Abstract
Objective
Previous studies suggest that nitric oxide (NO) may modulate insulin-induced uptake of glucose in insulin-sensitive tissues. Asymmetric dimethylarginine (ADMA) is an endogenous inhibitor of NO synthase (NOS). We hypothesized that a reduction in endogenous ADMA would increase NO synthesis and thereby enhance insulin sensitivity.
Methods and Results
To test this hypothesis we employed a transgenic mouse in which we overexpressed human dimethylarginine dimethylaminohydrolase (DDAH-I). The DDAH-I mice had lower plasma ADMA at all ages (22–70 weeks) by comparison to wild-type (WT) littermates. With a glucose challenge, WT mice showed a prompt increase in ADMA, whereas DDAH-I mice had a blunted response. Furthermore, DDAH-I mice had a blunted increase in plasma insulin and glucose levels after glucose challenge, with a 50% reduction in the insulin resistence index, consistent with enhanced sensitivity to insulin. In liver, we observed an increased Akt phosphorylation in the DDAH-I mice after i.p. glucose challenge. Incubation of skeletal muscle from WT mice ex vivo with ADMA (2μM) markedly suppressed insulin-induced glycogen synthesis in fast-twitch but not slow-twitch muscle.
Conclusions
These findings suggest that the endogenous NOS inhibitor ADMA reduces insulin sensitivity, consistent with previous observations that NO plays a role in insulin sensitivity.
Keywords: Arteriosclerosis, nitric oxide, asymmetric dimethylarginine, dimethylarginine dimethylaminohydrolase, glucose
Introduction
The insulin resistance syndrome (IRS) is associated with compensatory hyperinsulinemia, a constellation of metabolic perturbations, and increased risk for cardiovascular disease.1,2 The association of insulin resistance with cardiovascular disease may be mediated by its adverse effect on a variety of endothelial functions including those related to vascular reactivity, structure, inflammation and thrombosis.3 A decreased bioavailability of nitric oxide (NO) contributes to these endothelial dysfunctions, and to the development of arteriosclerosis. Intriguingly, a reduction in NO bioavailability may also play an important role in the development of insulin resistance. NO modulates insulin-mediated glucose disposal as well as reactivity of vessels in insulin-sensitive tissues (i.e. skeletal muscle, liver and adipose tissue).4 Furthermore, mice with gene disruption of either endothelial or neuronal NOS exhibit insulin resistance.5,6 Genetic disruption of NO synthesis may induce insulin resistance by reducing microvascular recruitment and/or muscle glucose uptake in response to insulin; indeed pharmacological inhibition of NOS by NG-monomethyl-L-arginine (L-NMMA) has the same effect.7
In this regard, it is intriguing that insulin resistance in humans is associated with increased levels of the endogenous NOS inhibitor asymmetric dimethylarginine (ADMA).8 ADMA inhibits all three NOS isoforms,9 impairs endothelium-dependent vasodilation, increases vascular resistance and is associated with hypertension.10 Plasma ADMA levels are elevated in patients with type 2 diabetes mellitus and insulin resistance.8,11 Metformin improves endothelial vasodilator function and decreases circulating ADMA in diabetic subjects and insulin-resistant rats.12,13 Interestingly, treatment with rosiglitazone improves insulin sensitivity and reduces plasma ADMA concentration in insulin-resistant subjects with hypertension, without having an effect on the mean blood pressure.8 In patients with type 2 diabetes, rosiglitazone ameliorates endothelial dysfunction independently of glucose control.14
The elevation of plasma or tissue ADMA and inhibition of NOS may explain in part the co-existence of insulin resistance and endothelial dysfunction. We hypothesized that ADMA(by inhibiting NO synthesis) may contribute to insulin resistance. To test this hypothesis, we studied insulin sensitivity in wild-type mice, and those with genetically reduced plasma and tissue ADMA levels.
Methods
Animals
The enzyme dimethylarginine dimethylaminohydrolase (DDAH) degrades ADMA, and is responsible for about 80% of the disposition of ADMA in healthy humans.15 Recently, DDAH-I transgenic mice have been generated in our laboratory.16 Male transgenic mice were mated with C57BL/6J (wild-type, i.e. WT) female mice (Jackson Laboratory, Bar Harbor, ME). Offspring were screened for transgene expression by PCR and Southern blot analysis using tail DNA as described earlier.16 Glucose tolerance tests (GTT) were performed on DDAH-I transgenic male mice, and age- and weight-matched WT littermates, at 22, 32, 44 and 70 weeks of age. Assessment of insulin-induced 14C-glucose uptake into glycogen in isolated mouse skeletal muscle was performed on male WT (at 5–9 weeks) and DDAH transgenic mice (at 10–11 months). Changes in liver insulin signaling were determined after i.p. administration of glucose in DDAH-I transgenic and WT littermates (12–16 weeks of age). All mice were housed in the animal care facility at Stanford University Medical Center (Stanford, CA), under standard temperature, humidity, and lighting conditions, and were provided mouse chow and water ad libitum. The investigation conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication No. 85-23, revised 1996). The study protocol was approved by the Administrative Panel on Laboratory Animal Care, Stanford University.
Assessment of glucose tolerance and insulin resistance
The changes in plasma glucose and insulin were measured after i.p. administration of glucose (1mg/10μl/g body weight). Mice were fasted overnight (16 hours) and weighed. Mice were wrapped in terry cloth to minimize the stress of handling and the tip of the tail was clipped to collect blood into heparinized capillary tubes. After collecting blood in the fasting condition, the tail wound was sealed with a strip of adhesive tape for hemostasis. The animal received an i.p. injection of glucose and tail blood sampled after removing the adhesive tape at 30, 60, 120 and 150 min.
