Abstract
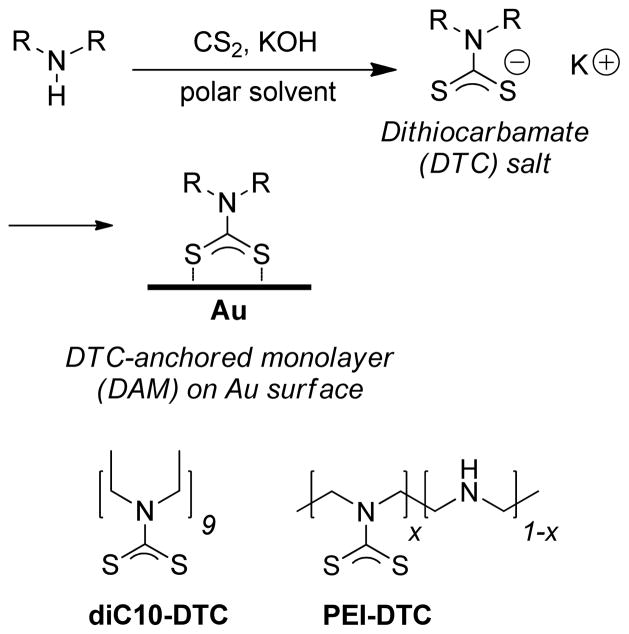
Dithiocarbamate (DTC)-anchored monolayers and polymers were investigated as positive resists for UV photolithography on planar and roughened Au surfaces. DTCs were formed in situ by the condensation of CS2 with monovalent or polyvalent amines such as linear polyethyleneimine (PEI) under mildly basic aqueous conditions, just prior to surface passivation. The robust adsorption of the polyvalent PEI-DTC to Au surfaces supported high levels of resistance to photoablation, providing opportunities to generate thin films with gradient functionality. Treatment of photopatterned substrates with alkanethiols produced binary coatings, enabling a direct visual comparison of DTC- and thiol-passivated surfaces against chemically induced corrosion using confocal microscopy.
Introduction
Surface chemistry is essential for the passivation and functionalization of metal substrates.1 Both physisorptive and chemisorptive surfactants have been employed, but the latter offers the benefit of reproducible monolayer formation and directional control. The best known examples of such chemisorptive systems are based on self-assembled monolayers (SAMs) of alkanethiols, with diverse applications ranging from stimuli-responsive surfaces to bioanalytical sensing.2 However, the chemical stability of alkanethiol-based SAMs is finite, as they are susceptible to oxidative degradation and displacement by competing surfactants. This places some limits on applications requiring variable environmental exposure.
In our search for robust methods in metal surface functionalization, we have determined that the adsorption of dithiocarbamates (DTCs) on Au can provide superior resistance to surface desorption under ambient conditions or in the presence of competing surfactants, while retaining the simplicity of self-assembly.3 DTCs can be formed by the in situ condensation of alkylamines with CS2 under ambient and mildly basic conditions, and are capable of forming monolayers with higher surface coverages than that reported for alkanethiol-based SAMs.4 In situ DTC formation has proven useful for anchoring a wide variety of amine-terminated molecules onto Au surfaces, ranging from low-molecular weight species to complex structures capable of biomolecular or supramolecular recognition.4,5,6,7,8,9,10,11,12, 13,14,15,16,17,18,19 Recently, in situ DTC formation has been extended toward the chemisorptive anchoring of polymeric amines such as polyethyeleneimine (PEI).20
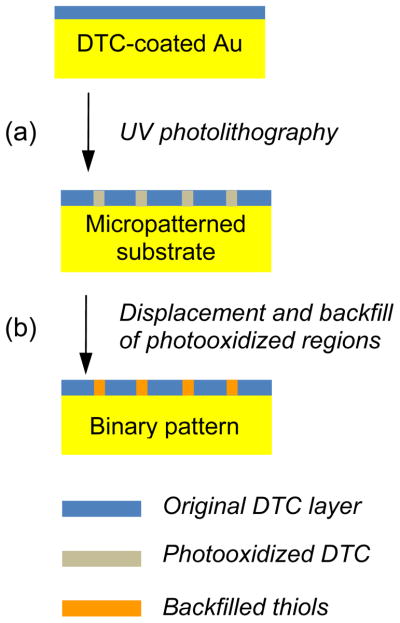
The utility of DTC-anchored monolayers (DAMs) and polymers can be further extended by developing methods that permit their etching into well-defined patterns. We were especially interested in UV photolithography, a method that has been successful to produce micropatterned substrates from alkanethiol-based SAMs by directed photooxidation.21,22 DAMs on Au surfaces should also be amenable to photolithographic patterning, as DTCs are strongly absorbing at UV wavelengths.4,23 In this article we apply UV photolithography as a method to produce patterned DTC-anchored coatings on Au, as well as binary patterns backfilled with alkanethiol-based SAMs. In addition, we find that DTC-anchored PEI on Au is degraded gradually by UV photooxidation, enabling surfaces to be patterned with a high degree of both chemical and spatial control. The binary patterned substrates permit a direct comparison of the ability of DTC- and thiol-passivated surfaces to resist chemical corrosion, as evaluated by confocal imaging of adjacent regions.
