Abstract
Among the purine and pyrimidine receptors, the discovery of small molecular allosteric modulators has been most highly advanced for the A1 and A3 ARs. These AR modulators have allosteric effects that are structurally separated from the orthosteric effects in SAR studies. The benzoylthiophene derivatives tend to act as allosteric agonists, as well as selective positive allosteric modulators (PAMs) of the A1 AR. A 2-amino-3-aroylthiophene derivative T-62 has been under development as a PAM of the A1 AR for the treatment of chronic pain. Several structurally distinct classes of allosteric modulators of the human A3 AR have been reported: 3-(2-pyridinyl)isoquinolines, 2,4-disubstituted quinolines, 1H-imidazo-[4,5-c]quinolin-4-amines, endocannabinoid 2-arachidonylglycerol and the food dye Brilliant Black BN. Site-directed mutagenesis of A1 and A3 ARs has identified residues associated with the allosteric effect, distinct from those that affect orthosteric binding. A few small molecular allosteric modulators have been reported for several of the P2X ligand-gated ion channels and the G protein-coupled P2Y receptor nucleotides. Metal ion modulation of the P2X receptors has been extensively explored. The allosteric approach to modulation of purine and pyrimidine receptors looks promising for development of drugs that are event-specific and site-specific in action.
Keywords: purines, G protein-coupled receptors, ion channels, allostery, adenosine receptors, P2Y receptors
I. Introduction
Drug design for cell surface receptors has largely focused on either competitive agonist or antagonist ligands that occupy the principal (orthosteric) binding sites of these receptors, i.e. the sites at which the native ligands for these receptors act. Recently interest has grown in the modulation of clinically validated receptors by small molecules that act at allosteric (from the Greek allos, “other,” and stereos, “space”) sites, i.e. those sites for binding on the receptor protein that are not identical with the orthosteric binding sites of the native ligands. Changeux and colleagues first introduced the concept of allosteric modulation of receptor action for the nicotinic cholinergic receptors, i.e. channels for cations that are activated by the neurotransmitter acetylcholine (Changeux, 2010). Now, the approach of allosteric modulation of the action of a native agonist has grown in importance for the ligand design and pharmacology of both GPCRs and ligand-gated ion channels. For example, two such agents that are already in clinical use for GPCR modulation are Cinacalcet (Harrington and Fotsch, 2007) and Maraviroc (Yang and Rotstein, 2010), which act as an allosteric agonist of the calcium sensing receptor and inhibitor of chemokine coreceptors required for HIV entry, respectively. The widely used benzodiazepines allosterically enhance the activation of GABAA chloride channels.
In the area of G protein-coupled receptors (GPCRs) in particular, many therapeutic agents currently in use act as orthosteric agonists and antagonists, but there is a need to expand the ways in which GPCRs and other cell surface receptors may be modulated. Two major types of allosteric modulators for GPCRs have been defined: positive allosteric modulators (PAMs), which increase the affinity, potency and/or efficacy of the agonist, and negative allosteric modulators (NAMs), which may decrease the above parameters (Christopoulos, 2002). Further divisions based on pharmacological parameters are also relevant. For example, a PAM may only modulate the action of the native agonists, i.e. magnify or enhance the effect of an endogenous molecular signal. Alternatively, it might have its own agonist action by binding to a site different from the binding site of the native agonist and would therefore be classified as an allosteric agonist.
There are several points of justification for studying allosteric modulation of cell surface receptors. First of all, the receptors are often widely distributed throughout the body – leading to problems of side effects when an orthosteric agonist is administered therapeutically. The action of a PAM would be expected to be more tissue-specific and event-specific than the action of a stable, exogenously administered orthosteric agonist, which would circulate throughout the body (Conn et al., 2009). Secondly, allosteric modulators may have an inherently greater chance of achieving subtype selectivity than the orthosteric ligands. In the case of some GPCRs, such as muscarinic acetylcholine receptors, the design of subtype-selective orthosteric agonists and antagonists has progressed very slowly until recently, largely because the amino acid residues within the orthosteric binding site are highly conserved if not identical across the subtypes. It is thought that greater subtype selectivity could be obtained by targeting other regions of the receptors, such as the extracellular loops (ELs) in Class A GPCRs where there is more structural variation than in transmembrane domains (TMs). In fact, this approach has resulted in muscarinic receptor modulators of great selectivity (Conn et al., 2009). Another possible advantage of PAMs over orthosteric agonists is the possibility to alter the spectrum of second messenger effects, or produce a bias toward a particular pathway based on conformational variation of the receptor in its activated state (Stewart et al., 2010).
Finally, an additional potential advantage of allosteric modulators is the preferential activation of receptors in areas of low receptor density, or low receptor reserve. A full agonist or a partial agonist will always activate areas where receptor density is highest. In contrast, it may be possible to have preferential action on areas of lower receptor density with PAMs, as was illustrated for the A1 AR (Childers et al., 2005).
Assay methods used to identify allosteric modulators of the ARs have included both radioligand binding and functional assays. Initially, screening typically has consisted of detecting an increase in the level of binding of radioligand to membranes expressing a given receptor subtype. Functional assays using an EC50 or EC80 concentration of an orthosteric ligand are used to screen for modulators, particularly in industry.
One commonly used screen is a single point dissociation assay with the goal to detect a change in koff as indicated by a change in the remaining radioligand after a fixed time of dissociation. This assay differs from a full dissociation kinetic experiment in the number of time-points included. Thus, the index of modulator activity known as the AE (allosteric enhancer) score, also called the K-score (Ferguson et al., 2009), is related to the percentage of specifically bound agonist remaining after a fix time of dissociation (e.g. 10 min). A more labor-intensive binding method has been to look for alteration of the dissociation rate of a radioligand. Thus, a decreased off-rate of an agonist ligand in the presence of a fixed concentration of the candidate modulator may indicate a PAM, and conversely an increased off-rate may indicate a NAM. Although these preliminary indicators are often validated in subsequent analysis, especially for the A1 AR, the feature of PAM or NAM depends on the overall effect of the modulator on the equilibrium binding of the probe, which is a ratio of the effect of the modulator on the dissociation (koff) and association (kon) rates of the tracer. Thus, the phenomenon of allosteric modulation of a receptor cannot be conclusively determined by binding alone, but rather at least one functional assay needs to be carried out in general. Such functional assays may consist of the enhancement (for a PAM) or reduction (for a NAM) of binding of a radiolabeled guanine nucleotide ([35S]GTPγS) in response to a known receptor agonist, or effects on agonist-induced changes in adenylate cyclase or other second messenger systems. The [35S]GTPγS assay measures receptor-mediated G protein activation.
For allosteric modulation in general, the experimental conditions may have a far greater influence on the outcome and the conclusions than is normally encountered in routine screening. Different conclusions may be reached for the same PAM in different binding assays, e.g. membrane versus whole cell studies, guanine nucleotide versus agonist radioligand, and cloned receptors (and their expression system) versus endogenous receptors. It is also worth noting that in vivo effects of PAMs are not necessarily predicted by cell-based assays. For example, tissue selectivites in vitro for allosteric enhancers have been described (Leung et al., 1995). However, the source of these differences, in receptor coupling or the tissue environment, remains to be determined. Also, to note is that many PAMs are poorly soluble in water and require DMSO for a stock solution, of which the stability and solubility when diluted may affect the results.
Another important concept is probe dependency, in which the allosteric properties of a particular PAM or NAM may be highly variable depending on which orthosteric ligand is used in the experiment. Slight differences in the region or mode of binding of orthosteric ligands within the same receptor are likely responsible for this phenomenon.
This review focuses on the purine and pyrimidine receptors: four subtypes of GPCRs that respond to extracellular adenosine (ARs), eight subtypes of GPCRs that respond to extracellular purine and pyrimidine nucleotides (P2Y receptors), and seven subtypes of cation-permeable ligand-gated ion channels (LGICs) that respond to extracellular adenine nucleotides (P2X receptors).
II. AR modulators
The subtypes of ARs are numbered A1, A2A, A2B, and A3. Activation of the A1 and A3 ARs leads to the inhibition of adenylate cyclase, while the other two subtypes are stimulatory (Fredholm et al., 2011). Endogenous adenosine acts as a mediator in numerous organs and tissues to protect against damaging effects of stress, such as in ischemia. Many novel drug concepts have been proposed based on administration of selective AR agonists and antagonists (Jacobson and Gao 2006). Fortunately, the lack of selective ligands that has plagued the muscarinic acetylcholine receptor field is not a limitation for the ARs, because both agonist and antagonist ligands that are thousand of fold selective have been reported for most of the subtypes. However, the ubiquity of the ARs throughout the body does present a problem of lack of selectivity for even highly selective agonists. Native adenosine is rapidly degraded and does not migrate beyond the target site. In stress situations, the extracellular concentration of adenosine is elevated locally, which avoids side effects in other organs. The action of a stable synthetic PAM of the ARs may be more selective than an orthosteric agonist because it boosts the effect of local adenosine elevation that occurs in response to a physiological need (i.e. stress) to an organ or tissue. Thus, allosteric enhancement of native adenosine in activating the ARs is a particularly attractive option for therapeutics – leading to site-specific and event-specific action. A similar rationale could be presented for allosteric modulation of receptors for nucleotides.
A second advantage of allosteric modulators of ARs concerns pharmacokinetics, giving such modulators a clear benefit in the central nervous system. Adenosine agonists, which are nearly always nucleoside derivatives and thus have a highly hydrophilic (i.e. ribose) region, tend not to readily penetrate the blood brain barrier (BBB). The brain entry of such nucleoside derivatives is typically only 1–2% of free passage across the BBB. Thus, for induction of AR activation in the brain, where adenosine levels can be greatly elevated in response to stress or hypoxia, a freely penetrating PAM (i.e. belonging to a different chemical class that might pass the BBB more easily) would be more effective than a nucleoside agonist.
The structure activity relationship (SAR) of small organic molecules as allosteric modulators is well explored for A1 and A3 ARs, with isolated reports of examples for allosteric or “noncompetitive” (i.e., potentially allosteric) ligands for other AR subtypes (Gao et al., 2005, Göblyös and IJzerman, 2009). Most of the examples of allosteric modulators of ARs are PAMs. The effects of metal ions as allosteric modulators of the ARs have also been reported (Gao and IJzerman, 2000).
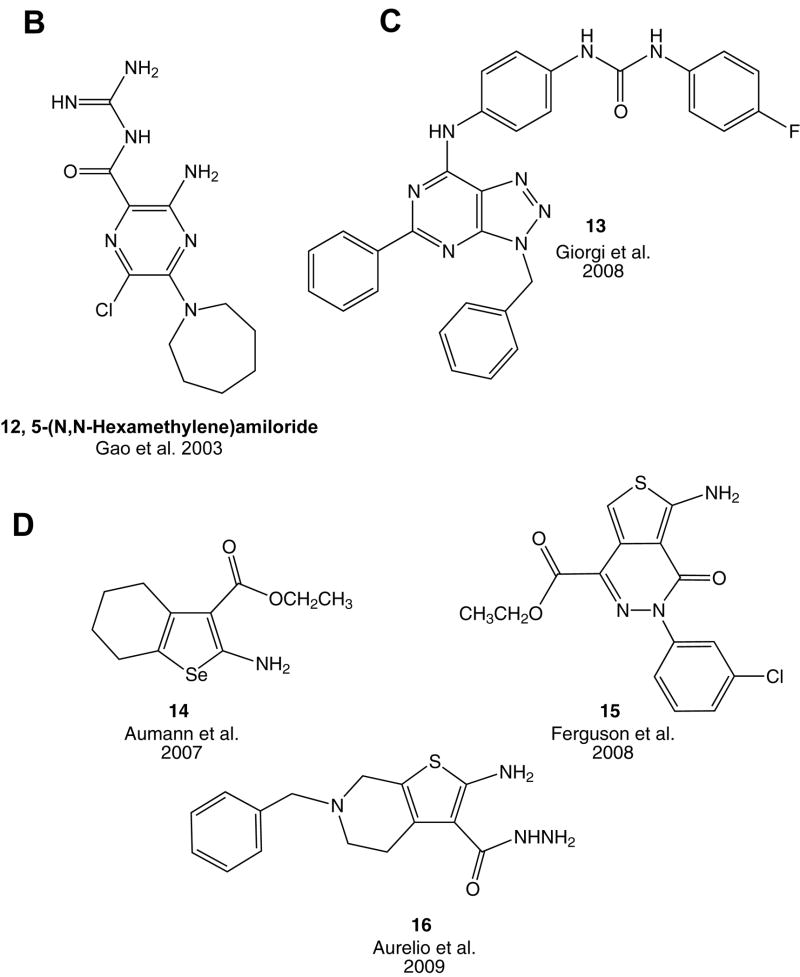
Some classes of GPCR allosteric modulators for example, amiloride analogues, affect multiple members of the AR family (Gao et al., 2003b). Amiloride analogues have been characterized as allosteric modulators of the A2A AR, although it is recognized that the also interact with other GPCRs, such as dopamine receptors. Thus, the amilioride analogues lack specificity due to interaction with many other protein sites, including other AR subtypes (Gao and IJzerman, 2000). At the A2A AR, 5-(N,N-dialkyl)amiloride derivatives containing a cyclic 5-(N,N-hexamethylene) group such as 12 (HMA, Fig. 1B), increase the dissociation rate of antagonist radioligand. Such amiloride analogues also allosterically modulate action of ligands at both A1 and A3 ARs (Gao et al., 2003b). At the A1 AR, their behavior is similar to the A2A AR. At the A3 AR, they additionally decrease the dissociation rate of agonist radioligand. They also compete for orthosteric binding at these three subtypes. Thus, amiloride analogues are not useful as selective allosteric pharmacological probes of specific AR subtypes.
