Abstract
In the avian embryo, endothelial cells originate from several sources, including the lateral plate and somite mesoderm. In this study, we show that Gata transcription factors are expressed in the lateral plate and in vasculogenic regions of the avian somite, and are able to promote a vascular endothelial fate when ectopically expressed in somite precursors. A fusion of GATA4 to the transcriptional activator VP16 promoted endothelium formation, indicating that GATA transcription factors promote vasculogenesis via activation of downstream targets, while a fusion of GATA4 to the transcriptional repressor engrailed repressed expression of Vascular Endothelial Growth Factor Receptor 2, a marker of endothelial precursors. These findings indicate a role for GATA transcription factors in the differentiation of the endothelium.
Keywords: Endothelial cell differentiation, GATA transcription factors, VEGF, Chick embryo
Introduction
Endothelial cells derive from multiple embryonic sources, including the lateral plate splanchnopleuric mesoderm and the somites (Ben-Yair and Kalcheim, 2008; Bertrand et al., 2010; Boisset et al., 2010; Dieterlen-Lievre and Jaffredo, 2009; Kissa and Herbomel, 2010; Pardanaud et al., 1996). The early splanchnopleuric mesoderm-derived endothelium is hemogenic: it can give rise to hematopoietic cells that are subsequently released into the circulation. In contrast, somite-derived endothelium appears to be restricted to an endothelial fate and does not give rise to hematopoietic tissue (Pardanaud et al., 1996). Some embryonic vessels, such as the aorta, are of dual origin, consisting of a lateral plate-derived hemogenic ventral side, and a somite-derived non-hemogenic dorsal side. After an initial period of hematopoiesis, the ventral wall of the aorta is replaced with somite-derived endothelium and ceases to be hemogenic (Pouget et al., 2006).
Multiple transcription factors have been implicated as playing roles in endothelial cell development (reviewed in (De Val and Black, 2009)), including Scl/Tal, MEF2C, and members of the Ets family (Ferdous et al., 2009; Lee et al., 2008; Lin et al., 1998; Patterson et al., 2005; Visvader et al., 1998). However, aside from the recently characterized Ets family member Etv2 (Ferdous et al., 2009; Lee et al., 2008), most of these factors appear to play roles in either the later differentiation and morphogenesis of already-specified endothelial cells, or in regulating the hemogenic properties of endothelium, rather than a specific role in the initial formation of endothelial cells per se. Members of the Gata family of transcription factors have been found to be required for hematopoiesis in mice (Tsai et al., 1994) or for formation of anterior hemangioblasts (a putative common precursor of endothelium and blood cells) in zebrafish (Peterkin et al., 2009), but a specific role for Gata factors in endothelial cell generation has not been described.
Several signaling pathways have been found to play roles during the early phases of endothelial cell differentiation. including the VEGF (Coultas et al., 2005; Shalaby et al., 1997; Shalaby et al., 1995), hedgehog (Byrd et al., 2002), and BMP (Reese et al., 2004; Winnier et al., 1995) pathways. BMPs can promote endothelial cell formation in the chick (Bressan et al., 2009; Nimmagadda et al., 2005; Reese et al., 2004), and have also been implicated in blood vessel formation and in the formation of hemangioblasts in Xenopus and zebrafish (Chen et al., 2008; Liu et al., 2008; Walmsley et al., 2002). BMP signaling also appears to be important for the vasculogenic properties of other signaling pathways. In mice, BMPs have been found to mediate the vasculogenic activity of Sonic Hedgehog (Shh) (Astorga and Carlsson, 2007; Byrd et al., 2002), and BMPs have also been found to regulate Vascular Endothelial Growth Factor receptor 2 (VEGFR2; flk1) expression during early vascular development (Ben-Yair and Kalcheim, 2008; He and Chen, 2005). However, the mechanisms by which BMP signaling mediates these endothelial-promoting effects have not been well characterized.
The current study reports that GATA transcription factors are expressed in vasculogenic regions of the embryo, can be induced by BMP signaling, can promote endothelial cell differentiation when ectopically expressed, and are required for normal expression of the endothelial cell marker VEGFR2. Taken together with previous data indicating that BMP signaling is required for induction of VEGFR2 and for the production of somite-derived endothelium (Ben-Yair and Kalcheim, 2008), these findings indicate a previously uncharacterized role for GATA factors during endothelial cell differentiation, and suggest that they may play a role in mediating the vasculogenic properties of BMP signaling.
Materials and Methods
Embryo culture and electroporation
Fertilized chick (Gallus gallus, white leghorn, Charles River Spafas) and Japanese quail (Coturnix japonica, Strickland Gamebird Farm) eggs were incubated at 38 degrees C to HH stage 3-5 (Hamburger and Hamilton, 1951) and then placed in modified New Culture as described (Sundin and Eichele, 1992). Electroporations were performed as described (James et al., 2006), and expression of Green fluorescent protein (GFP) in embryos was observed using a Leica dissecting microscope equipped with UV light source. Overall electroporation efficiency was between 50-75% of embryos. All plasmids were electroporated at a concentration of 0.6 mg/ml. If multiple plasmids were used, each plasmid was at a concentration of 0.6mg/ml. Control electroporations used either pCAGGS-GFP or pCAGGs empty vector. DiI-labeled Acetylated Low Density Lipoprotein (acLDL, Molecular Probes) was injected for 5 seconds directly into the heart using pulled glass needles and embryos were incubated for 30 minutes at 37 degrees Celsius before photographing and fixing as for immunostaining.
DNA Constructs
pCAGGS-mGATA4 and pCAGGS-mGATA5 contain the full length coding region of mouse GATA4 and mouse GATA5 respectively cloned into the pCAGGS vector (Niwa et al., 1991). pCAGGS GATA4-VP16 and pCAGGS GATA4-EnR encode for the mouse GATA DNA-binding domain (Zing finger domain) fused to either VP16 activator or Engrailed repressor sequences. All these pCAGGS constructs were obtained by subcloning inserts previously cloned into RCAS vectors, which will be described in detail elsewhere (Kempf et al., manuscript in preparation). pCAGGS-cGATA2 was constructed by isolating mRNA from 2 day old chicken embryos using the RNeasy kit (Qiagen) and producing cDNA using Transcriptor reverse transcriptase (Roche), followed by PCR at 55 degrees using Phusion polymerase (NEBiolabs) using a forward primer containing a XhoI restriction site 5′-ccg ctc gag agg ccc gag gcc tct a-3′ and a reverse primer containing an EcoRI restriction site 5′-ccg gat atc tct gcc acc ttt tgc tt-3′. PCR products were gel-purified, digested with EcoRI and XhoI and purified again using a Qiaprep kit (Qiagen) and cloned directly into pCIG, which is a pCAGGS vector containing an internal ribosome entry site upstream of GFP (gift from A. McMahon). pCAGGs H2B-GFP was a gift of C. Cepko.
