Abstract
The mammalian target of rapamycin (mTOR) is a downstream effector of the PI3-K/Akt/mTOR pathway. Allosteric inhibitors of mTOR, everolimus and temsirolimus, have shown promising clinical activity in advanced renal cell carcinoma but their effect is far from durable and only a subset of patients experience substantial benefit from these agents. The PI3-K/Akt/mTOR pathway represents an intricate network of fine regulation and feedback loops, and resistance to allosteric mTOR inhibitors may be embedded within this complexity. In this article we highlight the molecular elements of the PI3-K/Akt/mTOR pathway, the clinical experience with everolimus and temsirolimus in advanced renal cell carcinoma, and the future directions in terms of sequential therapy, combinational therapy and development of novel therapeutic agents.
Keywords: Akt, everolimus, mTOR, PI3-K, rapamycin, renal cancer, renal cell carcinoma, temsirolimus, TORC1
In the last decade a greater understanding of the molecular mechanisms involved in the pathogenesis of renal cell carcinoma (RCC) has identified specific targets for therapeutic intervention. Inhibition of the VEGF pathway, by blocking the binding of VEGF to its receptor (i.e., bevacizumab) or by inhibiting the tyrosine kinase activity of the intracellular domain of the VEGF receptor (VEGFR) with small molecules (i.e., sunitinib, sorafenib and pazopanib), has emerged as the primary therapeutic intervention for most patients with advanced RCC. The mammalian target of rapamycin (mTOR) is the second molecular target for which small molecule inhibitors (i.e., temsirolimus and everolimus) have demonstrated a significant clinical activity in patients with advanced RCC. In this article, we describe the mTOR signaling pathway, discuss potential mechanisms of action of mTOR inhibitors, outline the clinical data of these agents in RCC and speculate on future developments in agents targeting this pathway.
The mTOR pathway in RCC
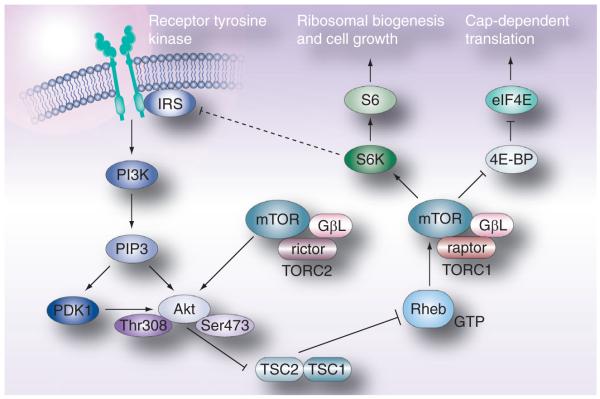
mTOR is a highly conserved serine/threonine kinase that regulates cell growth and meta bolism in response to environmental factors. It is activated downstream of the PI3-K/AKT pathway and executes its biologic functions as two distinct complexes, mTORC1 and mTORC2. mTOR, mLST8/GβL and the negative regulator Deptor are shared by both complexes [1]. mTORC1 is uniquely composed by the regulatory-associated protein of mTOR (raptor) and the proline-rich AKT substrate of 40 kDa (PRAS40), and is inhibited by rapamycin and the other rapalogs (everolimus and temsirolimus). mTORC2 includes rapamycin insensitive companion of TOR (rictor) and, in contrast to mTORC1, is largely resistant to inhibition by the rapalogs.
The mTOR pathway is most typically activated downstream of the PI3-K/Akt pathway in response to growth factors signaling through tyrosine kinase receptors (e.g., the binding of IGF to its receptor) (Figure 1). PI3-K mediates the generation of PIP3 from PIP2, an action which is opposed by the tumor suppressor PTEN. PIP3 binds directly to the PH domain of Akt and mediates its localization to the cell membrane where it is then activated by phosphorylation at two sites, threonine 308 and serine 473. One of the many functions of activated Akt is to directly phosphorylate tuberous sclerosis complex 2 (TSC2; tuberin) resulting in the inhibition of its GAP activity towards the small GTPase Rheb. Relief of this GAP activity enhances the GTP loading of Rheb, promoting its activity. Rheb has been shown to bind directly to the kinase domain of mTOR and activate mTOR kinase activity in a GTP-dependent manner [2].
Figure 1. The mTOR signaling pathway.
4E-BP: 4E-binding protein; Akt: Protein kinase B; eIF4E: Elongation initiation factor 4E; IRS: Insulin receptor substrate; mTOR: Mammalian target of rapamycin; PI3-K: Phosphatidylinositol 3-kinase; Raptor: Regulatory associated protein of TOR; Rheb: Ras homolog enriched in brain; Rictor: Rapamycin insensitive companion of TOR; S6K: P70 S6 kinase; TSC: Tuberous sclerosis complex.
