Abstract
Scanning transmission electron microscope (STEM) images of three-dimensional (3D) samples were simulated. The samples consisted of a micrometer(s)-thick substrate, and gold nanoparticles at various vertical positions. The atomic number (Z) contrast as obtained via the annular dark field detector was generated. The simulations were carried out using the Monte Carlo metihod in the Casino software (freeware). The software was adapted to include the STEM imaging modality, including the noise characteristics of the electron source, the conical shape of the beam, and 3D scanning. Simulated STEM images of nanoparticles on a carbon substrate revealed the influence of the electron dose on the visibility of the nanoparticles. The 3D datasets obtained by simulating focal-series showed the effect of beam broadening on the spatial resolution, and on the signal-to-noise-ratio. Monte Carlo simulations of STEM imaging of nanoparticles on a thick water layer were compared with experimental data by programming the exact sample geometry. The simulated image corresponded to the experimental image, and the signal-to-noise levels were similar. The Monte Carlo simulation strategy described here can be used to calculate STEM images of objects of an arbitrary geometry and amorphous sample composition. This information can then be used, for example, to optimize the microscope settings for imaging sessions where a low electron dose is crucial, for the design of equipment, or for the analysis of the composition of a certain specimen.
Keywords: Monte Carlo simulations, scanning transmission electron microscopy (STEM), shot noise, annular dark field (ADF) detector, three-dimensional STEM, liquid STEM, nanoparticles
INTRODUCTION
The STEM reaches atomic resolution on atoms placed on ultra-thin support materials (Crewe, et al., 1970), and sub-angstrom resolution is achievable on solid materials when the spherical aberration of the objective lens is corrected (Nellist, et al., 2004). The STEM can also be used for biological materials, for example, to determine the mass of proteins (Mueller & Engel, 2006). It was recently recognized that STEM imaging provides nanometer resolution on nanoparticles in micrometers-thick solid (Sousa, et al., 2008), or liquid specimens (de Jonge, et al., 2009), which is not possible with conventional transmission electron microscopy (TEM). The STEM is also useful for 3D tomography of thick biological specimens (Aoyama, et al., 2008; Hohmann-Marriott, et al., 2009), and for the recording of 3D datasets via focal-series (de Jonge, et al., 2010c; van Benthem, 2005). For the imaging of thick samples, the spatial resolution is not limited by the optics of the STEM, as is the case for ultra-thin amorphous substrates, but the interaction of the electron beam with the specimen plays a major role, and the resolution is limited by noise, or by beam broadening (de Jonge, et al., 2010a; Reimer & Kohl, 2008). Here, thick is defined in terms of the density of the material expressed by the mean-free-path length for electron scattering. It is crucial to have knowledge of the electron beam-specimen interactions, so that the imaging settings can be optimized for maximal resolution, especially when imaging radiation-sensitive samples. Knowledge of the beam broadening can potentially be used to enhance the resolution of 3D datasets by using deconvolution procedures. A precise way to study the interaction between an electron beam and a specific specimen geometry and composition is via the use of Monte Carlo simulations (Hovington, et al., 1997; Joy, 1995).
Here, we present the implementation of Monte Carlo simulations of STEM imaging of specific sample geometries and compositions in the CASINO software, which was previously used for the simulation of scanning electron microscope (SEM) imaging (Drouin, et al., 2007; Hovington, et al., 1997). The electron beam model included the Poisson noise, and a conical shape of the electron source, such that realistic STEM images were obtained with these simulations. By using the 3D geometry of the sample, and the 3D programming of the electron beam shape and focal point position, various STEM imaging sequences were simulated, including focal-series. The simulations used the annular dark field (ADF) detector, exhibiting atomic number (Z) contrast (Crewe, et al., 1970). The electron scattering physical models used in the previous version of CASINO were extended from the energy range of the SEM to energies of up to 300 keV as needed for STEM imaging. Simulations were carried out for three different specimens, 1) gold nanoparticles on a carbon substrate, 2) gold nanoparticles placed at several depths in/on a carbon substrate, and 3) gold nanoparticles placed on a water layer. For all samples we evaluated the achievable resolution, and signal-to-noise level. For the third sample, the simulated images were compared with experimental data.
METHODS
Software for Monte Carlo simulations
Monte Carlo numerical methods can be used to simulate electron trajectories in a sample of specified geometry and composition (Joy, 1995), and were used by different research groups to study the electron beam interactions in STEM imaging. For example, Reichelt and Engel compared the variation of the dark field signal with thickness for several organic materials (Reichelt & Engel, 1984). Hyun et al. studied the effect of beam spreading on X-Y projections of nanoparticles in STEM tomography (Hyun, et al., 2007; Hyun, et al., 2008). Sousa et al. studied STEM imaging of thick biological sections for several detector configurations (Sousa, et al., 2009).
