Abstract
Opioids have become the unequivocal therapy of choice in treating many varieties of chronic pain. With the increased prescription of opioids, some unintended consequences have occurred. After prolonged opioid exposure, opioid-induced hyperalgesia (OIH), the paradoxical effect that opioid therapy may in fact enhance or aggravate preexisting pain, may occur. Over the past several decades, an increasing number of laboratory and clinical reports have suggested lowered pain thresholds and heightened atypical pain unrelated to the original perceived pain sensations as hallmarks of OIH. However, not all evidence supports the clinical importance of OIH, and some question whether the phenomenon exists at all. Here, we present a nonexhaustive, brief review of the recent literature. OIH will be reviewed in terms of preclinical and clinical evidence for and against its existence; recommendations for clinical evaluation and intervention also will be discussed.
Keywords: Opioid-induced hyperalgesia, Pain hypersensitivity, Opioid dependence, Chronic pain, Individual differences, Quantitative sensory testing
Introduction
As the use of opioid medications for treatment of chronic noncancer pain has increased, there have been both intended and unintended consequences. Importantly, access to analgesic medications, including opioids, has risen. However, abuse of prescription opioids, with its associated morbidity and mortality, has concurrently increased, reaching epidemic levels in the United States [1]. Additionally, there has been increasing evidence for unintended pronociceptive consequences of long-term use of opioids. Worsening pain sensitivity without a new injury or exacerbation of an old injury in a person chronically exposed to opioids is termed opioid-induced hyperalgesia (OIH). This pain often can be diffuse, of a different quality, and unassociated with previous tissue damage. However, there remain many unanswered questions for OIH regarding its molecular mechanisms, pathophysiology, opioids with the highest risk for OIH, and the time frame involved from first use of an opioid to signs of clinically significant hyperalgesia.
OIH first was described in the peer-reviewed literature in 1943 [2], but has garnered increasing attention over the past two decades. Controlled preclinical experiments have defined OIH in animals as a decrease in pain threshold from baseline after chronic administration of opioids [3••]. There still is no accepted operational definition of OIH amongst researchers in human clinical trials; hyperalgesia is defined either as decrease in pain threshold or pain tolerance after chronic opioid exposure. Pain threshold is the first experience of pain from a given stimulus, and pain tolerance is the amount of pain from a given stimulus a person can handle before seeking relief (eg, the amount of time before stopping an experimental pain procedure due to pain, or amount of time before seeking pain-relieving therapy). Threshold and tolerance are two different constructs with, potentially, two different psychophysical mechanisms. Given the lack of consensus definition of OIH amongst pain researchers, it can be difficult to compare results across studies and draw firm conclusions.
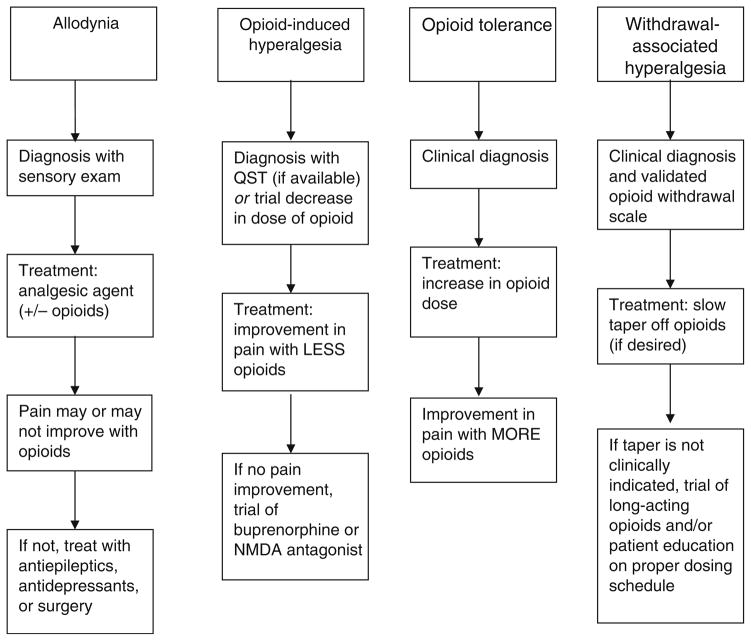
Additionally, OIH often is confused with opioid tolerance, allodynia, and withdrawal-associated hyperalgesia (WAH). These three syndromes can manifest similar symptoms, but need to be clinically differentiated from OIH due to differing effective interventions. Figure 1 offers a guideline for practitioners in the diagnosis and treatment strategies for all of them. Opioid tolerance occurs when increasing amounts of opioids are required to produce the same desired effect; an increase in opioid dose would be the intervention when opioid tolerance is associated with decreased analgesic efficacy. Allodynia is the experience of pain from a benign stimulus (eg, persons suffering from temporomandibular joint disorder will feel excruciating pain from light touch or cold foods). It can be treated with opioids, nonopioid analgesics, or surgical intervention. Finally, WAH is the experience of diffuse joint pain and body aches, which occur when detoxifying from chronic opioid use or skipping/missing scheduled doses; it is time-limited and can be treated with NSAIDs, clonidine, controlled taper of an opioid (if desired), or strict schedule of opioid dosing.
