen H, Dobson A, Kulathinal S. Effect on trend estimates of the difference between survey respondents and non-respondents: Results from 27 populations in the WHO MONICA Project. European Journal of Epidemiology. 2005;20:887–98. doi: 10.1007/s10654-005-2672-5. [DOI] [PubMed] [Google Scholar]
- Tsankova B, Renthal W, Kumar A, Nestler EJ. Epigenetic regulation in psychiatric disorders. Nature Reviews Neuroscience. 2007;8:355–367. doi: 10.1038/nrn2132. [DOI] [PubMed] [Google Scholar]
- Uher R. The implications of gene-environment interactions in depression: Will cause inform cure? Molecular Psychiatry. 2008;13:1070–1078. doi: 10.1038/mp.2008.92. [DOI] [PubMed] [Google Scholar]
- Uher R, McGuffin P. The moderation by the serotonin transporter gene of environmental adversity in the aetiology of mental illness: Review and methodological analysis. Molecular Psychiatry. 2008;13:131–146. doi: 10.1038/sj.mp.4002067. [DOI] [PubMed] [Google Scholar]
- Uher R, McGuffin P. The moderation by the serotonin transporter gene of environmental adversity in the aetiology of depression: 2009 update. Molecular Psychiatry. 2010 doi: 10.1038/mp.2009.123. [DOI] [PubMed] [Google Scholar]
- Weaver I, Cervoni N, Champagne F, D’Alessio A, Sharma S, Seckl J, et al. Epigenetic programming by maternal behavior. Nature Neuroscience. 2004;7(8):847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Wendland JR, Martin BJ, Kruse MR, Lesch KP, Murphy DL. Simultaneous genotyping of four functional loci of human SLC6A4, with a reappraisal of 5-HTTLPR and rs25531. Molecular Psychiatry. 2006;11:224–226. doi: 10.1038/sj.mp.4001789. [DOI] [PubMed] [Google Scholar]
- Wilhelm K, Mitchell P, Niven H, Finch A, Wedgwood L, Scimone A, et al. Life events, first depression onset and the serotonin transporter gene. British Journal of Psychiatry. 2006;188(3):210–215. doi: 10.1192/bjp.bp.105.009522. [DOI] [PubMed] [Google Scholar]
- Williams LM, Gatt JM, Schofield PR, Olivieri G, Peduto A, Gordon E. “Negativity bias” in risk for depression and anxiety: brain-body fear circuitry correlates, 5-HTT-LPR and early life stress. Neuroimage. 2009;47:804–814. doi: 10.1016/j.neuroimage.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Wong MY, Day NE, Luan JA, Chan KP, Wareham NJ. The detection of gene-environment interaction for continuous traits: Should we deal with measurement error by bigger studies or better measurement? International Journal of Epidemiology. 2003;32:51–57. doi: 10.1093/ije/dyg002. [DOI] [PubMed] [Google Scholar]
- Xie P, Kranzler H, Poling J, Stein M, Anton R, Brady K, et al. Interactive effect of stressful life events and the serotonin transporter 5-HTTLPR genotype on posttraumatic stress disorder diagnosis in 2 independent populations. Archives of General Psychiatry. 2009;66(11):1201–1209. doi: 10.1001/archgenpsychiatry.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalsman G, Huang Y, Oquendo M, Burke A, Hu X, Brent D, et al. Association of a Triallelic Serotonin Transporter Gene Promoter Region (5-HTTLPR) Polymorphism With Stressful Life Events and Severity of Depression. The American Journal of Psychiatry. 2006;163(9):1588–1593. doi: 10.1176/ajp.2006.163.9.1588. [DOI] [PubMed] [Google Scholar]