Calculating the insulin resistance index
The insulin resistance (IR) index was calculated as previously described.17,18 The IR index is the product of the average elevation in plasma glucose concentration over the fasting value times the average plasma insulin concentration during the GTT. This product decreases with enhanced insulin sensitivity. At 22 weeks, the data on DDAH and WT mice represent the average of 10 mice in each group. At 32 and 44 weeks, exclusion of mice receiving an inadequate i.p. injection of glucose left 9 mice per group. At 70 weeks of age, 2 mice in each group were excluded from testing due to spontaneous deaths and/or excessive weight loss.
Glucose uptake and glycogen synthesis activity in isolated mouse skeletal muscles
Soleus and extensor digitorum longus (EDL) muscle were isolated from overnight fasted mice (5–9 weeks of age), following an i.p. injection with 0.5 ketamine + 0.1 mg xylazine/10g body weight. The muscles were mounted on a curved sharpened staple to their resting length. Each muscle was placed into a glass scintillation vial containing 1.5ml of oxygenated Krebs Ringer bicarbonate (KRB) buffer supplemented with 6mM glucose and 0.05% bovine serum albumin in a shaking water bath (30°C). One half of the vials also contained ADMA (2μM). All vials (28) were gassed continuously with 95% O2 and 5% CO2 at a flow rate of 2L/min. A positive gas pressure was attained by sealing each vial with a rubber stopper containing a #18 gauge inflow needle and a #20 gauge outflow needle. After the initial incubations, timed additions of KRB buffer or insulin were made to each flask to provide insulin concentrations of 0, 25, 50, and 200μU/ml. Thirty min later, a 50μl aliquot of U-14C-glucose (approximately 0.31μC) was added to each vial. After another 30min (60min after the addition of insulin), the tissue was removed, blotted on absorbent paper, frozen in liquid nitrogen and transferred to a tared test tube containing 30% KOH in saturated Na2SO4. After weighing, the muscle tissue (2.5–8.0mg) was heated to 98°C, and after cooling, glycogen was precipitated with equal volumes of chilled 100% ethanol. After overnight refrigeration, free glucose was removed following serial (4X) centrifugation and washing with 50% ethanol. The recovered glycogen precipitate was dissolved in water and transferred to scintillation vials for determination of 14C radioactivity in a Beckman LS 6500 scintillation counter. Assessment of glucose concentration and 14C radioactivity in the incubation vial permitted assessment of nmoles glucose conversion to glycogen per g muscle.
Assessment of hepatic insulin signaling
Hepatic insulin signaling was assessed 30 and 60 min after i.p. administration of glucose (1mg/10μl/g body weight). Mice (12–16 weeks of age) were fasted overnight (16 hours) and weighed. The animals received an i.p. injection of glucose and were returned to their cage. The liver was isolated from mice, following an i.p. injection with 0.5 ketamine + 0.1 mg xylazine/10g body weight.
Whole liver lysates were prepared by homogenization of snap frozen liver tissue in T-Per (Pierce, Rockford, IL, USA) supplemented with NaF (10mM), EDTA (2mM), benzamidine (10mM), PMSF (1mM), leupeptin (1μg/ml), Na3VO4 (2mM), aprotinin (1,5μg/ml). Protein content was measured by Bradford-Assay and 20 – 60μg total protein was loaded onto 12% SDS/Page. Proteins were blotted onto nitrocellulose membranes (Schleicher&Schuell, Dassel, Germany), blocked in 5% dry non-fat milk and probed with primary antibodies and appropriate HRP-conjugated secondary antibodies. Detection was performed using an ECL kit (Roth, Karlsruhe, Germany). Densitometric analysis was assessed using Quantify-One Software (Bio-Rad, Munich, Germany). All chemicals were purchased from Sigma (St. Louis, IL, USA), if not otherwise stated. Antibodies for p-Akt-Ser473, Jun N-terminal kinase (JNK), p-JNK, insulin receptor substrate-1 (IRS-1), p-IRS-1-Ser307, anti-rabbit-IgG-HRP were purchased from Cell Signaling (Danvers, MA, USA). Antibodies for actin and Akt were purchased from Santa Cruz (Santa Cruz, CA, USA).
Measurement of plasma ADMA, insulin, glucose and total cholesterol
Plasma ADMA concentrations were measured by ELISA (DLD Diagnostika GmbH, Hamburg, Germany) as previously described19 in duplicate using a microtiter plate reader (Tecan GENios, Salzburg, Austria). Tail blood samples were collected in 1.5ml Eppendorf tubes, immediately centrifuged at 4°C (10min, 4000rpm), and plasma was stored at -20°C.
Plasma glucose was measured using a modification of the Trinder procedure,20 using reagents from Sigma-Aldrich (St. Louis, MO). Plasma insulin concentrations were measured using the Mercodia 1-2-3 ultrasensitive mouse insulin ELISA (ALPCO Diagnostics, Windham, NH), a solid phase two-site enzyme immunoassay. Total cholesterol concentrations were determined using the Infinity™ cholesterol liquid stable reagent (ThermoDMA, Louisville, CO) as described by Allain et al.21 with the modification of Roeschlau et al.22
Statistical analysis
The data are expressed as means ± SEM. Differences between groups were assessed by Students paired t test. Differences in densitometric analysis in liver tissue was assessed by Mann-Whitney, non-parametric, unpaired test. For multiple comparisons, a one way analysis of variance was used (i.e. to compare glucose, insulin, IR index and glucose incorporation between DDAH and WT animals at several insulin concentrations. The level for significance was set at p <0.05.