Materials and Methods
All materials were obtained from commercial sources and used as received, unless otherwise noted. Linear polyethyleneimine (PEI, 22 kDa, n~512) was kindly donated by Polymer Chemistry Innovations. Deionized water was obtained from an ultrafiltration system (Milli-Q, Millipore) with a measured resistivity above 18 MΩ·cm, and passed through a 0.22-μm filter to remove particulate matter. CS2 was freshly distilled from CaH2 before use.
Didecyl- (diC10) and PEI-DTCs were formed at room temperature in MeOH or water respectively, by treatment with 1 equiv of CS2 and KOH per amine (Scheme 1). In this study, we deliberately used potassium salts to minimize surface fouling by amines or ammonium species.4 DTC formation could be monitored by UV absorption spectroscopy based on their extinction peaks at 260 and 290 nm as described previously,4 and was essentially complete within 15 min of mixing. In the case of PEI-DTC, a 1 wt% suspension in water was treated with CS2 and KOH as described above (final pH 12), and stirred vigorously for 1 h at room temperature until a clear solution was formed.
Scheme 1.
In situ DTC formation and surface passivation. diC10 = didecyl; PEI= polyethyleneiminyl (x≥ 0.9).
Surface passivation was performed by immersing clean Au substrates in deaerated solutions of PEI-DTC (0.25 wt% in H2O, buffered at pH 10) for 12 h, or in deaerated solutions of diC10-DTC (1 mM in MeOH) for 2 h. Au-coated glass substrates (50 nm Au, 1×1 cm2, Reichart) were cleaned by immersion in freshly prepared piranha solution (2 parts 18 M H2SO4, 1 part 30% H2O2) for 3 minutes, then thoroughly rinsed and left to sit overnight in deionized water prior to immersion in DTC solutions. (Caution! Wear suitable protective apparel when handling highly corrosive acids.) Au substrates were also roughened by electroless Ag deposition and galvanic displacement to enable plasmon-enhanced fluorescence.19,24 Substrates were immersed in a 1.3 M citrate buffer (pH 3.5) containing AgNO3 (3.7 mM) and hydroquinone (120 mM) for 1.5 min, followed by a rinse and immersion in aqueous HAuCl4 solution (0.5 mM) for 30 min.
DTC-passivated Au substrates were subjected to photolithography using a Cd lamp (λmax=226 nm, 10 mW) and quartz photomasks (Advance Reproductions Corp.) having grating periods of 20 μm and linewidths of 10 or 15 μm, with exposure times ranging from 30 min to 1 h (Scheme 2). Substrates were placed in direct contact with the photomasks for optimum feature resolution. The photooxidized DTCs were displaced by treatment with methanolic solutions (1–3 mM) of 2-mercaptoethanol, dodecanethiol (C12-SH), 11-aminoundecanethiol (H2N–C11-SH), or methoxy(triethyleneglycol)undecanethiol (mEG3–C11-SH) for 12 h at room temperature. The latter was synthesized according to a previously published procedure.25 A confocal laser-scanning microscope (FV1000, Olympus) was used for optical transmission and fluorescence imaging, the former at a monochromatic wavelength of 635 nm and the latter at excitation and emission wavelengths of 488 and 525 nm, respectively. A standard optical microscope (BX51, Olympus) outfitted with a darkfield condensor was employed for darkfield imaging.