Microarray
Approximately 50 explants of presomitic mesoderm from Stage 7-8 chick embryos were cultured for 3 hours either in serum-free chick embryo medium [SF-CEM; (DMEM-F12, 5μg/ml human transferrin (Gibco), 100μg/ml conalbumin (Sigma), 1X insulin-transferrin-selenium (Gibco), 1% Pen-Strep, 1% Glutamine)] or in SF-CEM supplemented with 10 ng/ml of human recombinant BMP-2 (R & D Systems). Cultures were harvested in Trizol, and RNA was prepared as in the manufacturer’s instructions. Reverse Transcription and probe generation were carried out using the IVT Express kit (Affymetrix). Both control and BMP-treated probes were prepared in triplicate from three separate culture experiments. Probes were used to screen an Affymetrix Chick genome microarray, and data was analyzed with Affymetrix Gene Chip Operating software. A detailed description of the methods and results of the microarray experiments will be presented elsewhere.
Immunofluorescence and in situ hybridization
For immunofluorescence, embryos were fixed, processed and sectioned as previously described (James and Schultheiss, 2003; Schultheiss et al., 1997). Briefly, embryos were fixed in 4% paraformaldehyde for 30 minutes at room temperature, incubated in 5% sucrose/PBS briefly then 20% sucrose/PBS overnight before embedding in gelatin/15% sucrose/PBS for cryosectioning at 10 micron thickness and immunostaining. QH1 antibody ((Pardanaud et al., 1987), Developmental Studies Hybridoma Bank) ascites fluid was used at 1:100 dilution; rabbit anti-GFP antibody (Torrey Pines Biolabs) was used at 1:500 dilution; mouse anti-GFP antibody (Abcam) was used at 1:500 dilution; cleaved caspase3 antibody (Cell Signaling Technologies) was used at 1:100 dilution. Alexa-fluor 488 (Invitrogen) and Cy3 (Jackson Immunoresearch) conjugated secondary antibodies were used at 1:250-1:500 dilution. Slides were mounted and coverslipped with Vectashield (Vector Laboratories) and photographed using a Zeiss Axiophot or Nikon Eclipse E1000 microscope.
Whole mount in situ hybridization was carried out essentially as previously described (James and Schultheiss, 2005) with probes for chick Gata4 (Schultheiss et al., 1997), Gata5 (clone ChEST978f11 from the BBSRC Chick EST database, Ark Genomics (Boardman et al., 2002)), GATA2 (cloned by RT-PCR from embryonic chick cDNA using primers directed to the full-length GATA2 sequence) and VEGFR2 (Eichmann et al., 1993). Following development of signal, some embryos were cryosectioned (20 μm), mounted in Gelvatol (Sigma) and photographed using DIC optics using a Zeiss Axiophot microscope.
Quantification
GFP co-localization with acLdl or QH1 was quantified by taking 20x merged photographs of 5 representative sections for each embryo and normalizing by the total number of GFP labeled cells and expressing this ratio as a percentage. Apoptosis was quantified by taking 20x photographs of 3 representative sections for each embryo and counting the number of cells exhibiting cleaved caspase3 staining, normalizing by the number of GFP labeled cells, and expressing this ratio as a percentage. Neuroectodermal regions were excluded from the analysis. Data was analyzed by Student’s t-test (equal variance, 2 tails) and all error bars are one standard deviation above and below the mean.
Explant cultures, RCAS infection, and gene expression analysis
Presomitic mesoderm (PSM) and somite I-III were prepared from HH10 chicken embryos as described (Kempf et al., 2007; Zeng et al., 2002). For retroviral infection, freshly isolated explants were incubated with 10 μl of concentrated virus on ice for 2 h, before embedding in collagen gels. At day 5, individual explants were lysed and RNA was purified using a QIAGEN RNeasy mini kit, according to the manufacturer’s instructions. Gene expression analyses were performed by real time PCR using the 7900HT fast real-time PCR system (Applied Biosystems). GAPDH was used as an internal control. PCR conditions and primer sequences are available upon request.
Results
GATA factors are BMP-responsive genes and are expressed in vasculogenic regions of the embryo
As part of a project to identify genes involved in the patterning of the mesoderm downstream of BMP signaling in the avian embryo (James and Schultheiss, 2005), we exposed explants of paraxial mesoderm to BMP2 for 3 hours and screened a chicken genome microarray with probes synthesized from BMP-induced and uninduced tissues. Among the many genes significantly up-or down-regulated in response to BMP treatment, GATA5 was the second most strongly BMP-induced gene (9-fold induction, p<1×10−10). (See Materials and Methods for a description of the experiment; a full description of the microarray screen results will be published elsewhere.)
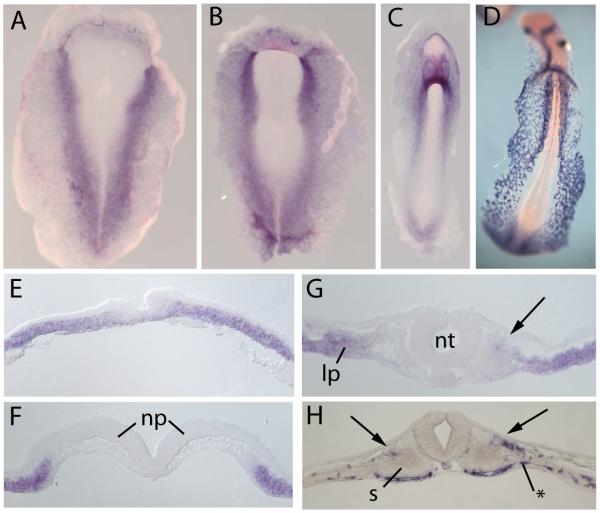
We examined the expression of GATA5 in chick embryos by whole mount in situ hybridization (Fig. 1). At early primitive streak stages, GATA5 were expressed in lateral regions of the embryo (Fig. 1D). At early somite stages, GATA5 became progressively more restricted to the splanchnic or ventral portion of the lateral plate mesoderm, with some expression in the endoderm. The expression of GATA5 in lateral plate mesoderm overlaps the major site of vascular precursors in the early embryo, which are located between the endoderm and the splanchnic layer of the lateral plate (Bressan et al., 2009; Reese et al., 2004). By Stage 10 (ten somite stage), GATA5 was also expressed in the dorsolateral quadrant of the epithelial stage somite (Fig. 1F). At this stage, significant overlap could be seen between the expression domains of GATA5 and of the early endothelial marker VEGFR2 in both the lateral plate and the lateral somite (Fig. 1; see also (Ben-Yair and Kalcheim, 2008; Pouget et al., 2006)).
Figure 1. Expression of GATA5 and VEGFR2 mRNA in early chick embryos.
