Abstract
The valid application of accelerometry and interpretation of its output (i.e., counts per unit time) for the measurement of walking behavior in persons with multiple sclerosis (MS) rests upon multiple untested assumptions. This study tested the assumption that a waist-worn accelerometer should capture the intra- and inter-person variation in walking behavior. Twenty-four participants with a neurologist-confirmed diagnosis of MS and who were ambulatory with minimal assistance undertook three 6-min periods of over-ground walking that involved comfortable (CWS) and then slower (SWS) and faster (FWS) walking speeds while wearing ActiGraph, model 7164, accelerometers around the waist and ankle. The experimental manipulation of walking was successful such that the CWS was 76.7 ± 13.0 m/min (range = 55.6–105.14), whereas the SWS and FWS were 64.3 ± 12.3 m/min (range = 44.5–90.1) and 89.1 ± 13.8 m/min (range = 60.9–116.4), respectively. Movement counts from the waist and ankle-worn accelerometer were strongly associated with the manipulation of speed, but the association was stronger for the waist than ankle based on both eta-squared estimates (η2 values = .78 and .46) and the average squared multiple correlations from individual regression analyses (R2 values = .97 ± .04 and .88 ± .21). The bivariate correlation between movement counts from the waist-worn accelerometer and speed of walking (r = .823, p = .001) was large in magnitude and significantly different (z = 3.22, p = .001) from that between movement counts from the ankle-worn unit and walking speed (r = .549, p = .001). This study provides novel evidence that an accelerometer worn around the waist captures intra- and inter-person variation in over-ground walking behavior in those with MS.
Keywords: Walking behavior, Impairment, Multiple sclerosis
Walking behavior is a common and important outcome in clinical research and practice involving persons with multiple sclerosis (MS). This behavior is typically measured in clinical and research settings using objective tests such as the timed 25-foot walk, 500-m walk, and 6-min walk (6MW) [1]. Recognizing the importance of ecologically valid walking measures, researchers have become interested in the objective measurement of walking in the context of real life [2,3] as such information might compliment the clinical and research assessment battery. Motion sensors such as accelerometers have the potential to become a “gold standard” measure of real-life walking behavior in persons with neurological conditions [1] including MS [4,5], although some research has questioned the reliability and validity of accelerometers as a measure of physical activity in MS [6]. The valid application of accelerometry and interpretation of its output (i.e., counts per unit time) for the measurement of walking behavior rests upon the untested assumptions that (a) an accelerometer worn around the waist (i.e., center of mass) will capture the signal associated with walking; (b) walking is a primary and significant form of movement undertaken by ambulatory persons with MS (i.e., ambulatory people with MS walk in everyday life); and (c) the intensity, duration, and frequency of walking in real-life are directly proportionate to temporal and spatial parameters of walking (e.g., speed, stride length and cadence, and double support time) [4]. The first assumption, in particular, implies that a waist-worn accelerometer should capture intra- and inter-person variation in walking behavior. To test this assumption, the present study experimentally manipulated walking behavior during three 6-min periods of over-ground walking (i.e., three 6MW tests) [7] that involved comfortable (CWS) and then slower (SWS) and faster (FWS) walking speeds; the SWS and FWS involved slower and faster walking, respectively, than CWS. This permitted an examination of the hypotheses that accelerometer output from a waist-worn unit would (a) better capture change as a function of the manipulation of walking speed within each person (i.e., intra-person variation) and (b) more strongly correlate with between person differences in walking speed across the 6MW tests (i.e., inter-person variation) than an unit worn around the ankle.