Reproduced with permission from [38].
mTORC1 acts through its downstream effectors, the S6K and the eukaryotic elongation factor 4E-BP, to regulate cell growth and proliferation in response to growth factors (i.e., IGF), nutrients (amino acids in particular), energy level and environmental stress (e.g., hypoxia, DNA damage and reducing conditions) [2]. The activation of S6K by mTOR is critical for ribosomal biogenesis [3], cell growth, anti-apoptosis and translation of the structured 5′ untranslated region (UTR) containing mRNA species, while the phosphorylation (and inactivation) of 4E-BP1 promotes cap-dependent translation. Phosphorylated 4E-BP1 releases eIF4E, a protein that binds the 5′ m7G cap structure of cellular mRNAs and facilitates translation by enhancing the association of the mRNA with the RNA helicase eIF4A and the ribosome-interacting scaffolding protein eIF4G [4]. The extent to which the translation of a particular transcript depends on mTORC1 (and eIF4E) activity is determined by the length and complexity of the mRNA 5′UTR. Approximately 3% of cellular mRNAs have 5′UTRs that extend to greater than a third of the transcript length. The 5′UTRs of these mRNAs often have high GC content and stem loop structures that preclude translation, except when mTORC1 is activated. Many of these ‘weak’, otherwise untranslated mRNAs, encode proteins essential for cell cycle progression (e.g., cyclins, c-Myc and ornithine decarboxylase), survival (e.g., the IAPs XIAP and survivin), and angiogenesis (e.g., VEGF and FGF-2) [5]. Not surprisingly, Dowling et al. have recently shown that phosphorylation of 4E-BP1 (and resultant activation of eIF4E) is the critical determinant in driving cell proliferation in response to TORC1 activation [6]. Furthermore, overexpression of eIF4E has been shown to be sufficient to drive malignant transformation in some cells [7]. Thus, while the precise mechanism of the therapeutic efficacy of mTOR inhibitors remains unknown, it is possible that attenuation of the translation of critical gene products driven by 4E-BP1 phosphorylation (and resultant eIF4E activation) by mTORC1 may be an important aspect of this effect.
The mTOR pathway may be of particular relevance to RCC as it has been shown that HIF protein expression is dependent on mTOR in certain cellular contexts. Inappropriate accumulation of HIF-1α and HIF-2α as a result of biallelic alterations in the von Hippel-Lindau (VHL) gene observed in the majority of clear cell RCC is believed to be a critical step in RCC tumorigenesis as a result of increased expression of HIF-regulated gene products including VEGF, PDGF and TGF-α. Toschi et al. have shown that mTOR activation, in conjunction with the presence of phospholipase D, enhances the expression of both HIF-1α and HIF-2α in RCC cells at a translational level rather than transcriptional [8]. Furthermore, treatment of mice bearing RCC xenograft models with temsirolimus has been shown to result in impaired expression of HIF-1α under both hypoxic and normoxic conditions, presenting another potential mechanism of action for the rapalogs in patient with RCC [9].
Rapamycin & its analogs
Both US FDA-approved mTOR inhibitors, temsirolimus and everolimus, are derivatives of the natural compound rapamycin, present in Streptomyces hygroscopius. Like rapamycin, temsirolimus and everolimus bind the cytoplasmic protein FK506 binding protein-12 (FKBP12) and together they bind mTOR adjacent to its kinase domain. The rapamycin-FKBP12 complex only interacts with unbound mTOR or mTOR in the mTORC1 complex. Consequently, the clinical activity of the rapalogs is believed to be primarily mediated by the inhibition of mTORC1. However, Sarbassov et al. showed that the prolonged exposure to rapamycin is able to inhibit mTORC2 in a tissue-specific manner possibly due to binding with the free mTOR [10]. Recently, Toschi et al. have shown that phosphatidic acid (PA; the metabolic product of phospholipase D) is required for the stable interaction of mTOR with either Rictor or Raptor and that the mechanism by which rapamycin may inhibit mTORC1 and mTORC2 activity is by competing with the interaction of these complexes with PA [11]. However, the levels of rapamycin required to compete with the interaction of PA with mTORC2 were much higher than that required to compete with that of PA and mTORC1. Therefore, the effect of the rapalogs on TORC2 activity may be significantly more dose-dependent. However, the effect of the rapalogs on mTORC1 has been extensively studied in multiple cell lines and a laboratory model. It is well established that rapamycin potently inhibits S6K1 activity in almost all cell types. Conversely, the ability of the rapalogs to inhibit the phosphorylation (and inactivation) of 4E-BP1 appears to be less reliable and more cell-dependent [12].
Rapamycin was initially developed as an immunosuppressive drug used to prevent rejection in solid organ transplant recipient. However, in the last few years, a very complex immunomodulatory role of mTOR inhibitors has been described. Araki et al. initially described in 2009 [13] that mTOR is a major regulator of memory CD8 T cell and that the rapalogs have immunostimulatory effects on the generation of memory CD8 T cells. Vγ2Vδ2 T cells are a subset of peripheral γδ T cells that exhibit MHC-unrestricted lytic activity against human tumors [14]. Rapamycin increases the proliferation of antigen exposed Vγ2Vδ2 cells. Moreover, Vδ2 T cells expanded with rapamycin show increased expression of the IL2 receptor (CD25) and Bcl-2 as well as decreased expression of the chemokine receptor CCR5, resulting in enhanced resistance to apoptosis [15]. These observations suggest the possible role of rapamycin and its derivatives as adjuvants to cancer vaccines. In mice treated with a heat shock protein cancer vaccine, CD8 T cells from mice concurrently treated with temsirolimus showed greater interferon-γ and cytotoxic T-cell responses than those from mice treated with vaccine alone. In addition, temsirolimus enhanced the formation of CD8 memory cells after vaccine administration [16]. The role that these immunomodulatory effects of rapalogs may play in the clinical e fficacy of these agents in RCC remains unknown.