Whole mount in situ hybridization for GATA5 (A-C, E-G) and VEGFR2 (D,H) in Stage 5 (A,E), Stage 7 (B, F), and Stage 10 (C,D,G,H) chick embryos. GATA5 is expressed in a broad region of the lateral plate, and at Stage 10 expression extends into the dorsolateral quadrant of the somite (arrow, G). VEGFR2 is expressed predominantly in a layer between the sphlanchnopleuric layer of the lateral plate (asterisk, H) and is also expressed in the lateral somite (arrow, H). Scale bar = 100 microns in sections. (np, neural plate; s, somite)
We also examined expression of the closely related gene GATA4, which has previously been found to be induced in the somites in response to BMP signaling (Reshef et al., 1998; Schultheiss et al., 1997). GATA4 was expressed in a partially overlapping pattern with GATA5 in the lateral plate, although it was not observed in the somites (Supplementary Figure 1). Interestingly, GATA5 expression consistently extends more medially than GATA4. This may indicate a greater sensitivity of GATA5 to BMP signals, and could explain why GATA5 but not GATA4 was identified as a BMP2-responsive gene in the microarray screen. Another GATA family member, GATA2, has been reported to be expressed in the dorsolateral quadrant of the somite, in a pattern similar to those observed for GATA5 and VEGFR2 (Pouget et al., 2006). Thus, multiple members of the GATA family of transcription factors are expressed in vasculogenic regions of the early embryo, in both the lateral plate and the somites.
Ectopic expression of GATA factors promotes endothelial cell formation
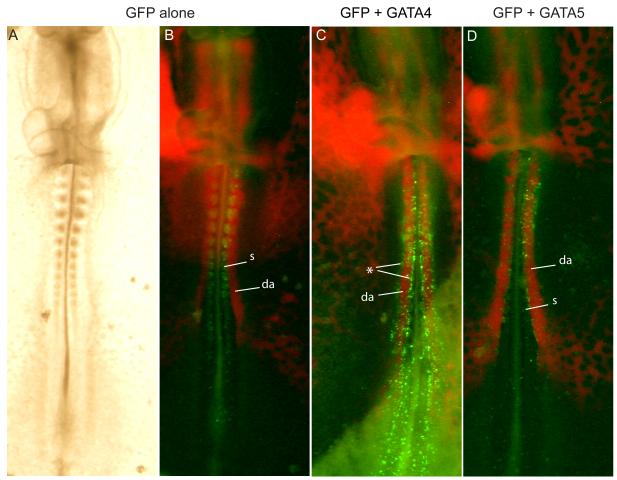
To investigate whether GATA5 could be involved in establishing cell fates downstream of BMP signaling, GATA5 was ectopically expressed in paraxial mesoderm, which includes the somitic precursors. Electroporation of a control GFP-expressing construct into chick anterior streak at HH3-5 (a region that contains somite precursors) resulted in labeling of numerous cells in the somite and presomitic mesoderm 24 hours later at about HH11. (Fig. 2A, B) In contrast, when a GATA5-expressing plasmid was co-electroporated with GFP, few GFP-expressing cells were visible in the somites, but GFP-positive cells were clearly associated with the vasculature, especially the aorta (Fig. 2D). Similar results were seen upon electroporation of GATA4 (Fig. 2C, see Table 1 for summary of electroporation results).
Figure 2. GATA-electroporated cells are associated with vasculature.
Gastrulating cells in chick anterior streak electroporated with GFP (A, B), GFP + GATA4 (C), or GFP + GATA5 (D). Injection with acLDL (red) reveals vasculature. GFP labeled cells are primarily in the somites and presomitic mesoderm in controls (B) but are found lining the aorta in GATA electroporated embryos (C, D). Note that because Gata-expressing cells do not remain in the somite, the somites are not strongly labeled by Gfp in C,D. In C, the asterisk marks rows of electroporated cells that have moved out from the somites and accumulated on their medial and lateral edges. (da, dorsal aorta; s, somite)
Table 1.
Electroporation Results. Fate of Gfp labeled cells quantified by observing native fluorescence using dissecting microscope at 10x magnification. Numbers indicate number of Chick (A) or Quail (B) embryos with Gfp in the indicated region divided by the total number of embryos electroporated
| A. Chick | ||
|---|---|---|
| Plasmid | Somites | Aorta |
| GFP | 16/27 (59%) | 3/27 (11%) |
| GATA4 | 7/35 (20%) | 25/35 (71%) |
| GATA5 | 2/24 (8%) | 17/24 (71%) |
| GATA-VP16 | 2/11 (18%) | 10/11 (91%) |
| GATA-EnR | 14/18 (78%) | 0/18 (0%) |
| B. Quail | ||
|---|---|---|
| Plasmid | Somites | Aorta |
| GFP | 16/23 (70%) | 0/23 (0%) |
| GATA4 | 2/5 (40%) | 4/5 (80%) |
| GATA5 | 0/24 (0%) | 23/24 (96%) |
| GATA-VP16 | 0/2 (0%) | 2/2 (100%) |
| GATA-EnR | 3/4 (75%) | 0/4 (0%) |
| GATA2 | 0/5 (0%) | 5/5 (100%) |
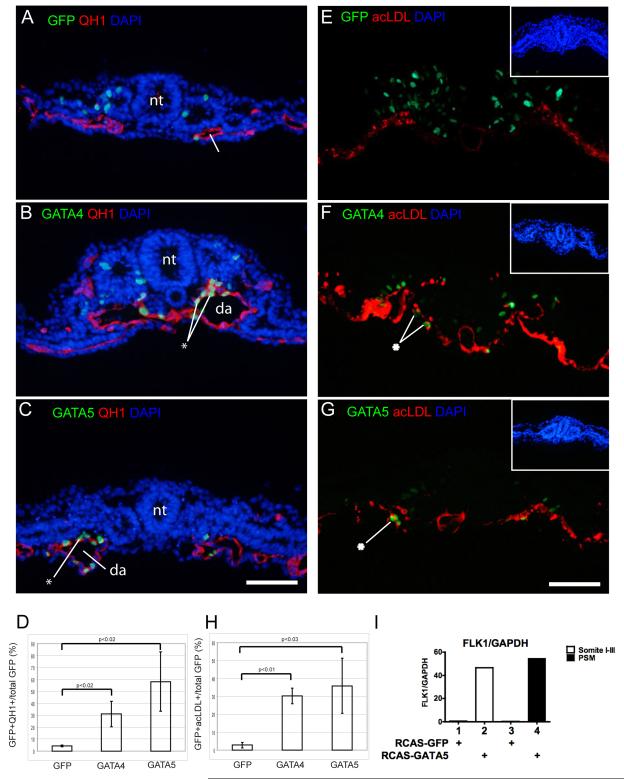
To determine the cell types produced by GATA misexpression in medial mesodermal cells, we used the quail embryo model system, and took advantage of the quail endothelial-specific antibody QH1 (Pardanaud et al., 1987). While only 4% of GFP-labeled quail somitic precursors were positive for QH1 staining (Fig. 3A, D), 31% of GATA4 and 58% of GATA5 ectopically expressing cells were vascular endothelial cells (Fig. 3B-D). Similar results were obtained with electroporation of GATA2, where 34% of electroporated cells were found in the endothelium (Supplementary Figure 2). GATA co-electroporated cells were found predominantly in the aorta, but could also be found in other vasculature in both ventral and dorsal regions of the embryo. Although there was an overall decrease in GFP labeling in GATA electroporated embryos (mean of 217 cells/control embryo vs. 108 cells/embryo and 81/embryo for GATA4 and GATA5, respectively) there was an overall increase in the absolute number of cells co-staining for GFP and endothelial markers (mean of 10 cells/control embryo vs. 30 cells/embryo and 47 cells/embryo for GATA4 and GATA5, respectively). GATA5 was also found to be able to strongly induce the expression of the endothelial cell marker VEGFR2 when ectopically expressed via a retroviral vector in cultured somites or paraxial mesoderm (Fig. 3I). Together, the increase in the number of GFP-positive endothelial cells in embryos overexpressing GATA2/4/5 in paraxial mesoderm and the induction of the vascular marker VEGFR2 by GATA5 in cultured somites implies that ectopic expression of GATA transcription factors either promotes the endothelial cell fate and/or promotes endothelial cell proliferation or viability.