Fig. 1.
Diagnosis and treatment strategies for opioid-induced hyperalgesia, opioid tolerance, allodynia, and withdrawal-associated hyperalgesia. NMDA N-methyl D-aspartate; QST quantitative sensory testing
It is not yet known what changes in the peripheral or central nervous systems cause OIH. Given that OIH is associated with chronic administration of opioids, it is theorized that neural plasticity in one or more aspects of the nervous system occurs before the onset of hyperalgesia. Likely neural candidates underlying OIH include the μ-opioid receptor, glutamate receptor, other excitatory neuropeptides, or the descending inhibitory pathway. This review explores recent research in OIH, looking at important preclinical and clinical experiments that are assisting scientists and clinicians in understanding this phenomenon. Additionally, important individual differences that impact on the experience of OIH are discussed. Finally, there will be a balanced discussion on the diagnosis, treatment, and clinical significance of OIH.
Molecular Mechanism of Opioid-Induced Hyperalgesia: The Importance of Preclinical Research
Kayan and colleagues [4] first described the phenomenon of hyperalgesia after acute morphine injection in rats in 1971. Chronic administration of opioids also was shown to cause a sustained pronociceptive (hyperalgesic) response [5, 6], defined as a lower threshold for pain as compared to baseline. OIH in these experiments depended both on the dose of opioid and on the experimental pain model (ie, thermal, mechanical, electrical, or chemical). Originally, OIH was thought to occur after varying opioid dosing regimens (including maintenance dosing, brief high doses, and brief low or ultra low doses). Early preclinical literature did suggest hyperalgesia after each of these regimens (Table 1), but there still are too few studies to suggest that these regimens represent different subtypes of OIH in humans. While there were variable circumstances under which OIH could be demonstrated, it became an accepted fact that opiates could induce hyperalgesia in rats and other animals.
Table 1.
Differential opioid dosing regimens and opioid-induced hyperalgesia
| Condition | Pain and diagnostic symptoms | Onset | Response to opioid treatment |
|---|---|---|---|
| OIH during chronic opioid maintenance | Pain is abnormal in presentation (often with concurrent allodynia) | Abrupt | Pain worsens |
| Pain may occur at a distant location from the original source or be widespread (panalgesia), and is poorly defined in terms of locality and quality | |||
| OIH can be associated with delirium, agitation, seizures, and myoclonus | |||
| OIH after brief high doses of opioids | Large opioid doses may increase tenderness in skin and soft tissue; seen often in postoperative periods or hospitalizations for pain | Abrupt | Pain worsens; may be appropriate to switch to a piperidine derivative (eg, fentanyl) |
| Inconsistent data and contradictory case reports | |||
| OIH after brief, low, or ultra-low doses of opioids | Little experimental work exists in humans, but rodent models suggest some evidence | Unknown | Pain may improve with increased dose |
Research over the past year has tried to elucidate the exact mechanism(s) for OIH, which still is (are) unknown. Four main hypotheses based on neuroanatomical location have emerged: sensitization of peripheral nerve endings; enhanced descending facilitation of nociceptive signal transmission; enhanced production, release, and diminished reuptake of nociceptive neurotransmitters; and/or sensitization of second-order neurons to nociceptive neurotransmitters. Although investigators have suggested chemokines could underlie the above neuroanatomical changes [7], the cause that has garnered the greatest attention is enhancement of the amount or response to excitatory neurotransmitters (eg, glutamate and glycine) via neural plasticity. To investigate this hypothesis, the following researchers have exposed animals to opioids while antagonizing glutamate receptors (specifically N-methyl D-aspartate [NMDA]) or the intracellular responses to glutamate in an attempt to inhibit the development of OIH (eg, calcium influx into postsynaptic neurons and activation of calcium/calmodulin-dependent protein kinases [CaMKs]).
