Abstract
It has long been known that cells can divide unequally by shifting the mitotic spindle to one side. Two recent reports identify an alternative way to generate daughter cells of different sizes.
All good cell biologists know that the mitotic spindle determines the plane of cytokinesis. Ray Rappaport, the godfather of cytokinesis [1], showed that experimentally moving a spindle could change the site of cytokinesis [2], and cytokinesis can be prevented by removing the spindle from a cell at least a few minutes before the cytokinetic furrow normally forms [3–4]. Recent work has begun to outline a mechanism for the mitotic spindle's furrow-inducing activity. Astral microtubules and midzone microtubules affect myosin distribution and actin architecture through local RhoA activation and Rac inactivation at the equatorial cortex, where the actin and myosin will form a contractile pursestring [5–7]. In nearly all cells, the spatial relationship between the spindle and the actomyosin-rich furrow is consistent with the above causal relationships: The spindle's position predicts accurately where furrowing will occur.
However, exceptions exist. In 2000, Kaltschmidt and colleagues reported live imaging of microtubules in Drosophila neuroblasts and showed a cell division plane that did not lie midway between the two spindle poles, but instead lied closer to one of the poles, resulting in daughter cells of two different sizes [8]. Now a new report from Cabernard and colleagues provides evidence that the furrow can be positioned independently of the spindle in these neuroblasts, by a mechanism that involves an asymmetric enrichment of cortical myosin in mitotic cells [9]. A second report from Ou and colleagues reports a similar mechanism in another system, a C. elegans neuroblast, and tests directly the role of asymmetric myosin enrichment in controlling daughter cell size [10]. The new results challenge the universality of the mitotic spindle as the primary determinant of furrow positioning, establishing an asymmetric cortical enrichment of myosin during mitosis as an alternative means to divide unequally in some cells.
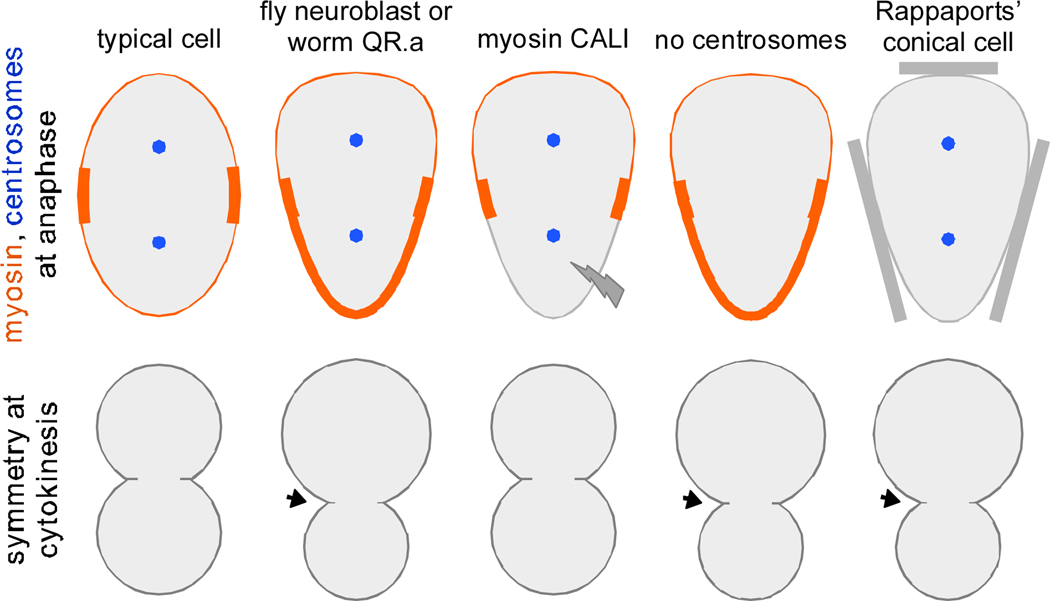
Drosophila neuroblasts divide asymmetrically, producing a larger daughter that retains stem cell characteristics and a smaller daughter that differentiates. Cabernard and colleagues showed by live imaging of neuroblasts that myosin localized in an unexpected pattern during mitosis, becoming enriched asymmetrically in the cell cortex on the side where the smaller daughter cell will form (Figure 1). Interestingly, this enrichment was established even before any mitotic spindle asymmetries were apparent, suggesting that the myosin asymmetry was not caused by any observed spindle asymmetries. Indeed, cells with spindles rotated out of their normal axis still had normal myosin enrichment on the basal side of the cell. The rotated spindle and the basal myosin each appeared to induce a furrow -- a double furrow! What does it mean? In Drosophila neuroblasts, the myosin crescent appears to provide an independent, parallel mechanism for cleavage furrow positioning, along with canonical spindle-derived cues.