Scheme 2.
(a) DTC-passivated Au substrates are exposed to UV irradiation through a micropatterned photomask. (b) Linear arrays of photooxidized DTCs are displaced and backfilled with alkanethiols to yield binary passivation layers.
Confocal fluorescence imaging of photopatterned PEI-DTC layers on roughened Au substrates (see above) was achieved by soaking patterned substrates for 30 min in aqueous solutions of fluorescein isothiocyanate (FITC; 100 μM) adjusted to pH 9, followed by careful rinsing with deionized water. A portion of the substrate was left unlabeled to establish baseline fluorescence intensities. For etching studies, smooth Au substrates supporting binary patterns of DTCs and alkanethiols were subjected to aqueous ethanol solutions containing 2-mercaptoethylamine (cysteamine, 0.1 M) and ammonium hydroxide (0.3 M), and evaluated by confocal microscopy using changes in relative transmittivity.
Results and Discussion
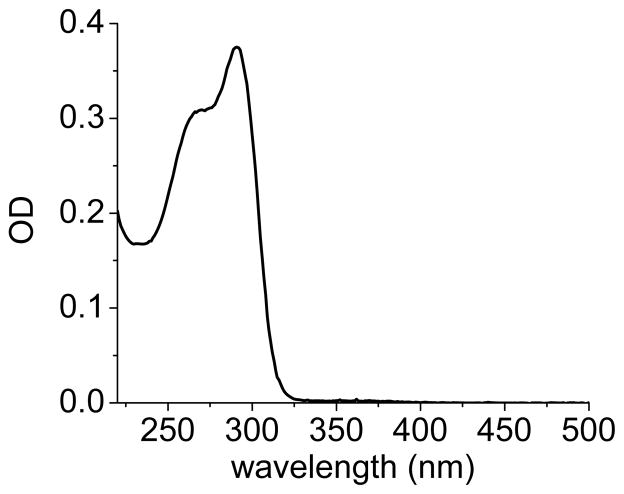
Monovalent and polyvalent DTCs were generated by the condensation of CS2 with didecylamine (diC10) and linear polyethyleneimine (PEI, 22 kDa), respectively. PEI is widely recognized as a versatile platform for mediating surface conjugation by electrostatic adsorption and by covalent crosslinking,20,26,27 and its multivalent nature is expected to contribute toward robust surface passivation. Although PEI is known to exist as a cationic aggregate in water, we observe that treatment of a 1 wt% PEI suspension (effective concentration of amine = 0.23 M) with a stoichiometric amount of CS2 and KOH produces a water-soluble species at pH 10 or above. The effective molar extinction coefficient of PEI-DTC based on monomer concentration is 8,040 M−1 cm−1 (Figure 1, λmax=290 nm); a comparison with the extinction values of other recently studied DTCs indicates that 90% or more of the amines in PEI have been converted into DTCs.4
Figure 1.
UV absorbance spectrum of aqueous PEI-DTC formed in situ (46 μM; ε = 8,040 M−1 cm−1). Molarity is based on monomer concentration (-CH2CH2NH-).
It is important to note that while DTCs can be generated using organic bases alone (i.e. amines), their adsorption onto freshly cleaned Au substrates may be impeded by the nonspecific adsorption of amines or their conjugate acids. The kinetics of DTC adsorption onto clean Au substrates (oxidized by treatment with piranha solution) has been characterized previously by second-harmonic generation analysis, which indicated that the first-order rate constants were retarded by the competitive adsorption of alkylammonium counterions.4 In this study, we prepared DTCs as potassium salts to minimize adventitious fouling by counterions.