1. Method
1.1. Participants
We recruited participants within our database who provided permission for being contacted about future research opportunities and resided within ~50 miles of the University of Illinois campus. The recruitment involved the delivery of flyers through the U.S. postal service, e-mail announcements, and scripted telephone calls. There were 50 people who expressed interest in the study and all of them underwent screening for inclusion criteria over the telephone. The inclusion criteria were age between 18 and 65 years, neurologist-confirmed diagnosis of MS, ambulatory with minimal assistance, relapse free in previous 30 days, and absence of self-reported risk-factors for undertaking strenuous physical activity including cardiovascular diseases, diabetes, hyperlipidemia, and hypertension. Twenty-four persons met those inclusion criteria, provided written consent, and completed the study protocol, whereas the 26 remaining persons who underwent screening did not meet inclusion criteria and were excluded from participation. The sample included 20 women and 4 men with a mean age of 43.0 (SD = 11.7) years. The mean duration of time since diagnosis of MS was 11.1 years (SD = 8.5) and all participants had relapsing-remitting MS. The median Patient Determined Disease Steps (PDDS) scale [8] score for the participants was 1 (range of 0–4). The median PDDS score corresponds with minimal gait disability whereas the upper end PDDS score corresponds with single point assistance, either early cane use or some other form of support (e.g., touching a wall or leaning on someone’s arm) for walking all the time or part of the time.
1.2. Accelerometer
This study included ActiGraph, model 7164, accelerometers, and all units were initially calibrated by the manufacturer and, based on our internal assessment using a 15-min period of walking on a treadmill by a member of the research team, there was less than 10% variation in output among the accelerometers. Research has further indicated that there is precision with this model of accelerometer based on 5% inter-monitor variation and 2% intra-monitor variation in controlled trials using a motorized turntable [9]. The model 7164 accelerometer is small (5.1 cm × 4.1 cm × 1.5 cm) and light weight (43 g) and contains a single, vertical axis piezoelectric bender element that generates an electrical signal proportional to the force acting on it. Acceleration detection ranges in magnitude from 0.05 to 3.2 Gs and the frequency response ranges from 0.25 to 2.5 Hz. Motion outside normal human movements is rejected by the aforementioned band-pass filter. The acceleration signal is digitized by an analog-to-digital converter and numerically integrated over a pre-programmed epoch interval. The integrated value is stored in RAM as movement counts and the integrator is reset at the end of each interval. The movement counts represent a digital integration of a bidirectional, positive and negative, acceleration signal over a given time window. This acceleration signal is proportional to the net external force generated during bodily movement associated with walking behavior (i.e., this is not the same as an accelerometer-based pedometer that only measures steps or strides). Some advantages of an accelerometer compared with a pedometer include increased accuracy, particularly with slow walking speeds, capacity for measuring amount and intensity of movement rather than simply steps taken, and prolonged monitoring, whereas the disadvantages might include difficulty of use, staff training, processing time, and data preparation [6].
Participants wore one accelerometer on an elastic belt that was positioned around the waist above the non-dominant hip that was identified as opposite of self-reported dominant handedness, and another accelerometer on an elastic belt that was positioned around the ankle above the ipsilateral lateral malleolus. The placement on the non-dominant hip was based on convention and evidence of non-significant differences in recordings of maximal motor activity by accelerometers placed on the dominant and non-dominant sides of the body [10]. We did not place accelerometers on central back because there is minimal difference in units from accelerometers between placements on the central back and waist (i.e., both locations correspond with the trunk and should capture motion of the center of mass) and wearing the accelerometer on the lower back would be uncomfortable in daily life (e.g., sitting in a chair with a back rest or a car seat). We set the recording epoch to be 1 s and the movement counts were expressed as the average of movement counts per minute (i.e., counts/min) across each of the 6MW tests.