Figure 3. GATA factors promote a vascular endothelial fate.
Gastrulating cells in quail (A-D) or chick (E-H) embryos were electroporated with GFP (A,E), GFP + GATA4 (B,F) or GFP + GATA5 (C,G). Endothelium was detected by the QH1 antibody (A-C) or by incorporation of DiI-labeled acLDL (E-G). Electroporated cells are primarily in somites in controls (A,E) but are found in the aorta in GATA electroporated embryos (B-C, E-F). Asterisk indicates Gfp-expressing cells in the aortic endothelium. In D and H, GFP positive cells in vasculature are expressed as a percentage of total GFP (n=3 for each condition). (I) Retroviral-driven expression of Gata5 in cultures of early somites or presomitic mesoderm (psm) induces strong upregulation of VEGFR2, as assessed by qRT-PCR. Scale bar = 100 microns. (da, dorsal aorta; nt, neural tube; psm, presomitic mesoderm).
To confirm these findings, similar experiments were performed using DiI-labeled acetylated low density lipoprotein (acLdl) as a marker of endothelial cells (Fig. 3 E-H) (Jaffredo et al., 1998). AcLdl specifically binds to scavenger receptors expressed on the surface of vascular endothelial cells and macrophages and is quickly endocytosed. Sections of chick embryos electroporated and then labeled with acLdl showed that while in controls about 3% of GFP-labeled somitic precursors can normally be found in the vascular endothelium, 30% of GFP-labeled cells are found in the vascular endothelium following GATA4 misexpression and 36% following GATA5 misexpression.
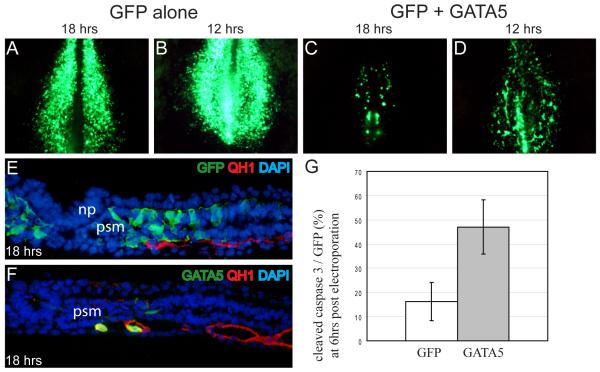
In an attempt to determine whether Gata-electroporated cells exhibited signs of endothelial differentiation while they were still in the somites, electroporated embryos were examined at earlier time points. At 12 or 18 hours after GATA4/5 electroporation, labeled cells were already visible in medial regions in the coalescing vascular plexus, while GFP-labeled cells in controls were distributed uniformly in the presomitic mesoderm. (Fig. 4A-D) Upon sectioning, co-localization of GFP and the endothelial marker QH1 was specifically observed in embryos that had been co-electroporated with GATA expression vehicles; GFP+/QH1+ cells were not seen in the somites themselves (Fig. 4E, F). At 6 hours after electroporation, GFP+/QH1+ cells were not yet seen (data not shown). Thus, it appears likely that Gata-electroporated cells initiate QH1 expression only after they have left the somite and become incorporated into blood vessels.
Figure 4. Dual response to Gata over-expression in the presomitic mesoderm: vasculogenesis and increased cell death.
Quail embryos electroporated at st3-5 with GFP (A, B) or GFP and GATA5 (C, D) and collected at 18hrs (A, C) or 12hrs (B, D) post electroporation. (E, F) Sections of embryos in A and C respectively stained for GFP (green), QH1 (red) and nuclei (blue). (G) Comparison of apoptosis detected by cleaved caspase3 staining at 6 hours post electroporation, a time when numbers of GFP positive cells are comparable (n=9 for both GFP and GATA5, p<0.01). Scale bar = 100 microns in sections. (np, neural plate; psm, presomitic mesoderm)
Ectopic expression of GATA factors in the somite promotes apoptosis in paraxial mesoderm cells
Overall, there seemed to be fewer GFP positive cells in embryos that had been co-electroporated with GATA expression vehicles than in control embryos that had been electroporated with only a GFP expression vehicle, raising the possibility that GATA misexpression could either block proliferation or induce apoptosis of paraxial mesodermal cells. This was investigated by staining for cleaved caspase3, an early marker of apoptosis. At 6 hours after electroporation, a time point at which GFP positive cells are detectable in similar numbers in the somites of both control and GATA electroporated embryos, cleaved caspase3-positive cells increased about 3-fold in GATA electroporated embryos. (Fig. 4G) Thus, ectopic expression of GATA4/5 in the paraxial mesoderm is accompanied by excess cell death, in addition to promotion of an endothelial cell differentiation program.