First, Minville and colleagues [8] investigated the effects of the NMDA-receptor antagonist ketamine on postoperative hyperalgesia in a mouse model of orthopedic pain. These experiments showed that mice given intraoperative opioid analgesia (sufentanil) and high-dose ketamine had significantly less thermal hyperalgesia (measured as the amount of time a hind paw could remain on a hot plate before withdrawal) for the first 4 postoperative days as compared to mice treated with sufentanil alone. Prior research with ketamine had shown similar results. Second, co-injection of fentanyl and magnesium (an NMDA antagonist) eliminated thermal hyperalgesia at 24 h that was seen with fentanyl-only injections in female rats [9]. Third, Chen and colleagues [10] looked at both the blockade of calcium/calmodulin-dependent protein kinase II (CaMKIIα) and knockout mice lacking CaMKIIα. In these two experiments, OIH did not occur in the mice lacking functional CaMKIIα (either because of blockade or genetically engineered lack of functional enzymes) after morphine exposure.
One recent experiment contradicted the above findings. Hay and colleagues [11] investigated the effect of chronic methadone administration with or without the NMDA antagonist memantine on thermal hyperalgesia in male rats. Methadone is both a μ-opioid–receptor agonist and an NMDA antagonist. The authors found that rats treated with 1 mg/kg methadone for 14 days had lower threshold for heat pain during treatment than before methadone was introduced. Memantine did not reverse the methadone-induced hyperalgesia, indicating that the excitatory NMDA receptors may not be involved in OIH from methadone. These results should be interpreted with caution because memantine has weaker affinity for the NMDA receptor; therefore, physiological amounts of glutamate potentially could overcome its blockade and result in excitatory neurotransmission and OIH.
Lastly, two novel experiments suggested that OIH paradoxically may not even require opioid receptors. Knockout mice without μ-, δ-, or κ-opioid receptors developed thermal hyperalgesia after acute and chronic (6 days) exposure to fentanyl [12]. This hyperalgesia could be blocked by NMDA antagonist MK-801. An additional experiment from the same lab produced hyperalgesia from morphine-6β-glucuronide (M6G; a metabolite of morphine with μ-receptor agonist activity) in knockout mice and wild-type mice treated concurrently with the opioid-receptor antagonist naloxone [13]. These experiments do not rule out that opioid receptor–like receptors (eg, ORL-1) are involved in OIH, but suggest that opioid metabolites or other endogenous responses to opioids can lead to OIH through opioid receptor–independent mechanisms.
Clinical Evidence for and against Opioid-Induced Hyperalgesia
Pain hypersensitivity after opioid administration in preclinical rodent models has been well documented, while human studies have met with more controversy. However, a growing clinical and experimental literature is documenting OIH in several diverse populations.
Evidence from Patient Populations
A handful of case reports and clinical studies have provided evidence of OIH outside of the laboratory. For example, Vorobeychik and colleagues [14] reported a case study where a patient experiencing symptoms of OIH experienced a substantial drop in self-reported pain intensity (from 8 to 3 out of 10 on a pain visual analogue scale) when the patient had their hydromorphone dose reduced by 40% to 50% and then started on methadone. Guignard and colleagues [15] found that adult patients undergoing major abdominal surgery who received remifentanil infusion intraoperatively reported significantly greater postoperative pain, needed rescue medication earlier, and required nearly twice as much morphine within the first 24-hour postoperative period than their control group counterparts.
Opioid-dependent individuals on replacement therapy also show evidence of OIH as compared to healthy control patients. In a novel comparison of methadone-maintained patients, buprenorphine-maintained patients, and matched control patients on cold pressor latency (a quantitative sensory testing technique where the participant typically places their hand in painfully cold water), Compton and colleagues [16, 17] found that methadone- and buprenorphine-maintained patients displayed shorter withdrawal latencies (were less tolerant) of the cold water procedure compared with matched control patients, and hinted that methadone-maintained patients were most sensitive when confronted with the cold pressor task. In a recent follow-up study, Compton et al. [18•] examined whether gabapentin (as prescribed for neuropathic pain) had the potential to reverse OIH in methadone-maintained patients. The authors assessed cold pressor pain threshold and tolerance after a 5-week trial of daily gabapentin. They found statistically significant improvement in cold pressor pain threshold and tolerance from baseline, and suggested that gabapentin may be effective in reducing OIH in stable methadone-maintained patients who take the medication as prescribed and abstain from illicit substance use. Moreover, a recent study in opiate-dependent patients suggested that individual differences in OIH were strongly related to important clinical outcomes [19]. Those who were most pain-sensitive reported the highest levels of clinical pain, the highest levels of distress, and the highest degree of cue-induced drug craving [19]. The link between OIH and opioid craving is particularly interesting because self-report of opioid craving was associated with opioid misuse in a 6-month prospective study of chronic pain patients maintained on oral opioids [20].