1.3. Protocol
The procedure was approved by a University Institutional Review Board and all participants first provided written informed consent. Participants completed a demographic questionnaire and the PDSS, were fitted with the accelerometers, and then walked the course for the 6MW tests as a familiarization protocol. The course for the 6MW tests was located in an accessible, rectangular hallway that was clear of obstructions and foot traffic. This was followed by the three 6MW tests that were interspersed with 5–10 min of rest. We opted for 6 min of walking as it is consistent with walking protocols in MS and is long enough for capturing variation in walking behavior between people of different ambulatory capacities [5]. The first 6MW involved the participant’s CWS, and the two remaining 6MW tests were undertaken above (FWS) and below (SWS) the participant’s CWS (i.e., ±13.4 m/min of CWS or ~0.5 mph). The order of the second and third 6MW tests was counter-balanced so as to minimize order effects, and there was between 5 and 10 min of seated rest between the 6MW tests with the duration varying depending on the participant’s readiness for the subsequent test. This was done as a strategy for minimizing fatigue between 6MW tests and we note that previous research has administered three sequential 6MW tests among persons with MS who have EDSS scores between 0 and 6.5 without undue fatigue [7]. One researcher followed approximately 1 m behind the participant during the first 6MW test that involved the participant’s CWS and recorded the speed using a measuring wheel (Stanley MW50, New Briton, CT) outfitted with a calibrated bicycle computer (Cateye Velo5, Osaka, Japan). The manipulation of walking speed in the second and third 6MW tests was accomplished by having the participant follow approximately 1 m behind a researcher who controlled the speed using the aforementioned measuring wheel. Participants received $20 remuneration for undertaking the study.
1.4. Data analysis
The analyses were performed using SPSS, version 17 (SPSS, Chicago, IL). Descriptive data are presented as mean ± standard deviation along with the actual range of scores. The intra-personal differences in actual walking speed (m/min) and movement counts (counts/min) across the three 6MW tests were examined using a series of one-way, within-subjects ANOVAs; speed was the single within-subjects factor with three levels of SWS, CWS, and FWS. The ANOVA on actual walking speed indicated the success of the speed manipulation across the three 6MW tests. The ANOVAs on movement counts provided eta-squared (η2) values that indicated the strength of the association between movement counts and speed for each participant. We further estimated the strength of the linear association between actual walking speed and movement counts as a squared multiple correlation (R2) from a linear regression analysis for each participant. The overall degree of association within each person (i.e., intra-person) was expressed using descriptive statistics along with the range of R2 values. The relationships between actual walking speed and movement counts overall (i.e., inter-person) and for each of the three 6MW tests were further estimated using Pearson product-moment correlations (r). We adopted criteria of .3, .5, and .7 as rules for judging the correlations as small, moderate, or large in magnitude [11]. The ANOVAs and multiple regression analyses provided an estimate of the intra-person variation, whereas the bivariate correlation analysis provided an estimate of the interperson variation.
2. Results
2.1. Accelerometer data
All 24 participants completed the three 6MW tests without stopping. There were no missing accelerometer data across the three tests in this study (i.e., no accelerometer malfunction nor download failures).
2.2. Check on manipulation of walking speed
The ANOVA identified a statistically significant main effect of speed on the actual speed of walking across the 6MW tests, F(2,46) = 454.69, p < .0001, η2 = .95. The CWS was 76.7 ± 13.0 m/min (range = 55.6–105.14), whereas the SWS and FWS were 64.3 ± 12.3 m/min (range = 44.5–90.1) and 89.1 ± 13.8 m/min (range = 60.9–116.4), respectively. The large η2 value indicated that there was a proportionate increase in actual walking speed across the three 6MW tests. This supports the success of the experimental manipulation of actual walking speed.
2.3. Intra-person variation
The ANOVA identified a statistically significant main effect of speed on movement counts from the waist-worn accelerometer across the 6MW tests, F(2,46) = 80.60, p < .0001, η2 = .78. The movement count for the CWS was 2988 ± 1125 counts/min (range = 1015–5055), whereas movement counts for the SWS and FWS were 2224 ± 882 counts/min (range = 685–3815) and 3980 ± 1509 counts/min (range = 1285–6921), respectively. The large η2 value indicated that there was a strong association between movement counts from a waist-worn accelerometer and the manipulation of walking speed during the three 6MWs.