Clinical experience with mTOR inhibitors in RCC
Temsirolimus
Temsirolimus was approved by the FDA for the first-line treatment of advanced RCC in May 2007 on the basis of a Phase III trial that randomized 626 patients with poor-prognosis metastatic RCC to receive temsirolimus (25 mg intravenous weekly), IFN-α (3 million U subcutaneously three-times weekly) or a combination of both (temsirolimus 15 mg weekly and 6 million U of IFN-α three-times weekly) [17]. The overall survival of patients treated with temsirolimus alone was statistically longer than those treated with IFN-α alone (0.73 hazard ratio [HR]; p = 0.0069). There was no statistical difference between patients treated with IFN-α alone and the combination of IFN-α and temsirolimus. The median overall survival time was 10.9 months in the temsirolimus group, 8.4 months in the combination group and 7.3 months in the interferon group. Temsirolimus was well tolerated with the most common adverse effects being asthenia, rash, anemia, nausea, peripheral edema, hyperlipidemia and hyperglycemia. This study enrolled only patients with metastatic RCC and at least three of six well-described poor prognostic features (Karnofsky performance status < 80, time from diagnosis to randomization <12 months, serum lactate dehydrogenase >1.5 upper limit of normal, Hb < lower limit of normal, corrected serum calcium >10 mg/dl and >1 metastatic site) based on the retrospective analysis of the Phase II study that suggested potentially unique efficacy in these populations [18]. Based on these results, temsirolimus is considered a first-line therapeutic option for patients with metastatic RCC who have poor risk prognostic features.
Everolimus
Everolimus is an orally administered rapalog, approved by the FDA in March 2009 for the treatment of patients with advanced RCC who failed either sorafenib, sunitinib or both based on the results of the Renal Cell Cancer Treatment With Oral RAD001 Given Daily (RECORD-1) [19]. In this Phase III, randomized, double-blind, placebo-controlled trial, 416 patients with advanced RCC (showing at least a component of clear cell histology) who had failed prior treatment with either sorafenib or sunitinib (VEGF-targeted tyrosine kinase inhibitors), or both within the last 6 months were randomized in a 2:1 fashion to receive either everolimus 10 mg PO once daily (n = 277) or placebo with best supportive care (n = 139) [20]. Primary end point was progression-free survival (PFS). Secondary end points included safety, objective response rate and overall survival. The trial was halted at the second interim analysis after 191 progression events had been observed. By central radiology review the median PFS for patients treated with everolimus was 4.9 months as compared with 1.9 months in the placebo group (HR: 0.33; 95% confidence interval [CI]: 0.25–0.43; p < 0.01). In the 124 patients previously treated with only sorafenib, the median PFS was 5.9 months for the everolimus group versus 2.8 months for the placebo group (HR: 0.25; 95% CI: 0.16–0.42). In the 184 patients previously treated with sunitinib alone the median PFS was 3.9 versus 1.8, respectively (HR 0.34; CI 0.23–0.51). Five patients (1.8%) in the everolimus group experienced partial responses versus 0% in the placebo group. Median overall survival was 14.8 in patients randomized to everolimus versus 14.4 months for the placebo arm (HR: 0.87; 95% CI: 0.65–1.15; p = 0.162). However, this end point was felt to be possibly contaminated by the allowance for patients exhibiting disease progression on placebo to crossover to open label everolimus. The side-effect profile was quite favorable with most common adverse events with everolimus being stomatitis (40%), rash (25%), fatigue (20%), hypercholesterolemia (76%), hyper triglyceridemia (71%) and hyperglycemia (50%). Pneumonitis was observed in 22 patients (8%) compared with 0 in the placebo group. Based on these results, everolimus is now considered a standard therapeutic option for patients who have experienced disease progression on VEGF-targeted tyrosine kinase inhibitors.
Ongoing clinical studies with mTOR inhibitors in RCC
Although both temsirolimus and everolimus are now approved by the FDA for the therapy of patients with advanced RCC, the role of these mTORC1 inhibitors will continue to evolve as many questions regarding their efficacy in specific therapeutic situations are addressed. Both temsirolimus and everolimus are being studied or considered in multiple other clinical scenarios and therapeutic strategies including in combinational regimens, sequential therapy with VEGF pathway inhibitors, the adjuvant setting and in patients with non-clear cell histology.
Combination therapy
Given the distinct molecular targets of VEGF and mTOR pathway inhibitors, there has been considerable interest in whether combinations of these two classes of agents may lead to additional therapeutic efficacy. For example, the combination of temsirolimus and bevacizumab (monoclonal antibody directed against VEGF) has shown encouraging efficacy in a Phase II trial in patients with refractory RCC, who have failed VEGF-targeted TKI, with a clinical benefit rates (partial response/stable disease) of 88% (partial response = 16% ) [21]. The combination of temsirolimus with bevacizumab is also investigated as one of the four arms of the BeST trial (a randomized Phase II study of VEGF, RAF kinase, and mTOR combination targeted therapy with bevacizumab, sorafenib and temsirolimus in advanced RCC – ECOG 2084) and in the open-label Phase IIIb, Investigation of Torisel and Avastin Combination Therapy (INTORACT) trial, in which temsirolimus plus bevacizumab is compared with bevacizumab plus IFN-α for the first-line treatment of patients with advanced RCC.