Planar Au substrates were first cleaned by immersion in piranha solution, then soaked overnight in deionized water prior to DTC exposure, allowing the reactive surface oxide to revert to metallic Au.28 The reduced Au substrates were then immersed in deaerated DTC solutions for 12 hours at room temperature, rinsed with copious amounts of methanol or water, and dried under a stream of nitrogen, to produce passivated surfaces that were optically smooth upon visual inspection. Chemically roughened Au substrates (see Materials and Methods) were washed and immediately immersed in DTC solutions as described above to produce samples having optically diffuse reflectance. Low-molecular weight species such as diC10-DTC can assemble as monolayers (i.e. DAMs) on Au substrates, as indicated by x-ray photoelectron spectroscopy (XPS),3,4 however the polymer PEI-DTC is unlikely to form uniform layers at the molecular scale. The chemisorption of multivalent polymers can be expected to proceed with lower surface coverages than monovalent species,29 and the physisorption of polyelectrolytes on smooth substrates is well known to produce mushroom-like islands with nanoscale roughness.30 Nevertheless, the deposition of PEI-DTC onto Au produces optically uniform films suitable for photopatterning.
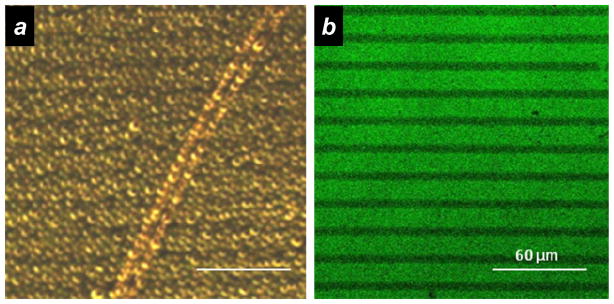
DTC-coated substrates were subjected to UV photolithography by exposure through a quartz photomask under ambient conditions, and further treated with alkanethiols for the preparation of mixed or binary monolayers (Scheme 2). The photopatterned substrates were not directly visible by optical microscopy under brightfield conditions, but could be readily imaged by alternative optical imaging methods. The simplest of these involved a “breath test” based on the size-dependent light scattering of condensed microdroplets, using darkfield microscopy to image regional differences in droplet nucleation and growth (Figure 2a). Photopatterned PEI-DTCs on roughened Au substrates could also be imaged by conjugation with fluorescent dyes such as fluorescein isothiocyanate (FITC), for direct visualization by fluorescence microscopy (Figure 2b). Although Au substrates are well known for their efficient quenching of excited-state fluorophores, this can be prevented and even inverted by introducing nanoscale roughness surface, which generates localized surface plasmon modes that can modulate excited-state lifetimes and enhance the rate of photoemission.24,31 We note that while roughened metal films can also be produced by electrochemical or metal-evaporation techniques,32,33,34 the electroless deposition of Ag followed by galvanic displacement with Au is more convenient and practical for converting smooth Au surfaces into substrates that are compatible with fluorescence imaging. The nanoscale corrugation may lead to some local differences in optical intensities resulting in a grainy image quality, but overall the fluorescence intensities appear quite uniform (Figure 2b).
Figure 2.
Optical imaging of photopatterned PEI-DTC layers (15-μm lines), backfilled with 2-mercaptoethanol (5-μm lines). (a) “Breath test” of binary patterned layers on smooth Au substrate, based on darkfield imaging of condensed microdroplets. Bar = 60 μm. (b) Fluorescence image of photopatterned PEI-DTCs on roughened Au substrate, with subsequent labeling by FITC.