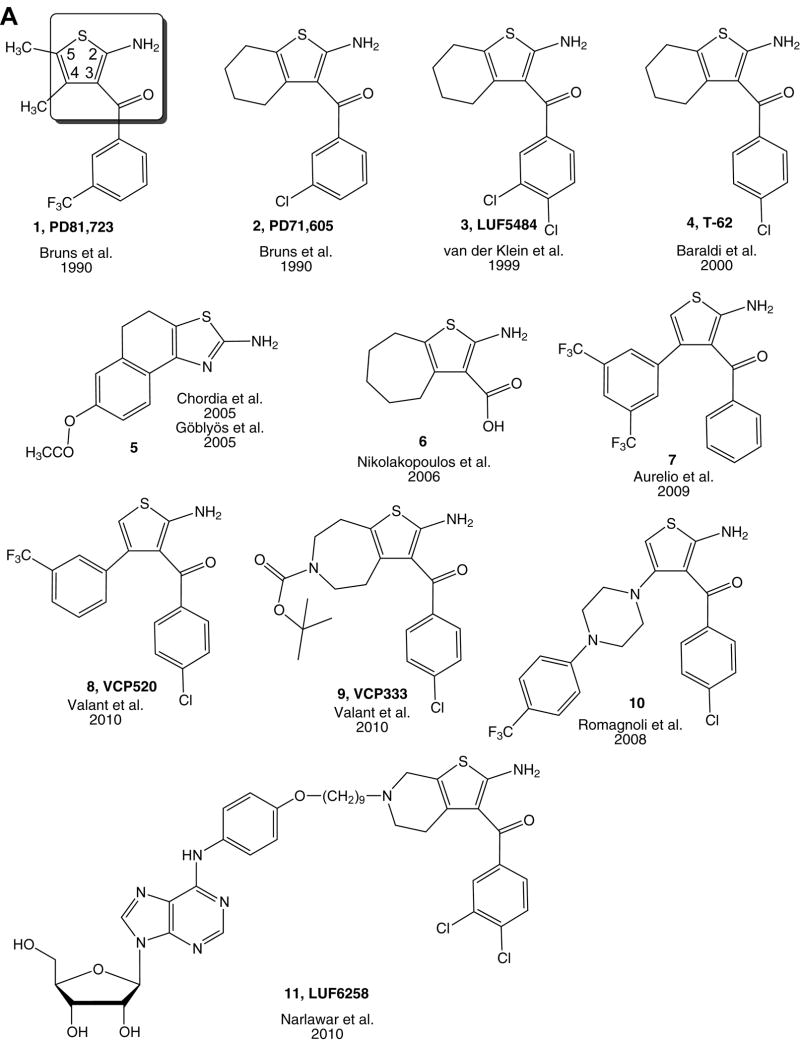
Fig. 1.
Allosteric modulators of the A1 AR, including benzoylthiophene derivatives (A) and an amiloride derivative lacking subtype selectivity (B), and of the A2A AR (C). The miminal, principal pharmacophore of the class of aroylthiophenes as A1 AR PAMs is shown in a box on PD81,723 2. Compound 11 is a bitopic modulator, containing an agonist (6-phenyl substituted adenosine) moiety. In part (D) there are recently published 2-amino-3-substituted thiophene derivatives as allosteric modulators for A1 adenosine receptors. Numbering of ring substitutions is shown for compound 1.
Other non-selective AR allosteric modulators include: SCH-202676 (N-(2,3-diphenyl-1,2,4-thiadiazol-5-(2H)-ylidene)methanamine), which affects a wide range of structurally unrelated G protein–coupled receptors, has highly divergent effects on purine receptors (Gao et al., 2004b; van den Nieuwendijk et al., 2004). However, later studies suggested that SCH-202676 is a chemical modifying agent, rather than a true allosteric modulator (Göblyös et al., 2005; Lewandowicz et al., 2006). Another nonselective agent, Brillant black G, was shown to decrease antagonist affinity at both A1 and A3 ARs (May et al., 2010a).
A. SAR of A1 AR allosteric modulators
1. Benzoylthiophenes and related allosteric modulators of the A1 AR
The benzoylthiophenes were the first A1 AR allosteric modulators to be identified (Bruns et al., 1990). They were identified as PAMs of the A1 AR initially by increasing the level of agonist radioligand ([3H]CHA) bound to the receptor in rat brain membranes. Among the various analogues coming out of a chemical library screen, the prototypical benzoylthiophene determined to act as an A1 AR allosteric modulator is PD81,723 1 (Fig. 1A). This compound is still used extensively as a pharmacological standard.
The structure of 1 has been extensively modified in subsequent studies, and the SAR of benzoylthiophene derivatives as PAMs has been documented (Romagnoli et al., 2010). Many analogues have been prepared and found to have comparable or more favorable activity as allosteric modulators (Kourounakis et al., 2000; Gao et al., 2005; Baraldi et al., 2007; Baraldi et al., 2003; Baraldi et al., 2004; Romagnoli et al., 2006). The 2-amino-3-carbonyl thiophene moiety is required as a minimal pharmacophore (shown in box). The aroyl group can be substituted with other phenyl and heteroaromatic groups. The 4,5-alkyl substituents of the thiophene ring may be cyclized, with cycloalkyl chains. Cyclohexyl groups as in 2–4 have been incorporated in various analogues (van der Klein et al., 1999), although cycloheptyl rings as in 6 are also tolerated (Nikolakopoulos et al., 2006). In certain cases, the aroyl group can be simplified in the form of a carboxylic acid as in 6. The synthesis of novel benzoylthiophene analgoues VCP520 8 and VCP333 9 has been described (Valant et al., 2010).
An atypical structural class, 2-aminothiazoles including 5, were reported as PAMs of the A1 AR, but their allosteric activity is not always evident and appears to be limited to specific salt forms (Göblyös et al., 2005, Chordia et al., 2005). The 3-piperazinyl derivative 10 was found to act as a PAM of the A1 AR (Romagnoli et al., 2008).
An allosteric modulator of the A1 AR, the benzoylthiophene analogue T-62 4 (Baraldi et al., 2000), has been in clinical trials for treating chronic pain (Kiesman et al., 2009). T-62 is active in the central nervous system, and produces a beneficial effect in several in vivo pain models. An anti-nociceptive effect has been studied following the intrathecal administration of T-62 in carrageenan-inflamed rats. This allosteric AR modulation reduced hypersensitivity following peripheral inflammation by a central mechanism (Li et al., 2003). In investigation of the mode of action of T-62 in brain slices, it was found to selectively enhance the Gi protein coupling of the A1 AR (Childers et al., 2005). T-62 also raises the basal levels of [35S]GTPγS binding in brain slices and therefore acts as an allosteric agonist. T-62 has been radiolabeled, and its binding properties are indicative of allosteric binding (Romagnoli et al., 2006).
A new 3,5-di(trifluoromethyl) benzoylthiophene derivative 7 from the Scammells lab was shown to act as an allo-agonist of the A1AR (Aurelio et al., 2009). Activation of the ERK phosphorylation pathway required higher concentrations of the derivative than for G protein modulation (based on [35S]GTPγS binding), suggesting the possibility of signaling bias, pending clarification in additional studies.
Valant et al. (2010) used a combination of membrane-based and intact-cell radioligand binding, multiple signaling assays, and a native tissue bioassay, to characterize the allosteric interaction between benzoylthiophenes and various radiolabeled agonists and antagonists of the A1 AR. The findings were consistent with a ternary complex model involving binding of the benzoylthiophene modulator at a single extracellular allosteric site. As noted previously, the benzoylthiophenes can also serve as allosteric agonists, and the consequent signaling pathways are biased with respect to signaling from a standard orthosteric agonist. However, when allowed access to the intracellular milieu, the benzoylthiophenes have a secondary action as direct G protein inhibitors, which was also seen after stimulation of another GPCR. Thus, there are multiple modes of interaction with the A1 AR, which should be taken into account in pharmacological experiments.
Interestingly, 2-aminoselenophene-3-carboxylates also proved to be PAMs of the A1 AR. Compound 14 (Fig. 1D) had an AE score of 64%, and it was significantly more potent as a PAM of the A1 AR than its thiophene analogue. However, it is noteworthy that this compound is not stable under mildly acidic conditions (Aumann et al., 2007).
In a pharmacophore-based library screen, ethyl 5-amino-3-(4-tert-butylphenyl)-4-oxo-3,4-dihydrothieno[3,4-d]pyridazine-1-carboxylate was identified as a new allosteric modulator of the A1 AR. On the basis of this lead compound, various derivatives were prepared and evaluated for activity at the human (h) A1 AR. However these compounds turned out to be a new class of hA1 AR antagonists that can also recognize the receptor’s allosteric site with lower potency. Among them, compound 15 proved to be the most potent (Ferguson et al., 2008). Similar results were found for a series of 2-amino-4,5,6,7-tetrahydrothieno[2,3-c]pyridines, e. g. compound 16 with an AE score of 83% (Aurelio et al., 2009). These findings emphasized the caveat that changes in orthosteric ligand dissociation kinetics induced by a test compound cannot guarantee that the predominant pharmacological effect will be allosteric. Compounds that act allosterically and/or orthosterically at the hA1 AR often have close structural resemblance, which suggests that the allosteric site on the A1 AR is closer or more similar to the orthosteric site on the A1 AR than in other Class A GPCRs (see Fig 1. D). Novel conformationally rigid analogues of the benzoylthiophenes were screened at the hA1 AR, and (2-aminoindeno[2,1-b]thiophen-3-yl)(phenyl)methanones with para-chloro substitution displayed considerable PAM activity (Aurelio et al., 2010).
2. Bitopic allosteric modulators of the A1 AR
The concept of bitopic allosteric modulators, which bridge orthosteric and allosteric binding regions on a given GPCR protein, has been introduced (Valant et al., 2009; Mohr et al., 2010). IJzerman and coworkers recently designed such a bitopic ligand for the A1 AR by tethering pharmacophores using spacers of varying lengths (Narlawar et al., 2010). The bivalent ligand N6-[2-amino-3-(3,4-dichlorobenzoyl)-4,5,6,7-tetrahydrothieno[2,3-c]pyridin-6-yl-9-nonyloxy-4-phenyl]-adenosine 11 (LUF6258) with a 9 carbon atom spacer did not show significant changes in affinity or potency in the presence of PD81,723, indicating that this ligand bridged both sites on the A1 AR. Furthermore, this bitopic ligand displayed an increase in efficacy, but not potency, compared to the parent, monovalent agonist. Molecular modeling and docking of this ligand suggested that the allosteric site could be located both in proximity to the orthosteric site and in the vicinity of EL2.
B. A2A AR allosteric modulators
Allosteric modulation of the Gs-coupled A2A AR is much less well advanced than allosteric modulation of the A1 AR. In an early abstract, Bruns et al. reported that a benzopyran-2-one derivative named PD120,918 (not shown) was an enhancer of agonist radioligand ([3H]NECA in the presence of an A1 AR agonist CPA – prior to the development of A2A AR-selective radioligands) binding at the A2A AR in rat striatal membranes (Bruns and Lu, 1989; Gao et al., 2005), but this lead was not subsequently explored.
Amiloride and its analogues were demonstrated to be allosteric inhibitors for the A2A AR too. Among the derivatives tested HMA proved to be the most potent allosteric inhibitor (Fig. 1B). Amiloride analogues increased the dissociation rate of the antagonist [3H]ZM 241385 from the A2A AR, however they did not show any effect on the dissociation rate of the agonist [3H]CGS21680 (Gao and IJzerman, 2000). Sodium ions (high concentrations of NaCl) rather decreased the dissociation rate of the antagonist [3H]ZM241385 from the A2A AR in a concentration-dependent manner.
Recently, a 2-phenyl-9-benzyl-8-azaadenine derivative 13 (Fig. 1C) was reported to be a PAM of both agonist and antagonist radioligand binding at the A2A AR, and it increased the potency of an A2A AR agonist to induced relaxation of rat aortic rings (Giorgi et al., 2008).
Addex Pharmaceuticals is developing PAMs of the A2A AR for treatment of inflammatory diseases, such as psoriasis and osteoarthritis, but so far the compounds remain in the preclinical phase (http://www.addexpharma.com/press-releases/press-release-details/article/addex-rd-day-highlights-broadened-therapeutic-potential-of-allosteric-modulation-platform-to-includ/).
A cholesterol-sequestering cyclodextrin molecule, enhanced adenosine A2A AR-activated transepithelial short circuit current from the basolateral side of colonic epithelial cells (Lam et al., 2009). Thus, cholesterol content modulates agonist-selective signaling at this receptor.
C. A2B AR allosteric modulators
Allosteric modulators for the A2B AR have not yet been reported. Until recently, there was no suitable radioligand for this receptor subtype that could be obtained commercially. However, with the advent of the radiolabeled antagonist [3H]MRS1754 and other radioligands such as [3H]PSB603 (Borrmann et al., 2009) these studies are now feasible. However, a radiolabeled agonist is not available, and therefore identification of PAMs of agonist binding is still hampered.