GATA factors act as transcriptional activators in order to promote endothelial cell formation
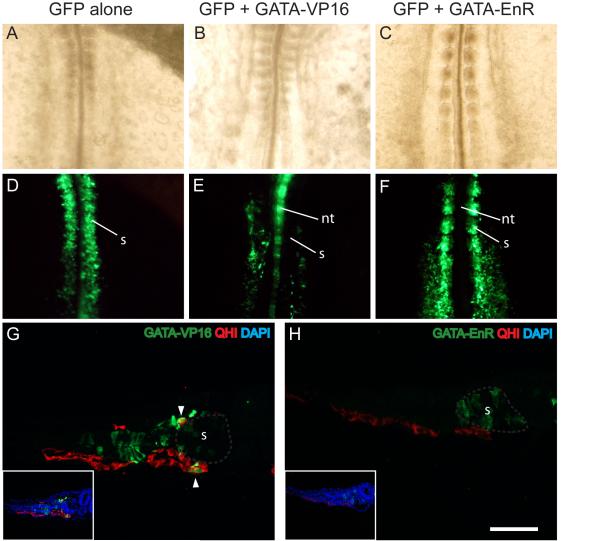
To determine whether GATA factors were acting as transcriptional activators or repressors to induce vascular endothelial cells, we employed constructs expressing the GATA4 DNA binding domain fused to either the VP16 transcriptional activation domain (GATA-VP16) or the Engrailed transcriptional repressor domain (GATA-EnR). Similar to what was obtained with the misexpression of wild-type GATAs, embryos co-electroporated with GATA-VP16 and GFP displayed labeled cells predominantly in the aorta (Fig. 5B, E, G). Electroporation of GATA-VP16 also resulted in up-regulation of the endothelial cell marker VEGFR2 (6 of 6 embryos, Fig. 6A-D). In contrast, cells that had been co-electroporated with GATA-EnR remained in either the somites or presomitic mesoderm and failed to migrate to the aortae. (Fig. 5C, F, H). We also noted that GATA-VP16 expressing cells seemed to be excluded from the somite, but were present in either intermediate mesoderm or vasculature, while GATA-EnR expressing cells were found in both the somite or presomitic mesoderm but not in the vasculature. (Fig. 5G, H) These findings indicate that GATA4/5 act as transcriptional activators to promote an endothelial cell fate.
Figure 5. GATAs act as a transcriptional activators during initiation of vasculogenesis.
Chick embryos (A-F) and sections of quail embryos (G, H) electroporated with GFP (A, D) or GFP + GATA4-VP16 (B, E, G) or GFP + GATA4-EnR (C, F, H). GFP labeling reveals cells primarily in the somites in controls and with the GATA-EnR construct (D, F, H) but in vasculature when the GATA-VP16 construct is expressed (E, G). Note in E that neural tube and aortae are labeled without labeling of intervening somites. Sections (G,H) are stained for GFP (green), QH1 (red) and DAPI (blue). The main photos show GFP and QH1 only, whereas DAPI is added in the insets. Gray outlines in G and H indicate somite. Arrowheads indicate co-staining in G. Scale bar = 100 microns for sections. (nt, neural tube; s, somite)
Figure 6. VEGFR2 expression is responsive to GATA activity in vivo.
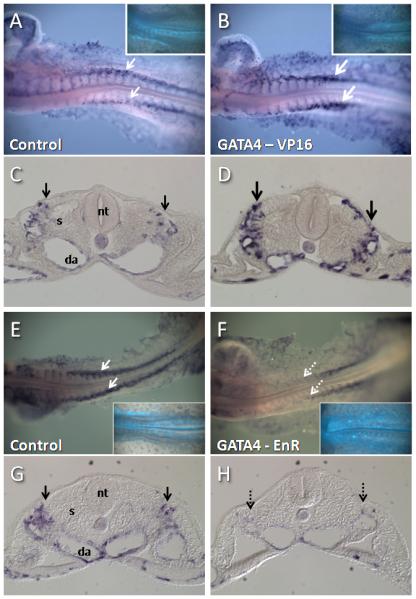
Embryos were electroporated with control (A,C,E,G) Gata4-VP16 (B,D), or GATA4-enR constructs at Stage 4 and examined for expression of VEGFR2 transcripts at Stage 12-13. C,D,G, and H are sections of embryos in A,B, E, and F, respectively. Insets in A,B,E, and F show Gfp expression, which indicates electroporated cells. Gata4-VP16 produced an increase in VEGFR2 expression (compare bold arrows in B,D with arrows in A,C), while Gata4-enR produced a reduction of VEGFR2 expression (compare dashed arrows in F,H with arrows in E,G), particularly in anterior areas of the embryo. Embryos in A-D were stained in parallel and photographed under identical conditions, as were embryos in E-H. (Da, dorsal aorta; nt, neural tube; s, somite).
Inhibition of GATA activity leads to loss of expression of the endothelial cell marker VEGFR2
Since GATA factors appear to act as transcriptional activators in the context of embryonic vasculogenesis, the GATA-enR construct was used to investigate whether repression of GATA target genes had an effect on normal embryonic vasculogenesis. Electroporation of the Gata4-enR construct resulted in marked inhibition of VEGFR2 expression in Stage 12-13 embryos (Fig. 6 E-H) (7 of 7 EnR-electroporated embryos showed reduction of VEGFR2 expression (dashed arrows in Figure 6 F,H), compared with 1 of 5 control-electroporated embryos). The reduction in VEGFR2 expression was most pronounced in anterior regions of the embryo. Electroporation of Gata4-enR did not appear to cause large-scale malformations of blood vessels, and a few endothelial cells co-expressing Gata-enR and the endothelial cell marker QH1 could be detected (data not shown). Thus, wild type levels of Gata activity seem to be required for normal expression of VEGFR2, but not necessarily for expression of all other endothelial cell markers or for blood vessel formation itself.
Discussion
In the current experiments, ectopic expression of GATA factors in somitic precursors was found to promote the formation of endothelium. Conversely, inhibition of Gata factor activity by means of a reverse function Gata4-engrailed repressor construct led to reduction in expression of the endothelial marker VEGFR2. Given the similarity in the DNA recognition motifs of GATA factors (Orkin, 1992) and the demonstrated functional redundancy within at least some members of this class (Blobel et al., 1995; Holtzinger and Evans, 2007), it is not possible to specify with any certainty which GATA factor(s) regulate endothelial cell formation in vivo. However, taken together, the current results suggest a role for GATA-binding transcription factors during endothelium formation.
Several previous loss- and gain-of-function studies of Gata factors have been reported. GATA4−/− or GATA5−/− mice do not exhibit deficiencies in blood vessel formation. However, GATA4/5/6 have highly similar DNA-binding domains and partially overlapping expression patterns (reviewed in (Burch, 2005)), and it is possible that a vascular phenotype would not be revealed until two or more family members are eliminated. A GATA4/6 double knockout mouse does form endothelial cells (Zhao et al., 2008), however these mice do not generate a heart, and thus do not survive long enough to determine whether there are defects later in vascular development. The more distantly-related GATA2 is expressed in the common precursor of hematopoietic tissue and endothelial cells. However GATA2−/− mice have defects in hematopoiesis and not vasculogenesis (Tsai et al., 1994). Vasculogenic regions of the embryo express multiple GATA factors. Expression of GATA4 and GATA5 overlaps in the lateral plate (this study), while GATA5 and GATA2 are both expressed in the dorso-lateral quadrant of the somite (this study, (Ben-Yair and Kalcheim, 2008; Pouget et al., 2006)) Because of these overlapping expression patterns, it is possible that demonstration of a requirement for GATA factors in vasculature development using mutational analysis will require more extensive targeting of GATA genes. The current results with the GATA-engrailed construct at least suggest that GATA activity may be required for normal expression of the endothelial marker VEGFR2.