The ANOVA further identified a statistically significant main effect of speed on movement counts from the ankle-worn accelerometer across the 6MW tests, F(2,46) = 19.47, p < .0001, η2 = .46. The movement counts for the CWS were 7590 ± 2831 counts/min (range = 3260–14,057), whereas movement counts for the SWS and FWS were 6755 ± 2807 counts/min (range = 2555–13,260) and 8750 ± 3536 counts/min (range = 3687–18,984), respectively. The η2 value indicates that there was a strong association between movement counts from an ankle-worn accelerometer and the manipulation of walking speed during the three 6MWs. The qualitative comparison of η2 values between ANOVAs suggested that the output from the ankle-worn accelerometer was not as tightly coupled with speed as was the output from the waist-worn accelerometer.
The linear regression analysis indicated that movement counts from the waist-worn accelerometer were tightly coupled with speed for each participant (R2 = .97 ± .04, range = .84–1.00). The analysis further indicated that movement counts from the ankle-worn accelerometer were tightly coupled with speed for each participant (R2 = .88 ± .21, range = .40–1.00). The average R2 was significantly larger for the waist-worn accelerometer than the ankle-worn unit based on a paired-samples t-test, t(23) = 2.17, p = .04. This quantitatively demonstrated that the output from the ankle-worn accelerometer was not as tightly coupled with speed as was the output from the waist-worn accelerometer.
2.4. Inter-personal variation
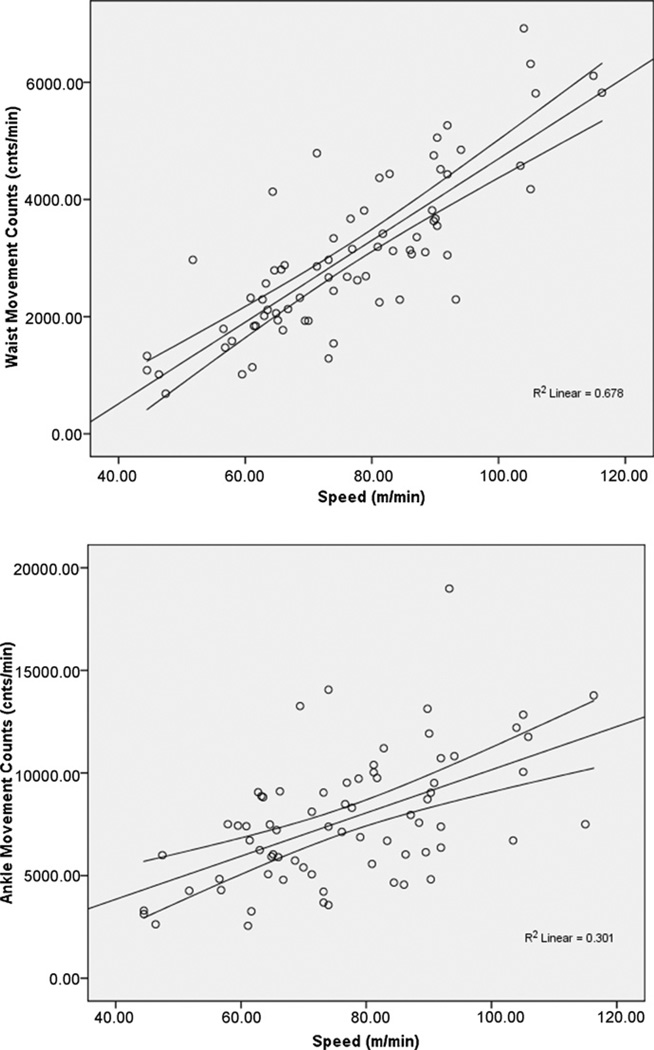
Scatter plots along with 95% confidence intervals of the overall association between movement counts and speed of walking are provided in Fig. 1. The bivariate correlation between movement counts from the waist-worn accelerometer and speed of walking was large in magnitude and statistically significant (r = .823, p = .001). By comparison, the association between movement counts from the ankle-worn unit and walking speed was moderate in magnitude, but still statistically significant (r = .549, p = .001). Those two correlations were significantly different based on Fisher’s z-test (z = 3.22, p = .001) and the movement counts from the waist had substantially more shared variance with speed than did movement counts from the ankle.