D. SAR of A3 AR allosteric modulators
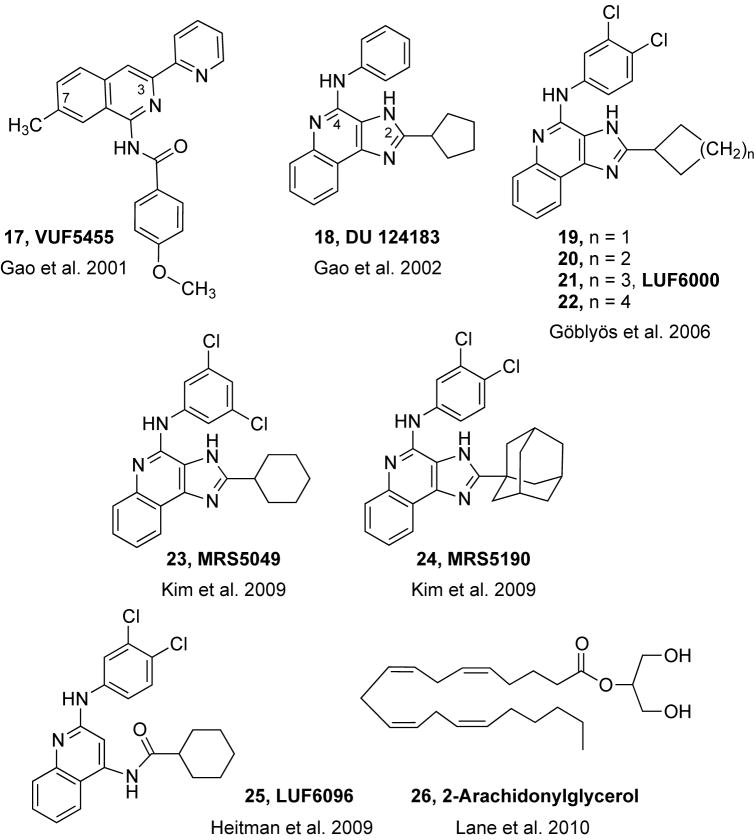
Lead compounds for allosteric modulators of the A3 AR were discovered in the course of screening structurally diverse chemical libraries in binding at this subtype (Gao et al., 2001, 2002). Just as in the initial discovery of PD81,723 1 as a PAM at the A1 AR, certain lead molecules were found to incease the level of agonist radioligand ([125I]AB-MECA) binding at this other Gi-coupled subtype. Classes of heterocyclic ligands that became prototypical PAMs of the A3 AR include 3-(2-pyridinyl)isoquinolines (e.g. VUF5455 17) and 1H-imidazo-[4,5-c]quinolin-4-amines (e.g. DU124183 18 and LUF6000 21).
1. 3-(2-Pyridinyl)isoquinoline derivatives as as allosteric modulators of the A3 AR
It is to be noted that some members of the same chemical classes identified as PAMs are pure antagonists of radioligand binding at the A3 AR, and only certain members of these groups of heterocycles were found to decrease the rate of agonist dissociation in addition to displacing the radioligand. Thus, the interaction of these compound classes with the A3 AR is complex. For example, IJzerman and coworkers reported the pyridinylisoquinoline derivative VUF5455 17 (N-(2-methoxyphenyl)-N′-[2-(3-pyrindinyl)-4-quinazolinyl]-urea) to be a selective antagonist of the A3AR with a potent Ki value of 4 nM. Other members of this chemical series were found to be less potent antagonists of the A3 AR, leading to a distinct SAR in the inhibition of radioligand binding at the orthosteric site.
The effects of the reference A3 AR agonist Cl-IB-MECA on forskolin-induced cAMP formation were significantly enhanced by several 3-(2-pyridinyl)isoquinoline derivatives, previously identified as potential antagonists for the hA3 AR (Muijlwijk-Koezen et al., 1998). VUF5455 was shown to be selective for the agonistic state of the A3 AR. In competitive binding studies on cloned hA3 AR, VUF5455 displayed modest affinity as an orthosteric antagonist (Ki = 1680 nM). Replacement of the 7-methyl group of VUF5455 by H (VUF8504) had no influence on the allosteric activity but increased the A3 AR affinity nearly 100-fold (Ki = 17.3 nM). Exchanging the 4′-methoxy group of VUF8504 by methyl (VUF8502) or H (VUF8507) lowered the A3 AR affinity (Ki values of 96 nM and 204 nM) without affecting the allosteric activity. The corresponding imino instead of carboxamido analogues displayed moderate A3 AR affinity (Ki values 300–700 nM), but were devoid of allosteric properties. Thus, although VUF5455 is not devoid of A3 AR antagonistic activity, the compound might be used as a lead for the design of pure PAMs of the A3 AR.
Subsequently, various pyridinylisoquinolines were also found to exhibit allosteric properties, mainly enhancement but in some cases inhibition, with respect to the binding of radiolabeled A3 AR agonist (Fig. 2). The SAR of pyridinylisoquinolines in orthosteric binding to the A3 AR is distinct from SAR in allosteric enhancement. It was proposed that the displacement of orthosteric radioligand at the A3 AR was competitive, but this assumption has not been conclusively established. Since many structural homologues of the pyridinylisoquinolines were already available, it was feasible to characterize their profile as PAMs of A3 AR.
Fig. 2.
Structures of pyridinylisoquinoline and imidizoquinolinamine derivatives that act as PAMs of of the human A3 AR and structure of 2-AG, which acts as a NAM of the A3 AR. Numbering of ring substitutions is shown for compounds 17 and 18.
In a recent study with single living cells, it was shown that both the A3 AR enhancer VUF5455 and the A1 AR enhancer PD81,723 increased the dissociation rate of a fluorescent-tethered nucleoside agonist ABA-X-BY630 from the A3 AR and decreased it at the A1 AR (May et al., 2010b). The latter finding was surprising, and may have to do with the probe dependency of allosteric modulators. These results in whole cells are in contrast to previous findings in membrane preparations and emphasize the complexity of the interactions involved in allosteric modulation.
2. 1H-Imidazo-[4,5-c]quinolin-4-amine derivatives as allosteric modulators of the A3 AR
Another structural class of AR antagonists that was subsequently found to include PAMs of the A3 AR is the imidazoquinolinamines (Gao et al., 2002). IJzerman and coworkers originally introduced the imidazoquinolinamines as A1 AR antagonists (van Galen et al., 1991). In addition to inhibiting the binding of competitive radioligands, the compound DU124183 was found to be a PAM of agonist binding at the A3 AR. This action was initially shown by a reduction in the off-rate of bound agonist radioligand and then conclusively by a functional enhancement at the A3 AR.
The SAR of a further series of imidazoquinolinamines as PAMs of the A3 AR has been explored in detail (Göblyös et al., 2006; Gao et al., 2008b). Allosteric enhancement of the A3 AR was demonstrated by reducing the dissociation rate of agonist radioligand and by enhancement of maximal guanine nucleotide binding in the presence of the reference agonist Cl-IB-MECA. There was clearly a divergence of the structural requirements for allosteric action and the inhibition of binding of orthosteric radioligands. Modification of the 2 and 4 positions was most useful for demonstrating the difference in SAR. The most favorable groups for allosteric enhancement of the A3 AR were found to be 2-cyclohexyl and 4-phenylamino. By structural modification in the series, i.e. altering the size of the 2-cycloalkyl ring (19 – 22) and by substitution of the 4-phenylamino group, the allosteric effects were enhanced without increasing the orthosteric inhibition. The combination of favorable structural modifications resulted in the prototypical A3 AR PAM of the imidazoquinolinamine class, LUF6000 21 (N-(3,4-dichlorophenyl)-2-cyclohexyl-1H-imidazo[4,5-c]quinolin-4-amine). The potency of Cl-IB-MECA in binding to the hA3 AR was not affected by LUF6000, but the maximal functional effect of the agonist was increased.
Further exploration of steric and electronic effects of substitution at the 2 and 4 positions of a series of imidazoquinolinamines as PAMs of the A3AR was reported (Kim et al., 2009). The enhancing ability with minimal inhibition of orthosteric radioligand binding was maintained with 3,5-dichloro substitution in MRS5049 23. Enhancement was observed by two bridged derivatives containing 2-bicycloalkyl groups as in the adamantyl derivative MRS5190 24, indicating that rigid steric bulk is tolerated at the 2 position. Hydrophobicity is also a requirement at that position. Introduction of nitrogen atoms in the 2-cycloalkyl substituents abolished the allosteric enhancement.
Scission of the imidazole ring in the structure of LUF6000 led to a series of 2,4-disubstituted quinolines as PAMs of the A3 AR (Heitman et al., 2009). The same substitution pattern as in LUF6000 led to the most potent allosteric modulator in the series, LUF6096 25, with negligible orthosteric activity on A1 and A3 ARs, even less than observed for LUF6000.
It has been shown that adenosine derivatives are highly structure-dependent in activating as well as binding to the A3 AR. It is even possible to convert nucleoside agonists to A3 AR antagonists. The PAM LUF6000 was found to convert the nucleoside antagonist of the A3 AR, MRS542, into an A3 AR agonist, which is an example of a complete reversal of the nature of the action of an antagonist by a PAM of a GPCR. The experimental result is consistent with the prediction from a mathematical model (Gao et al., 2008b). This phenomenon was not observed with non-nucleoside antagonists of the A3 AR such as MRS1220.
Lane and coworkers recently reported that some endogenous cannabinoid ligands also modulate the A3 AR (Lane et al., 2010). 2-Arachidonylglycerol 26 (2-AG) was able to inhibit agonist [125I]AB-MECA binding and increase the rate of [125I]AB-MECA dissociation, suggesting that 2-AG acts as a NAM. The presence of A3 AR in astrocytes and microglia suggests that this finding may be relevant to cerebral ischemia, a pathological condition in which levels of 2-AG are raised.
The synthetic azo food dye Brilliant Black BN (500 μM, tetrasodium (6Z)-4-acetamido-5-oxo-6-[[7-sulfonato-4-(4-sulfonatophenyl)azo-1-naphthyl]hydrazono]naphthalene-1,7-disulfonate) decreased the affinity of certain antagonists acting at the A1 AR and at the A3 AR, but had no effect on calcium mobilization stimulated by the nonselective adenosine receptor agonist NECA (May et al., 2010a). This allosteric effect was ascribed to a significant increase in dissociation rate of the antagonist.
E. Hypotheses for binding modes of AR allosteric modulators
The potential binding modes of AR allosteric modulators have been probed in site-directed mutagenesis studies (Barbhaiya et al., 1996; Kourounakis et al., 2001; De Ligt et al., 2005; Heitman et al., 2006; Gao et al., 2003a). However, the residues studied only yield a partial view of the amino acids involved in the allosteric regulation of ARs. Chimeras of the A1 AR having A2A AR substitutions (Bhattacharya et al., 2006) have also provided some insight into the structural basis of allosteric modulation. Asp55(2.50) [using in parentheses the Ballesteros numbering for each TM (Ballesteros and Weinstein, 1995)] is probably responsible for allosteric regulation of ligand binding by sodium ions and amilorides, but in G14T(1.37) and T277A(7.42) mutant A1 ARs PD81,723 loses its enhancing activity with respect to CPA. As the potency of CPA alone is also drastically diminished by these mutations, it is not clear yet whether these two amino acids are also part of the PD 81,723 binding site or not.
Gao and colleagues studied the E13Q(1.39) and H278Y(7.43) mutations of the A2A AR. The authors concluded the two residues Glu13 and His278, which are closely linked spatially, are the most important for agonist recognition and partly responsible for the allosteric regulation by sodium ions (Gao and IJzerman, 2000; Gao et al., 2003).
Mutagenesis of the hA3 AR has shown that the PAMs imidazoquinolinamine DU124183 and pyridinylisoquinoline VUF5455 lost their allosteric effects upon F182A(5.43) and N274A(7.45) mutation. The D107N(3.49) mutation eliminated the effects of DU124183, but not of VUF5455. Other residues, such as Trp243(6.48) and Asn30(1.50), were modulatory. Asn274 in TM7 was required for allosteric binding of the imidazoquinolinamine but not for maintaining the orthosteric binding site. His95(3.37) and Phe182(5.43) are important for orthosteric binding of the DU124183 18 (Gao et al., 2003a). A conserved His272(7.43) residue in TM7 is required for A3 AR radioligand binding, and therefore it was not possible to establish the effect of its mutation to Ala on allosteric enhancement. A docking study for a PAM, VUF5455 17, in the agonist-occupied hA3 AR molecular model was reported based on an energetically favorable interaction of this heterocyclic derivative with the outer portions of the receptor – near the ELs (Gao et al., 2003a). This would allow the simultaneous binding of both agonist and PAM to different regions of the receptor protein, as has been shown for allosteric modulation of muscarinic receptors (Conn et al., 2009). The determination of the A2A AR crystal structure (Jaakola et al., 2008) has not yet had an impact on the determination of the allosteric binding site(s) on the ARs. It has also been hypothesized that agonists and PAMs might bind on opposite protomers of homodimeric receptor pairs (Schwartz and Holst, 2006). However, it is not clear if this hypothesis is applicable to the binding of PAMs at the ARs.
III. ‘Translational’ assessment of a prototypical PAM of A1 AR
The characteristics of an allosteric effect depend on the nature of both receptor and orthosteric ligand, on the cellular context and on the pharmacological read-out (Christopoulos and Kenakin, 2002). Interestingly, the PAM PD81,723 has been evaluated in a great number of pharmacological assays, and hence a ‘translational’ assessment of its modulatory potency is now possible for the first time. This analysis follows its effect on the signaling cascade of receptor, G protein and second messengers, down to various organ and tissue systems, and finally in vivo.
A. Receptor effects
The modulatory effects of PD81,723 were first discovered in radioligand binding assays by Bruns and colleagues. When three different tritiated agonists, CHA, NECA and R-PIA, were used, PD81,723 enhanced their binding to the A1 AR from rat brain tissue in a highly similar way (approx. 40% at 10–30 μM). The binding of the tritiated inverse agonist DPCPX was inhibited, approx. 30% at 10 μM PD81,723 (Bruns et al., 1990). This behavior was replicated in brain tissue from dog and guinea-pig (Jarvis et al., 1999). However, adipocyte membranes from all these species, which also express the A1 AR, appeared insensitive to PD81,723.
Not all of the data in the early reports on benzoyl thiophenes were internally consistent. For example, certain data were explained by the antagonist properties for the prototypical PAMs, which were not confirmed in later studies. For example, Childers et al. (2005) did not observe antagonist properties of T-62 in autoradiographic studies. This may relate to the fact that a steady state increase in agonist binding in the presence of a PAM is not necessarily associated with a measured decrease in koff for the agonist. Similarly, compounds that decreased Koff may not necessary show an increase in agonist binding at steady state or equilibrium conditions.