Figure 1.
Diagram of myosin and spindle pole (centrosome) positions at anaphase (top), and the resulting cytokinetic furrow position (bottom).
Ou and colleagues investigated the asymmetric division of another cell, a C. elegans neuroblast. Division of a neuroblast called QR.a produces daughter cells of different sizes and fates, with the larger daughter becoming a neuron, and the smaller daughter undergoing apoptosis. Despite this asymmetry of size and fate, the mitotic spindle of this cell is aligned in the center at metaphase, just as in Drosphila neuroblasts [8, 10]. And just as in Drosophila neuroblasts, the authors show that myosin becomes enriched asymmetrically in the cortex of one side of the cell during anaphase, on the side that will form the smaller daughter cell.
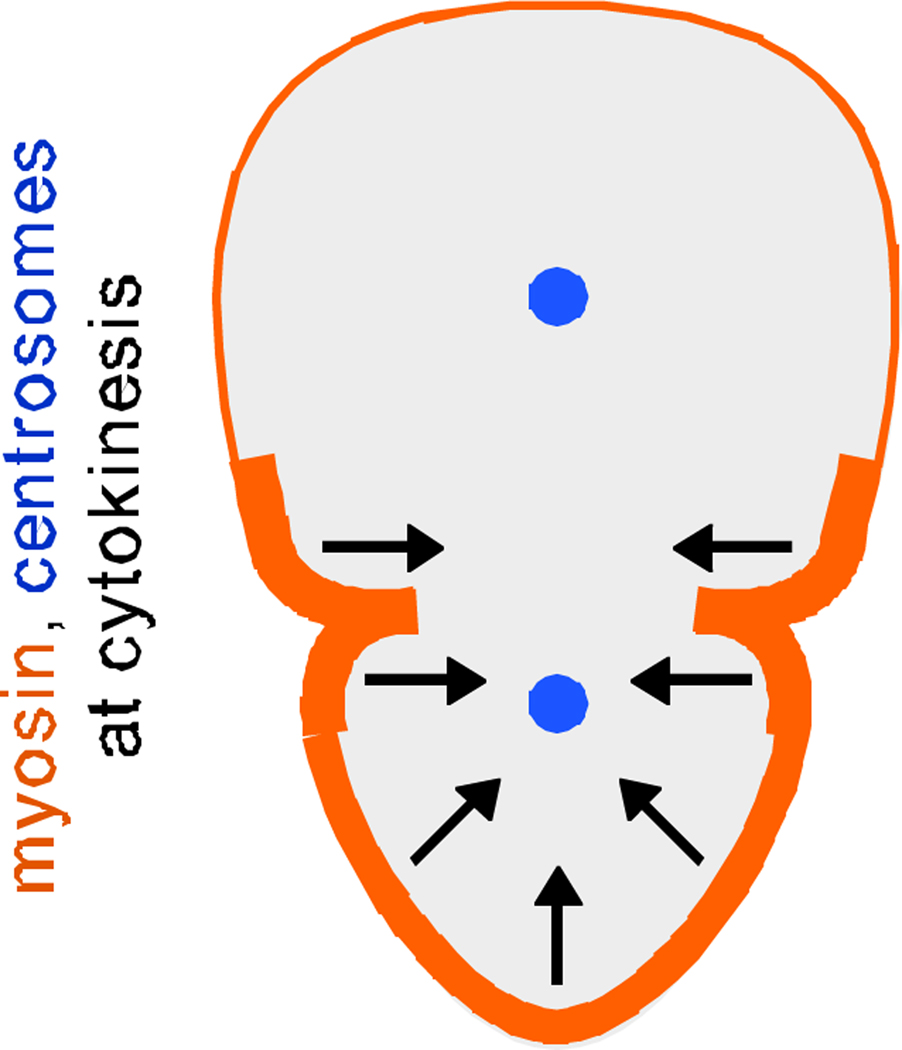
Ou et al. propose a mechanism for how asymmetric myosin might drive unequal cell division: Cortical contractility driven by the myosin crescent could shrink one hemisphere of the dividing cell, driving cytoplasmic flow through the ingressing cleavage furrow and resulting in two differently-sized daughter cells (Figure 2). To test myosin's role in specific regions of the cell, they used chromophore-assisted laser inactivation (CALI), a technique that uses reactive products emitted upon fluorophore excitation to locally inactivate proteins [11–13]. They found that CALI of GFP-myosin in the region where it is enriched could prevent that side of the dividing cell from shrinking normally, leading in some cases to equal cell division (Figure 1), whereas CALI of a control GFP-tagged molecule could not. Interestingly, in some cases in which daughter cell size was affected, cell fate was also affected. The results show that asymmetric enrichment of myosin in mitosis can locally affect the size and the fate of a nascent daughter cell.
Figure 2.
Model proposing how an asymmetric myosin crescent can affect daughter cell size, after reference [10]. Arrows represent actomyosin-driven contractions shrinking one end of the cell during cytokinesis.