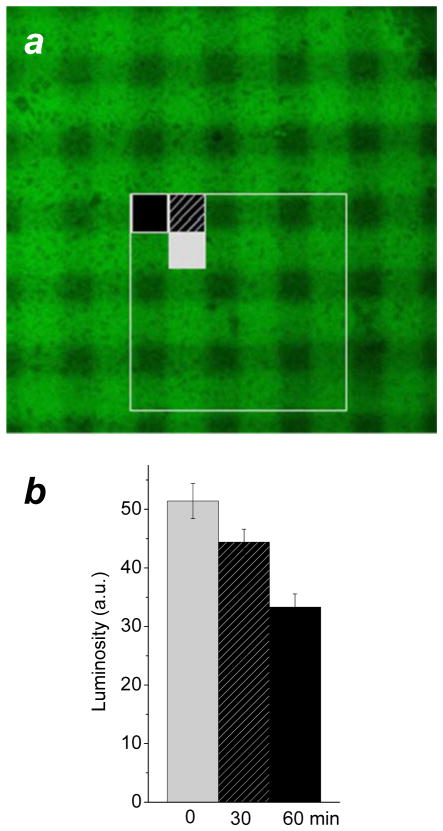
DTCs on Au are more resistant to degradation and desorption than simple alkanethiols under various environmental conditions due to their strong chemisorption,3 and may thus require a higher exposure to UV irradiation for complete ablation. In this regard, we observed PEI-DTCs on Au to be remarkably tenacious under photooxidative conditions, with incomplete removal even after a 60-min exposure to an unfiltered Cd lamp (λmax=226 nm). The resistance of PEI-DTC layers to photooxidation enabled us to use UV photolithography to create monolayers with gradient functionality (Figure 3a).35 PEI-DTC layers on roughened Au substrates were exposed to UV irradiation through a 10-μm grating in two 30-min sittings, with the photomask rotated 90 degrees in between exposure times, to produce a crosshatched pattern with three different densities of amines in decreasing order of UV exposure time. The photopatterned substrate was then treated with mEG3-C11-SH for displacement and backfilling of the UV-exposed regions, in order to evaluate the gradation in amine density within the crosshatched regions. Electrostatic adsorption of FITC-labeled bovine serum albumin (BSA-FITC) and imaging by confocal fluorescence microscopy provided a simple comparison of the mean luminosities from different regions of the photopatterned substrate (Figure 3b). It should be noted that the UV-exposed regions retained significantly higher affinity for BSA-FITC than reference substrates coated only with mEG3-C11 SAMs (not shown), indicating incomplete displacement of photooxidized PEI-DTC.
Figure 3.
(a) Confocal fluorescence image of BSA-FITC adsorbed onto a crosshatched PEI-DTC layer. A photopatterned PEI-DTC on a roughened Au substrate was backfilled with mEG3-C11-SH, then treated with BSA-FITC for a qualitative evaluation of amine density. (b) Relative luminosities (8-bit green channel) from three different regions of the crosshatched PEI-DTC, correlating BSA-FITC adsorption with UV total exposure times of 0, 30 and 60 min. Mean luminosities and standard deviations were taken from 9 separate regions within boxed area in (a).
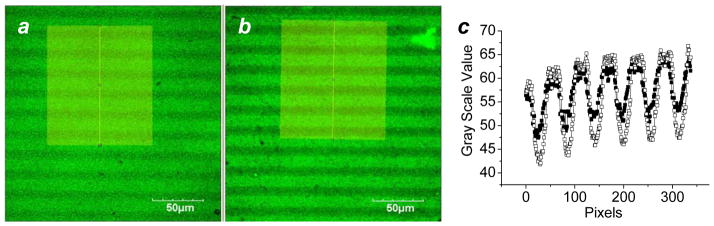
The robust character of PEI-DTC on roughened Au substrates was also confirmed by a direct comparison with an alkanethiol-based SAM. Roughened Au substrates passivated with PEI-DTC was photopatterned then backfilled with H2N-C11 thiol, stored for one week in air at 4 °C (protected from light), then treated with an aqueous FITC solution and examined by confocal fluorescence microscopy. The luminosity difference between PEI-DTC- and thiol-passivated regions was greater after a one-week storage period, relative to that measured from a freshly prepared substrate (Figure 4). We attribute the increase in contrast to desorption of oxidized H2N-C11 thiols during FITC labeling and washing.
Figure 4.
Confocal fluorescence image of a binary passivation layer comprised of PEI-DTC (15-μm lines) and H2N-C11 thiol (5-μm lines). (a) Freshly prepared binary patterns labeled with FITC; (b) binary patterns labeled with FITC after 1 week exposure to air at 4 °C. (c) Fluorescence intensity linescans of images (a) and (b), freshly labeled (■) and 1 week later (□), respectively.
Encouraged by the apparent robustness of DTC passivation, we further investigated their potential to protect the underlying Au substrate from chemical corrosion. Chemically induced corrosion requires that the etchant diffuse through the passivating layer at an appreciable rate, and depends both on the quality of monolayer packing and the stability of the chemisorbed monolayer. In the binary DTC/thiol passivation system, the former is more strongly chemisorptive but less likely to be close-packed and possibly permeable to molecular agents, whereas the latter should form densely packed SAMs but are more prone to oxidation and surface desorption.