Two recent prospective studies examined OIH in chronic pain patients without a history of addiction. In a small sample, Chu and colleagues [21] examined analgesic tolerance and hyperalgesia before and 1 month after oral morphine treatment in patients with chronic low back pain who were opioid naïve at study entry. The authors found that 1 month of morphine treatment was associated with significant hyperalgesia using cold models, but not heat pain models. A more recent cross-sectional study compared pain threshold, tolerance, and temporal summation (a measure of central sensitization) among participants in three groups: chronic pain with opioid therapy, chronic pain without opioid therapy, and healthy control patients (no pain/no opioids) [22]. They found that individuals with chronic pain and on opioid therapy significantly differed from the other two groups by displaying decreased heat pain thresholds and enhanced temporal summation responses. The authors also noted that opioid dose correlated with these responses.
However, the clinical relevance of OIH has been disputed by some [3••], and a few studies examining OIH have been met with negative findings. For example, Reznikov and colleagues [23] conducted a large-scale trial of 224 patients with chronic pain (142 opioid-treated and 82 non–opioid-treated) using quantitative sensory testing (QST), including von Frey filaments, mechanical pressure sensitivity, heat pain threshold, and suprathreshold tonic heat pain (46.5°C applied for 1 min), and found no differences in QST between groups. They also examined subgroups within their sample and found that “weak” opioid-treated individuals (self-reported mild-moderate pain) did not differ from “strong” opioid-treated participants (self-reported moderate-severe pain). This study did not have a healthy control group, mixed persons with cancer and noncancer pain, and allowed use of multiple analgesic, antidepressant, and antianxiety agents, so the results should be interpreted with caution. In addition, Cortinez et al. [24] found no difference in pain scores or morphine requirements after gynecological surgery between a group of participants assigned to receive remifentanil and sevoflurane. Despite these mixed findings, many researchers still suggest that OIH likely plays a role in poor clinical outcomes, and published case studies demonstrate effectiveness of opioid withdrawal in reducing chronic pain levels in some individuals [25].
Evidence from Nonclinical Samples
In an exhaustive systematic review, Fishbain and colleagues [3••] examined the results and quality of literature focused on OIH. They found the most evidence for OIH in opioid-infusion studies where secondary hyperalgesia was measured in normal, healthy volunteers. Authors have refined the acute remifentanil infusion model of OIH and have shown mechanical hyperalgesia [26], electrical hyperalgesia [27], pressure hyperalgesia [28], and hyperalgesia from a capsaicin (the active ingredient in hot chili peppers) pain model [29], but not cold hyperalgesia, after acute doses of intravenous remifentanil. A very recent prospective, randomized, placebo-controlled, two-way crossover study in healthy volunteers examined secondary hyperalgesia to electrical pain stimulation after brief remifentanil or saline exposure. The postinfusion period witnessed an increase in the area of secondary hyperalgesia. The authors went on to examine the effect of a bolus dose of naloxone on the area and found no effect in the remifentanil or the saline group, potentially suggesting that the endogenous opioid system may not play a role in the modulation of OIH produced in this model [30•]. Although most remifentanil studies have consistently shown hyperalgesia to a wide variety of pain stimuli, the model does not account for the neural plasticity changes thought to underlie OIH from chronic opioid use. Therefore, these results should be interpreted with caution and may represent acute physical dependence with subsequent WAH.
Individual Differences: Complicating the Already Complicated Picture
Similar to the tremendous variability inherent in pain perception [31], individual differences in the development of OIH appear to be substantial [3••, 32]. To date, it is not understood why some individuals suffer from OIH while others on even larger chronic doses of opioids do not. Chronic opioid dose does appear to be one relevant factor in determining OIH susceptibility, although hyperalgesia after acute doses of opioids may not depend on the dose. Many other individual difference variables also are likely to play a role.
For example, over the past decade, several reports have noted sex differences in OIH both in nonhuman animal and human studies. As recently reviewed by Bodnar and Kest [33], sex differences in OIH have been characterized in both quantitative and qualitative assessments. Female rats have been found to have decreased tail-flick latencies and hyperalgesia to mechanical stimuli after acute administration of low subcutaneous morphine compared to male rats [34]. Juni et al. [35] also found that morphine produced hyperalgesia earlier in females and also lasted longer than in their male counterparts. Differences were absolved in ovariectomized females on estrogen replacement, suggesting a potential role for sex steroids/hormones in the development or maintenance of OIH. While NMDA receptors have been identified as contributing to opioid hyperalgesia, NMDA-receptor antagonists may reduce opioid analgesia in male mice only [36]. In a follow-up study designed to elucidate the neurochemical system mediating morphine hyperalgesia and relationship with NMDA, Juni and colleagues [37] found that the melanocortin-1 receptors make a substantial contribution to morphine hyperalgesia in female mice.