The electron trajectory calculation model used in this study was implemented in the CASINO software, which is described in detail elsewhere (Drouin, et al., 2007). The program calculates electron trajectories through a material. The latest version of CASINO (version 3) includes an editor with a graphic user interface (GUI) to define complex three-dimensional (3D) sample geometries (Drouin & Couture, 2002). The sample was programmed using a combination of basic objects, i.e., planes, boxes, and spheres, with geometrical properties such as size, position and orientation. The material properties (density, atomic number, and atomic weight) were defined for each object. These material properties determined how electrons interacted within each region in the sample via the mean-free-path-length between collisions of elastic scattering lel:
| (1) |
where N0 is Avogadro’s number, ρ is the mass density, σel is the total electron elastic cross section, and A is the atomic weight. In the case of a compound, lel was obtained by using the mixing formula (Kyser, 1979):
| (2) |
where ρ is the mass density of a molecule with n elements, N0 is Avogadro’s number, Ci is the mass fraction, σiel is the total electron elastic cross section, and Ai is the atomic weight of element i. The Monte Carlo simulation stepped through an electron trajectory from collision to collision. The distance L between two successive elastic collisions was evaluated using:
| (3) |
where R is a random number between 0 and 1. After each collision, the angle of the electron was changed to account for elastic scattering (Drouin, et al., 2007). The energy of the electron was also changed as needed (see below).
The CASINO code was modified to include the following STEM parameters: the electron beam semi-angle α, the opening angle of the annular dark field (ADF) detector β, the noise characteristics of the electron source, and the 3D position of the focal point. The modified CASINO software was used to simulate STEM Z-contrast images of high atomic number nanoparticles in micrometers-thick low atomic number substrates. Figure 1 shows the schematic of sample geometry used in this work. Nanoparticles with a diameter d are placed on the top, inside, or at the bottom of the substrate with a thickness T.
Figure 1.
Schematic of the sample geometry (not to scale) used for STEM imaging simulations. One or more nanoparticles of diameter d were placed on the top surface, inside, or at the bottom surface of a substrate with a thickness T.
Electron Beam Properties
The electron beam of the STEM consists of a conical shape focused at a specific 3D position in the sample, with the electron probe radius rf at the focal point, and the beam semi-angle α. Using these parameters, the initial position (xa, ya, za) and the initial direction (dx, dy, dz) at the aperture plane were calculated for each electron trajectory as follows. All parameters used for the calculation in this section are illustrated schematically in Figure 2. A point (x′f, y′f, zf) in a horizontal circular probe shape was generated randomly around the focal point (xf, yf, zf) and defined by the radius r′f:
| (4) |
Figure 2.
Schematic of the electron beam parameters used to calculate the initial position (xa, ya, za) and direction (dx, dy, dz) of the electron for each simulated electron trajectory, with the electron probe radius rf at the focal point and the beam semi-angle α See text for the description of all parameters. The dimensions and angles are not to scale.
The electron probe in vacuum was simulated on the basis of a uniform distribution of the electrons at the beam-limiting aperture, which is allowed since the probe size in vacuum was much smaller than the objects used in the simulations presented here. For a uniform distribution, xR and yR were random numbers uniformly distributed between 0 and rf with the condition that lateral radius was smaller than or equal to the beam radius (r′f ≤ rf). Note that the CASINO software included the option to change to a Gaussian beam profile. Other beam profiles can be implemented if needed, for example, to account for effects of the various lens aberrations. The electron trajectory was initiated at the position (xa, ya, za) in the circle of the objective aperture of radius ra. This radius was calculated from the point at the focal plane (x′f, y′f, zf) as follows:
| (5) |
where the position za is a constant value above the sample (objective aperture) and zf is the focus position of the electron beam in the sample. From this radius, we obtained the scaling factor ra/rf to calculate the initial trajectory position (xa, ya, za) at the aperture:
| (6) |
The initial electron direction between the aperture point and the focus point was obtained from:
| (7) |
where L is the distance between the two points.
The above equations defined the trajectories to simulate one pixel of a STEM image. The CASINO software included the capability to simulate lines, 2D-, and 3D images, by changing the scan point position (xf, yf, zf). The software also permitted a scan in the XZ or YZ planes, as needed to study, for example, the influence of over- or under-focus on the imaging of nanoparticles.
To simulate the signal-to-noise-ratio SNR obtained in the STEM images, the number of electrons per pixel and the noise characteristics of the electron source were taken into account. For each scan point, the number of electrons simulated N was calculated from the beam current I and pixel dwell time τ as follows:
| (8) |
where e is the electron charge. The CASINO software included the variation of the number of electrons from pixel to pixel in order to model the shot noise of the electron gun (Reimer, 1998), which results in noise in the STEM image. The number of electrons for a specific pixel Ni was obtained from a Poisson distribution random number generator with:
| (9) |
These beam properties allowed the simulation of STEM images with realistic microscope parameters.