A recent paper reports that in zebrafish, GATA 4/5/6 are necessary but not sufficient for hemangioblast development in the anterior region of zebrafish embryos (Peterkin et al., 2009). While we concur that Gata factors appear to be necessary for normal endothelial cell gene expression, in our studies ectopic expression of GATA’s 2/4/5 alone was sufficient to promote endothelial cell formation in paraxial mesodermal tissue. An explanation for the differences in these findings may be that in zebrafish study, ectopic GATA5 and 6 was expressed at relatively low levels because of a general embryonic disruption caused by ubiquitous high levels of ectopic GATA misexpression (Peterkin et al., 2009). In contrast, because the chick electroporation system generates mosaic expression in targeted regions of the embryo, embryos can tolerate relatively high levels of ectopic gene expression, which may have allowed for the observation of a vasculogenic effect of ectopic GATA factor expression.
Ben-Yair and Kalcheim have reported that the VEGF receptor Flk-1, a marker of endothelial cell precursors, is expressed in the dorsolateral quadrant of the epithelial stage somite, and that BMP signals are required both for the expression of Flk-1 in this region and for the proper differentiation and/or migration of endothelial cells into the cardinal vein (Ben-Yair and Kalcheim, 2008). In the current work we find that Gata5 is a Bmp-responsive gene, is expressed (along with Gata2) in the dorsolateral somite, can induce the expression of VEGFR2 in somites cultured in vitro, and that expression of a GATA-Engrailed repressor domain fusion protein in paraxial mesoderm leads to loss of dorsolateral somite expression of VEGFR2. A reasonable inference based upon these findings is that GATA activity is required for mediating the vasculogenic effects of BMP signaling in the somite. Consistent with this notion, a GATA binding site in the first intron of VEGFR2 has been shown to be required for endothelial-specific expression in vivo, and is bound by GATA2 in vitro, indicating that it is a direct target of GATA factors (Kappel et al., 2000).
The GATA-induced endothelial cells observed in the current experiments were predominantly found in the aorta and other normal vascular structures, and not in the somites themselves. This suggests that the GATA-expressing cells either emigrated from the somites to join existing vascular structures or moved out of the presomitic mesoderm prior to somite epithelialization. One candidate gene that could potentially mediate such migration is Flk-1 itself. VEGF is expressed in the endoderm (Breier et al., 1995; Dumont et al., 1995; Flamme et al., 1995), and has been found to regulate migration of Flk-1-expressing cells (Cleaver and Krieg, 1998). Taken together with the data linking BMP signaling and VEGFR2 discussed above, one possible scenario is that BMP-mediated induction of GATA5 (and possibly GATA2) in the dorsolateral quadrant of the epithelial somite leads to subsequent expression of Flk-1 in these cells and consequent responsiveness to the endothelial inducer VEGF.
In addition to endothelial cell formation, we also noted a significant elevation of cell death in GATA4/5-electroporated paraxial mesoderm cells. How might GATA misexpression lead to both induction of endothelial cells and simultaneously increase cell death in paraxial mesodermal cells? One plausible model is that a subpopulation of GATA-electroporated cells that experience a threshold level of VEGF signaling take part in the formation of vascular structures, as occurs during normal aorta formation, while GATA-electroporated cells that fail to receive sufficient VEGF signals undergo programmed cell death. In recent work, we have found that ectopic expression of GATA factors alters the response of somitic cells to Sonic hedgehog (Shh) signaling and severely attenuates the expression of a subset of hedgehog target genes (H.K., G.D., and A.B.L, unpublished data). Since Shh is a known survival factor for somitic cells (Teillet et al., 1998), it is possible that GATA-electoporated somite cells which express VEGFR2 and are exposed to sufficient levels of VEGF signaling to differentiate as endothelial cells are therefore protected from apoptosis that would otherwise occur due to loss of Shh signal transduction.
In the current study, we identify GATA4 and 5 as BMP-responsive genes that have endothelium-promoting properties, in particular with respect to regulating expression of VEGFR2. In future studies, it will be important to understand the role of Gata factors in regulating other endothelial cell genes, in particular the transcription factors that establish endothelial cell identity, and to evaluate whether Gata factors have a role in regulating the hemopoietic properties of endothelium.
Supplementary Material
Supplemental Figure 1: Expression of GATA4 mRNA in early chick embryos Whole mount in situ hybridization for GATA4 at Stage 5 (A,D), Stage 7 (B,E) and Stage 10 (C,F). Lines in A-C indicate approximate level of sections in D-F. Arrowhead indicates medial-most extension of expression. GATA4 is expressed later and in a more restricted region than GATA5 and is not expressed in the dorso-lateral quadrant of the somite (compare with Figure 1). Scale bar = 100 microns in sections. (np, neural plate; nt, neural tube; s, somite).
Supplemental Figure 2: Ectopic expression of GATA2 promotes a vascular endothelial fate. Gastrulating cells in quail (A) were electroporated with a vector coexpressing GATA2 and GFP. QH1 antibody detects vascular endothelium in aortae and smaller vessels. GFP positive cells in vasculature are expressed as a percentage of total GFP (B). Arrowheads indicate costaining. Scale bar = 100 microns. (p<0.05). (np, neural plate, s, somite)
Research Highlights.
The main novel finding of the manuscript is the finding of a link between GATA transcription factors and endothelium formation. This is demonstrated through both gain-of-function and loss-of-function studies in the avian embryo. Previously, GATA factors have been linked to hematopoiesis and to the generation of hemangioblasts (the putative common precursor of endothelial cells and blood cells), but not to the formation of blood vessels themselves. We also provide evidence that GATA factor expression is induced by Bone Morphogenetic Proteins (BMPs) and is required for Vascular Endothelial Growth Factor Receptor 2 (VEGFR2) expression in the somite, a known BMP-dependent event, and thus provide a framework for thinking about GATA factors as key mediators of the known vasculogenic properties of BMP signaling.