In a study evaluating patients with chronic pain treated with or without oral opioids, Ram and colleagues [38] found no differences between the groups in cold pressor threshold, tolerance, or pain intensity levels. However, the magnitude of the diffuse noxious inhibitory controls (DNIC: an assessment involving two simultaneous pain-induction procedures) effect, a marker of pain inhibition, was larger for non–opioid-treated patients when compared to those on opioids. Interestingly, men in the nonopioid group had a significantly larger DNIC effect compared to men in the opioid group, while there was no difference among women. Additionally, in the opioid group, a trend toward significance emerged where those treated for a shorter time on opioids had an increased DNIC response compared to those with a longer duration of treatment. The authors also found that both opioid dosage and treatment duration negatively predicted DNIC in men in the opioid analgesic group. Pain regulatory processes that may contribute to the development of OIH among patients with chronic pain treated with opioids appear to differ from those on nonopioid analgesics in a sex-dependent fashion.
A recent clinical study of healthy volunteers found that a genetic predisposition to OIH may exist, as polymorphisms of the catechol-O-methyl transferase gene affected acute changes in pain sensitivity after administration of a potent parenteral opioid [39].
Collectively, it is clear that individual patients vary in the induction and severity of OIH. In an interesting study aimed at identifying links between individual differences in risk for opioid misuse and individual differences in hyperalgesia, Edwards and colleagues [40] found that pain-related distress (eg, anxiety and catastrophizing about pain) predicted hyperalgesia in patients on opioids, and those in the “high risk” abuse potential group reported higher levels of clinical pain, lower pressure threshold, and thermal pain threshold and tolerance, and rated temporal summation procedures as more painful. While individual differences in OIH have been examined only recently, these studies indicate vast variability in the susceptibility to OIH and suggest that further study into individual differences within these variables, including genetic and psychological factors, is warranted.
Clinical Implications
A recently published guideline for future opioid pharmacotherapy research called specifically for increased investigation into OIH [41•]. The expert authors noted that specific clinical guidelines are lacking for pain practitioners. The following important questions still remain unanswered:
How is OIH diagnosed?
Which patient populations are at greatest risk for developing OIH?
Do diverse opioid medications, doses, or duration of treatment have differing risk profiles for OIH?
If OIH is suspected, would opioid switching, addition of NMDA-blocking agent, or decrease in opioid dose lead to resolution of hyperalgesia?
If patients require maintenance treatment of opioid dependence, would methadone or buprenorphine have decreased risk for the development of OIH?
Although these questions still remain, there are a few guidelines to assist today’s pain practitioners based upon current literature and clinical experience.
Diagnosis of Opioid-Induced Hyperalgesia
QST performed before initiation of opioid treatment and then repeated at set intervals can guide clinical decision making [22]. Demonstrated changes in pain threshold on QST strongly suggest OIH if other causes have been eliminated (Fig. 1). Office-based QST currently is limited to major research institutions, but the availability for other settings is increasing. Diagnosis of OIH also may be demonstrated by a reduction in opioid dose. If the dose reduction results in better pain control, OIH is the most likely cause of the hyperalgesia; if pain worsens, opioid tolerance likely has developed.
Treatment of Opioid-Induced Hyperalgesia
If opioids are required for adequate analgesia and dose reductions are unacceptable to the patient, there are some medications that may help treat OIH. Ketamine has been most successful in the treatment of OIH in outpatient case reports and clinical trials of surgically related analgesia, but a recent retrospective cohort study did not find evidence in support of outpatient weekly ketamine infusions for the treatment of chronic pain [42•]. Additionally, this medication is only available as an injection in the United States, which limits its use in the everyday management of pain. However, there are ongoing clinical trials of intranasal, oral, and topical ketamine for the treatment of a wide variety of pain states. Gabapentin, an antiepileptic medication (AED), also was shown to increase pain threshold (by reducing OIH) in methadone-maintained individuals; yet, the relatively small magnitude of increase as compared to baseline may have little clinical benefit for OIH treatment [18•]. If neuropathic pain is the source of chronic pain, other AEDs and antidepressants may be combined to allow a dose reduction in opioids with better pain control. Finally, opioid switching has a theoretical benefit but no currently demonstrated effect on OIH in controlled clinical trials. For example, methadone is administered as a racemic mixture, and its d-isomer has NMDA antagonist activity. There are anecdotal reports that methadone allows for a decreased opioid dose and is associated with less OIH, but this medication often needs to be given two to three times daily for adequate analgesia and has been associated with increases in overdose fatalities. Intramuscular and intravenous buprenorphine has been approved by the US Food and Drug Administration for pain control, but the sublingual formulation is only approved for the office-based treatment of opioid dependence in the United States. As a partial μ opioid–receptor agonist and ORL-1 agonist, buprenorphine may provide less risk for OIH and decrease risk for abuse when taken as prescribed. Its widespread use as an analgesic agent is limited in the United States unless concurrent opioid dependence exists, though clinical trials of transdermal buprenorphine for the treatment of pain are proceeding.