Results
Baseline metabolic values
The metabolic characteristics of fasting male DDAH transgenic mice and their littermate WT controls are listed in Table 1. Body weights were comparable between DDAH and WT mice with both groups showing a slight increase from 22 to 70 weeks of age. Plasma glucose levels were also comparable between groups and were lower in younger mice than in older animals. Fasting plasma insulin levels tended to be lower in DDAH animals. Plasma cholesterol levels were comparable between groups from 22 to 70 weeks of age. Plasma ADMA values were reduced in DDAH mice by 37, 38, 33 and 26% at 22, 32, 44 and 70 weeks respectively.
Table 1.
Baseline metabolic values in DDAH transgenic and wild-type mice
| Age | 22 weeks | 32 weeks | 44 weeks | 70 weeks | ||||
|---|---|---|---|---|---|---|---|---|
| Group | DDAH | WT | DDAH | WT | DDAH | WT | DDAH | WT |
| N | 10 | 10 | 9 | 9 | 9 | 9 | 8 | 8 |
| Weight [g] | 26.4±0.4 | 26.6±0.6 | 27.5±0.4 | 28.4±0.8 | 29.0±0.7 | 28.4±1.2 | 28.7±0.9 | 31.0±1.3 |
| Glucose [mg/dl] | 96±4.1 | 92±4.4 | 87±6.2* | 104±5.0 | 128±4.9 | 129±5.3 | 124±6.2 | 133±8.3 |
| Insulin [ng/ml] | 0.53±0.17 | 0.87±0.19 | 0.22±0.04 | 0.28±0.04 | 0.21±0.03 | 0.24±0.04 | 0.19±0.04 | 0.32±0.10 |
| Cholesterol [mg/dl] | 104±4.5 | 107±2.1 | 108±6.3 | 115±9.0 | 108±4.3 | 97±5.2 | 103±4.9 | 112±4.8 |
| ADMA [μmol/l] | 0.63±0.03† | 1.00±0.04 | 0.65±0.03† | 1.05±0.05 | 0.56±0.02† | 0.84±0.02 | 0.63±0.03† | 0.85±0.03 |
Each value is the mean ± SEM for 10 animals from 22 weeks of age, 9 animals from 32 and 44 weeks of age, and 8 animals/each group at 70 weeks of age. DDAH = DDAH-I transgenic mice, WT = wild-type littermates;
p<0.05 DDAH vs. WT,
p<0.01 DDAH vs. WT
Assessment of insulin sensitivity during a glucose tolerance test
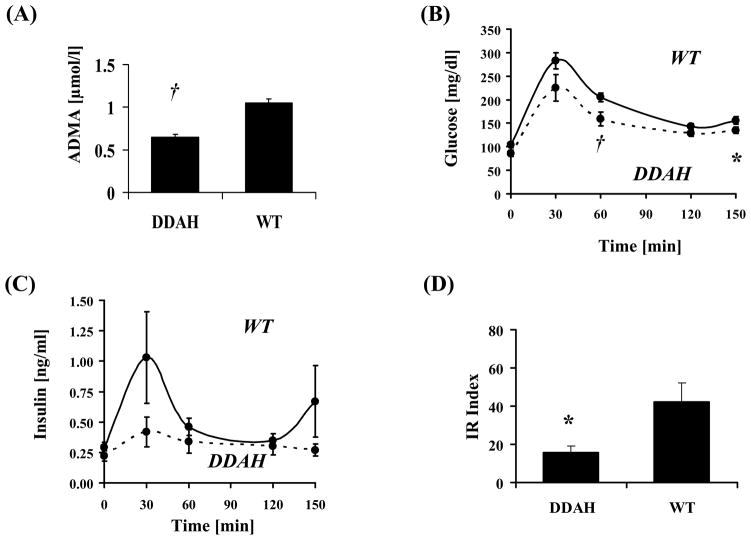
The plasma glucose and insulin responses of DDAH and WT mice (32 weeks old) to an i.p. glucose challenge are illustrated in Fig. 1. Plasma ADMA was significantly lower in DDAH animals (Fig. 1A). Glucose and insulin levels in response to the glucose challenge were attenuated in DDAH animals (Fig. 1B+C) and the IR index, representing the product of the average glucose elevation times the average insulin concentration, was significantly reduced (by 63%; p<0.025) in DDAH animals (Fig. 1D).
Figure 1. Alterations in plasma ADMA, glucose, insulin, and IR index.
Plasma ADMA levels (A) were reduced in DDAH transgenic mice. Furthermore, glucose (B) and insulin (C) levels trended lower in DDAH animals and the IR index (D) was significantly reduced by 63%. Values are mean ± SEM for 9 animals in each group. *p<0.05 DDAH vs. WT, †p<0.025 vs. WT.
Similar findings were observed at 22 and at 44 weeks. At 22 weeks, the IR index was significantly reduced by 43%. At 44 weeks, both basal plasma glucose (124±6.2 vs 133±8.3mg/dl) and insulin (0.19±0.04 vs 0.32±0.10ng/ml) tended to be reduced in the DDAH transgenic mice. Two hours after the glucose challenge, plasma glucose levels (143±6.7 vs 155±16.1mg/dl) tended to be less and plasma insulin levels (0.71±0.11 vs 1.34±0.21ng/ml; p<0.05) were significantly less. To summarize, at all ages (22, 32 and 44 weeks) the same metabolic pattern was observed in response to a glucose challenge, with attenuated increases in plasma glucose and insulin, and a reduced IR index, observed in the DDAH transgenic animals.