With mitotic cells constricted at one end by cortical actomyosin-derived forces, the resulting cell shape resembles one of the classic Rappaport experiments. After his retirement as a professor, Ray Rappaport and his wife Barbara, both in their 70's at the time, published a paper in which they reported the effect of squeezing mitotic cells into conical shapes [14]. Why squeeze cells into conical shapes? A computer model developed by Albert Harris and Sally Gewalt had predicted that cells of this shape could be used to distinguish between existing models for spindle positioning [15]. Interestingly, the result of changing cell shape was similar to that shown in worm and fly neuroblasts: The furrow formed closer to the narrow end of the cell, instead of midway between the two spindle poles (Figure 1). The authors interpreted this as resulting from a more effective interaction between the spindle and the cortex at the narrow end of the cell, as the cortex in this end of the cell lies closer to the spindle.
The Rappaports' result shows that tapering one end of a cell can result in the furrow forming closer to the spindle pole at that end of the cell. Might the asymmetric myosin observed in worm and fly neuroblasts affect furrow position in this way? Myosin is itself a key furrow component, so an indirect effect of myosin on furrow positioning through cell shape -- allowing the spindle and cortex to more effectively interact on one end of the cell -- might seem circuitous. Indeed, in fly neuroblasts, Cabernard et al. were able to eliminate the spindle altogether by colcemid treatment and then genetically bypass the spindle checkpoint, and they found that the basal myosin enrichment and asymmetric cytokinesis still occurred. This result establishes the new mechanism as a truly independent mechanism, not requiring the mitotic spindle. It will be interesting to learn the extent to which this will stand as an independent mechanism in other systems.
How does myosin localize asymmetrically in mitotic cells? Temporal and spatial mechanisms must be involved. Metaphase-arrested Drosophila neuroblasts failed to localize myosin asymmetrically, suggesting that myosin localization must be temporally linked to mitotic progression, as asymmetric spindle positioning is in certain cells [9, 16]. The authors show that spatial regulation of myosin depends on familiar players, a PAR-1-like kinase called PIG-1 in C. elegans neuroblasts, and the asymmetric Pins protein in Drosophila, which has well-established roles in spindle positioning [9–10, 16–18]. These molecular links are likely to serve as key steps toward dissecting the mechanisms of asymmetric myosin distribution in mitotic cells.