Conclusions
OIH has been reliably shown in the preclinical literature, and most often is defined as a decrease in pain threshold after opioid exposure. Recent evidence points to the importance of NMDA-receptor activation in this process, and opioid receptors may not be necessary for the development of OIH. Although evidence is not entirely consistent, OIH in humans appears to pose a significant clinical challenge in acute, chronic, and cancer pain settings. However, not all persons develop OIH, and its clinical implications are not yet well described. QST assessing patients’ pain sensitivity before and during opioid pharmacotherapy likely should be used as tools for monitoring the onset and extent of OIH. The conclusions of the Ram et al. [38] study highlight the importance of examining DNIC and point to potential mechanisms whereby OIH can have an impact on clinical pain. A prospective study assessing DNIC and other experimental pain modalities in healthy control patients as well as patients with pain before opioid/nonopioid prescription would be of interest and help establish the implications of OIH. For example, if prolonged exposure to opioid analgesics produced decreased pain inhibition (measured through DNIC), and low DNIC is associated with poor outcomes, these patients may have an increased risk profile for further deterioration/exacerbation of their pain conditions. The next 10 years hopefully will bring more answers to the mechanisms of OIH and open the field for better treatments with the goal of pain reduction and increased quality of life for patients.
Footnotes
Disclosures Dr. D. Andrew Tompkins has received a grant from the National Institute of Drug Abuse. Dr. Claudia Campbell has received travel expense compensation from Arcion Therapeutics, Inc.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Mendelson J, Flower K, Pletcher MJ, Galloway GP. Addiction to prescription opioids: characteristics of the emerging epidemic and treatment with buprenorphine. Exp Clin Psychopharmacol. 2008;16:435–441. doi: 10.1037/a0013637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrews HL. The effect of opiates on the pain threshold in post-addicts. J Clin Invest. 1943;22:511–516. doi: 10.1172/JCI101420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3••.Fishbain DA, Cole B, Lewis JE, et al. Do opioids induce hyperalgesia in humans? An evidence-based structured review. Pain Med. 2009;10:829–839. doi: 10.1111/j.1526-4637.2009.00653.x. This is a comprehensive review on human studies in opioid-induced hyperalgesia. The authors conclude that the strength of the evidence for opioid-induced hyperalgesia is limited but did not refute its existence. [DOI] [PubMed] [Google Scholar]
- 4.Kayan S, Woods LA, Mitchell CL. Morphine-induced hyperalgesia in rats tested on the hot plate. J Pharmacol Exp Ther. 1971;177:509–513. [PubMed] [Google Scholar]
- 5.Celerier E, Laulin JP, Corcuff JB, et al. Progressive enhancement of delayed hyperalgesia induced by repeated heroin administration: a sensitization process. J Neurosci. 2001;21:4074–4080. doi: 10.1523/JNEUROSCI.21-11-04074.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laulin JP, Celerier E, Larcher A, et al. Opiate tolerance to daily heroin administration: an apparent phenomenon associated with enhanced pain sensitivity. Neuroscience. 1999;89:631–636. doi: 10.1016/s0306-4522(98)00652-6. [DOI] [PubMed] [Google Scholar]
- 7.White F, Wilson N. Opiate-induced hypernociception and chemokine receptors. Neuropharmacology. 2010;58:35–37. doi: 10.1016/j.neuropharm.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Minville V, Fourcade O, Girolami JP, Tack I. Opioid-induced hyperalgesia in a mice model of orthopaedic pain: preventive effect of ketamine. Br J Anaesth. 2010;104:231–238. doi: 10.1093/bja/aep363. [DOI] [PubMed] [Google Scholar]
- 9.Mert T, Gunes Y, Ozcengiz D, Gunay I. Magnesium modifies fentanyl-induced local antinociception and hyperalgesia. Naunyn Schmiedebergs Arch Pharmacol. 2009;380:415–420. doi: 10.1007/s00210-009-0447-3. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y, Yang C, Wang ZJ. Ca2+/calmodulin-dependent protein kinase II alpha is required for the initiation and maintenance of opioid-induced hyperalgesia. J Neurosci. 2010;30:38–46. doi: 10.1523/JNEUROSCI.4346-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hay JL, Kaboutari J, White JM, et al. Model of methadone-induced hyperalgesia in rats and effect of memantine. Eur J Pharmacol. 2010;626:229–233. doi: 10.1016/j.ejphar.2009.09.056. [DOI] [PubMed] [Google Scholar]