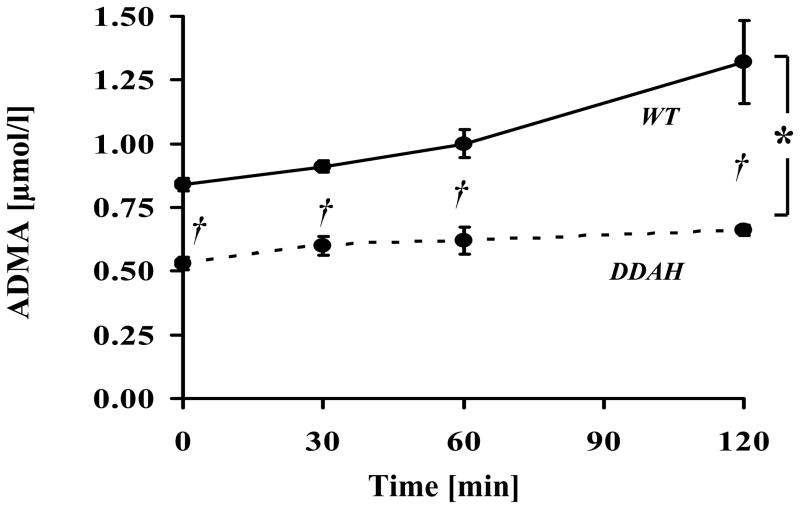
Glucose challenge increases plasma ADMA concentrations
In one group of animals (44 week old DDAH and WT mice), plasma ADMA levels were measured in serial fashion after a glucose challenge. Intriguingly, plasma ADMA increased by 0.44μM (165±18%) in the WT mice two hours after the glucose challenge (Fig. 2). Notably, in DDAH transgenic mice the absolute (0.18μM) or relative (124±7 %) increase in plasma ADMA levels was significantly less (both p<0.05) than observed in the WT mice at the same timepoint.
Figure 2. Plasma ADMA increases after a glucose challenge.
Basal ADMA plasma concentrations were significantly lower in DDAH animals. Plasma ADMA levels increased in WT animals following an intraperitoneal glucose challenge. The increase in plasma ADMA levels was blunted in the DDAH transgenic mice. Values are mean ± SEM for 9 animals in each group. WT = wild-type littermates, DDAH = DDAH-1 transgenic mice. Curve for DDAH animals is expressed as dashed line. *p<0.05 DDAH vs. WT (AUC analysis). †p<0.01 vs. WT, for each timepoint.
To confirm these results, a modified GTT was performed in 70 week old mice (n=8 in each group). Under fasting conditions, plasma glucose (124±6.2 vs 133±8.3mg/dl) and plasma insulin levels (0.19±0.04 vs. 0.32±0.10ng/ml) tended to be less in the DDAH transgenic mice. Two hours after the glucose challenge, plasma glucose levels (143±6.7 vs 155±16.1mg/dl) tended to be less in the DDAH transgenic, and plasma insulin levels (0.71±0.11 vs 1.34±0.21ng/ml; p<0.05) were significantly less. The increase in plasma insulin levels after glucose challenge in the DDAH transgenic mice was only 51% of that observed in WT. Under fasting conditions, plasma ADMA levels were less in the DDAH transgenic mice (0.63±0.03 vs 0.85±0.03μmol/l, p<0.01). After the glucose challenge at two hours plasma ADMA levels increased by 59% in WT mice. By contrast, in the DDAH transgenic mice, ADMA levels only increased by 22%, and remained significantly different from WT (0.77±0.02 vs 1.35±0.07μmol/l; p<0.01).