References
- 1.Canman JC, Wells WA. Rappaport furrows on our minds: the ASCB Cytokinesis Meeting Burlington, VT July 22–25, 2004. J Cell Biol. 2004;166:943–948. doi: 10.1083/jcb.200409019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rappaport R. Repeated furrow formation from a single mitotic apparatus in cylindrical sand dollar eggs. J. Exp. Zool. 1985;234:167–171. doi: 10.1002/jez.1402340120. [DOI] [PubMed] [Google Scholar]
- 3.Hiramoto Y. Cell division without mitotic apparatus in sea urchin eggs. Exp. Cell Res. 1956;11:630–636. doi: 10.1016/0014-4827(56)90171-9. [DOI] [PubMed] [Google Scholar]
- 4.Rappaport R. Cytokinesis - Cleavage Furrow Establishment in Cylindrical Sand Dollar Eggs. Journal of Experimental Zoology. 1981;217:365–375. [Google Scholar]
- 5.Glotzer M. The molecular requirements for cytokinesis. Science. 2005;307:1735–1739. doi: 10.1126/science.1096896. [DOI] [PubMed] [Google Scholar]
- 6.Canman JC. Cytokinetic astralogy. J Cell Biol. 2009;187:757–759. doi: 10.1083/jcb.200911084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bement WM, Miller AL, von Dassow G. Rho GTPase activity zones and transient contractile arrays. Bioessays. 2006;28:983–993. doi: 10.1002/bies.20477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaltschmidt JA, Davidson CM, Brown NH, Brand AH. Rotation and asymmetry of the mitotic spindle direct asymmetric cell division in the developing central nervous system. Nat Cell Biol. 2000;2:7–12. doi: 10.1038/71323. [DOI] [PubMed] [Google Scholar]
- 9.Cabernard C, Prehoda KE, Doe CQ. A spindle-independent cleavage furrow positioning pathway. Nature. 2010;467:91–94. doi: 10.1038/nature09334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ou G, Stuurman N, D'Ambrosio M, Vale RD. Polarized Myosin Produces Unequal-Size Daughters During Asymmetric Cell Division. Science. 2010 doi: 10.1126/science.1196112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diefenbach TJ, Latham VM, Yimlamai D, Liu CA, Herman IM, Jay DG. Myosin 1c and myosin IIB serve opposing roles in lamellipodial dynamics of the neuronal growth cone. J Cell Biol. 2002;158:1207–1217. doi: 10.1083/jcb.200202028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobson K, Rajfur Z, Vitriol E, Hahn K. Chromophore-assisted laser inactivation in cell biology. Trends Cell Biol. 2008;18:443–450. doi: 10.1016/j.tcb.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang FS, Wolenski JS, Cheney RE, Mooseker MS, Jay DG. Function of myosin-V in filopodial extension of neuronal growth cones. Science. 1996;273:660–663. doi: 10.1126/science.273.5275.660. [DOI] [PubMed] [Google Scholar]
- 14.Rappaport R, Rappaport BN. Cleavage in conical sand dollar eggs. Dev Biol. 1994;164:258–266. doi: 10.1006/dbio.1994.1196. [DOI] [PubMed] [Google Scholar]
- 15.Harris AK, Gewalt SL. Simulation testing of mechanisms for inducing the formation of the contractile ring in cytokinesis. J Cell Biol. 1989;109:2215–2223. doi: 10.1083/jcb.109.5.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCarthy Campbell EK, Werts AD, Goldstein B. A cell cycle timer for asymmetric spindle positioning. PLoS Biol. 2009;7:e1000088. doi: 10.1371/journal.pbio.1000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cordes S, Frank CA, Garriga G. The C. elegans MELK ortholog PIG-1 regulates cell size asymmetry and daughter cell fate in asymmetric neuroblast divisions. Development. 2006;133:2747–2756. doi: 10.1242/dev.02447. [DOI] [PubMed] [Google Scholar]
- 18.Siller KH, Doe CQ. Spindle orientation during asymmetric cell division. Nat Cell Biol. 2009;11:365–374. doi: 10.1038/ncb0409-365. [DOI] [PubMed] [Google Scholar]