Binary patterns comprised of DTCs and thiols were prepared on smooth, semitransparent Au substrates (t=50 nm) for characterization by confocal brightfield (transmission) imaging. In this study, diC10- and PEI-DTCs were used to address the issue of binding valency in DTC-passivated regions, whereas C12 and mEG3-C11 thiols were used to address the issue of hydrophilicity in thiol-passivated regions. Changes in transmittivity were monitored as a function of exposure time to an ethanolic solution of cysteamine (0.1 M) and NH4OH (0.3 M) at room temperature, a condition known to etch thiol-passivated Au surfaces.36
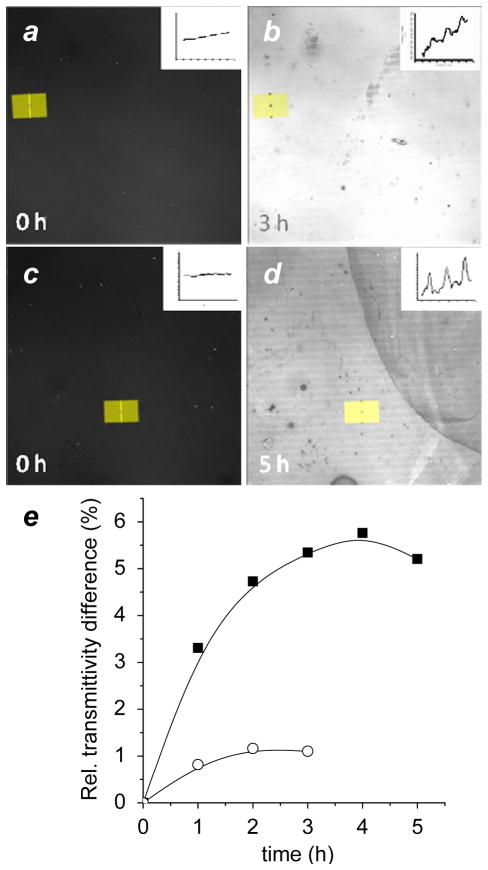
In the case of binary patterns comprised of diC10-DTC and C12 thiol, the alternating regions exhibited modest but discernible differences in optical transmission, with the former showing more resistance to etching (Figure 5a,b). A control experiment involving photopatterned diC10-DAMs without backfill with C12 thiols revealed a similar level of optical contrast, confirming that the thiol-based SAMs provided negligible protection against cysteamine. A greater difference in transmittivity was observed for binary patterns comprised of PEI-DTC and mEG3-C11 thiol (Figure 5c,d), indicating that the polyvalent PEI-DTC conferred additional resistance against chemical etching. Maximum contrast was achieved at 4 hours, after which the DTC-passivated regions degraded more rapidly (Figure 5e). We believe further protection against chemical etchants by DTC passivation is possible, either by reducing the number of surface defects during DTC chemisorption or by forming a chemically impermeable polymer coating, such as that created by emulsion polymerization.37
Figure 5.
Transmission confocal images revealing differential resistance of DTC- and thiol-passivated Au substrates to chemical etchant (cysteamine). (a,b) Binary patterns comprised of diC10-DTC and C12 thiol, before and 3 h after exposure to etchant. (c,d) Binary patterns comprised of PEI-DTC and mEG3-C11 thiol, before and 5 h after exposure to etchant. Representative linescan profiles (yellow) shown at upper right. (e) Relative rate of substrate etching as a function of exposure time to etchant.
Acknowledgments
The authors gratefully acknowledge financial support from the National Science Foundation (CHE-0957738), the National Institutes of Health (RC1 CA147096), the Department of Defense (W911SR-08-C-0001) administered through the U.S. Army RDECOM (Edgewood Contracting Division), and the Center for Sensing Science and Technology at Purdue University. We thank Prof. Ron Reifenberger and David Lyvers for providing the photomask and for helpful discussions.