Effect of ADMA on glycogen synthesis in incubated muscle
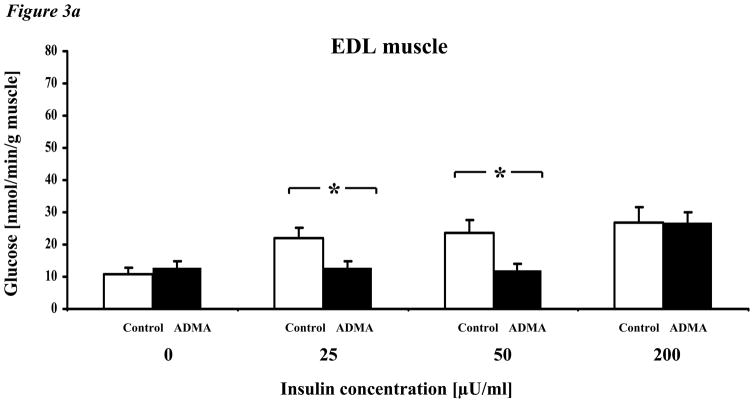
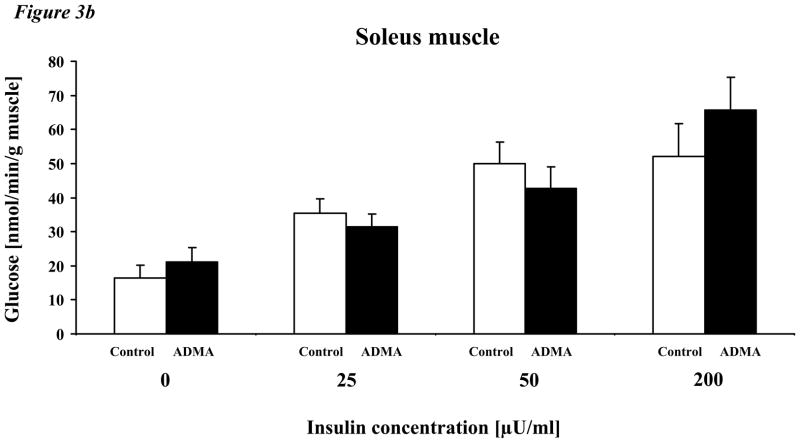
To understand the mechanisms by which ADMA may affect insulin sensitivity in adult mice, we assessed the effect of exogenous ADMA on the insulin sensitivity of isolated skeletal muscle from C57BL/6J mice (5–9 weeks of age). Specifically, we measured skeletal muscle uptake of glucose and conversion to glycogen in response to clinically relevant concentrations of insulin, ranging from 25–200μU/ml (167 – 1333pM) ex vivo using isolated EDL and soleus muscles. In the presence of vehicle alone, glucose incorporation into glycogen was increased in a dose-dependent fashion by insulin (Fig. 3a). By contrast, in the presence of ADMA (2μM), glucose uptake and incorporation into glycogen was shifted rightward, indicating that ADMA significantly increased resistance to insulin. In contrast to EDL muscle, soleus muscle manifested a higher basal incorporation of glucose into glycogen and a greater sensitivity to insulin (Fig. 3b). By contrast to EDL, incubation with ADMA did not affect insulin sensitivity.
Figure 3. Effect of ADMA on glycogen synthesis in incubated EDL and soleus muscle.
a. Exogenous ADMA (2μM) significantly reduced insulin-induced glucose conversion to glycogen in isolated EDL muscles ex vivo. Glucose incorporation into glycogen was significantly increased by insulin at all concentrations in vehicle-treated EDL muscle. By contrast, insulin-induced glucose incorporation was abrogated by ADMA except at the highest concentration of insulin.
b. Soleus muscle, in contrast to EDL muscle, exhibited a higher basal incorporation of glucose into glycogen and overall a greater response to insulin. The effect of insulin to increase glycogen synthesis was unaffected by ADMA.
Values are mean ± SEM. Control = C57BL/6J mice, ADMA = C57BL/6J mice with 2μM ADMA concentration in the incubation media. *p<0.05 ADMA vs. control,
We performed additional studies in 10–11 months old DDAH transgenic and WT mice using EDL muscle. Tissue was incubated with 2 μM ADMA before adding insulin (50 μU/ml) and radioactively labeled glucose. There was a trend for increased glucose uptake in EDL muscle from DDAH transgenic mice compared to WT littermates at baseline (12.6±1.9 vs. 8.7±0.8 nmol/min/g muscle), consistent with increased insulin sensitivity in the DDAH transgenic mice. However, after adding exogenous insulin no differences in glucose uptake were observed between the groups (11.0±1.2 vs.14.2±1.4 nmol/min/g muscle).
Insulin signaling cascade in liver tissue following an i.p. glucose challenge
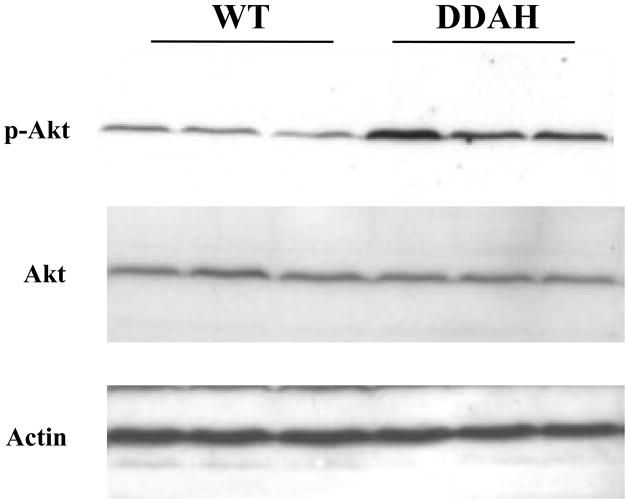
To evaluate the contribution of another insulin-sensitive tissue to the accelerated glucose disposal in DDAH transgenic mice, we assessed glucose-stimulated insulin signaling in the liver. Despite lower blood insulin levels (0.42±0.12 vs 1.03±0.38ng/ml) 30 min post glucose challenge, we observed an increased Akt phosphorylation in the DDAH transgenic mice compared to WT littermates indicative of a more effective insulin signaling in the liver (276±15 vs 100±13 p-Akt/total Akt in %, p<0.03; Fig. 4). This increase in insulin signaling was not due to differences in JNK activation or inhibitory serine-phosphorylation of IRS-1 (data not shown). A trend for increased Akt phosphorylation in the DDAH transgenic mice remained at 60 minutes after glucose challenge (121±5 vs 100±7 p-Akt/total Akt in %, p = n.s.).
Figure 4. Glucose-stimulated insulin signaling in whole liver lysates.
Western analysis reveals that hepatic Akt phosphorylation was increased to a greater degree in the DDAH transgenic mice 30 min post glucose challenge. This enhancement of insulin signaling was not due to changes in Jun N-terminal kinase (JNK) activation or inhibitory serine-phosphorylation of insulin receptor substrate-1 (IRS-1) (dns).