Acknowledgements
We thank T. Matsuda (C. Cepko lab), A. McMahon, and C. Kalcheim for plasmids, and E. McGlinn and D. Herzlinger for critical reading of the manuscript. This work was supported by NIH grant DK071041 to TMS, NIH grant HD45499 to CJT, NIH grant GM054879 to ABL, and a predoctoral fellowship from the NSF to CNK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Astorga J, Carlsson P. Hedgehog induction of murine vasculogenesis is mediated by Foxf1 and Bmp4. Development. 2007;134:3753–61. doi: 10.1242/dev.004432. [DOI] [PubMed] [Google Scholar]
- Ben-Yair R, Kalcheim C. Notch and bone morphogenetic protein differentially act on dermomyotome cells to generate endothelium, smooth, and striated muscle. J Cell Biol. 2008;180:607–18. doi: 10.1083/jcb.200707206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand JY, Chi NC, Santoso B, Teng S, Stainier DY, Traver D. Haematopoietic stem cells derive directly from aortic endothelium during development. Nature. 2010;464:108–11. doi: 10.1038/nature08738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel GA, Simon MC, Orkin SH. Rescue of GATA-1-deficient embryonic stem cells by heterologous GATA-binding proteins. Mol Cell Biol. 1995;15:626–33. doi: 10.1128/mcb.15.2.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boardman PE, Sanz-Ezquerro J, Overton IM, Burt DW, Bosch E, Fong WT, Tickle C, Brown WR, Wilson SA, Hubbard SJ. A comprehensive collection of chicken cDNAs. Curr Biol. 2002;12:1965–9. doi: 10.1016/s0960-9822(02)01296-4. [DOI] [PubMed] [Google Scholar]
- Boisset JC, van Cappellen W, Andrieu-Soler C, Galjart N, Dzierzak E, Robin C. In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature. 2010;464:116–20. doi: 10.1038/nature08764. [DOI] [PubMed] [Google Scholar]
- Breier G, Clauss M, Risau W. Coordinate expression of vascular endothelial growth factor receptor-1 (flt-1) and its ligand suggests a paracrine regulation of murine vascular development. Dev Dyn. 1995;204:228–39. doi: 10.1002/aja.1002040303. [DOI] [PubMed] [Google Scholar]
- Bressan M, Davis P, Timmer J, Herzlinger D, Mikawa T. Notochord-derived BMP antagonists inhibit endothelial cell generation and network formation. Dev Biol. 2009;326:101–11. doi: 10.1016/j.ydbio.2008.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch JB. Regulation of GATA gene expression during vertebrate development. Semin Cell Dev Biol. 2005;16:71–81. doi: 10.1016/j.semcdb.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Byrd N, Becker S, Maye P, Narasimhaiah R, St-Jacques B, Zhang X, McMahon J, McMahon A, Grabel L. Hedgehog is required for murine yolk sac angiogenesis. Development. 2002;129:361–72. doi: 10.1242/dev.129.2.361. [DOI] [PubMed] [Google Scholar]
- Chen VC, Stull R, Joo D, Cheng X, Keller G. Notch signaling respecifies the hemangioblast to a cardiac fate. Nat Biotechnol. 2008;26:1169–78. doi: 10.1038/nbt.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleaver O, Krieg PA. VEGF mediates angioblast migration during development of the dorsal aorta in Xenopus. Development. 1998;125:3905–14. doi: 10.1242/dev.125.19.3905. [DOI] [PubMed] [Google Scholar]
- Coultas L, Chawengsaksophak K, Rossant J. Endothelial cells and VEGF in vascular development. Nature. 2005;438:937–45. doi: 10.1038/nature04479. [DOI] [PubMed] [Google Scholar]
- De Val S, Black BL. Transcriptional control of endothelial cell development. Dev Cell. 2009;16:180–95. doi: 10.1016/j.devcel.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieterlen-Lievre F, Jaffredo T. Decoding the hemogenic endothelium in mammals. Cell Stem Cell. 2009;4:189–90. doi: 10.1016/j.stem.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Dumont DJ, Fong GH, Puri MC, Gradwohl G, Alitalo K, Breitman ML. Vascularization of the mouse embryo: a study of flk-1, tek, tie, and vascular endothelial growth factor expression during development. Dev Dyn. 1995;203:80–92. doi: 10.1002/aja.1002030109. [DOI] [PubMed] [Google Scholar]
- Eichmann A, Marcelle C, Breant C, Le Douarin NM. Two molecules related to the VEGF receptor are expressed in early endothelial cells during avian embryonic development. Mech Dev. 1993;42:33–48. doi: 10.1016/0925-4773(93)90096-g. [DOI] [PubMed] [Google Scholar]
- Ferdous A, Caprioli A, Iacovino M, Martin CM, Morris J, Richardson JA, Latif S, Hammer RE, Harvey RP, Olson EN, Kyba M, Garry DJ. Nkx2-5 transactivates the Ets-related protein 71 gene and specifies an endothelial/endocardial fate in the developing embryo. Proc Natl Acad Sci U S A. 2009;106:814–9. doi: 10.1073/pnas.0807583106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamme I, Breier G, Risau W. Vascular endothelial growth factor (VEGF) and VEGF receptor 2 (flk-1) are expressed during vasculogenesis and vascular differentiation in the quail embryo. Dev Biol. 1995;169:699–712. doi: 10.1006/dbio.1995.1180. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. Journal of Morphology. 1951;88:49–92. [PubMed] [Google Scholar]
- He C, Chen X. Transcription regulation of the vegf gene by the BMP/Smad pathway in the angioblast of zebrafish embryos. Biochem Biophys Res Commun. 2005;329:324–30. doi: 10.1016/j.bbrc.2005.01.133. [DOI] [PubMed] [Google Scholar]
- Holtzinger A, Evans T. Gata5 and Gata6 are functionally redundant in zebrafish for specification of cardiomyocytes. Dev Biol. 2007;312:613–22. doi: 10.1016/j.ydbio.2007.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffredo T, Gautier R, Eichmann A, Dieterlen-Lievre F. Intraaortic hemopoietic cells are derived from endothelial cells during ontogeny. Development. 1998;125:4575–83. doi: 10.1242/dev.125.22.4575. [DOI] [PubMed] [Google Scholar]
- James RG, Kamei CN, Wang Q, Jiang R, Schultheiss TM. Odd-skipped related 1 is required for development of the metanephric kidney and regulates formation and differentiation of kidney precursor cells. Development. 2006;133:2995–3004. doi: 10.1242/dev.02442. [DOI] [PubMed] [Google Scholar]
- James RG, Schultheiss TM. Patterning of the avian intermediate mesoderm by lateral plate and axial tissues. Dev Biol. 2003;253:109–24. doi: 10.1006/dbio.2002.0863. [DOI] [PubMed] [Google Scholar]
- James RG, Schultheiss TM. Bmp signaling promotes intermediate mesoderm gene expression in a dose-dependent, cell-autonomous and translation-dependent manner. Dev Biol. 2005;288:113–25. doi: 10.1016/j.ydbio.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Kappel A, Schlaeger TM, Flamme I, Orkin SH, Risau W, Breier G. Role of SCL/Tal-1, GATA, and ets transcription factor binding sites for the regulation of flk-1 expression during murine vascular development. Blood. 2000;96:3078–85. [PubMed] [Google Scholar]