Physical Models for 100 – 300 keV Electrons
The electron scattering properties were calculated from the electron elastic cross section (EECS). The EECS determined the path length before the first collision, or the path length between collisions, and the electron deflection angle after a collision. An accurate EECS model is important to simulate a STEM Z-contrast image. The basic EECS model is the relativistic Rutherford EECS (Newbury & Myklebust, 1981) where the total cross section σiel (in cm2) for element i at electron energy E is given by:
| (10) |
with the screening parameter δi given by:
| (11) |
This model has been widely used for the calculation of the contrast obtained with STEM imaging (Reimer & Kohl, 2008). However, the approximations used in the Rutherford model are imprecise for heavier elements (Z > 30) and it is recommended to use a more accurate model (Williams & Carter, 1996). Therefore, a numerical EECS model was implemented in CASINO. The model was based on the software ELSEPA (Salvat, et al., 2005), which is used by the Electron Elastic Scattering Cross Section Database (Jablonski, et al., 2003) available from NIST (Gaithersburg, MD). This EECS model involves the calculation of the relativistic (Dirac) partial-wave for scattering by a local central interaction potential, using the software ELSEPA (Salvat, et al., 2005). The total and partial electron elastic cross sections were pre-calculated using ELSEPA for all chemical elements in the energy range 100 eV to 300 keV. The calculations of the cross sections used the default parameters suggested by the authors of the software ELSEPA (Salvat, et al., 2005). These pre-calculated values were then tabulated and included in CASINO to allow accurate simulations of the electron scattering.
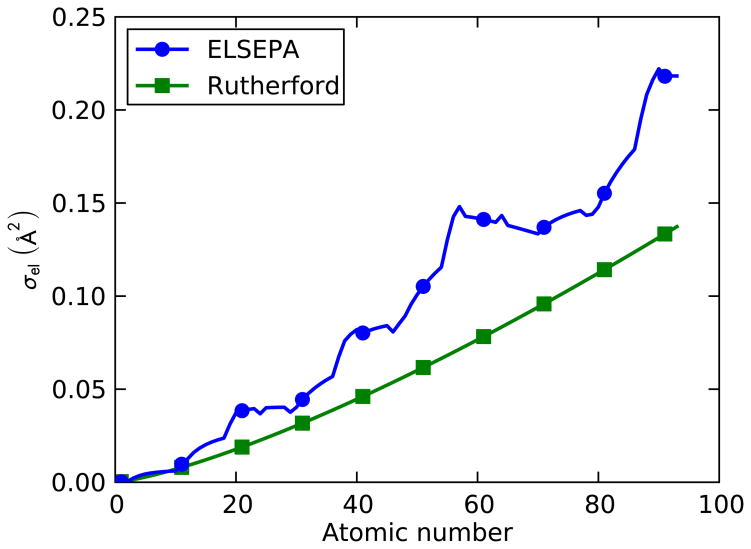
Figure 3 compares the total electron elastic cross section σel as function of the atomic number for the two models at an electron energy of 200 keV. This figure shows how the scattering chance generally increases with atomic number as predicted by the two models. But, the ELSEPA model shows the complex variation of the cross section between adjacent atoms, due to differences in the interaction potentials between the atoms. Because of the difference between the two models, the simulated STEM contrast could be different in some particular cases. For example, the contrast obtained on a CdSe quantum dot would be stronger as calculated with ELSEPA model than predicted by the Rutherford model.
Figure 3.
The total electron elastic cross section σel plotted as a function of atomic number for an electron energy of 200 keV. Comparison of two models of the elastic scattering cross section: 1) the relativistic Rutherford formula, and 2) tabulated values calculated with the ELSEPA software (Salvat, et al., 2005).
In these Monte Carlo simulations we also accounted for possible energy loss of the electrons during their trajectory through the specimen. The energy loss ΔE between collisions was calculated using the following equation:
| (12) |
where dE/ds is the rate of energy loss and L is the distance between two successive elastic collisions. The amount of energy loss was calculated by the relativistic Bethe equation (Bethe, 1930; Bethe, 1933). We neglected the effect of inelastic scattering on electron deviation and grouped all the electron energy loss events in a continuous energy loss function (Joy & Luo, 1989). For STEM applications, the angular deviation by the inelastic scattering events would have a small contribution on the electron trajectories and were ignored (Sousa, et al., 2009).
Transmitted Electron Detector
STEM images are typically obtained from the transmitted electron signal (in some cases secondary electrons are detected as well). For Z-contrast, an annular dark field (ADF) detector is used to collect electrons scattered out of the cone of the electron probe as shown in Figure 4. The ADF detector was defined in CASINO with an inner semi-angle β1, and an outer semi-angle β2, that allowed the calculation of the detected transmitted electron intensity from the final exit angle of each electron trajectory. The quantum detection efficiency (QDE) of the ADF detector was assumed to equal unity in the calculations presented here.