Similarly, everolimus has also been studied in combination with bevacizumab in a Phase II study of 50 treatment-naive patients and 30 patients previously treated with sunitinib and/or sorafenib. The combination of everolimus and bevacizumab showed activity in both groups with a median PFS of 9.1 months in previously untreated patients and 7.1 in previously treated patients. A total of 30% of the previously untreated group and 23% of the previously treated patients had objective response to treatment [22]. These encouraging data have led to the ongoing CALGB 90802 trial, which is a large Phase III randomized trial comparing everolimus plus placebo versus everolimus plus bevacizumab for patients with advanced RCC progressing after treatment with TKIs.
Sequential therapy
The currently available VEGF pathway and mTOR inhibitors only extremely rarely produce complete or durable remissions that can be maintained off therapy in patients with advanced RCC. Prospective studies and retrospective analysis have suggested that there is no absolute crossresistance amongst TKIs and this appears to also be true between VEGF pathway inhibitors and mTOR inhibitors as well. Currently, sequential single-agent therapy with targeted therapy has become the standard of care for metastatic RCC. However, the optimal sequence of agents that would result in maximal disease control duration and minimal toxicity is unknown and under active investigation. The RECORD-3 is an ongoing randomized Phase II trial investigating this very specific issue comparing the efficacy and safety of the sequence of first-line everolimus (RAD001) followed by second-line sunitinib versus the reverse sequence of first-line sunitinib followed by second-line everolimus in patients with metastatic clear-cell RCC who have received no prior systemic therapy for their disease [23]. Similarly the Torisel 404 study is a Phase III study investigating temsirolimus versus sorafenib in patients who have failed initial therapy with sunitinib. While these studies may identify more promising sequencing regimens, they will not address the question of whether sequential therapy is in fact superior to upfront combinational therapy.
Adjuvant therapy
At present, no therapy has been shown to decrease relapse rate and increase survival after surgical management of early stage RCC and no approved therapies are approved for use in the adjuvant setting. A large Phase III trial (ECOG 2805) randomizing patients with advanced RCC and high risk of recurrence following neph rectomy to receive sorafenib, sunitinib or placebo for 1 year has finished accrual, but mature results will not be available for several years. Given the demonstrated efficacy of mTOR inhibitors in the advanced disease setting, combined with the favorable toxicity profile, these agents may be particularly attractive in the adjuvant setting. A large randomized, placebo-controlled Phase III trial is being planned within the US Intergroup mechanism to formally assess the role of adjuvant everolimus in patients with resected RCC at high risk of recurrence.
Nonclear cell RCC
Although the efficacy of mTORC1 inhibitors has primarily been established in clear cell RCC, further ana lysis of the pivotal Phase III trial leading to the FDA approval of temsirolimus suggested this mTORC1 inhibitor may be even more effective compared with interferon in patients with non-clear cell RCC than clear cell RCC [24]. The median overall survival of temsirolimus versus interferon was 11.6 versus 4.3 months in patients with non-clear cell histology (75% of which were of papillary subtype) compared with 10.7 versus 8.2 months in patients with clear cell RCC. The possibility that mTORC1 inhibitors in general may have unique efficacy in non-clear cell RCC has prompted the initiation of a randomized Phase II trial of temsirolimus versus sunitinib in European patients with metastatic non-clear cell RCC. Likewise, everolimus will also be studied in a Phase II trial in 60 European patients with metastatic papillary RCC (RAD001 in Advanced Papillary Tumor Program in Europe [RAPTOR] trial). These two Phase II trials should provide critical information regarding the efficacy of mTORC1 inhibitors in patients with non-clear cell histology RCC.
Mechanisms of resistance to mTOR inhibitors
Despite the established efficacy of mTORC1 inhibitors in RCC, responses to these agents are generally not durable and all patients will eventually experience disease progression while on treatment. Identification of mechanisms of resistance to mTORC1 inhibitors in RCC will be critical to improve clinical outcomes. While the exact mechanisms for resistance have yet to be elucidated, several possibilities have already been proposed.
One potential mechanism of resistance to mTORC1 inhibitors is the feedback activation of critical upstream signaling pathways induced in some cells following treatment with rapamycin or its analogs. It is well known that mTORC1 activation exerts a negative feedback on PI3-K through S6K. Activated S6K represses, through phosphorylation and subsequent proteasomal degradation, insulin responsive substrate 1 (IRS-1), which is required for PI3-K activation. In preclinical models, it has been shown that tumors with activated PI3-K/Akt are very sensitive to mTOR inhibitors, but in some cases the biologic activity of these agents seems attenuated by the fact that mTORC1 inhibition releases this negative feedback and results in the activation of Akt upstream of mTOR [25]. It has also been suggested that the immediate effect of mTORC1 inhibition is to drive the formation of mTORC2, an effect which would be expected to increase phosphorylation of Akt on its Ser473 site and enhance its activity [26]. As the PI3-K/Akt pathway controls numerous kinases, transcription factors and other proteins associated with cell growth and survival in addition to mTOR, the activation of this pathway induced by mTORC1 inhibition may undermine the clinical efficacy of mTORC1 inhibitors. Finally, an additional mTORC1-MAPK feedback loop dependent on S6K/PI3-K/Ras was recently described by Carracedo et al. [27]. Tumor specimens from both patients and animal models treated with everolimus showed evidence of increased activation of MAPK. The loss of these negative feedback loops may represent the basis of important mechanisms of resistance to rapalogs.