Allosteric enhancement by PD81,723 was also noticed when radioligand agonist ([125I]ABA) binding to the hA1 AR stably expressed in Chinese hamster ovary (CHO) cells was studied (Bhattacharya and Linden, 1995; see also Figler et al., 2003). PD81,723 tested at 20 μM caused a 3-fold increase in the fraction of receptors found in a high-affinity G protein-coupled conformation. When [3H]CCPA, another agonist radioligand, was studied no significant changes in KD values were noted in the absence or presence of 10 μM PD81,723, not only for stably expressed receptors in CHO cells (Kourounakis et al., 2001), but also in human and rat brain membranes (Baraldi et al., 2004). Bmax values, however, appeared somewhat increased in the presence of PD81,723 (Baraldi et al., 2003). This latter finding had been reported previously by Kollias-Baker et al. (1997) using [3H]CHA, yet another radiolabeled agonist. In radioligand displacement studies with [3H]DPCPX (Bhattacharya and Linden, 1995) a 2.4-fold increase in the potency of the agonist R-PIA was observed, confirmed in later observations (Kourounakis et al., 2001). This enhanced affinity was easily reconciled with the observation that PD81,723 caused a 1.5-fold increase of the dissociation half-life of [125I]ABA from the receptor; a similar 2-fold increase was found for COS-7 cell membranes expressing the canine A1 AR (Mizumura et al., 1996). Bhattacharya and Linden were among the first to suggest that PD81,723 binds to the A1 AR at a site distinct from the agonist (i.e. orthosteric) binding site and stabilizes agonist-R-Gi complexes. This applied also to studies of an A1/A2A AR chimera having the third intracellular loop of the A1 AR replaced by that of the A2A AR (Bhattacharya et al., 2006). On this receptor construct PD81,723 caused a comparable increase of the dissociation half-life of [125I]ABA from the receptor. Heitman et al. (2006) also studied the effects of PD81,723 on the hA1 AR. The authors first examined its influence on equilibrium saturation experiments with the radiolabeled inverse agonist [3H]DPCPX. The KD value of [3H]DPCPX was increased 5.5 times in the presence of 10 μM PD81,723, suggesting that PD81,723 promotes a receptor state that is unfavorable for this inverse agonist. In equilibrium displacement experiments with the agonist CPA, PD81,723 increased the affinity of CPA for the low-affinity state of the receptor by 3.6 fold, while the affinity for the high-affinity state was not altered. A similar finding was observed in another cellular background, i.e. COS cells expressing the hA1 AR (De Ligt et al., 2005). These effects were somewhat different from the findings by Linden and coworkers described above, in which an increase in the fraction of receptors in the high-affinity state was noticed. The effect in the Heitman study was probe-dependent; PD81,723 slightly decreased the affinity of LUF5831, a non-ribose agonist for the receptor, whereas another ribose-containing agonist (N6-4-methoxyphenyladenosine) again showed a 4-fold increase in affinity (Narlawar et al., 2010). This enhancing effect vanished in the latter study when hybrid (‘bitopic’) agonist ligands were studied, in which the classic adenosine-like agonist was linked and coupled to a PD81,723-like pharmacophore. Using lower concentrations of PD81,723, like 3 μM in a study by Musser et al. (1999), did not cause significant changes in Bmax or KD values of radiolabeled agonists and antagonists on rat brain membranes or CHO cells expressing the rat A1 AR.
B. G protein effects
The G protein-dependency of some of the receptor interactions of PD81,723 was revealed by a number of research groups. It was first found that the effects of (stable derivatives of) GTP on agonist binding to the A1 AR were influenced by PD81,723. Bhattacharya and Linden (1995) demonstrated that the PAM caused a 2.2-fold increase in the concentration of GTPγS required to half-maximally uncouple receptor-G-protein complexes. In a similar experimental setup (Kollias-Baker et al., 1994), the IC50 values for GppNHp to reduce specific binding of [3H]CHA to guinea pig cardiac membranes increased from 1.5 μM in the absence of PD81,723 (30 μM) to 10 μM in its presence. The effects of PD81,723 were also examined in direct [35S]GTPγS binding experiments. In a recent study, the potency of the agonist N6-4-methoxyphenyladenosine to stimulate GTPγS binding on cell membranes expressing the hA1 AR was increased 4.9-fold by 10 μM PD81,723 (Narlawar et al., 2010). Remarkably, Kollias-Baker et al. (1997) showed that PD81,723 also caused a direct stimulation of GTPγS binding to cell membranes expressing the hA1 AR, i.e. in the absence of an added agonist. This increase in binding was abrogated in the presence of DPCPX and not influenced by the addition of adenosine deaminase, excluding a role for endogenous adenosine in these observations. In yet another approach, Klaasse et al. (2004) studied the behavior of PD81,723 on receptor-Gαi protein fusion products. The effects of 10 μM PD81,723 on ligand binding were rather similar for both the unfused A1 AR (expressed in G protein-poor COS cells) and the different fusion proteins (mutations in the α-subunit and expressed in the same cell line). In the presence of PD81,723, CPA’s affinity for the unfused receptor increased 4-fold. The allosteric modulator increased the affinity of CPA for the various fusion proteins to a varying but overall quite similar extent, namely 2- to 6-fold. The binding of the inverse agonist DPCPX to the fusion products was not much affected by PD81,723. In another ‘fusion’ study, Bhattacharya et al. (2006) examined chimaeric constructs between A1 and A2A ARs. The authors showed that the allosteric effect of PD81,723 was maintained in a construct in which the third intracellular loop of the Gi-coupled A1 AR was replaced with the analogous sequence of the Gs-coupled A2A AR. PD81,723 increased the potency of N6-cyclopentyladenosine to increase cAMP accumulation in cells expressing this chimaeric receptor with or without pretreatment with pertussis toxin. The results suggest that the recognition site for PD81,723 is on the A1 AR, and that the enhancer directly stabilizes the receptor in a conformation capable of coupling to Gi or Gs.
C. Second messengers and intracellular pathways
1. cAMP
In intact CHO cells expressing the hA1 AR, PD81,723 increased the potency of the reference agonist R-PIA to decrease forskolin-stimulated cAMP accumulation by 3.3-fold (Bhattacharya and Linden, 1995). Musser et al. (1999) further studied its effects on adenylate cyclase and cAMP production. The compound inhibited basal adenylyl cyclase (AC) activity as well as forskolin-, cholera toxin-, and pertussis toxin-stimulated AC activity in ‘empty’ CHO cells and CHO cells carrying the rat A1 AR gene. For instance, basal AC activity was significantly inhibited in both cell lines by a high concentration of PD81,723 (30 μM). In CHO-A1 cells, half-maximal inhibition of forskolin-stimulated AC occurred at 5 μM PD81,723 compared to 10 μM in CHO cells. Some of these effects may actually occur at the level of adenylate cyclase itself, because [3H]forskolin was displaced from purified enzyme from rat liver by PD81,723 with an IC50 of 96 μM. Apparently, two mechanisms appear to contribute to the observed effects of PD81,723: allosteric enhancement of A1 AR function and direct effect on adenylate cyclase. Kollias-Baker et al. (1997) studied CHO cells stably expressing the hA1 AR in which PD81,723 acted in synergism with the agonist (R)-PIA to inhibit forskolin-stimulated cAMP formation. In a more extensive study by Kourounakis et al. (2001), PD81,723 (10 μM) alone inhibited cAMP production to approx. 70–80% of forskolin-stimulated levels. The agonist CPA decreased cAMP production to 70% at a concentration of 1 nM, while PD81,723 further decreased the cAMP production in combination with CPA. In this cell line DPCPX increased the cAMP production by approx. 30%. In the additional presence of PD81,723 cAMP production decreased to forskolin-only levels. For the agonist CPA a dose–response curve was recorded in the absence (EC50 4.2 nM) and presence (EC50 0.6 nM) of 10 μM PD81,723.
2. MAP kinase
PD81,723 (10 μM) enhanced the potency of an A1 AR agonist, N6-4-methoxyphenyladenosine, to stimulate ERK1/2 phosphorylation in CHO cells expressing the hA1 AR by 4-fold. It also enhanced the efficacy of the agonist by 30% (Narlawar et al., 2010).
3. DNA synthesis
PD81,723 (3 μM) enhanced the potency of the selective A1 AR agonist CCPA (1 μM) to stimulate DNA synthesis in pig coronary artery smooth muscle cells. A similar effect was seen with adenosine as the agonist (100 μM). Control experiments showed that treatment of the cells with DPCPX or with pertussis toxin abolished the stimulatory effects on DNA synthesis (Shen et al., 2005)
4. Receptor desensitization and internalization
Bhattacharya and Linden (1996) studied the desensitization and down-regulation of the hA1 AR in CHO cells. Pretreatment with 20 μM PD81,723 or 10 μM CPA caused a 1.5- and 4.0-fold desensitization measured as a reduced potency of CPA to lower cAMP levels in the cells. Pretreatment with these agents did not modify the acute effect of PD81,723 to increase the potency of CPA 5-fold. Radioligand binding experiments were performed to measure receptor down-regulation in cell membranes and in intact cells. Pretreatment of the cells with PD81,723 had no significant effect on the number of receptors. Pretreatment of cells with CPA produced large reductions in the binding of agonist and antagonist radioligands to both membranes and intact cells. The authors speculated that the relatively small degree of functional desensitization and down-regulation of receptors caused by long term exposure of cells to PD81,723 is encouraging in terms of the therapeutic potential of such PAMs. Klaasse et al. (2005) studied the long-term effect of PD81,723 on receptor internalization. To visualize this process the receptor was engineered to contain a C-terminal YFP tag. The introduction of this marker did not affect the radioligand binding properties of the receptor. CHO cells stably expressing this receptor were subjected during 16 h to varying concentrations of the agonist CPA in the absence or presence of 10 μM of PD81,723. CPA itself was able to internalize 25% and 40% of the receptors at a concentration of 400 nM or 4 μM, respectively. Addition of PD81,723 alone had no effect on internalization. However, a slight amount of internalization induced by with PD81,723 was obtained already at 40 nM of CPA, and 59% of the receptors internalized at 400 nM CPA.
D. Effects on Tissues
1. Heart
One of the first studies of cardiac effects of PD81,723 was performed by Mudumbi et al. (1993). The authors investigated the effects of PD81,723 in spontaneously contracting right atria and electrically stimulated left atria isolated from Sprague-Dawley rats. The reference A1 AR agonist CPA produced a concentration-dependent inhibition of heart rate in right atria (chronotropy) and contractile parameters in left atria (inotropy). In both right and left atrium PD81,723 (5 μM) significantly left-shifted the concentration-response curves for CPA. In the same year, Amoah-Apraku et al. (1993) studied PD81,723 as an enhancer of the negative dromotropic effect of exogenous adenosine in guinea pig isolated and in situ hearts. In isolated hearts, PD81,723 alone produced only a small stimulus-to-His bundle (S-H) interval prolongation of 1.5 to 4 msec, which could be blocked by the A1-selective antagonist 8-cyclopentyltheophylline (CPT). Under hypoxia, leading to an increase of interstitial adenosine levels, the S-H interval was also prolonged, and this was increased 2-fold in the presence of 5 μM PD81,723 (Kollias-Baker et al., 1994b). PD81,723 (5 μM) significantly increased the potency of adenosine for prolongation of the S-H interval from 7 to 4 μM. This potentiation by PD81,723 was also dose-dependent (Kollias-Baker et al., 1994a). The effect was A1 AR-dependent; PD81,723 had no effect when e.g., carbachol was used. In in situ hearts, PD81,723 (2 μmol/kg i.v.) caused a significant leftward and upward shift of the adenosine dose-response curve for inducing atrium-to-His bundle (A-H) interval prolongation. As a consequence the degree of atrioventricular block caused by adenosine was also increased. Martynyuk et al. (2002) studied the molecular mechanisms underlying the adenosine-induced slowing of atrioventricular nodal conduction in guinea pig isolated hearts and in single atrial myocytes. This is a rate-dependent process, of which the authors analyzed the atrium-to-His bundle (A-H) interval (heart) or patch-clamp recordings (myocytes). A decrease in atrial cycle length from 300 to 190 ms decreased the concentration of adenosine needed to cause atrioventricular nodal block from 8 to 3 μM. PD81,723 (5 μM) potentiated the negative dromotropic effect of adenosine. In atrial myocytes adenosine augmented a time- and voltage-dependent K+ current, which was also potentiated by PD81,723. It should be mentioned though that PD81,723 appears to act as a direct inhibitor of some K+ channels in guinea-pig atrial myocytes too, most notably inward rectifying ones (Brandts et al., 1997). Mizimura et al. (1996) performed in vivo studies in dogs and examined the phenomenon of cardiac preconditioning. To determine if PD81,723 lowers the threshold for ischemic preconditioning, anesthetized dogs were subjected to coronary artery occlusion and subsequent reperfusion. Myocardial infarct size was significantly decreased by a combination of PD81,723 and preconditioning (a short period of artery occlusion preceding the main event), which beneficial effect could be blocked by the intravenous administration of DPCPX.