- 12.Waxman AR, Arout C, Caldwell M, et al. Acute and chronic fentanyl administration causes hyperalgesia independently of opioid receptor activity in mice. Neurosci Lett. 2009;462:68–72. doi: 10.1016/j.neulet.2009.06.061. [DOI] [PubMed] [Google Scholar]
- 13.van Dorp EL, Kest B, Kowalczyk WJ, et al. Morphine-6beta-glucuronide rapidly increases pain sensitivity independently of opioid receptor activity in mice and humans. Anesthesiology. 2009;110:1356–1363. doi: 10.1097/ALN.0b013e3181a105de. [DOI] [PubMed] [Google Scholar]
- 14.Vorobeychik Y, Chen L, Bush MC, Mao J. Improved opioid analgesic effect following opioid dose reduction. Pain Med. 2008;9:724–727. doi: 10.1111/j.1526-4637.2008.00501.x. [DOI] [PubMed] [Google Scholar]
- 15.Guignard B, Bossard AE, Coste C, et al. Acute opioid tolerance: intraoperative remifentanil increases postoperative pain and morphine requirement. Anesthesiology. 2000;93:409–417. doi: 10.1097/00000542-200008000-00019. [DOI] [PubMed] [Google Scholar]
- 16.Compton P, Charuvastra VC, Ling W. Pain intolerance in opioid-maintained former opiate addicts: effect of long-acting maintenance agent. Drug Alcohol Depend. 2001;63:139–146. doi: 10.1016/s0376-8716(00)00200-3. [DOI] [PubMed] [Google Scholar]
- 17.Compton P, Charuvastra VC, Kintaudi K, Ling W. Pain responses in methadone-maintained opioid abusers. J Pain Symptom Manage. 2000;20:237–245. doi: 10.1016/s0885-3924(00)00191-3. [DOI] [PubMed] [Google Scholar]
- 18•.Compton P, Kehoe P, Sinha K, et al. Gabapentin improves cold-pressor pain responses in methadone-maintained patients. Drug Alcohol Depend. 2010;109:213–219. doi: 10.1016/j.drugalcdep.2010.01.006. This report describes how one medication may improve opioid-induced hyperalgesia in stable patients on methadone. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ren ZY, Shi J, Epstein DH, et al. Abnormal pain response in pain-sensitive opiate addicts after prolonged abstinence predicts increased drug craving. Psychopharmacology (Berl) 2009;204:423–429. doi: 10.1007/s00213-009-1472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wasan AD, Butler SF, Budman SH, et al. Does report of craving opioid medication predict aberrant drug behavior among chronic pain patients? Clin. J Pain. 2009;25:193–198. doi: 10.1097/AJP.0b013e318193a6c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chu LF, Clark DJ, Angst MS. Opioid tolerance and hyperalgesia in chronic pain patients after one month of oral morphine therapy: a preliminary prospective study. J Pain. 2006;7:43–48. doi: 10.1016/j.jpain.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 22.Chen L, Malarick C, Seefeld L, et al. Altered quantitative sensory testing outcome in subjects with opioid therapy. Pain. 2009;143:65–70. doi: 10.1016/j.pain.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reznikov I, Pud D, Eisenberg E. Oral opioid administration and hyperalgesia in patients with cancer or chronic nonmalignant pain. Br J Clin Pharmacol. 2005;60:311–318. doi: 10.1111/j.1365-2125.2005.02418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cortinez LI, Brandes V, Munoz HR, et al. No clinical evidence of acute opioid tolerance after remifentanil-based anaesthesia. Br J Anaesth. 2001;87:866–869. doi: 10.1093/bja/87.6.866. [DOI] [PubMed] [Google Scholar]
- 25.Baron MJ, McDonald PW. Significant pain reduction in chronic pain patients after detoxification from high-dose opioids. J Opioid Manag. 2006;2:277–282. doi: 10.5055/jom.2006.0041. [DOI] [PubMed] [Google Scholar]
- 26.Koppert W, Sittl R, Scheuber K, et al. Differential modulation of remifentanil-induced analgesia and postinfusion hyperalgesia by S-ketamine and clonidine in humans. Anesthesiology. 2003;99:152–159. doi: 10.1097/00000542-200307000-00025. [DOI] [PubMed] [Google Scholar]