Discussion
The major finding of this study is that DDAH transgenic mice are insulin-sensitive. We documented the insulin sensitivity of the DDAH transgenic mice throughout adulthood (22 through 70 weeks). Our study is consistent with previous reports that eNOS or nNOS deficient mice are insulin-resistant.5,6 Together, these observations support our hypothesis that endogenous ADMA modulates insulin sensitivity, presumably by decreasing NO synthesis. Furthermore, we suggest two possible mechanisms by which ADMA may modulate insulin sensitivity, i.e. by reducing glucose uptake in skeletal muscle and/or by diminishing insulin signaling in the liver.
We have previously shown that the overexpression of human DDAH reduces tissue and plasma levels of the endogenous NOS inhibitor ADMA.16 The increased degradation of ADMA in these transgenic animals is associated with an increased production of NO, reflected by increased plasma and urinary nitrogen oxide levels, as well as a reduction in systemic vascular resistance and blood pressure.16 Accumulating evidence suggests that ADMA is a critical modulator of NOS activity in humans.10 Plasma levels of ADMA are elevated by cardiovascular risk factors and associated with endothelial vasodilator dysfunction and with cardiovascular disease.10 Notably, plasma ADMA levels are increased in conditions associated with insulin resistance, such as hypertension, hypertriglyceridemia, and diabetes mellitus.3,10 Plasma ADMA is increased in type 2 diabetic subjects, and is reduced by metformin treatment in humans and insulin-resistant rats.11–13 In insulin-resistant subjects, treatment with rosiglitazone is associated with a reduction in plasma ADMA levels and an improvement in insulin sensitivity.8 However, in these previous studies, it was suggested that elevations in plasma ADMA were a consequence of insulin resistance.
However, earlier studies by Baron and colleagues have suggested a role for NOS activity in insulin sensitivity. Insulin-induced uptake of glucose was impaired by infusion of the NOS inhibitor L-NMMA.7,23 The relationship between NOS and insulin resistance has been further clarified using mice genetically deficient in NOS. Within skeletal muscle, NOS is present in myocytes (μ-NOS, a variant of nNOS) as well as the endothelium (eNOS) of the skeletal muscle microvasculature.24–26 Mice lacking the endothelial (eNOS) or neuronal (nNOS) isoform of NOS each develop insulin resistance. However, there are subtle phenotypic differences in the insulin resistance associated with these knockouts. In hyperinsulinemic-euglycemic clamp studies, the glucose infusion rate is reduced to a greater extent in the eNOS knockout mice compared with the nNOS knockout mice.5 The endogenous glucose output is completely suppressed in the nNOS knockout and WT mice during insulin infusion, but not in the eNOS knockout mice. In addition, the eNOS knockout mice display significantly reduced whole-body glucose disposal rates compared with WT mice. These data suggest that different NOS isoforms may modulate insulin sensitivity with different tissue preferences. Duplain et al.6 made similar observations in eNOS-deficient mice. They observed that eNOS−/− mice were hypertensive and had fasting hyperinsulinemia, hyperlipidemia, and a 40% lower insulin-stimulated glucose uptake than control mice. The development of insulin resistance in the eNOS−/− mice was related to impaired NO synthesis rather than hypertension per se, since equally hypertensive 1-kidney/1-clip mice manifested normal insulin-stimulated glucose uptake.6
Insulin enhances glucose transport and stimulates glycogen synthase in skeletal muscle fibers.27,28 We observed that a physiologically relevant concentration of ADMA (2μM) markedly inhibited insulin-induced glycogen synthesis in isolated EDL skeletal muscle of young wild-type mice. In aged DDAH transgenic mice, there was an enhanced basal glycogen synthesis in EDL skeletal muscle, but no difference in insulin-induced glycogen synthesis. Intriguingly, whereas the fast-twitch EDL muscle was sensitive to alterations in endogenous or exogenous ADMA, glycogen synthesis in slow-twitch soleus muscle was not. These discordant responses may be due to differences in muscle composition and/or enzyme content. As a slow-twitch muscle, the soleus is largely composed of type I fibers, whereas the EDL (a fast-twitch muscle) is primarily composed by glycolytic type IIb fibers and to a lesser extent oxidative type IIa fibers. This difference in composition may be important because nNOS expression is greater in type IIb.25,26 Kapur and coworkers noted that nNOS protein was only detected in EDL muscles whereas eNOS protein content was comparable in soleus and EDL muscle.26 The modulation by ADMA of basal and insulin-induced skeletal muscle glycogen synthesis appears to be dependent upon age and fiber type.