2. Brain
Janusz et al. (1991) were the first to evaluate the actions of PD81,723 in brain slices. PD81,723 dose-dependently enhanced the inhibitory effects of exogenously applied adenosine in hippocampal brain slices as indicated by two parameters, the amplitude of the population spike and paired-pulse facilitation. PD81,723 had no effect when administered alone, but required the presence of adenosine. In a different experimental setup on the same preparation adenosine reduced the duration of epileptiform bursting in a dose-dependent manner. Application of PD81,723 at concentrations as high as 100 μM also resulted in a dose-dependent reduction in the duration of the triggered burst (Janusz and Berman, 1993). Phillis and coworkers (1994) studied the effects of PD81,723 in ischemia-evoked amino acid transmitter release from rat cerebral cortex. When administered at 10 mg/kg i.p., PD81,723 significantly depressed glutamate efflux but not of GABA. However, in a gerbil model of forebrain ischemia PD81,723, studied at three dosages, failed to protect against ischemia/reperfusion evoked cerebral injury (Cao and Phillis, 1995). Bueters et al. (2002) characterized the effects of PD81,723 on striatal acetylcholine release. Upon local administration in conscious rats via a microdialysis probe the compound (0.1 – 100 μM) caused a concentration-dependent increase of extracellular acetylcholine levels of approximately 40%, which was similar to that obtained by the selective A1 AR antagonist CPT. In competition experiments PD81,723 did not change the inhibition of acetylcholine release by CPA, whereas CPT caused an eightfold rightward shift of the CPA dose-response curve. Apparently, the putative antagonistic action of PD81,723 in this animal model appeared to counteract its allosteric action. Meno et al. (2003) investigated the effects of PD81,723 (3 or 10 mg/kg i.p.) on hippocampal injury and Morris water maze performance following hyperglycemic cerebral ischemia and reperfusion in the rat. Only at the lower dose a significant reduction of hippocampal injury was observed, in line with an improved water maze performance suggesting that ‘reinforcement’ of endogenously produced adenosine provides neuroprotection in this animal model.
Therefore, PD81,723 enhances A1 AR agonist binding and function in many but not all tissues (e.g., adipocytes, see Jarvis et al., 1999) examined. Its effects are relatively modest, i.e. agonist potencies tend to be increased by a factor of 2–5 with PD81, 723 at micromolar (3–100 μM) concentrations. Probe dependency has been established, for instance non-ribose agonists are less sensitive, if at all, than adenosine-like derivatives to the influence of PD81,723. In in vivo and ex vivo experimental results vary, as a differentiation between enhancing and antagonistic effects of PD81,723 is not always easily observed.
IV. Allosteric modulators of P2Y and P2X receptors for nucleotides
A. P2Y receptor modulation
P2Y receptors respond to various extracellular nucleotides, including ATP, UTP, ADP, UDP, and UDP-glucose. The subtypes of P2Y receptors are numbered P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13, and P2Y14. The three P2Y12 - P2Y14 subtypes inhibit adenylate cyclase through Gi protein, and the other five subtypes activate phospholipase Cβ through Gq protein.
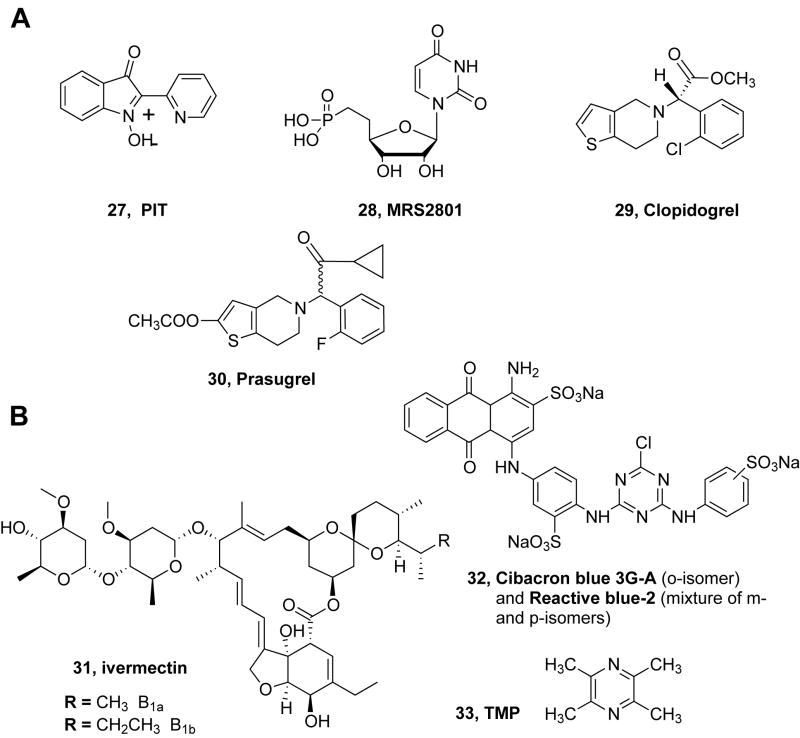
The P2Y1 receptor is activated by endogenous ADP to induce platelet aggregation, muscle relaxation, and vasodilation. The inhibitory effect of 2,2′-pyridylisatogen tosylate 27 (PIT) on the hP2Y1 receptor is allosteric (Spedding et al., 2000, Gao et al., 2004a). PIT blocked the accumulation of inositol phosphates induced by the potent synthetic agonist 2-methylthio-ADP (2-MeSADP) and by ADP in 1321N1 astrocytoma cells stably expressing the hP2Y1 receptor. The antagonism occurred in a concentration-dependent manner but was non-competitive, and it did not inhibit the binding of a selective P2Y1 receptor antagonist radioliogand. PIT had no significant effect on agonist activation of other P2Y receptors examined. Thus, PIT selectively and non-competitively blocked P2Y1 receptor signaling without affecting nucleotide binding.
SCH-202676 was shown to inhibit ATP-induced Na+-K+ pump activity mediated via the P2Y1 receptor in depolarized skeletal muscle (Broch-Lips et al., 2010), although it did not inhibit the binding of the selective radioligand [3H]MRS2279 to the P2Y1 receptor (Gao et al., 2004). It has been reported that SCH-202676 affects a number of G protein coupled receptors (Fawzi et al., 2001). However, later studies suggested SCH-202676 modulates some GPCRs via thiol modification rather than via true allosteric mechanisms (Göblyös et al., 2005; Lewandowicz et al., 2006).
A uridine 5′-methylene-phosphonate derivative 28 was a relatively potent (EC50 = 1.6 ± 0.4 μM) agonist at the P2Y2 receptor and had no effect on the P2Y4 and P2Y6 receptors (Cosyn et al., 2009). However, the maximal agonist effect observed was <50% of that observed with the native agonist UTP. UMP itself was inactive at this receptor. High concentrations of 28 failed to antagonize activation of the P2Y2 receptor by UTP, suggesting that it potentially activates the P2Y2 receptor through an allosteric mechanism. At the P2Y4 receptor, diadenosine polyphosphates potentiated the UTP agonist response, which was not observed at other P2Y subtypes (Patel et al., 2001).
Antagonists of the P2Y12 receptor are in clinical use as antithrombotic agents. Several of these agents, clopidogrel 29 and prasugrel 30, are thienopyridine derivatives that contain masked thiol groups that are liberated in vivo. The reactive metabolites act as irreversible inhibitors of the binding of ADP at this receptor, and their site of action might prove to be allosteric. They have been shown to bind covalently to form disulfides with Cys residues that normally form a bridge between EL2 and TM3 (Algaier et al., 2008).
Other modulators of P2Y receptor signaling that are possibly allosteric modulators are cysteinyl leukotriene antagonists (Mamedova et al., 2005) and bicyclic diketopiperazines (Besada et al., 2005).
B. P2X receptor modulation
The subtypes of P2X receptors are numbered P2X1 through P2X7. These subunits combine to form trimeric LGICs that are activated by extracellular ATP at various concentrations. The recent determination of the zebrafish P2X4 crystal structure (Kawate et al., 2009) has provided insights into the structure of this family of ion channels.
Zn2+ ions, Cu2+ ions, and pH can act as allosteric modulators of action at the P2X receptors (Evans, 2009). Other metals, such as lanthanides, may also modulate P2X receptors, and ethanol has been shown to reduce the potency of ATP at various P2X receptors (reviewed in Coddou et al., 2011). The sedative propofol and various lipids also modulate action at P2X receptors.
The differential sensitivity of various P2X receptor subtypes to metal ions and to protons has been probed using mutagenesis. Zn2+ at low micromolar concentrations increased the channel activity of P2X2 and P2X4 receptors (Brake et al., 1994; Seguela et al., 1996; Soto et al., 1996; Xiong et al., 1999). The same treatment decreased the activity of P2X1 and P2X7 receptors (Wildman et al., 1999; Virginio et al., 1997). The effects of Cu2+ ions (Xiong et al., 1999) and protons (King et al., 1996; Li et al., 1996; Stoop et al., 1997; Wildman et al., 1998, 1999) of various P2X receptor subtypes have been extensively explored. Various His and Asp residues have been found to be involved in these effects using site-directed mutagenesis. The potentiation of the P2X4 receptor activity by Zn2+ is dependent on Cys132 and to a lesser extent on Thr133 (Coddou et al., 2007). Neither of these residues affects inhibition by Cu2+, which is dependent on Asp138 and His140. Thus, this receptor region contains a pocket for trace metal coordination with two distinct and separate sites for di-cations as a PAM or NAM. For the P2X2 receptor, His120 and His213 were identified as part of an intersubunit binding site that accounts for Zn2+ potentiation (Nagaya et al., 2005); these residues are also involved in Cu2+ potentiation (Lorka et al., 2005).
The anti-parasitic drug ivermectin 31 (a mixture of macrocyclic lactone disaccharides 22,23-dihydroavermectin B1a + 22,23-dihydroavermectin B1b) has been reported to enhance currents at the P2X4 receptor but not at other P2X receptors (Khakh et al., 1999). Its mechanism of action and specific amino acid residues involved in the effect have been investigated (Priel and Silberberg, 2004, Toulmé et al., 2006; Silberberg et al., 2007; Zemkova et al., 2010; Coddou et al., 2011).
Cibacron blue 30 allosterically modulates the rat P2X4 receptor (Miller et al., 1998). It was also found to be a PAM of the P2X3 receptor that also restored the ATP responsiveness to acutely desensitized receptors (Alexander et al., 1999). There has been confusion about which isomer of this dye is designated Cibacron blue. Cibacron blue 3GA refers to the ortho-isomer of 32, but many studies have assumed Reactive blue-2 (mixture of meta- and para-isomers) to be synonymous with Cibacron blue.
Tetramethylpyrazine (TMP, 33), an alkaloid in traditional Chinese medicine, inhibits the effects of nucleotides at the P2X3 receptor in primary afferent transmission in neuropathic pain states (Gao et al., 2008a). It has been proposed to bind at an allosteric site on the large extracellular region of the P2X receptor.
IV. Conclusion
Allosteric modulators have been most highly developed for the A1 and A3 ARs among the purine and pyrimidine receptors. In fact, a 2-amino-3-aroylthiophene derivative T-62 has been under development as a PAM of the A1 AR for the treatment of chronic pain. The prototypic PAM of the A1 AR, PD81,723, has been evaluated in a great number of pharmacological assays, which makes possible a ‘translational’ assessment of its modulatory potency. The benzoylthiophene derivatives tend to act as allosteric agonists as well as pure PAMs of this subtype and lack action at other AR subtypes. Two classes of A3 AR allosteric modulators have been explored: 3-(2-pyridinyl)isoquinolines (e.g. VUF5455) and 1H-imidazo-[4,5-c]quinolin-4-amines (e.g. DU124183 and LUF6000), which selectively decreased the agonist dissociation rate at the hA3ARs, but not at A1 and A2A ARs. These A3 AR modulators have allosteric effects that can be structurally separated from the orthosteric effects in SAR studies. Nucleoside derivatives that are A3 selective antagonists and low efficacy agonists can be converted into full agonists by coadministration of the PAM LUF6000. Site-directed mutagenesis of A1 and A3 receptors has identified residues associated with the allosteric effect. Distinct amino acid residues affect orthosteric vs. allosteric binding. Thus, there are clear advantages to the design of allosteric modulators of ARs. Small molecular allosteric modulators have been reported for several of the P2Y nucleotide receptors and P2X receptors, but there is much room for exploration of this approach into the nucleotide receptor field. Allosteric modulation of the P2X receptors by metal ions and protons has been extensively studied by site directed mutagenesis. In conclusion, allosteric modulation of purine and pyrimidine receptors looks promising for development of drugs that are event-specific and site-specific in action.
Fig. 3.
Structures of compounds found to act as allosteric modulators of P2Y (A) and P2X (B) receptors.
Acknowledgments
KAJ acknowledges support from the Intramural Research Program of the NIH, National Institute of Diabetes & Digestive & Kidney Diseases. APIJ thanks Dutch Top Institute Pharma (project The GPCR forum, D1.105) for generous financial support.