- 27.Koppert W, Angst M, Alsheimer M, et al. Naloxone provokes similar pain facilitation as observed after short-term infusion of remifentanil in humans. Pain. 2003;106:91–99. doi: 10.1016/s0304-3959(03)00294-x. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt S, Bethge C, Forster MH, Schafer M. Enhanced postoperative sensitivity to painful pressure stimulation after intraoperative high dose remifentanil in patients without significant surgical site pain. Clin J Pain. 2007;23:605–611. doi: 10.1097/AJP.0b013e318122d1e4. [DOI] [PubMed] [Google Scholar]
- 29.Hood DD, Curry R, Eisenach JC. Intravenous remifentanil produces withdrawal hyperalgesia in volunteers with capsaicin-induced hyperalgesia. Anesth Analg. 2003;97:810–815. doi: 10.1213/01.ANE.0000078811.80093.88. [DOI] [PubMed] [Google Scholar]
- 30•.Chu LF, Dairmont J, Zamora AK, et al. The endogenous opioid System is not involved in modulation of opioid-induced hyperalgesia. J Pain. 2010 doi: 10.1016/j.jpain.2010.05.006. This report provides evidence that the known opioid receptors are not needed for the development of opioid-induced hyperalgesia. [DOI] [PubMed] [Google Scholar]
- 31.Fillingim RB. Individual differences in pain responses. Curr Rheumatol Rep. 2005;7:342–347. doi: 10.1007/s11926-005-0018-7. [DOI] [PubMed] [Google Scholar]
- 32.Colvin LA, Fallon MT. Opioid-induced hyperalgesia: a clinical challenge. Br J Anaesth. 2010;104:125–127. doi: 10.1093/bja/aep392. [DOI] [PubMed] [Google Scholar]
- 33.Bodnar RJ, Kest B. Sex differences in opioid analgesia, hyperalgesia, tolerance and withdrawal: central mechanisms of action and roles of gonadal hormones. Horm Behav. 2010;58:72–81. doi: 10.1016/j.yhbeh.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 34.Holtman JR, Jr, Wala EP. Characterization of morphine-induced hyperalgesia in male and female rats. Pain. 2005;114:62–70. doi: 10.1016/j.pain.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 35.Juni A, Klein G, Kowalczyk B, et al. Sex differences in hyperalgesia during morphine infusion: effect of gonadectomy and estrogen treatment. Neuropharmacology. 2008;54:1264–1270. doi: 10.1016/j.neuropharm.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 36.Kavaliers M, Choleris E. Sex differences in N-methyl-D-aspartate involvement in kappa opioid and non-opioid predator-induced analgesia in mice. Brain Res. 1997;768:30–36. doi: 10.1016/s0006-8993(97)00569-6. [DOI] [PubMed] [Google Scholar]
- 37.Juni A, Cai M, Stankova M, et al. Sex-specific mediation of opioid-induced hyperalgesia by the melanocortin-1 receptor. Anesthesiology. 2010;112:181–188. doi: 10.1097/ALN.0b013e3181c53849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ram KC, Eisenberg E, Haddad M, Pud D. Oral opioid use alters DNIC but not cold pain perception in patients with chronic pain—new perspective of opioid-induced hyperalgesia. Pain. 2008;139:431–438. doi: 10.1016/j.pain.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 39.Jensen KB, Lonsdorf TB, Schalling M, et al. Increased sensitivity to thermal pain following a single opiate dose is influenced by the COMT val(158)met polymorphism. PLoS One. 2009;4:e6016. doi: 10.1371/journal.pone.0006016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Edwards RR, Wasan A, Jamison R. Hyperalgesia in pain patients at elevated risk for opioid misuse. J Pain. 2010;11:S45. doi: 10.1016/j.jpain.2011.02.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41•.Chapman CR, Lipschitz DL, Angst MS, et al. Opioid pharmacotherapy for chronic non-cancer pain in the United States: a research guideline for developing an evidence-base. J Pain. 2010;11:807–829. doi: 10.1016/j.jpain.2010.02.019. This is a report on the specific research needs for the pain treatment field for the future. [DOI] [PubMed] [Google Scholar]
- 42•.Kapural L, Kapural M, Bensitel T, Sessler DI. Opioid-sparing effect of intravenous outpatient ketamine infusions appears short-lived in chronic-pain patients with high opioid requirements. Pain Physician. 2010;13:389–394. This report provides evidence that ketamine infusions can reduce pain and opioid requirements in persons with chronic pain, but the benefits remit 6 months after ending treatment. [PubMed] [Google Scholar]
- 43.Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology. 2006;104:570–87. doi: 10.1097/00000542-200603000-00025. [DOI] [PubMed] [Google Scholar]