Insulin signalling in the liver may also be modulated by alterations in DDAH activity. In the DDAH transgenic mice, we observed evidence of enhanced hepatic insulin signalling. Specifically, an increase in glucose-induced Akt phosphorylation was observed in liver tissue from the DDAH transgenic mice. This effect occurred in spite of lower blood insulin levels in the DDAH transgenic mice after the glucose challenge. This insulin sensitivity was not associated with alterations in the JNK pathway or inhibitory phosphorylation sites of IRS-1 – each of which may also be involved in insulin sensitivity.29
We suggest that the relationship between endogenous ADMA and insulin resistance may be reciprocal. Specifically, the metabolic perturbations associated with insulin resistance can increase plasma ADMA levels, but it also seems true that ADMA may increase insulin resistance. With respect to this reciprocal relationship, we observed that the glucose challenge acutely increased plasma ADMA levels in wild-type animals (an effect that was attenuated in the DDAH transgenic mice). We have previously documented that exposure of cultured endothelial cells to high glucose reduces the activity of DDAH and increases the accumulation of ADMA in the conditioned medium. This effect is reversed by antioxidants.30 The sensitivity of DDAH to oxidative stress is likely conferred by a critical sulfhydryl moiety in its catalytic site.31
In conclusion, we demonstrate that the DDAH transgenic mouse is insulin-sensitive. This observation is consistent with previous studies suggesting a link between NOS activity and insulin sensitivity. We suggest that the enhanced sensitivity to insulin in DDAH mice is due to reduced plasma and/or tissue levels of ADMA. ADMA may affect skeletal muscle glycogen synthesis in an age and fiber type-dependent manner. Furthermore, DDAH overexpression is associated with enhanced insulin signaling in the liver. Accordingly, modification of DDAH expression or activity may provide a new therapeutic avenue for treatment of insulin resistance, diabetes mellitus and cardiovascular disease.
Acknowledgments
Dr. Sydow has been a recipient of a research grant provided by the Deutsche Forschungsgemeinschaft (DFG; Sy 41/1-1). This work was supported by grants to Dr. Cooke from the National Institutes of Health (R01 HL-63685; RO1 HL-75774; R01 CA098303 and P01 AG18784; and PO1AI50153) and the Tobacco Related Disease Research Program (11RT-0147). Dr. Cooke is an inventor and receives royalties from patents owned by Stanford University relating to diagnostic and therapeutic applications of the NOS pathway.
References
- 1.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 2.Reaven G. Insulin resistance, type 2 diabetes mellitus, and cardiovascular disease: the end of the beginning. Circulation. 2005;112:3030–3032. doi: 10.1161/CIRCULATIONAHA.105.504670. [DOI] [PubMed] [Google Scholar]
- 3.Cooke JP. The endothelium: a new target for therapy. Vasc Med. 2000;5:49–53. doi: 10.1177/1358836X0000500108. [DOI] [PubMed] [Google Scholar]
- 4.Baron AD. Vascular reactivity. Am J Cardiol. 1999;84:25J–27J. doi: 10.1016/s0002-9149(99)00354-9. [DOI] [PubMed] [Google Scholar]
- 5.Shankar RR, Wu Y, Shen HQ, Zhu JS, Baron AD. Mice with gene disruption of both endothelial and neuronal nitric oxide synthase exhibit insulin resistance. Diabetes. 2000;49:684–687. doi: 10.2337/diabetes.49.5.684. [DOI] [PubMed] [Google Scholar]
- 6.Duplain H, Burcelin R, Sartori C, Cook S, Egli M, Lepori M, Vollenweider P, Pedrazzini T, Nicod P, Thorens B, Scherrer U. Insulin resistance, hyperlipidemia, and hypertension in mice lacking endothelial nitric oxide synthase. Circulation. 2001;104:342–345. doi: 10.1161/01.cir.104.3.342. [DOI] [PubMed] [Google Scholar]
- 7.Steinberg HO, Brechtel G, Johnson A, Fineberg N, Baron AD. Insulin-mediated skeletal muscle vasodilation is nitric oxide dependent. A novel action of insulin to increase nitric oxide release. J Clin Invest. 1994;94:1172–1179. doi: 10.1172/JCI117433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stuhlinger MC, Abbasi F, Chu JW, Lamendola C, McLaughlin TL, Cooke JP, Reaven GM, Tsao PS. Relationship between insulin resistance and an endogenous nitric oxide synthase inhibitor. Jama. 2002;287:1420–1426. doi: 10.1001/jama.287.11.1420. [DOI] [PubMed] [Google Scholar]
- 9.Tsikas D, Boger RH, Sandmann J, Bode-Boger SM, Frohlich JC. Endogenous nitric oxide synthase inhibitors are responsible for the L-arginine paradox. FEBS Lett. 2000;478:1–3. doi: 10.1016/s0014-5793(00)01686-0. [DOI] [PubMed] [Google Scholar]
- 10.Cooke JP. Asymmetrical dimethylarginine: the Uber marker? Circulation. 2004;109:1813–1818. doi: 10.1161/01.CIR.0000126823.07732.D5. [DOI] [PubMed] [Google Scholar]
- 11.Abbasi F, Asagmi T, Cooke JP, Lamendola C, McLaughlin T, Reaven GM, Stuehlinger M, Tsao PS. Plasma concentrations of asymmetric dimethylarginine are increased in patients with type 2 diabetes mellitus. Am J Cardiol. 2001;88:1201–1203. doi: 10.1016/s0002-9149(01)02063-x. [DOI] [PubMed] [Google Scholar]