Abbreviations
- AR
adenosine receptor
- BBB
blood brain barrier
- CPA
N6-cyclopentyladenosine
- CCPA
2-chloro-N6-cyclopentyladenosine
- CHA
N6-cyclohexyladenosine
- CGS21680
2-p-(2-carboxyethyl)phenethylamino-5′-N-ethylcarboxamidoadenosine hydrochloride
- Cl-IB-MECA
2-chloro-N6-(3-iodobenzyl)-adenosine-5′-N-methyluronamide
- CPT
8-cyclopentyltheophylline
- DU124183
N-phenyl-2-cyclopentyl-1H-imidazo[4,5-c]quinolin-4-amine
- EL
extracellular loop
- GPCR
G protein-coupled receptor
- HMA
5-(N,N-hexamethylene)amiloride
- LGIC
ligand-gated ion channel
- LUF6000
N-(3,4-dichlorophenyl)-2-cyclohexyl-1H-imidazo[4,5-c]quinolin-4-amine
- LUF6258
N(6)-[2-amino-3-(3,4-dichlorobenzoyl)-4,5,6,7-tetrahydrothieno[2, 3-c]pyridin-6-yl -9-nonyloxy-4-phenyl]-adenosine
- MRS1220
N-[9-chloro-2-(2-furanyl)[1,2,4]triazolo[1,5-c]quinazolin-5-yl]benzeneacetamide
- PIT
pyridyl isatogen tosylate
- MRS 542
2-chloro-N6-(3-iodobenzyl)-adenosine
- MRS5049
2-cyclohexyl-N-(3,5-dichlorophenyl)-1H-imidazo[4,5-c]quinolin-4-amine
- MRS5190
2-(1-adamantanyl)-N-(3,4-dichlorophenyl)-1H-imidazo[4,5-c]quinolin-4-amine
- NECA
5′-(N-ethylcarboxamido)adenosine
- PD120,918
4-methyl-2-oxo-2H-chromen-7-yl methylcarbamate
- LUF5484
(2-amino-4,5,6,7-tetrahydrobenzo[b]thiophen-3-yl)(3,4-dichlorophenyl)methanone
- PAM
positive allosteric modulator
- PD71,605
(2-amino-4,5,6,7-tetrahydro-benzo[b]thiophen-3-yl)-(2-chlorophenyl)-methanone
- PD81,723
(2-amino-4,5-dimethyl-3-thienyl)-[3-(trifluoromethyl)phenyl]methanone
- SAR
structure activity relationship
- SCH-202676
N-(2,3-diphenyl-1,2,4-thiadiazol-5-(2H)-ylidene)methanamine
- T62
2-amino-3-(4-chlorobenzoyl)-5,6,7,8-tetrahydrobenzothiophene
- TM
transmembrane domain
- VUF5455
(N-(2-methoxyphenyl)-N′-[2-(3-pyrindinyl)-4-quinazolinyl]-urea
- VUF8502
4-methyl-N-[3-(2-pyridinyl)-1-isoquinolinyl]benzamide
- VUF8504
methoxy-N-[3-(2-pyridinyl)-1-isoquinolinyl]benzamide
- VUF8507
N-[3-(2-pyridinyl)-1-isoquinolinyl]benzamide
- ERK
extracellular-signal-regulated kinase
- VCP520
2-amino-4-(3,5-bis(trifluoromethyl)phenyl)thiophen-3-yl)(phenyl)methanone
- VCP333
tert-butyl 2-amino-3-(4-chlorobenzoyl)-7,8-dihydro-4H-thieno[2,3-d]azepine-6(5H)-carboxylate
- ZM241385
4-{2-[7-amino-2-(2-furyl)-1,2,4-triazolo[1,5-a]1,3,5]triazin-5-yl-amino]ethyl}phenol
Footnotes
Conflict of interest: The authors are inventors on patents for LUF6000 and LUF6096.
References
- Alexander K, Niforatos W, Bianchi B, Burgard EC, Lynch KJ, Kowaluk EA, Jarvis MF, van Biesen T. Allosteric Modulation and Accelerated Resensitization of Human P2X3 Receptors by Cibacron Blue. J Pharmacol Exp Ther. 1999;291:1135–1142. [PubMed] [Google Scholar]
- Algaier I, Jakubowski JA, Asai F, von Kügelgen I. Interaction of the active metabolite of prasugrel, R-138727, with cysteine 97 and cysteine 175 of the human P2Y12 receptor. J Thromb Haemost. 2008;6:1908–1914. doi: 10.1111/j.1538-7836.2008.03136.x. [DOI] [PubMed] [Google Scholar]
- Amoah-Apraku B, Xu J, Lu JY, Pelleg A, Bruns RF, Belardinelli L. Selective potentiation by an A1 adenosine receptor enhancer of the negative dromotropic action of adenosine in the guinea pig heart. J Pharmacol Exp Ther. 1993;266:611–7. [PubMed] [Google Scholar]
- Aumann KM, Scammells PJ, White JM, Schiesser CH. On the stability of 2-aminoselenophene-3-carboxylates: potential dual-acting selenium-containing allosteric enhancers of A1 adenosine receptor binding. Org Biomol Chem. 2007;5:1276–1281. doi: 10.1039/b700812k. [DOI] [PubMed] [Google Scholar]
- Aurelio L, Valant C, Flynn BL, Sexton PM, Christopoulos A, Scammells PJ. Allosteric modulators of the adenosine A1 receptor: Synthesis and pharmacological evaluation of 4-substituted 2-amino-3-benzoylthiophenes. J Med Chem. 2009;52:4543–4547. doi: 10.1021/jm9002582. [DOI] [PubMed] [Google Scholar]
- Aurelio L, Valant C, Figler H, Flynn BL, Linden J, Sexton PM, Christopoulos A, Scammells PJ. 3- and 6-Substituted 2-amino-4,5,6,7-tetrahydrothieno[2,3-c]pyridines as A1 adenosine receptor allosteric modulators and antagonists. Bioorg Med Chem. 2009;17:7353–7361. doi: 10.1016/j.bmc.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aurelio L, Valant C, Flynn BL, Sexton PM, White JM, Christopoulos A, Scammells PJ. Effects of conformational restriction of 2-amino-3-benzoylthiophenes on A1 adenosine receptor modulation. J Med Chem. 2010;53:6550–6559. doi: 10.1021/jm1008538. [DOI] [PubMed] [Google Scholar]
- Ballesteros JA, Weinstein H. Integrated methods for the construction of three-dimensional models and computational probing of structure-function relations in G protein-coupled receptors. Methods in Neurosciences. 1995;25:366–428. [Google Scholar]
- Baraldi PG, Zaid AN, Lampronti I, Fruttarolo F, Pavani MG, Tabrizi MA, Shryock JC, Leung E, Romagnoli R. Synthesis and biological effects of a new series of 2-amino-3-benzoylthiophenes as allosteric enhancers of A1 adenosine receptor. Bioorg Med Chem Lett. 2000;10:1953–1957. doi: 10.1016/s0960-894x(00)00379-6. [DOI] [PubMed] [Google Scholar]
- Baraldi PG, Iaconinoto MA, Moorman AR, Carrion MD, Cara CL, Preti D, Lopez OC, Fruttarolo F, Tabrizi MA, Romagnoli R. Allosteric enhancers for A1 adenosine receptor. Mini Rev Med Chem. 2007;7:559–569. doi: 10.2174/138955707780859459. [DOI] [PubMed] [Google Scholar]
- Baraldi PG, Romagnoli R, Pavani MG, Nunez MC, Tabrizi MA, Shryock JC, Leung E, Moorman AR, Uluoglu C, Iannotta V, Merighi S, Borea PA. Synthesis and biological effects of novel 2-amino-3-naphthoylthiophenes as allosteric enhancers of the A1 adenosine receptor. J Med Chem. 2003;46:794–809. doi: 10.1021/jm0210212. [DOI] [PubMed] [Google Scholar]
- Baraldi PG, Pavani MG, Shryock JC, Moorman AR, Iannatta V, Borea PA, Romagnoli R. Synthesis of 2-amino-3-heteroaroylthiophenes and evalutaion of their activity as potential allosteric enhancers at the human A1 receptor. Eur J Med Chem. 2004;39:855–865. doi: 10.1016/j.ejmech.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Barbhaiya H, McClain R, IJzerman A, Rivkees SA. Site-directed mutagenesis of the human A1 adenosine receptor: Influences of acidic and hydroxy residues in the first four transmembrane domains on ligand binding. Mol Pharmacol. 1996;50:1635–1642. [PubMed] [Google Scholar]
- Besada P, Mamedova L, Thomas CJ, Costanzi S, Jacobson KA. Design and synthesis of new bicyclic diketopiperazines as scaffolds for receptor probes of structurally diverse functionality. Org Biomol Chem. 2005;3:2016–2025. doi: 10.1039/b416349d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya S, Linden J. The allosteric enhancer, PD 81,723, stabilizes human A1 adenosine receptor coupling to G proteins. Biochim Biophys Acta. 1995;1265:15–21. doi: 10.1016/0167-4889(94)00204-r. [DOI] [PubMed] [Google Scholar]
- Bhattacharya S, Linden J. Effects of long-term treatment with the allosteric enhancer, PD81,723, on Chinese hamster ovary cells expressing recombinant human A1 adenosine receptors. Mol Pharmacol. 1996;50:104–111. [PubMed] [Google Scholar]
- Bhattacharya S, Youkey RL, Ghartey K, Leonard M, Linden J, Tucker AL. The allosteric enhancer PD 81,723 increases chimaeric A1/A2A adenosine receptor coupling with Gs. Biochem J. 2006;396:139–146. doi: 10.1042/BJ20051422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrmann T, Hinz S, Bertarelli DC, Li W, Florin NC, Scheiff AB, Müller CE. 1-Alkyl-8-(piperazine-1-sulfonyl)phenylxanthines: development and characterization of adenosine A2B receptor antagonists and a new radioligand with subnanomolar affinity and subtype specificity. J Med Chem. 2009;52:3994–4006. doi: 10.1021/jm900413e. [DOI] [PubMed] [Google Scholar]
- Brake AJ, Wagenbach MJ, Julius D. New structural motif for ligand-gated ion channels defined by an ionotropic ATP receptor. Nature. 1994;371:519–523. doi: 10.1038/371519a0. [DOI] [PubMed] [Google Scholar]
- Broch-Lips M, Pedersen TH, Nielsen OB. Effect of purinergic receptor activation on Na+-K+ pump activity, excitability, and function in depolarized skeletal muscle. Am J Physiol Cell Physiol. 2010;298:C1438–C1444. doi: 10.1152/ajpcell.00361.2009. [DOI] [PubMed] [Google Scholar]
- Brandts B, Bünemann M, Hluchy J, Sabin GV, Pott L. Inhibition of muscarinic K+ current in guinea-pig atrial myocytes by PD 81,723, an allosteric enhancer of adenosine binding to A1 receptors. Br J Pharmacol. 1997;121:1217–1223. doi: 10.1038/sj.bjp.0701254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns RF, Lu GH. In: Adenosine Receptors in the Nervous System. Ribeiro JA, editor. Taylor and Francis; London: 1989. p. 192. [Google Scholar]
- Bruns RF, Fergus JH. Allosteric enhancement of adenosine A1 receptor binding and function by 2-amino-3-benzoylthiophenes. Mol Pharmacol. 1990;38:939–949. [PubMed] [Google Scholar]
- Bruns RF, Fergus JH, Coughenour LL, Courtland GG, Pugsley TA, Dodd JH, Tinney FJ. Structure-activity relationships for enhancement of adenosine A1 receptor binding by 2-amino-3-benzoylthiophenes. Mol Pharmacol. 1990;38:950–958. [PubMed] [Google Scholar]
- Bueters TJ, van Helden HP, Danhof M, IJzerman AP. Effects of the adenosine A1 receptor allosteric modulators PD 81,723 and LUF 5484 on the striatal acetylcholine release. Eur J Pharmacol. 2002;454:177–182. doi: 10.1016/s0014-2999(02)02494-9. [DOI] [PubMed] [Google Scholar]
- Cao X, Phillis JW. Adenosine A1 receptor enhancer, PD 81,723, and cerebral ischemia/reperfusion injury in the gerbil. Gen Pharmacol. 1995;26:1545–1548. doi: 10.1016/0306-3623(95)00042-9. [DOI] [PubMed] [Google Scholar]
- Changeux JP. Allosteric receptors: from electric organ to cognition. Ann Rev Pharmacol Toxicol. 2010;50:1–38. doi: 10.1146/annurev.pharmtox.010909.105741. [DOI] [PubMed] [Google Scholar]
- Childers SR, Li XL, Xiao R, Eisenach JC. Allosteric modulation of adenosine A1 receptor coupling to G-proteins in brain. J Neurochem. 2005;93:715–723. doi: 10.1111/j.1471-4159.2005.03044.x. [DOI] [PubMed] [Google Scholar]
- Christopoulos A, Kenakin T. G protein-coupled receptor allosterism and complexing. Pharmacol Rev. 2002;54:323–74. doi: 10.1124/pr.54.2.323. [DOI] [PubMed] [Google Scholar]
- Chordia MD, Zigler M, Murphree LJ, Figler H, Macdonald TL, Olsson RA, Linden J. 6-aryl-8H-indeno[1,2-d]thiazol-2- ylamines: A1 adenosine receptor agonist allosteric enhancers having improved potency. J Med Chem. 2005;48:5131–5139. doi: 10.1021/jm049132j. [DOI] [PubMed] [Google Scholar]
- Christopoulos A. Allosteric binding sites on cell-surface receptors: novel targets for drug discovery. Nat Rev Drug Discov. 2002;1:198–210. doi: 10.1038/nrd746. [DOI] [PubMed] [Google Scholar]
- Coddou C, Acuna-Castillo C, Bull P, Huidobro-Toro JP. Dissecting the facilitator and inhibitor allosteric metal sites of the P2X4 receptor channel: critical roles of Cys-132 for zinc-potentiation and Asp-138 for copper-inhibition. J Biol Chem. 2007;282:36879–36886. doi: 10.1074/jbc.M706925200. [DOI] [PubMed] [Google Scholar]
- Coddou C, Yan Z, Obsil T, Huidobro-Toro JP, Stojilkovic SS. Activation and regulation of purinergic P2X receptor channels. Pharmacol Rev. 2011 doi: 10.1124/pr.110.003129. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn PJ, Christopoulos A, Lindsley CW. Allosteric modulators of GPCRs: a novel approach for the treatment of CNS disorders. Nat Rev Drug Discov. 2009;8:41–54. doi: 10.1038/nrd2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosyn L, Van Calenbergh S, Joshi BV, Ko H, Carter RL, Harden TK, Jacobson KA. Synthesis and P2Y receptor activity of nucleotide 5′-phosphonate derivatives. Bioorg Med Chem Lett. 2009;19:3002–3005. doi: 10.1016/j.bmcl.2009.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans RJ. Orthosteric and allosteric binding sites of P2X receptors. Eur Biophys J. 2009;38:319–327. doi: 10.1007/s00249-008-0275-2. [DOI] [PubMed] [Google Scholar]