References
- 1.Ulman A. Chem Rev. 1996;96:1533–1554. doi: 10.1021/cr9502357. [DOI] [PubMed] [Google Scholar]
- 2.(a) Flink S, van Veggel FCJM, Reinhoudt DN. Adv Mater. 2000;12:1315–1328. [Google Scholar]; (b) Love JC, Estroff LA, Kriebel JK, Nuzzo RG, Whitesides GM. Chem Rev. 2005;105:1103–1170. doi: 10.1021/cr0300789. [DOI] [PubMed] [Google Scholar]
- 3.Zhao Y, Pérez-Segarra W, Shi Q, Wei A. J Am Chem Soc. 2005;127:7328–7329. doi: 10.1021/ja050432f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu H, Coleman DM, Dehen CJ, Geisler IM, Zemlyanov D, Chmielewski J, Simpson GJ, Wei A. Langmuir. 2008;24:8660–8666. doi: 10.1021/la801254b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.(a) Huff TB, Hansen MN, Zhao Y, Cheng JX, Wei A. Langmuir. 2007;23:1596–1599. doi: 10.1021/la062642r. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Huff TB, Tong L, Zhao Y, Hansen MN, Cheng JX, Wei A. Nanomedicine. 2007;2:125–132. doi: 10.2217/17435889.2.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Tong L, Zhao Y, Huff TB, Hansen MN, Wei A, Cheng JX. Adv Mater. 2007;19:3136–3141. doi: 10.1002/adma.200701974. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Wei Q, Song HM, Leonov AP, Hale JB, Oh D, Ong QK, Ritchie KP, Wei A. J Am Chem Soc. 2009;131:9728–9734. doi: 10.1021/ja901562j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharma J, Chhabra R, Yan H, Liu Y. Chem Commun. 2008;2140–2142 doi: 10.1039/b800109j. [DOI] [PubMed] [Google Scholar]
- 7.Hansen MN, Chang L-S, Wei A. Supramol Chem. 2008;20:35–40. doi: 10.1080/10610270701647927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li M, Gao F, Yang P, Wang L, Fang B. Surface Sci. 2008;602:151–155. [Google Scholar]
- 9.(a) Vickers MS, Cookson J, Beer PD, Bishop PT, Thiebaut B. J Mater Chem. 2006;16:209–215. [Google Scholar]; (b) Cormode DP, Davis JJ, Beer PD. J Inorg Organomet Polym Mater. 2008;18:32–40. [Google Scholar]
- 10.(a) Guerrini L, Garcia-Ramos JV, Domingo C, Sanchez-Cortes S. Langmuir. 2006;22:10924–10926. doi: 10.1021/la062266a. [DOI] [PubMed] [Google Scholar]; (b) Guerrini L, Garcia-Ramos JV, Domingo C, Sanchez-Cortes S. Anal Chem. 2009;81:953–960. doi: 10.1021/ac801709e. [DOI] [PubMed] [Google Scholar]; (c) Guerrini L, Garcia-Ramos JV, Domingo C, Sanchez-Cortes S. Phys Chem Chem Phys. 2009;11:1787–1793. doi: 10.1039/b812811a. [DOI] [PubMed] [Google Scholar]
- 11.Patel G, Kumar A, Pal U, Menon S. Chem Commun. 2009:1849–1851. doi: 10.1039/b822734a. [DOI] [PubMed] [Google Scholar]
- 12.Knight ER, Cowley AR, Hogarth G, Wilton-Ely J. Dalton Trans. 2009:607–609. doi: 10.1039/b814476a. [DOI] [PubMed] [Google Scholar]
- 13.Murphy FA, Suarez S, Figgemeier E, Schofield ER, Draper SM. Chem Eur J. 2009;15:5740–5748. doi: 10.1002/chem.200801930. [DOI] [PubMed] [Google Scholar]
- 14.(a) Wessels JM, Nothofer HG, Ford WE, von Wrochem F, Scholz F, Vossmeyer T, Schroedter A, Weller H, Yasuda A. J Am Chem Soc. 2004;126:3349–3356. doi: 10.1021/ja0377605. [DOI] [PubMed] [Google Scholar]; (b) Morf P, Raimondi F, Nothofer HG, Schnyder B, Yasuda A, Wessels JM, Jung TA. Langmuir. 2006;22:658–663. doi: 10.1021/la052952u. [DOI] [PubMed] [Google Scholar]; (c) Morf P, Ballav N, Putero M, von Wrochem F, Wessels JM, Jung TA. J Phys Chem Lett. 2010;1:813–816. [Google Scholar]