Although it has been shown in some cases that exposure of some cell lines to rapamycin can lead to the suppression of mTORC2 formation, this effect appears to be a function of either dose or chronicity of exposure [10,11]. Although rapamycin is well known to have wide intrapatient pharmacokinetic variability, the newer rapalogs such as everolimus and temsirolimus appear to have more predictable pharmaco kinetic properties. However, dose may remain an important factor given the aforementioned competitive interaction of the rapalogs and PA, and that RCC has been shown to overexpress phospho lipase D2 [28]. Whether the differential expression of phospholipase D2 can predict for tumors that are responsive to the rapalogs remains to be seen.
Regardless, as the suppression of mTORC2 activity requires significantly higher doses as compared with that of mTORC1, the rapalogs are, in general, thought to function primarily as selective allosteric inhibitors of mTORC1 activity. This selective activity may be of particular relevance to RCC given the recent assertion that the expression of HIF-2α, argued by many to be the more relevant HIF in RCC [29], is dependent only on mTORC2 activity [30]. As it has recently been shown that a substantial fraction of VHL−/− RCC express only HIF-2α [31], the inability of selective mTORC1 inhibitors to suppress HIF-2α expression may be another mechanism for resistance to this class of agents.
Finally, it is also clear that the activity of the primary executors of TORC1, S6K and 4E-BP1, are not equally sensitive to the rapalogs. While the phosphorylation S6K is extremely sensitive to inhibition by rapamycin, Choo et al. have recently shown that the suppression of 4E-BP1 phosphorylation by rapamycin in some cells is reversed within a few hours of drug exposure [12]. Therefore, the efficacy of rapalogs in patients with RCC may be dependent upon their ability to effectively and continuously inhibit 4E-BP1 phosphorylation. Ideally, enhanced knowledge of the mechanisms of resistance to mTORC1 inhibitors may be used to direct combinational therapy with other molecularly targeted agents as well as identify opportunities to improve on the first generation allosteric inhibitors of mTORC1.
Patient selection strategies
As with other targeted therapies there is great interest in developing patient selection strategies for mTOR inhibitors so as to direct the most appropriate patient towards therapy with these agents. Similar to that observed with other molecularly targeted agents, not all patients with advanced RCC experience clinical benefit from treatment mTOR inhibitors and at present there are no clinically validated clinicopathologic or molecular biomarkers predictive of benefit from therapy. Studies have suggested that the pre treatement activation status of the PI3-K/Akt/mTOR signaling pathway may be one such predictor for the likelihood of clinical benefit from mTOR inhibitors. For example, in vitro, multiple myeloma cells that had lost PTEN expression showed an enhanced antiproliferative response to treatment with rapamycin [32]. The analysis of tumor samples from a subset of patients within a randomized Phase II trial of temsirolimus in patients with RCC suggested that pretreatment phospho-S6 and phospho-Akt expression may be predictive biomarkers of response to temsirolimus [33]. This retrospective observation is currently under prospective investigation in a Phase II study in which biopsies of metastases of RCC will be obtained from 40 patients, before initiating therapy with everolimus. The status of the PI3-K/Akt signaling pathway will be analyzed and correlated with the clinical response to treatment to the mTOR inhibitor in order to validate its role as a reliable predictor of clinical benefit.
One of the expected pharmacodynamic effects of mTOR inhibitors is the downmodulation of glucose import. In fact, changes in glucose uptake as measured by 18-fluoro-deoxyglucose positron emission tomography (FDG-PET) imaging have been used to optimize everolimus dosing in vivo [34]. To address the possibility that changes in FDG-PET scanning may serve as an early predictor of response or resistance to everolimus treatment, a Phase II trial has been completed in which 60 patients with metastatic RCC underwent FDG-PET imaging before and after initiating therapy with everolimus. The primary objective of this study is to determine if high basal FDG-avidity is predictive of greater likelihood of response to everolimus as determined by changes in RECIST-based tumor measurements after 8 weeks of therapy. The study will also investigate whether changes in FDG-avidity as a result of therapy are associated with clinical responses. The results of this Phase II trial will be reported at the 2011 ASCO annual meeting and will hopefully shed light on whether this imaging modality can be used to either identify which patients a priori should be treated with mTOR inhibitors or as a pharmacodynamic predictor of response or resistance following the initiation of therapy.
Conclusion
The PI3-K/Akt/mTOR pathway has a central role in cell proliferation and homeostasis in response to environmental conditions. The upregulation of this pathway has been described to be a common hallmark of many tumors. Allosteric inhibitors of mTOR have demonstrated promising activity in the treatment of advanced RCC, and two such agents, temsirolimus and everolimus, are now approved in the first- and second-line settings for the treatment of metastatic RCC. Despite these developments, it is clear that only a subset of patients experience substantial benefit. The investigation of the PI3-K/Akt/mTOR intricate network and its regulation has suggested multiple mechanisms of resistance to the mTORC1 inhibitors and offered the basis for development of novel agents (i.e., direct mTOR kinase inhibitors or dual PI3-K/mTOR inhibitors) and novel combination strategies that may result in greater clinical efficacy. Given the crowded therapeutic landscape in RCC, efforts must continue to identify predictive biomarkers of response to both currently available mTORC1 inhibitors and similarly targeted novel agents.