- Fawzi AB, Macdonald D, Benbow LL, Smith-Torhan A, Zhang H, Weig BC, Ho G, Tulshian D, Linder ME, Graziano MP. SCH-202676: an allosteric modulator of both agonist and antagonist binding to G protein-coupled receptors. Mol Pharmacol. 2001;59:30–37. doi: 10.1124/mol.59.1.30. [DOI] [PubMed] [Google Scholar]
- Ferguson GN, Valant C, Horne J, Figler H, Flynn BL, Linden J, Chalmers DK, Sexton PM, Christopoulos A, Scammells PJ. 2-Aminothienopyridazines as novel adenosine A1 receptor modulators and antagonists. J Med Chem. 2008;51:6165–6172. doi: 10.1021/jm800557d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figler H, Olsson RA, Linden J. Allosteric enhancers of A1 adenosine receptors increase receptor-G protein coupling and counteract guanine nucleotide effects on agonist binding. Mol Pharmacol. 2003;64:1557–1564. doi: 10.1124/mol.64.6.1557. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, IJzerman AP, Jacobson KA, Linden J, Müller C. Nomenclature and classification of adenosine receptors – An update. Pharmacol Rev. 2011 doi: 10.1124/pr.110.003285. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Xu C, Liang S, Zhang A, Mu S, Wang Y, Wan F. Effect of tetramethylpyrazine on primary afferent transmission mediated by P2X3 receptor in neuropathic pain states. Brain Res Bull. 2008a;77:27–32. doi: 10.1016/j.brainresbull.2008.02.026. [DOI] [PubMed] [Google Scholar]
- Gao ZG, IJzerman AP. Allosteric modulation of A2A adenosine receptors by amiloride analogues and sodium ions. Biochem Pharmacol. 2000;60:669–676. doi: 10.1016/s0006-2952(00)00360-9. [DOI] [PubMed] [Google Scholar]
- Gao ZG, van Muijlwijk-Koezen JE, Chen A, Müller CE, IJzerman AP, Jacobson KA. Allosteric modulation of A3 adenosine receptors by a series of 3-(2-pyridinyl)isoquinoline derivatives. Mol Pharmacol. 2001;60:1057–1063. [PMC free article] [PubMed] [Google Scholar]
- Gao ZG, Kim SG, Soltysiak KA, Melman N, IJzerman AP, Jacobson KA. Selective allosteric enhancement of agonist binding and function at human A3 adenosine receptors by a series of imidazoquinoline derivatives. Mol Pharmacol. 2002;62:81–89. doi: 10.1124/mol.62.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao ZG, Kim SK, Gross AS, Chen A, Blaustein J, Jacobson KA. Identification of essential residues involved in the allosteric modulation of the human A3 adenosine receptor. Mol Pharmacol. 2003a;63:1021–1031. doi: 10.1124/mol.63.5.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao ZG, Melman N, Erdmann A, Kim SG, Muller CE, IJzerman AP, Jacobson KA. Differential allosteric modulation by amiloride analogues of agonist and antagonist binding at A1 and A3 adenosine receptors. Biochem Pharmacol. 2003b;65:525–534. doi: 10.1016/s0006-2952(02)01556-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao ZG, Mamedova L, Tchilibon S, Gross AS, Jacobson KA. 2,2′-Pyridylisatogen tosylate antagonizes P2Y1 receptor signaling without affecting nucleotide binding. Biochem Pharmacol. 2004a;68:231–237. doi: 10.1016/j.bcp.2004.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao ZG, Gross AS, Jacobson KA. Effects of SCH-202676 on adenosine and P2Y receptors. Life Sci. 2004b;74:3173–3180. doi: 10.1016/j.lfs.2003.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao ZG, Kim SK, IJzerman AP, Jacobson KA. Allosteric modulation of the adenosine family of receptor. Mini Rev Med Chem. 2005;5:545–553. doi: 10.2174/1389557054023242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao ZG, Kim S-K, Gross AS, Chen A, Blaustein JB, Jacobson KA. Identification of essential residues involved in the allosteric modulation of the human A3 adenosine receptor. Mol Pharmacol. 2003;63:1021–1031. doi: 10.1124/mol.63.5.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao ZG, Ye K, Göblyös A, IJzerman AP, Jacobson KA. Flexible modulation of agonist efficacy at the human A3 adenosine receptor by an imidazoquinoline allosteric enhancer LUF6000 and its analogues. BMC Pharmacol. 2008b;8:20. doi: 10.1186/1471-2210-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgi I, Biagi G, Bianucci AM, Borghini A, Livi O, Leonardi M, Pietra D, Calderone V, Martelli A. N6–1,3-diphenylurea derivatives of 2-phenyl-9-benzyladenines and 8-azaadenines: synthesis and biological evaluation as allosteric modulators of A2A adenosine receptors. Eur J Med Chem. 2008;43:1639–1647. doi: 10.1016/j.ejmech.2007.10.021. [DOI] [PubMed] [Google Scholar]
- Göblyös A, IJzerman AP. Allosteric modulation of adenosine receptors. Purinergic Signal. 2009;5:51–61. doi: 10.1007/s11302-008-9105-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göblyös A, Santiago SN, Pietra D, Mulder-Krieger T, von Frijtag Drabbe Künzel J, Brussee J, IJzerman AP. Synthesis and biological evaluation of 2-aminothiazoles and their amide derivatives on human adenosine receptors. Lack of effect of 2-aminothiazoles as allosteric enhancers. Bioorg Med Chem. 2005;13:2079–2087. doi: 10.1016/j.bmc.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Göblyös A, Gao ZG, Brussee J, Connestari R, Neves Santiago S, Ye K, IJzerman AP, Jacobson KA. Structure activity relationships of 1H-imidazo[4,5-c]quinolin-4-amine derivatives new as allosteric enhancers of the A3 adenosine receptor. J Med Chem. 2006;49:3354–3361. doi: 10.1021/jm060086s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington PE, Fotsch C. Calcium sensing receptor activators: calcimimetics. Curr Med Chem. 2007;14:3027–3034. doi: 10.2174/092986707782794096. [DOI] [PubMed] [Google Scholar]
- Heitman LH, Göblyös A, Zweemer AM, Bakker R, Mulder-Krieger T, van Veldhoven JP, de Vries H, Brussee J, IJzerman AP. A series of 2,4-disubstituted quinolines as a new class of allosteric enhancers of the adenosine A3 receptor. J Med Chem. 2009;52:926–931. doi: 10.1021/jm8014052. [DOI] [PubMed] [Google Scholar]
- Heitman LH, Mulder-Krieger T, Spanjersberg RF, von Frijtag Drabbe Künzel JK, Dalpiaz A, IJzerman AP. Allosteric modulation, thermodynamics and binding to wild-type and mutant (T277A) adenosine A1 receptors of LUF5831, a novel nonadenosine-like agonist. Br J Pharmacol. 2006;147:533–541. doi: 10.1038/sj.bjp.0706655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaakola VP, Griffith MT, Hanson MA, Cherezov V, Chien EY, Lane JR, IJzerman AP, Stevens RC. The 2.6 angstrom crystal structure of a human A2A adenosine receptor bound to an antagonist. Science. 2008;322:1211–1217. doi: 10.1126/science.1164772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, Gao ZG. Adenosine receptors as therapeutic targets. Nature Rev Drug Discovery. 2006;5:247–264. doi: 10.1038/nrd1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janusz CA, Berman RF. The adenosine binding enhancer, PD 81,723, inhibits epileptiform bursting in the hippocampal brain slice. Brain Res. 1993;619:131–136. doi: 10.1016/0006-8993(93)91604-q. [DOI] [PubMed] [Google Scholar]
- Janusz CA, Bruns RF, Berman RF. Functional activity of the adenosine binding enhancer, PD 81,723, in the in vitro hippocampal slice. Brain Res. 1991;567:181–187. doi: 10.1016/0006-8993(91)90794-v. [DOI] [PubMed] [Google Scholar]
- Jarvis MF, Gessner G, Shapiro G, Merkel L, Myers M, Cox BF, Martin GE. Differential effects of the adenosine A1 receptor allosteric enhancer PD 81,723 on agonist binding to brain and adipocyte membranes. Brain Res. 1999;840:75–83. doi: 10.1016/s0006-8993(99)01747-3. [DOI] [PubMed] [Google Scholar]
- Kawate T, Michel JC, Birdsong WT, Gouaux E. Crystal structure of the ATP-gated P2X4 ion channel in the closed state. Nature. 2009;460:592–599. doi: 10.1038/nature08198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakh BS, Proctor WR, Dunwiddie TV, Labarca C, Lester HA. Allosteric control of gating and kinetics at P2X4 receptor channels. J Neurosci. 1999;19:7289–99. doi: 10.1523/JNEUROSCI.19-17-07289.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiesman WF, Elzein E, Zablocki J. A1 adenosine receptor antagonists, agonists and allosteric enhancers. In: Wilson CN, Mustafa SJ, editors. Adenosine receptors in health and disease, Handbook of experimental pharmacology. Springer-Verlag; Berlin, Heidelberg: 2009. pp. 25–58. [DOI] [PubMed] [Google Scholar]
- Kim Y, de Castro S, Gao ZG, IJzerman AP, Jacobson KA. Novel 2- and 4-substituted 1H-imidazo[4,5-c]quinolin-4-amine derivatives as allosteric modulators of the A3 adenosine receptor. J Med Chem. 2009;52:2098–2108. doi: 10.1021/jm801659w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King BF, Ziganshin LE, Pintor J, Burnstock G. Full sensitivity of P2X2 purinoceptor revealed by changing extracellular pH. Br J Pharmacol. 1996;117:1371–1373. doi: 10.1111/j.1476-5381.1996.tb15293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaasse E, de Ligt RA, Roerink SF, Lorenzen A, Milligan G, Leurs R, IJzerman AP. Allosteric modulation and constitutive activity of fusion proteins between the adenosine A1 receptor and different 351Cys-mutated Gi alpha-subunits. Eur J Pharmacol. 2004;499:91–98. doi: 10.1016/j.ejphar.2004.07.108. [DOI] [PubMed] [Google Scholar]
- Klaasse EC, van den Hout G, Roerink SF, de Grip WJ, IJzerman AP, Beukers MW. Allosteric modulators affect the internalization of human adenosine A1 receptors. Eur J Pharmacol. 2005;522:1–8. doi: 10.1016/j.ejphar.2005.08.052. [DOI] [PubMed] [Google Scholar]
- van der Klein PAM, Kourounakis AP, IJzerman AP. Allosteric modulation of the adenosine A1 receptor: Synthesis and biological evaluation of novel 2-amino-3-benzoylthiophenes as allosteric enhancers of agonist binding. J Med Chem. 1999;42:3629–3635. doi: 10.1021/jm991051d. [DOI] [PubMed] [Google Scholar]
- Kollias-Baker C, Ruble J, Dennis D, Bruns RF, Linden J, Belardinelli L. Allosteric enhancer PD 81,723 acts by novel mechanism to potentiate cardiac actions of adenosine. Circ Res. 1994a;75:961–971. doi: 10.1161/01.res.75.6.961. [DOI] [PubMed] [Google Scholar]
- Kollias-Baker C, Xu J, Pelleg A, Belardinelli L. Novel approach for enhancing atrioventricular nodal conduction delay mediated by endogenous adenosine. Circ Res. 1994b;75:972–980. doi: 10.1161/01.res.75.6.972. [DOI] [PubMed] [Google Scholar]
- Kollias-Baker CA, Ruble J, Jacobson M, Harrison JK, Ozeck M, Shryock JC, Belardinelli L. Agonist-independent effect of an allosteric enhancer of the A1 adenosine receptor in CHO cells stably expressing the recombinant human A1 receptor. J Pharmacol Exp Ther. 1997;281:761–768. [PubMed] [Google Scholar]
- Kourounakis AP, van de Klein PAM, IJzerman AP. Elucidation of stucture-activity relationships of 2-amino-3-benzoylthiophenes: study of their allosteric enhanceing vs. antagonistic activity on adenosine A1 receptors. Drug Dev Res. 2000;49:227–237. [Google Scholar]
- Kourounakis A, Visser C, de Groote M, IJzerman AP. Differential effects of the allosteric enhancer (2-amino-4,5-dimethyltrienyl)[ 3-(trifluoromethyl)phenyl] methanone (PD 81,723) on agonist and antagonist binding and function at the human wild-type and a mutant (T277A) adenosine A1 receptor. Biochem Pharmacol. 2001;61:137–144. doi: 10.1016/s0006-2952(00)00536-0. [DOI] [PubMed] [Google Scholar]
- Lam RS, Nahirny D, Duszyk M. Cholesterol-dependent regulation of adenosine A2A receptor-mediated anion secretion in colon epithelial cells. Exp Cell Res. 2009;315:3028–3035. doi: 10.1016/j.yexcr.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Lane JR, Beukers MW, Mulder-Krieger T, IJzerman AP. The endocannabinoid 2-arachidonylglycerol is a negative allosteric modulator of the human A3 adenosine receptor. Biochem Pharmacol. 2010;79:48–56. doi: 10.1016/j.bcp.2009.07.024. [DOI] [PubMed] [Google Scholar]
- Leung E, Walsh LK, Kim EJ, Lazar DA, Seran CS, Wong EH, Eglen RM. Enhancement of the adenosine A receptor functions by benzoylthiophenes in guinea pig tissues in vitro. Naunyn-Schmiedebergs-Arch Pharmacol. 1995;352:206–212. doi: 10.1007/BF00176776. [DOI] [PubMed] [Google Scholar]