Fig. 1.
Scatter plot along with 95% confidence interval and squared multiple correlation (R2) of the linear associations between movement counts and speed for accelerometer worn on the waist (top) and ankle (bottom) in sample of 24 persons with MS.
Additional bivariate correlations indicated that the associations between movement counts from the waist-worn accelerometer and actual walking speed were statistically significant and large in magnitude for the SWS (r = .824, p = .001), CWS (r = .740, p = .001), and FWS (r = .738, p = .001) 6MW tests. By comparison, the bivariate correlations between movement counts from the ankle-worn unit and walking speed were statistically significant, but generally moderate in magnitude, for the SWS (r = .579, p = .003), CWS (r = .501, p = .013), and FWS (r = .474, p = .019) 6MW tests. The bivariate correlations between movement counts from the waist- and ankle-worn units were statistically significant for the SWS (r = .507, p = .01) and CWS (r = .525, p = .008) 6MW tests, but not the FWS (r = .313, p = .136) 6MW test.
We conducted another analysis of the association between PDDS scores, movement counts from the waist- and ankle-worn accelerometers, and velocity across the three 6MW tests. PDDS scores were neither associated with accelerometer counts from the waist-worn unit for the SWS (r = −.021, p = .92), CWS (r = −.082, p = .70), and FWS (r = −.205, p = .34) 6MW tests nor accelerometer counts from the ankle-worn unit for the SWS (r = .049, p = .82), CWS (r = .032, p = .88), and FWS (r = .008, p = .97) 6MW tests. We further note that PDDS scores were not significantly associated with velocity of the SWS (r = −.170, p = .43), CWS (r = −.274, p = .20), and FWS (r = −.235, p = .27) 6MW tests.
3. Discussion
The major accomplishment of the present study involved a test of an important assumption (i.e., accelerometer worn around the waist should capture intra- and inter-person variation in walking behavior) underlying the valid application and interpretation of accelerometry for the objective measurement of real-life walking behavior in persons with MS. To that end, the results indicated that movement counts from a waist-worn accelerometer were more tightly coupled with intra- and inter-personal variation in over-ground walking behavior (i.e., speed of walking) than were movement counts from an ankle-worn accelerometer. Indeed, the initial analyses of variance indicated that movement counts from a waist-worn accelerometer were strongly associated with the manipulation of over-ground walking speed across the 6MW tests. This was supported by the linear regression analyses on each person’s data and the resulting average R2 value. The final analysis indicated that movement counts from a waist-worn accelerometer were strongly associated with the over-ground walking speed overall and for each of the 6MW tests. The associations were all stronger for the waist-worn accelerometer than the ankle-worn unit and this is because the placement is closest to the center of mass and should better capture whole body movements that accompany intra- and inter-individual variability in ambulation. This study, therefore, provides novel evidence that an accelerometer worn around the waist better captures intra- and inter-person variation in over-ground walking behavior in those with MS than an ankle-worn unit.
There has been an increased interest in the application of motion sensors such as accelerometers for the objective measurement of real-life walking behavior in persons with MS. The existing research indicates that the average of total daily movement counts across a 7-day period from this accelerometer has correlated with scores from the Multiple Sclerosis Walking Scale-12, Expanded Disability Status Scale, mobility subscale of the Performance Scales, and Symptom Inventory [3,5,12–14]. Those data support the observation that motion sensors such as accelerometers have the potential to become a “gold standard” measure of real-life walking behavior in persons with neurological conditions [1] including MS [4,5]. Interestingly, the present study reported that PDDS scores were not significantly correlated with accelerometer counts from the units worn on the waist and ankle during the three 6MW tests. Such an observation might be considered inconsistent with that previous body of research and undermine the application of accelerometers as a measure of walking behavior. We do not believe this to be the case. The most straight-forward explanation for the non-significant associations is the relative lack of gait disability in the study sample. Indeed, the median PDDS score was 1.0 (i.e., noticeable symptoms with minor impact on every day life) and PDDS scores did not correlate with velocity of the 6MW tests (i.e., walking speed is a common marker of gait impairments in MS). To that end, the lack of associations between PDDS scores and accelerometer counts was likely driven by the presence of minimal gait impairment in the present sample.