- 15.Cao R, Jr, Diaz A, Cao R, Otero A, Cea R, MCR-A, Serra C. J Am Chem Soc. 2007;129:6927–6930. doi: 10.1021/ja068349v. [DOI] [PubMed] [Google Scholar]
- 16.(a) Park MH, Ofir Y, Samanta B, Arumugam P, Miranda OR, Rotello VM. Adv Mater. 2008;20:4185–4188. [Google Scholar]; (b) Park MH, Ofir Y, Samanta B, Rotello VM. Adv Mater. 2009;21:2323–2325. [Google Scholar]; (c) Park MH, Duan X, Ofir Y, Creran B, Patra D, Ling XY, Huskens J, Rotello VM. ACS Appl Mater Interfaces. 2010;2:795–799. doi: 10.1021/am9009007. [DOI] [PubMed] [Google Scholar]
- 17.Zhao Y, Newton JN, Liu J, Wei A. Langmuir. 2009;25:13833–13839. doi: 10.1021/la902087e. [DOI] [PubMed] [Google Scholar]
- 18.Eckermann AL, Shaw JA, Meade TJ. Langmuir. 2010;26:2904–2913. doi: 10.1021/la902839r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adak AK, Leonov AP, Ding N, Thundimadathil J, Kularatne S, Low PS, Wei A. Bioconjugate Chem. 2010;21:2065–2075. doi: 10.1021/bc100288c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Subramani C, Ofir Y, Patra D, Jordan BJ, Moran IW, Park MH, Carter KR, Rotello VM. Adv Funct Mater. 2009;19:2937–2942. [Google Scholar]
- 21.Tarlov MJ, Burgess DRF, Gillen G. J Am Chem Soc. 1993;115:5305–5306. [Google Scholar]
- 22.Ryan D, Parviz BA, Linder V, Semetey V, Sia SK, Su J, Mrksich M, Whitesides GM. Langmuir. 2004;20:9080–9088. doi: 10.1021/la048443u. [DOI] [PubMed] [Google Scholar]
- 23.Lee AWM, Chan WH, Chiu CML, Tang KT. Anal Chim Acta. 1989;218:157–160. [Google Scholar]
- 24.Shankar SS, Rizzello L, Cingolani R, Rinaldi R, Pompa PP. ACS Nano. 2009;3:893–900. doi: 10.1021/nn900077s. [DOI] [PubMed] [Google Scholar]
- 25.Pale-Grosdemange C, Simon ES, Prime KL, Whitesides GM. J Am Chem Soc. 1991;113:12–20. [Google Scholar]
- 26.Sadtler B, Wei A. Chem Commun. 2002:1604–1605. doi: 10.1039/b204760h. [DOI] [PubMed] [Google Scholar]
- 27.Moon JM, Wei A. J Phys Chem B. 2005;109:23336–23341. doi: 10.1021/jp054405n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsai H, Hu E, Perng K, Chen M, Wu JC, Chang YS. Surface Sci. 2003;537:L447–450. [Google Scholar]
- 29.Johnson PA, Levicky R. Langmuir. 2004;20:9621–9627. doi: 10.1021/la048458s. [DOI] [PubMed] [Google Scholar]
- 30.Holmberg K, Jonsson B, Kronberg B, Lindman B. Surfactants and Polymers in Aqueous Solution. John Wiley & Sons; Chichester, U.K: 2003. [Google Scholar]
- 31.Lakowicz JR. Anal Biochem. 2001;298:1–24. doi: 10.1006/abio.2001.5377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hupp JT, Larkin D, Weaver MJ. Surface Sci. 1983;125:429–451. [Google Scholar]
- 33.Norrod KL, Sudnik LM, Rousell D, Rowlen KL. Appl Spectrosc. 1997;51:994–1001. [Google Scholar]
- 34.Oates TWH, Shiratori Y, Noda S. J Phys Chem C. 2009;113:9588–9594. [Google Scholar]
- 35.Toh CR, Fraterman TA, Walker DA, Bailey RC. Langmuir. 2009;25:8894–8898. doi: 10.1021/la9019537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ducker RE, Leggett GJ. J Am Chem Soc. 2006;128:392–393. doi: 10.1021/ja0555771. [DOI] [PubMed] [Google Scholar]
- 37.Quaroni L, Chumanov G. J Am Chem Soc. 1999;121:10642–10643. [Google Scholar]