- Kempf H, Ionescu A, Udager AM, Lassar AB. Prochondrogenic signals induce a competence for Runx2 to activate hypertrophic chondrocyte gene expression. Dev Dyn. 2007;236:1954–62. doi: 10.1002/dvdy.21205. [DOI] [PubMed] [Google Scholar]
- Kissa K, Herbomel P. Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature. 2010;464:112–5. doi: 10.1038/nature08761. [DOI] [PubMed] [Google Scholar]
- Lee D, Park C, Lee H, Lugus JJ, Kim SH, Arentson E, Chung YS, Gomez G, Kyba M, Lin S, Janknecht R, Lim DS, Choi K. ER71 acts downstream of BMP, Notch, and Wnt signaling in blood and vessel progenitor specification. Cell Stem Cell. 2008;2:497–507. doi: 10.1016/j.stem.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Q, Lu J, Yanagisawa H, Webb R, Lyons GE, Richardson JA, Olson EN. Requirement of the MADS-box transcription factor MEF2C for vascular development. Development. 1998;125:4565–74. doi: 10.1242/dev.125.22.4565. [DOI] [PubMed] [Google Scholar]
- Liu F, Walmsley M, Rodaway A, Patient R. Fli1 acts at the top of the transcriptional network driving blood and endothelial development. Curr Biol. 2008;18:1234–40. doi: 10.1016/j.cub.2008.07.048. [DOI] [PubMed] [Google Scholar]
- Nimmagadda S, Geetha Loganathan P, Huang R, Scaal M, Schmidt C, Christ B. BMP4 and noggin control embryonic blood vessel formation by antagonistic regulation of VEGFR-2 (Quek1) expression. Dev Biol. 2005;280:100–10. doi: 10.1016/j.ydbio.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193–9. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- Orkin SH. GATA-binding transcription factors in hematopoietic cells. Blood. 1992;80:575–81. [PubMed] [Google Scholar]
- Pardanaud L, Altmann C, Kitos P, Dieterlen-Lievre F, Buck CA. Vasculogenesis in the early quail blastodisc as studied with a monoclonal antibody recognizing endothelial cells. Development. 1987;100:339–49. doi: 10.1242/dev.100.2.339. [DOI] [PubMed] [Google Scholar]
- Pardanaud L, Luton D, Prigent M, Bourcheix LM, Catala M, Dieterlen-Lievre F. Two distinct endothelial lineages in ontogeny, one of them related to hemopoiesis. Development. 1996;122:1363–71. doi: 10.1242/dev.122.5.1363. [DOI] [PubMed] [Google Scholar]
- Patterson LJ, Gering M, Patient R. Scl is required for dorsal aorta as well as blood formation in zebrafish embryos. Blood. 2005;105:3502–11. doi: 10.1182/blood-2004-09-3547. [DOI] [PubMed] [Google Scholar]
- Peterkin T, Gibson A, Patient R. Common genetic control of haemangioblast and cardiac development in zebrafish. Development. 2009;136:1465–74. doi: 10.1242/dev.032748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouget C, Gautier R, Teillet MA, Jaffredo T. Somite-derived cells replace ventral aortic hemangioblasts and provide aortic smooth muscle cells of the trunk. Development. 2006;133:1013–22. doi: 10.1242/dev.02269. [DOI] [PubMed] [Google Scholar]
- Reese DE, Hall CE, Mikawa T. Negative regulation of midline vascular development by the notochord. Dev Cell. 2004;6:699–708. doi: 10.1016/s1534-5807(04)00127-3. [DOI] [PubMed] [Google Scholar]
- Reshef R, Maroto M, Lassar AB. Regulation of dorsal somitic cell fates: BMPs and Noggin control the timing and pattern of myogenic regulator expression. Genes Dev. 1998;12:290–303. doi: 10.1101/gad.12.3.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultheiss TM, Burch JB, Lassar AB. A role for bone morphogenetic proteins in the induction of cardiac myogenesis. Genes Dev. 1997;11:451–62. doi: 10.1101/gad.11.4.451. [DOI] [PubMed] [Google Scholar]
- Shalaby F, Ho J, Stanford WL, Fischer KD, Schuh AC, Schwartz L, Bernstein A, Rossant J. A requirement for Flk1 in primitive and definitive hematopoiesis and vasculogenesis. Cell. 1997;89:981–90. doi: 10.1016/s0092-8674(00)80283-4. [DOI] [PubMed] [Google Scholar]
- Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, Schuh AC. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–6. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- Sundin O, Eichele G. An early marker of axial pattern in the chick embryo and its respecification by retinoic acid. Development. 1992;114:841–52. doi: 10.1242/dev.114.4.841. [DOI] [PubMed] [Google Scholar]
- Teillet M, Watanabe Y, Jeffs P, Duprez D, Lapointe F, Le Douarin NM. Sonic hedgehog is required for survival of both myogenic and chondrogenic somitic lineages. Development. 1998;125:2019–30. doi: 10.1242/dev.125.11.2019. [DOI] [PubMed] [Google Scholar]
- Tsai FY, Keller G, Kuo FC, Weiss M, Chen J, Rosenblatt M, Alt FW, Orkin SH. An early haematopoietic defect in mice lacking the transcription factor GATA-2. Nature. 1994;371:221–6. doi: 10.1038/371221a0. [DOI] [PubMed] [Google Scholar]
- Visvader JE, Fujiwara Y, Orkin SH. Unsuspected role for the T-cell leukemia protein SCL/tal-1 in vascular development. Genes Dev. 1998;12:473–9. doi: 10.1101/gad.12.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walmsley M, Ciau-Uitz A, Patient R. Adult and embryonic blood and endothelium derive from distinct precursor populations which are differentially programmed by BMP in Xenopus. Development. 2002;129:5683–95. doi: 10.1242/dev.00169. [DOI] [PubMed] [Google Scholar]
- Winnier G, Blessing M, Labosky PA, Hogan BL. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 1995;9:2105–16. doi: 10.1101/gad.9.17.2105. [DOI] [PubMed] [Google Scholar]
- Zeng L, Kempf H, Murtaugh LC, Sato ME, Lassar AB. Shh establishes an Nkx3.2/Sox9 autoregulatory loop that is maintained by BMP signals to induce somitic chondrogenesis. Genes Dev. 2002;16:1990–2005. doi: 10.1101/gad.1008002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R, Watt AJ, Battle MA, Li J, Bondow BJ, Duncan SA. Loss of both GATA4 and GATA6 blocks cardiac myocyte differentiation and results in acardia in mice. Dev Biol. 2008;317:614–9. doi: 10.1016/j.ydbio.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Expression of GATA4 mRNA in early chick embryos Whole mount in situ hybridization for GATA4 at Stage 5 (A,D), Stage 7 (B,E) and Stage 10 (C,F). Lines in A-C indicate approximate level of sections in D-F. Arrowhead indicates medial-most extension of expression. GATA4 is expressed later and in a more restricted region than GATA5 and is not expressed in the dorso-lateral quadrant of the somite (compare with Figure 1). Scale bar = 100 microns in sections. (np, neural plate; nt, neural tube; s, somite).
Supplemental Figure 2: Ectopic expression of GATA2 promotes a vascular endothelial fate. Gastrulating cells in quail (A) were electroporated with a vector coexpressing GATA2 and GFP. QH1 antibody detects vascular endothelium in aortae and smaller vessels. GFP positive cells in vasculature are expressed as a percentage of total GFP (B). Arrowheads indicate costaining. Scale bar = 100 microns. (p<0.05). (np, neural plate, s, somite)