This application of accelerometry and the valid interpretations of its output (i.e., counts per unit time) for the measurement of walking behavior rests upon the assumptions that (a) an accelerometer worn around the waist (i.e., center of mass) will capture the signal associated with walking; (b) walking is a primary and significant form of movement undertaken by ambulatory persons with MS; and (c) the intensity, duration, and frequency of walking are directly proportionate to impairments in walking parameters (e.g., speed, stride length and cadence, and double support time). The present study supports the first assumption whereby an accelerometer worn around the waist captures intra- and inter-person variation in walking behavior in persons with MS. Such evidence provides a stronger basis for the application of accelerometry for the objective measurement of real-life walking behavior and we await additional research that further replicates our findings and tests the other two assumptions underlying this application.
The waist-worn accelerometer was more strongly associated with variation in walking behavior than was the ankle-worn unit in this sample of persons with MS. This difference in strength of association is largely consistent with the notion that an accelerometer should be placed around the waist as this placement is closest to the center of body mass and should capture whole-body movement during ambulation. By comparison, placement of an accelerometer around the ankle would be considerably farther from the center of mass and does not capture whole-body movement during ambulation. This placement, instead, would measure leg movements alone during walking. To that end, the accelerometer should be placed on the body in a manner that is consistent with the movement under investigation. If the application is for measuring real-life walking behavior, placement around the center of mass (i.e., the waist) is most appropriate and should be the preferential site in subsequent applications and tests of this technology as a measure of real-life walking behavior in MS.
There are other assumptions that require examination for the further application of accelerometer as a measure of walking behavior in persons with MS. One of the untested assumptions is that the primary contribution into the accelerometer signal is variation associated with the amount of ambulation undertaken during one’s daily life. This straight-forward assumption is that those who ambulate more throughout the day should have greater accelerometer movement counts than those who ambulate less throughout the day. Another of the untested assumptions is that the amount of daily ambulation should be inversely associated with temporal and spatial parameters of ambulation such as double-support time and step length, respectively. This assumption implies that persons who have greater gait abnormalities should accumulate less accelerometer movement counts based on a decrease in the amount of daily walking behavior. This is important as those who have extremely ataxic gait or severe bilateral leg weakness might have excessive bodily movement during ambulation and this might affect both walking behaviors and the output of accelerometers. We further note that some researchers have questioned the reliability and validity of accelerometers as a measure of physical activity in persons with MS [6]. Accordingly, to the extent that the assumptions hold in subsequent research, there will be an even stronger basis for the application of a waist-worn accelerometer as a marker of real-life walking behavior in persons with MS.
There are several limitations of the study that should be considered in the interpretation of our research. The first limitation is that we only examined the association between accelerometer movement counts and speed of walking during a 6-min period of walking. This brief period of walking might not represent the variation in walking speed that occurs in the context of daily community living. Another limitation was that the study included a narrowly defined sample of persons with relapsing-remitting MS who had a relatively short disease duration and low degree of disability. This limits generalization of our results more broadly among those who have MS. The final limitation is that we included a single brand and model of accelerometer, namely the ActiGraph, model 7164. We do not have data that would support the generalizability of our results across brands and models of accelerometers. We further note that the Actigraph, model 7164 was a uni-axial accelerometer and our results should be replicated using a tri-axial accelerometer that measures acceleration in three planes.
In summary, the novel finding of the present study was that an accelerometer worn around the waist captured intra- and inter-person variation in walking behavior, and this supports one of the assumptions underlying the valid application and interpretation of accelerometry for the objective measurement of real-life walking behavior in persons with MS. We are pursuing additional research designed to address the remaining assumptions as this will provide a stronger basis for the application of accelerometry as a marker of real-life walking impairments for clinical research and practice involving persons with MS.