- 12.Asagami T, Abbasi F, Stuelinger M, Lamendola C, McLaughlin T, Cooke JP, Reaven GM, Tsao PS. Metformin treatment lowers asymmetric dimethylarginine concentrations in patients with type 2 diabetes. Metabolism. 2002;51:843–846. doi: 10.1053/meta.2002.33349. [DOI] [PubMed] [Google Scholar]
- 13.Katakam PV, Ujhelyi MR, Hoenig M, Miller AW. Metformin improves vascular function in insulin-resistant rats. Hypertension. 2000;35:108–112. doi: 10.1161/01.hyp.35.1.108. [DOI] [PubMed] [Google Scholar]
- 14.Pistrosch F, Passauer J, Fischer S, Fuecker K, Hanefeld M, Gross P. In type 2 diabetes, rosiglitazone therapy for insulin resistance ameliorates endothelial dysfunction independent of glucose control. Diabetes Care. 2004;27:484–490. doi: 10.2337/diacare.27.2.484. [DOI] [PubMed] [Google Scholar]
- 15.MacAllister RJ, Parry H, Kimoto M, Ogawa T, Russell RJ, Hodson H, Whitley GS, Vallance P. Regulation of nitric oxide synthesis by dimethylarginine dimethylaminohydrolase. Br J Pharmacol. 1996;119:1533–1540. doi: 10.1111/j.1476-5381.1996.tb16069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dayoub H, Achan V, Adimoolam S, Jacobi J, Stuehlinger MC, Wang BY, Tsao PS, Kimoto M, Vallance P, Patterson AJ, Cooke JP. Dimethylarginine dimethylaminohydrolase regulates nitric oxide synthesis: genetic and physiological evidence. Circulation. 2003;108:3042–3047. doi: 10.1161/01.CIR.0000101924.04515.2E. [DOI] [PubMed] [Google Scholar]
- 17.Levine R, Haft DE. Carbohydrate homeostasis. N Engl J Med. 1970;283:237–246. doi: 10.1056/NEJM197007302830506. [DOI] [PubMed] [Google Scholar]
- 18.Mondon CE, Dolkas CB, Oyama J. Enhanced skeletal muscle insulin sensitivity in year-old rats adapted to hypergravity. Am J Physiol. 1981;240:E482–488. doi: 10.1152/ajpendo.1981.240.5.E482. [DOI] [PubMed] [Google Scholar]
- 19.Schulze F, Wesemann R, Schwedhelm E, Sydow K, Albsmeier J, Cooke JP, Boger RH. Determination of asymmetric dimethylarginine (ADMA) using a novel ELISA assay. Clin Chem Lab Med. 2004;42:1377–1383. doi: 10.1515/CCLM.2004.257. [DOI] [PubMed] [Google Scholar]
- 20.Trinder P. Determination of blood glucose using an oxidase-peroxidase system with a non-carcinogenic chromogen. J Clin Pathol. 1969;22:158–161. doi: 10.1136/jcp.22.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allain CC, Poon LS, Chan CS, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20:470–475. [PubMed] [Google Scholar]
- 22.Roeschlau P, Bernt E, Gruber W. Enzymatic determination of total cholesterol in serum. Z Klin Chem Klin Biochem. 1974;12:226. [PubMed] [Google Scholar]
- 23.Baron AD, Tarshoby M, Hook G, Lazaridis EN, Cronin J, Johnson A, Steinberg HO. Interaction between insulin sensitivity and muscle perfusion on glucose uptake in human skeletal muscle: evidence for capillary recruitment. Diabetes. 2000;49:768–774. doi: 10.2337/diabetes.49.5.768. [DOI] [PubMed] [Google Scholar]
- 24.Kobzik L, Reid MB, Bredt DS, Stamler JS. Nitric oxide in skeletal muscle. Nature. 1994;372:546–548. doi: 10.1038/372546a0. [DOI] [PubMed] [Google Scholar]
- 25.Silvagno F, Xia H, Bredt DS. Neuronal nitric-oxide synthase-mu, an alternatively spliced isoform expressed in differentiated skeletal muscle. J Biol Chem. 1996;271:11204–11208. doi: 10.1074/jbc.271.19.11204. [DOI] [PubMed] [Google Scholar]
- 26.Kapur S, Bedard S, Marcotte B, Cote CH, Marette A. Expression of nitric oxide synthase in skeletal muscle: a novel role for nitric oxide as a modulator of insulin action. Diabetes. 1997;46:1691–1700. doi: 10.2337/diab.46.11.1691. [DOI] [PubMed] [Google Scholar]
- 27.Lawrence JC, Jr, Roach PJ. New insights into the role and mechanism of glycogen synthase activation by insulin. Diabetes. 1997;46:541–547. doi: 10.2337/diab.46.4.541. [DOI] [PubMed] [Google Scholar]
- 28.Azpiazu I, Manchester J, Skurat AV, Roach PJ, Lawrence JC., Jr Control of glycogen synthesis is shared between glucose transport and glycogen synthase in skeletal muscle fibers. Am J Physiol Endocrinol Metab. 2000;278:E234–243. doi: 10.1152/ajpendo.2000.278.2.E234. [DOI] [PubMed] [Google Scholar]
- 29.Cohen P. The twentieth century struggle to decipher insulin signalling. Nat Rev Mol Cell Biol. 2006;7:867–873. doi: 10.1038/nrm2043. [DOI] [PubMed] [Google Scholar]
- 30.Lin KY, Ito A, Asagami T, Tsao PS, Adimoolam S, Kimoto M, Tsuji H, Reaven GM, Cooke JP. Impaired nitric oxide synthase pathway in diabetes mellitus: role of asymmetric dimethylarginine and dimethylarginine dimethylaminohydrolase. Circulation. 2002;106:987–992. doi: 10.1161/01.cir.0000027109.14149.67. [DOI] [PubMed] [Google Scholar]
- 31.Murray-Rust J, Leiper J, McAlister M, Phelan J, Tiley S, Santa Maria J, Vallance P, McDonald N. Structural insights into the hydrolysis of cellular nitric oxide synthase inhibitors by dimethylarginine dimethylaminohydrolase. Nat Struct Biol. 2001;8:679–683. doi: 10.1038/90387. [DOI] [PubMed] [Google Scholar]