Future perspective
It is likely that in the next several years, the role of mTOR inhibitors in RCC will continue to evolve. Efforts to improve patient selection may allow the identification of a subset of patients who would most benefit from agents targeting this pathway. At the same time, insights into mechanisms of resistance to mTOR inhibitors will likely inform combinational regimens that enhance the efficacy of mTOR inhibitors in RCC. Although the confirmatory studies are pending, it appears likely that mTOR inhibitors will have a role in non-clear cell RCC for which few therapies have established efficacy. Finally, the agents targeting the PI3-K/Akt/mTOR pathway are likely to change as well and improve upon the first generation mTOR inhibitors.
Several agents that directly inhibit the kinase function of mTOR regardless of whether it is complexed with mTORC1 or mTORC2 are in clinical development. These active site inhibitors of mTOR appear to infer additional benefit to allosteric inhibitors of mTOR by decreasing AKT phosphorylation and ultimately potentiating mTORC1 inhibition. At the same, agents directly inhibiting kinases upstream of mTOR, such as Akt and PI3-K, are also in active clinical development. These agents would have the advantage of not being susceptible to the feedback activation of upstream pathways which may limit mTORC1 inhibitors. Furthermore, the catalytic domain of mTOR and p110α subunit of PI3-K are structurally related, a characteristic that has been exploited to develop compounds with dual activity against PI3-K and mTOR. These drugs have shown potent anticancer activity in preclinical studies [35] and have now entered Phase I–II clinical trials alone or in combination [36,37] and trials in RCC are planned. Whether these novel agents will prove to have superior clinical activity to the rapalogs in patients with advanced RCC will be an area of active and exciting investigation in RCC in the years to come.
Executive summary.
Introduction
▪ The development of agents targeting VEGF signaling has been a major breakthrough in renal cell carcinoma (RCC) therapy.
▪ mTOR is the second kinase towards which molecularly targeted inhibitors have shown significant clinical efficacy in RCC.
The mTOR pathway in RCC
▪ Activated downstream of PI3-K/Akt, mTOR is a highly conserved serine/threonine kinase that regulates cell growth and metabolism in response to environmental factors.
▪ mTOR executes its biologic functions as a part of two functionally distinct complexes, mTORC1 (including mTOR and Raptor) and mTORC2 (including mTOR and Rictor).
▪ mTORC1 plays a central role in regulating the cap-dependent translation of mRNA of genes critical to malignant transformation and progression.
▪ Both mTORC1 and mTORC2 also regulate the expression of the HIFs.
Rapamycin & its analogs
▪ Both temsirolimus and everolimus are derivatives of rapamycin (i.e., rapalogs) and are primarily allosteric inhibitors of mTORC1 function.
▪ Although the rapalogs were originally developed as immunosuppressive agents, it is now becoming apparent they these agents have other immunomodulatory effects which in some circumstances can enhance cellular immune responses.
Clinical experience with mTOR inhibitors in RCC
▪ Temsirolimus was approved by the US FDA after showing a survival advantage compared with interferon in a large randomized Phase III trial in patients with advanced RCC and poor prognostic factors.
▪ Everolimus was approved by the FDA for therapy following the failure of VEGF-targeted tyrosine kinase inhibitors after demonstrating superior efficacy to placebo in a large randomized placebo-controlled Phase III trial in patients with advanced RCC who had failed prior treatment with sorafenib, sunitinib or both.
Ongoing clinical studies with mTOR inhibitors in RCC
▪ Everolimus and temsirolimus are currently being investigated given sequentially with VEGF-targeted tyrosine kinase inhibitors.
▪ The mTOR inhibitors are also actively being investigated in combinational regimens with other molecularly targeted and anti-angiogenic agents in RCC.
▪ Everolimus will be evaluated in the adjuvant setting in a large intergroup trial.
▪ Both everolimus and temsirolimus will be investigated in non-clear cell RCC as well.
Mechanisms of resistance to mTOR inhibitors
▪ Preclinical studies have shown that inhibition of mTORC1 can result in the upstream activation of PI3-K, Akt and MAP-kinase.
▪ As the expression of HIF-2α, believed to be the more relevant HIF in RCC, is dependent primarily upon mTORC2 activity, allosteric inhibitors of mTORC1 such as the rapalogs may have minimal activity against HIF-2α.
Patient selection strategies
▪ As patients benefiting from mTOR inhibitors may not necessarily be the same patients benefiting from VEGF inhibitors, efforts are underway to identify predictive biomarkers of response to these agents.
▪ In particular, the pretreatment activation of the PI3-K/Akt/mTOR pathway may serve as a promising predictor of response.
Conclusion
▪ Allosteric inhibitors of mTOR have clear activity in RCC.
▪ The place in these agents in the crowded therapeutic landscape of RCC will continue to evolve as more is learned regarding predictive models and the efficacy of these agents in combinational and sequential regimens.
▪ A new generation of agents targeting mTOR and upstream at PI3-K and Akt is emerging and will be investigated actively in RCC.
Acknowledgments
D Cho has served on advisory boards for Novartis, Wyeth, and Genentech Pharmaceuticals. Work discussed in this article is supported by the NIH (K08CA142890).