Acknowledgments
Funding
None.
Footnotes
Conflict of interest statement
None.
References
- 1.Pearson OR, Busse ME, van Deursen RW, Wiles CM. Quantification of walking mobility in neurological disorders. QJM. 2004;97:463–475. doi: 10.1093/qjmed/hch084. [DOI] [PubMed] [Google Scholar]
- 2.Gijbels D, Alders G, Van Hoof E, Charlier C, Roelants M, Broekmans T, et al. Predicting habitual walking performance in multiple sclerosis: relevance of capacity and self-report measures. Mult Scler. 2010;16:618–626. doi: 10.1177/1352458510361357. [DOI] [PubMed] [Google Scholar]
- 3.Snook EM, Motl RW, Gliottoni RC. The effect of walking mobility on the measurement of physical activity using accelerometry in multiple sclerosis. Clin Rehabil. 2009;23:248–258. doi: 10.1177/0269215508101757. [DOI] [PubMed] [Google Scholar]
- 4.Goldman MD, Motl RW, Rudick RA. Possible clinical outcome measures for clinical trials in patients with multiple sclerosis. Ther Adv Neurol Disord. 2010;3:229–239. doi: 10.1177/1756285610374117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weikert M, Motl RW, Suh Y, McAuley E, Wynn D. Accelerometry in persons with multiple sclerosis: measurement of physical activity or walking mobility? J Neurol Sci. 2009;290:6–11. doi: 10.1016/j.jns.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 6.Kayes NM, Schluter PJ, McPherson KM, Leete M, Mawston G, Taylor D. Exploring actical accelerometers as an objective measure of physical activity in people with multiple sclerosis. Arch Phys Med Rehabil. 2009;90:594–601. doi: 10.1016/j.apmr.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 7.Goldman MD, Marrie RA, Cohen JA. Evaluation of the six-minute walk in multiple sclerosis subjects and healthy controls. Mult Scler. 2008;14:383–390. doi: 10.1177/1352458507082607. [DOI] [PubMed] [Google Scholar]
- 8.Hadjimichael O, Kerns RB, Rizzo MA, Cutter G, Vollmer T. Persistent pain and uncomfortable sensations in persons with multiple sclerosis. Pain. 2007;127:35–41. doi: 10.1016/j.pain.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 9.Metcalf BS, Curnow JSH, Evans C, Voss LD, Wilkin TJ. Technical reliability of the CSA activity monitor: The EarlyBird Study. Med Sci Sports Exerc. 2002;34:1533–1537. doi: 10.1097/00005768-200209000-00022. [DOI] [PubMed] [Google Scholar]
- 10.Lehnkering H, Strauss A, Wegner B, Siegmund R. Actigraphic investigations on the activity-rest behavior of right- and left-handed students. Chronobiol Int. 2006;23:593–605. doi: 10.1080/07420520600724094. [DOI] [PubMed] [Google Scholar]
- 11.Munro BH. Statistical methods for health care research. 5th ed. Baltimore, MD: Lippincott Williams, and Wilkins; 2005. [Google Scholar]
- 12.Motl RW, Schwartz C, Vollmer T. Continued validation of the symptom inventory in multiple sclerosis. J Neurol Sci. 2009 doi: 10.1016/j.jns.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 13.Motl RW, Snook EM. Confirmation and extension of the validity of the Multiple Sclerosis Walking Scale-12 (MSWS-12) J Neurol Sci. 2008;268:69–73. doi: 10.1016/j.jns.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Motl RW, Snook EM, Wynn DR, Vollmer T. Physical activity correlates with neurological impairment and disability in multiple sclerosis. J Nerv Ment Dis. 2008;196:492–495. doi: 10.1097/NMD.0b013e318177351b. [DOI] [PubMed] [Google Scholar]