- Lewandowicz AM, Vepsäläinen J, Laitinen JT. The ‘allosteric modulator’ SCH-202676 disrupts G protein-coupled receptor function via sulphydryl-sensitive mechanisms. Br J Pharmacol. 2006;147:422–429. doi: 10.1038/sj.bjp.0706624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Peoples RW, Weight FF. Proton potentiation of ATP-gated ion channel responses to ATP and Zn2+ in rat nodose ganglion neurons. J Neurophys. 1996;76:3048–3058. doi: 10.1152/jn.1996.76.5.3048. [DOI] [PubMed] [Google Scholar]
- Li X, Conklin D, Pan H-L, Eisenach JC. Allosteric adenosine receptor modulation reduces hypersensitivity following peripheral inflammation by a central mechanism. J Pharmacol Exp Ther. 2003;305:950–955. doi: 10.1124/jpet.102.047951. [DOI] [PubMed] [Google Scholar]
- De Ligt RAF, Rivkees SA, Lorenzen A, Leurs R, IJzerman AP. A “locked-on,” constitutively active mutant of the adenosine A1 receptor. Eur J Pharmacol. 2005;510:1–8. doi: 10.1016/j.ejphar.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Lorca RA, Coddou C, Gazitua MC, Bull P, Arredondo C, Huidobro-Toro JP. Extracellular histidine residues identify common structural determinants in the copper/zinc P2X2 receptor modulation. J Neurochem. 2005;95:499–512. doi: 10.1111/j.1471-4159.2005.03387.x. [DOI] [PubMed] [Google Scholar]
- Mamedova L, Capra V, Accomazzo MR, Gao ZG, Ferrario S, Fumigalli M, Abbracchio MP, Rovati GE, Jacobson KA. CysLT1 leukotriene receptor antagonists inhibit the effects of nucleotides acting at P2Y receptors. Biochem Pharmacol. 2005;71:115–125. doi: 10.1016/j.bcp.2005.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martynyuk AE, Zima A, Seubert CN, Morey TE, Belardinelli L, Dennis DM. Potentiation of the negative dromotropic effect of adenosine by rapid heart rates: possible ionic mechanism. Basic Res Cardiol. 2002;97:295–304. doi: 10.1007/s00395-002-0354-y. [DOI] [PubMed] [Google Scholar]
- May LT, Briddon SJ, Hill SJ. Antagonist Selective Modulation of Adenosine A1 and A3 Receptor Pharmacology by the Food Dye Brilliant Black BN: Evidence for Allosteric Interactions. Mol Pharmacol. 2010a;77:678–686. doi: 10.1124/mol.109.063065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May LT, Self TJ, Briddon SJ, Hill SJ. The effect of allosteric modulators on the kinetics of agonist-G protein-coupled receptor interactions in single living cells. Mol Pharmacol. 2010b;78:511–523. doi: 10.1124/mol.110.064493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meno JR, Higashi H, Cambray AJ, Zhou J, D’Ambrosio R, Winn HR. Hippocampal injury and neurobehavioral deficits are improved by PD 81,723 following hyperglycemic cerebral ischemia. Exp Neurol. 2003;183:188–196. doi: 10.1016/s0014-4886(03)00162-6. [DOI] [PubMed] [Google Scholar]
- Miller KJ, Michel AD, Chessell IP, Humphrey PP. Cibacron blue allosterically modulates the rat P2X4 receptor. Neuropharmacology. 1998;37:1579–1586. doi: 10.1016/s0028-3908(98)00153-1. [DOI] [PubMed] [Google Scholar]
- Mizumura T, Auchampach JA, Linden J, Bruns RF, Gross GJ. PD 81,723, an allosteric enhancer of the A1 adenosine receptor, lowers the threshold for ischemic preconditioning in dogs. Circ Res. 1996;79:415–423. doi: 10.1161/01.res.79.3.415. [DOI] [PubMed] [Google Scholar]
- Mohr K, Tränkle C, Kostenis E, Barocelli E, De Amici M, Holzgrabe U. Rational design of dualsteric GPCR ligands: quests and promise. Br J Pharmacol. 2010;159:997–1008. doi: 10.1111/j.1476-5381.2009.00601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudumbi RV, Montamat SC, Bruns RF, Vestal RE. Cardiac functional responses to adenosine by PD 81,723, an allosteric enhancer of the adenosine A1 receptor. Am J Physiol. 1993;264:H1017–H1022. doi: 10.1152/ajpheart.1993.264.3.H1017. [DOI] [PubMed] [Google Scholar]
- van Muijlwijk-Koezen JE, Timmerman H, Link R, van der Goot H, IJzerman AP. A novel class of adenosine A3 receptor ligands. 1 3-(2-Pyridinyl)isoquinoline derivatives. J Med Chem. 1998;41:3987–3993. doi: 10.1021/jm980036q. [DOI] [PubMed] [Google Scholar]
- Musser B, Mudumbi RV, Liu J, Olson RD, Vestal RE. Adenosine A1 receptor-dependent and -independent effects of the allosteric enhancer PD 81,723. J Pharmacol Exp Ther. 1999;288:446–454. [PubMed] [Google Scholar]
- Nagaya N, Tittle RK, Saar N, Dellal SS, Hume RI. An intersubunit zinc binding site in rat P2X2 receptors. J Biol Chem. 2005;280:25982–25993. doi: 10.1074/jbc.M504545200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narlawar R, Lane JR, Doddareddy M, Lin J, Brussee J, IJzerman AP. Hybrid ortho/allosteric ligands for the adenosine A1 receptor. J Med Chem. 2010;53:3028–3037. doi: 10.1021/jm901252a. [DOI] [PubMed] [Google Scholar]
- van den Nieuwendijk AM, Pietra D, Heitman L, Göblyös A, IJzerman AP. Synthesis and biological evaluation of 2,3,5-substituted [1,2,4]thiadiazoles as allosteric modulators of adenosine receptors. J Med Chem. 2004;47:663–672. doi: 10.1021/jm030863d. [DOI] [PubMed] [Google Scholar]
- Nikolakopoulos G, Figler H, Linden J, Scammels PJ. 2-Aminothiophene-3-carboxylates and carboxamides as adenosine A1 receptor allosteric enhancers. Bioorg Med Chem. 2006;14:2358–2365. doi: 10.1016/j.bmc.2005.11.018. [DOI] [PubMed] [Google Scholar]
- Patel K, Barnes A, Camacho J, Paterson C, Boughtflower R, Cousens D, Marshall F. Activity of diadenosine polyphosphates at P2Y receptors stably expressed in 1321N1 cells. Eur J Pharmacol. 2001;430:203–210. doi: 10.1016/s0014-2999(01)01401-7. [DOI] [PubMed] [Google Scholar]
- Phillis JW, Smith-Barbour M, Perkins LM, O’Regan MH. Characterization of glutamate, aspartate, and GABA release from ischemic rat cerebral cortex. Brain Res Bull. 1994;34:457–466. doi: 10.1016/0361-9230(94)90019-1. [DOI] [PubMed] [Google Scholar]
- Priel A, Silberberg SD. Mechanism of ivermectin facilitation of human P2X4 receptor channels. J Gen Physiol. 2004;123:281–293. doi: 10.1085/jgp.200308986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagnoli R. Synthesis and biological characterization of [3H](2-amino-4,5,6,7-tetrahydrobenzo[b]thiophen-3-yl)-(4-chlorophenyl)-methanone, the first radiolabelled adenosine A1 allosteric enhancer. Bioorg Med Chem. 2006;16:1402–1404. doi: 10.1016/j.bmcl.2005.11.037. [DOI] [PubMed] [Google Scholar]
- Romagnoli R, Baraldi PG, Carrion MD, Cara CL, Cruz-Lopez O, Iaconinoto MA, Preti D, Shryock JC, Moorman AR, Vincenzi F, Varani K, Borea PA. Synthesis and biological evaluation of 2-amino-3-(4-chlorobenzoyl)-4-[N-(substituted)piperazin-1-yl]thiophenes as potent allosteric enhancers of the A1 adenosine receptor. J Med Chem. 2008;51:5875–5879. doi: 10.1021/jm800586p. [DOI] [PubMed] [Google Scholar]
- Romagnoli R, Baraldi PG, Moorman AR, Iaconinoto MA, Carrion MD, Cara CL, Tabrizi MA, Preti D, Fruttarolo F, Baker SP, Varani K, Borea PA. Microwave-assisted synthesis of thieno[2,3-c]pyridine derivatives as a new series of allosteric enhancers at the adenosine A1 receptor. Bioorg Med Chem. 2006;16:5530–5533. doi: 10.1016/j.bmcl.2006.08.041. [DOI] [PubMed] [Google Scholar]
- Romagnoli R, Baraldi PG, Tabrizi MA, Gessi S, Borea PA, Merighi S. Allosteric enhancers of A1 adenosine receptors: state of the art and new horizons for drug development. Current Med Chem. 2010;17:3488–3502. doi: 10.2174/092986710792927831. [DOI] [PubMed] [Google Scholar]
- Schwartz TW, Holst B. Ago-allosteric modulation and other types of allostery in dimeric 7TM receptors. J Receptors and Signal Transduction. 2006;26:107–128. doi: 10.1080/10799890600567570. [DOI] [PubMed] [Google Scholar]
- Seguela P, Haghighi A, Soghomonian J-J, Cooper E. A novel neuronal P2X ATP receptor ion channel with widespread distribution in the brain. J Neurosci. 1996;16:448–455. doi: 10.1523/JNEUROSCI.16-02-00448.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Halenda SP, Sturek M, Wilden PA. Novel mitogenic effect of adenosine on coronary artery smooth muscle cells: role for the A1 adenosine receptor. Circ Res. 2005;96:982–990. doi: 10.1161/01.RES.0000165800.81876.52. [DOI] [PubMed] [Google Scholar]
- Silberberg SD, Li M, Swartz KJ. Ivermectin interaction with transmembrane helices reveals widespread rearrangements during opening of P2X receptor channels. Neuron. 2007;54:263–274. doi: 10.1016/j.neuron.2007.03.020. [DOI] [PubMed] [Google Scholar]
- Soto F, Garcia-Guzman M, Gomez-Hernandez JM, Hollmann M, Karschin C, Stuhmer W. P2X4: an ATP-activated ionotropic receptor cloned from rat brain. Proc Natl Acad Sci USA. 1996;93:3684–3688. doi: 10.1073/pnas.93.8.3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spedding M, Menton K, Markham A, Weetman DF. Antagonists and the purinergic nerve hypothesis: 2, 2′-pyridylisatogen tosylate (PIT), an allosteric modulator of P2Y receptors. A retrospective on a quarter century of progress. J Auton Nerv Syst. 2000;81:225–227. doi: 10.1016/s0165-1838(00)00142-9. [DOI] [PubMed] [Google Scholar]
- Stewart GD, Sexton PM, Christopoulos A. Prediction of functionally selective allosteric interactions at an M3 muscarinic acetylcholine receptor mutant using Saccharomyces cerevisiae. Mol Pharmacol. 2010;78:205–214. doi: 10.1124/mol.110.064253. [DOI] [PubMed] [Google Scholar]
- Stoop R, Surprenant A, North RA. Different sensitivities to pH of ATP-induced currents at four cloned P2X receptors. J Neurophysiol. 1997;78:1837–40. doi: 10.1152/jn.1997.78.4.1837. [DOI] [PubMed] [Google Scholar]
- Toulmé E, Soto F, Garret M, Boué-Grabot E. Functional properties of internalization-deficient P2X4 receptors reveal a novel mechanism of ligand-gated channel facilitation by ivermectin. Mol Pharmacol. 2006;69:576–587. doi: 10.1124/mol.105.018812. [DOI] [PubMed] [Google Scholar]
- Valant C, Sexton PM, Christopoulos A. Orthosteric/allosteric bitopic ligands: going hybrid at GPCRs. Mol Interv. 2009;9:125–135. doi: 10.1124/mi.9.3.6. [DOI] [PubMed] [Google Scholar]
- Valant C, Aurelio L, Urmaliya VB, White P, Scammells PJ, Sexton PM, Christopoulos A. Delineating the mode of action of adenosine A1 receptor allosteric modulators. Mol Pharmacol. 2010;78:444–455. doi: 10.1124/mol.110.064568. [DOI] [PubMed] [Google Scholar]
- van Galen PJ, Nissen P, van Wijngaarden I, IJzerman AP, Soudijn W. 1H-imidazo[4,5-c]quinolin-4-amines: novel non-xanthine adenosine antagonists. J Med Chem. 1991;34:1202–1206. doi: 10.1021/jm00107a046. [DOI] [PubMed] [Google Scholar]
- Virginio C, Church D, North RA, Surprenant A. Effects of divalent cations, protons and calmidazolium at the rat P2X7 receptor. Neuropharmacology. 1997;36:1285–1294. doi: 10.1016/s0028-3908(97)00141-x. [DOI] [PubMed] [Google Scholar]
- Wildman SS, King BF, Burnstock G. Zn2+ modulation of ATP responses at recombinant P2X2 receptors and its dependence on extracellular pH. Br J Pharmacol. 1998;123:1214–1220. doi: 10.1038/sj.bjp.0701717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildman SS, King BF, Burnstock G. Modulatory activity of extracellular H+ and Zn2+ on ATP-responses at rP2X1 and rP2X3 receptors. Br J Pharmacol. 1999;128:486–492. doi: 10.1038/sj.bjp.0702802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong K, Peoples RW, Montgomery JP, Chiang Y, Stewart RR, Weight FF, Li C. Differential modulation by copper and zinc of P2X2 and P2X4 receptor function. J Neurophysiol. 1999;81:2088–2094. doi: 10.1152/jn.1999.81.5.2088. [DOI] [PubMed] [Google Scholar]
- Yang H, Rotstein DM. Novel CCR5 antagonists for the treatment of HIV infection: a review of compounds patented in 2006 – 2008. Expert Opin Ther Pat. 2010;20:325–354. doi: 10.1517/13543770903575674. [DOI] [PubMed] [Google Scholar]
- Zemkova H, Kucka M, Li S, Gonzalez-Iglesias AE, Tomic M, Stojilkovic SS. Characterization of purinergic P2X4 receptor channels expressed in anterior pituitary cells. Am J Physiol Endocrinol Metab. 2010;298:E644–E651. doi: 10.1152/ajpendo.00558.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]