Footnotes
Financial & competing interests disclosure The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Guertin DA, Sabatini DM. The pharmacology of mTOR Inhibition. Sci. Signal. 2009;2(67):PE24. doi: 10.1126/scisignal.267pe24. [DOI] [PubMed] [Google Scholar]
- 2.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124(3):471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 3.Jastrzebski K, Hannan KM, Tchoubrieva EB, Hannan RD, Pearson RB. Coordinate regulation of ribosome biogenesis and function by the ribosomal protein S6 kinase, a key mediator of mTOR function. Growth Factors. 2007;25(4):209–226. doi: 10.1080/08977190701779101. [DOI] [PubMed] [Google Scholar]
- 4.Holland EC, Sonenberg N, Pandolfi PP, Thomas G. Signaling control of mRNA translation in cancer pathogenesis. Oncogene. 2004;23(18):3138–3144. doi: 10.1038/sj.onc.1207590. [DOI] [PubMed] [Google Scholar]
- 5.Konicek BW, Dumstorf CA, Graff JR. Targeting the eIF4F translation initiation complex for cancer therapy. Cell Cycle. 2008;7(16):2466–2471. doi: 10.4161/cc.7.16.6464. [DOI] [PubMed] [Google Scholar]
- 6.Dowling RJ, Topisirovic I, Alain T, et al. mTORC1-mediated cell proliferation, but not cell growth, controlled by the 4E-BPs. Science. 2010;328(5982):1172–1176. doi: 10.1126/science.1187532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fredrickson RM, Mushynski WE, Sonenberg N. Phosphorylation of translation initiation factor eIF-4E is induced in a ras-dependent manner during nerve growth factor-mediated PC12 cell differentiation. Mol. Cell. Biol. 1992;12(3):1239–1247. doi: 10.1128/mcb.12.3.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toschi A, Edelstein J, Rockwell P, Ohh M, Foster DA. HIFα expression in VHL-deficient renal cancer cells is dependent on phospholipase D. Oncogene. 2008;27(19):2746–2753. doi: 10.1038/sj.onc.1210927. [DOI] [PubMed] [Google Scholar]
- 9.Thomas GV, Tran C, Mellinghoff IK, et al. Hypoxia-inducible factor determines sensitivity to inhibitors of mTOR in kidney cancer. Nat. Med. 2006;12(1):122–127. doi: 10.1038/nm1337. [DOI] [PubMed] [Google Scholar]
- 10.Sarbassov DD, Ali SM, Sengupta S, et al. Prolonged rapamycin treatment inhibits mMTORC2 assembly and Akt/PKB. Mol. Cell. 2006;22(2):159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 11.Toschi A, Lee E, Xu L, Garcia A, Gadir N, Foster DA. Regulation of mTORC1 and mTORC2 complex assembly by phosphatidic acid: competition with rapamycin. Mol. Cell. Biol. 2009;29(6):1411–1420. doi: 10.1128/MCB.00782-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choo AY, Yoon SO, Kim SG, Roux PP, Blenis J. Rapamycin differentially inhibits S6Ks and 4E-BP1 to mediate cell-type-specific repression of mRNA translation. Proc. Natl Acad. Sci. USA. 2008;105(45):17414–17419. doi: 10.1073/pnas.0809136105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Araki K, Turner AP, Shaffer VO, et al. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460(7251):108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alexander AA, Maniar A, Cummings JS, et al. Isopentenyl pyrophosphate-activated CD56+ {γ}{δ} T lymphocytes display potent antitumor activity toward human squamous cell carcinoma. Clin. Cancer Res. 2008;14(13):4232–4240. doi: 10.1158/1078-0432.CCR-07-4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li H, Pauza CD. Rapamycin increases the yield and effector function of human γδ T cells stimulated in vitro. Cancer Immunol. Immunother. 2011;60(3):361–370. doi: 10.1007/s00262-010-0945-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, Wang XY, Subjeck JR, Shrikant PA, Kim HL. Temsirolimus, an mTOR inhibitor, enhances anti-tumour effects of heat shock protein cancer vaccines. Br. J. Cancer. 2011;104(4):643–652. doi: 10.1038/bjc.2011.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon α, or both for advanced renal-cell carcinoma. N. Engl. J. Med. 2007;356(22):2271–2281. doi: 10.1056/NEJMoa066838. ▪ Describes the data leading to the US FDA approval of temsirolimus for patients with advanced renal cell carcinoma (RCC).
- 18.Atkins MB, Hidalgo M, Stadler WM, et al. Randomized Phase II study of multiple dose levels of CCI-779, a novel mammalian target of rapamycin kinase inhibitor, in patients with advanced refractory renal cell carcinoma. J. Clin. Oncol. 2004;22(5):909–918. doi: 10.1200/JCO.2004.08.185. [DOI] [PubMed] [Google Scholar]
- 19.Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled Phase III trial. Lancet. 2008;372(9637):449–456. doi: 10.1016/S0140-6736(08)61039-9. ▪ Describes the data leading to the FDA approval of everolimus for patients with advanced RCC.
- 20.Motzer RJ, Escudier B, Oudard S, et al. Phase 3 trial of everolimus for metastatic renal cell carcinoma: final results and analysis of prognostic factors. Cancer. 2010;116(18):4256–4265. doi: 10.1002/cncr.25219. [DOI] [PubMed] [Google Scholar]
- 21.Merchan JR, Pitot HC, Qin R, et al. Phase I/II trial of CCI 779 and bevacizumab in advanced renal cell carcinoma (RCC): safety and activity in RTKI refractory RCC patients. Presented at: 45th Annual Meeting of the American Society of Clinical Oncology (ASCO); Orlando, FL, USA. 29 May–2 June 2009. [Google Scholar]
- 22.Hainsworth JD, Spigel DR, Burris HA, 3rd, Waterhouse D, Clark BL, Whorf R. Phase II trial of bevacizumab and everolimus in patients with advanced renal cell carcinoma. J. Clin. Oncol. 2010;28(13):2131–2136. doi: 10.1200/JCO.2009.26.3152. [DOI] [PubMed] [Google Scholar]
- 23.Knox JJ, Kay AC, Schiff E, et al. First-line everolimus followed by second-line sunitinib versus the opposite treatment sequence in patients with metastatic renal cell carcinoma (mRCC). Presented at: 46th Annual Meeting of the American Society of Clinical Oncology (ASCO); Chicago, IL, USA. 4–8 June 2010. [Google Scholar]
- 24.Dutcher JP, de Souza P, McDermott D, et al. Effect of temsirolimus versus interferon-α on outcome of patients with advanced renal cell carcinoma of different tumor histologies. Med. Oncol. 2009;26(2):202–209. doi: 10.1007/s12032-009-9177-0. [DOI] [PubMed] [Google Scholar]
- 25.Sun SY, Rosenberg LM, Wang X, et al. Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res. 2005;65(16):7052–7058. doi: 10.1158/0008-5472.CAN-05-0917. ▪ The first description of the feedback activation of PI3-K/Akt in response to treatment with rapalogs.
- 26.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 27.Carracedo A, Ma L, Teruya-Feldstein J, et al. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3-K-dependent feedback loop in human cancer. J. Clin. Invest. 2008;118(9):3065–3074. doi: 10.1172/JCI34739. ▪ The first description of the feedback activation of the MAP-K pathway in response to treatment with rapalogs.
- 28.Zhao H, Ehara H, Akao Y, et al. Increased activity and intranuclear expression of phospholipase D2 in human renal cancer. Biochem. Biophys. Res. Commun. 2000;278(1):140–143. doi: 10.1006/bbrc.2000.3719. [DOI] [PubMed] [Google Scholar]
- 29.Kondo K, Kim WY, Lechpammer M, Kaelin WG. Inhibition of HIF2α is sufficient to suppress pVHL-defective tumor growth. PLoS Biol. 2003;1(3):439–444. doi: 10.1371/journal.pbio.0000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Toschi A, Lee E, Gadir N, Ohh M, Foster DA. Differential dependence of hypoxia-inducible factors 1 α and 2 α on mTORC1 and mMTORC2. J. Biol. Chem. 2008;283(50):34495–34499. doi: 10.1074/jbc.C800170200. ▪▪ Describes the critical dependence of HIF-2α expression on TORC2 activity rather than TORC1.
- 31.Gordan JD, Lal P, Dondeti VR, et al. HIF-α effects on c-Myc distinguish two subtypes of sporadic VHL-deficient clear cell renal carcinoma. Cancer Cell. 2008;14(6):435–446. doi: 10.1016/j.ccr.2008.10.016. ▪▪ Describes three major molecular phenotypes of clear cell RCC. VHL wild-type, VHL mutant HIF-1/2 expressing, and VHL mutant HIF-2 only.
- 32.Shi Y, Gera J, Hu L, et al. Enhanced sensitivity of multiple myeloma cells containing PTEN mutations to CCI-779. Cancer Res. 2002;62(17):5027–5034. [PubMed] [Google Scholar]
- 33.Cho D, Signoretti S, Dabora S, et al. Potential histologic and molecular predictors of response to temsirolimus in patients with advanced renal cell carcinoma. Clin. Genitourin. Cancer. 2007;5(6):379–385. doi: 10.3816/CGC.2007.n.020. [DOI] [PubMed] [Google Scholar]
- 34.Cejka D, Kuntner C, Preusser M, et al. FDG uptake is a surrogate marker for defining the optimal biological dose of the mTOR inhibitor everolimus in vivo. Br. J. Cancer. 2009;100:1739–1745. doi: 10.1038/sj.bjc.6605076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cho DC, Cohen MB, Panka DJ, et al. The efficacy of the novel dual PI3-kinase/mTOR inhibitor NVP-BEZ235 compared with rapamycin in renal cell carcinoma. Clin. Cancer Res. 2010;16(14):3628–3638. doi: 10.1158/1078-0432.CCR-09-3022. ▪ Discusses potential shortcoming of the rapalogs and provides preclinical evidence that a dual PI3-K/mTOR inhibitor may have superior efficacy in RCC.
- 36.Serra V, Markman B, Scaltriti M, et al. NVP-BEZ235, a dual PI3-K/mTOR inhibitor, prevents PI3-K signaling and inhibits the growth of cancer cells with activating PI3-K mutations. Cancer Res. 2008;68(19):8022–8030. doi: 10.1158/0008-5472.CAN-08-1385. [DOI] [PubMed] [Google Scholar]
- 37.Vilar E, Perez-Garcia J, Tabernero J. Pushing the envelope in the mTOR pathway. The second generation of inhibitors. Mol. Cancer Ther. 2011;10(3):395–403. doi: 10.1158/1535-7163.MCT-10-0905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cho DC, Atkins MB. Everolimus: emerging evidence of its therapeutic impact in patients with advanced renal cell carcinoma. Clin. Med. Rev. Oncol. 2010 (In press) [Google Scholar]