Figure 4.
Schematic representation of the annular dark field (ADF) detector (not to scale). The electrons are incident on the sample in a cone with a semi-angle α. Electrons scattered by the sample with an exit angle between the inner-, and outer detector semi-angles, β1, and β2, respectively, are considered as detected.
RESULTS AND DISCUSSION
Calculation of the lateral resolution of gold nanoparticles on a thick carbon substrate
For the imaging of radiation sensitive samples, such as biological materials or polymers, it is important to optimize the achievable resolution in terms of the maximal electron dose. This optimization requires the calculation of the resolution, and signal-to-noise level obtained for a specific sample and STEM imaging settings. The Monte Carlo simulation of STEM imaging presents a precise method to conduct such calculations. As an example, we have simulated linescans (one-dimensional images) for a sample consisting of gold nanoparticles placed on the top surface —with respect to the electron beam propagation direction— of a 1 μm thick carbon support substrate, see Figure 5A. The linescans were generated in the lateral direction, i.e., perpendicular to the propagation direction of the electron beam. The sample consisted of spherical gold nanoparticles of diameters of d = 1, 5, and 10 nm. The linescans were simulated for E = 200 keV, α = 20 mrad, df = 0.1 nm, a pixel size of 0.1 nm, β1 = 94 mrad, β2 = 611 mrad, and for two different probe current and pixel dwell time conditions. For Figure 5B, the conditions were I = 500 pA, and τ = 20 μs, resulting in a total number of electrons per pixel N = 62415. For Figure 5C, the conditions were I = 100 pA, and τ = 2 μs, resulting in N = 1248. The focus of the simulated electron probe was located at the vertical position of the nanoparticles. The main characteristics of the linescans are signal peaks, originating from the increased scattering at the locations of nanoparticles, on a background level with noise caused by the scattering in the carbon substrate. Figure 5C shows an increased noise level compared to Figure 5B as expected, as the number of electrons has been reduced by a factor of 50.
Figure 5.
Monte Carlo simulation of linescans recorded with the ADF detector of a STEM. (A) Schematic of the sample geometry (not to scale) used for the simulation. The sample consisted of 1, 5, and 10 nm diameter gold nanoparticles located on the top surface of a 1 μm thick carbon substrate. (B) and (C) Linescans showing the number of detected electrons N versus horizontal position x, simulated for 62415 and 1248 electrons per pixel, respectively. The dashed line indicates the Rose criterion (SNR = 5) for nanoparticle visibility.
To analyze the linescans we have calculated the signal-to-noise-ratio (SNR) and full-width at half-maximum (FWHM) for each peak. The average amount of electrons in the background NB and standard deviation std(NB) of the background were calculated from the flat regions at both sides of the peak. The maximal signal at the position of a nanoparticle NS was obtained from the maximum of the peak. Following the definition of Rose (Rose, 1948), the SNR was then calculated as:
| (13) |
Nanoparticles were assumed to be visible in the noise of the background when the Rose criterion (Rose, 1948) was satisfied, i.e., when SNR ≥ 5. In Figure 5B and C, the dashed horizontal line indicates the signal level for SNR = 5. The smallest nanoparticle (d = 1 nm) is just visible in Figure 5B, while it vanished in the noise in Figure 5C. We calculated the FWHM values for the visible nanoparticles. The half-maximum (HM) value was calculated by HM = (NS - NB)/2, and the two positions x1 and x2 on each side of the central peak were obtained. The FWHM was given by the difference of these positions FWHM = x2 – x1. Note that a smoothing function may be applied prior to the FWHM determination in the case of a small SNR.
Table 1 presents the SNR and FWHM values obtained for the two simulated linescans, including error margins. With the highest current, all three nanoparticles were visible above the noise. The FWHM values were about 20% smaller than the diameters of the nanoparticles. This difference can partly be explained on the basis of the imaging process, which is mathematically described by a convolution of the shape of the probe with the shape of the nanoparticles. The convolution of a Gaussian beam profile with a spherical object results in a line profile with a 10% smaller FWHM than the diameter of the nanoparticle. In addition, the calculated values are influenced by the simulated image noise. As expected on the basis of electron scattering, the SNR decreased with decreasing diameter. When imaged using fifty times fewer electrons, only the 10 nm nanoparticle was still visible above the noise level. The 5 nm nanoparticle approached the limit of detection, but the smallest nanoparticle (d = 1 nm) was completely hidden in the noise. The FWHM values were about 1 nm smaller at the lower current than at the higher current. It was also tested if the FWHM could be determined with more precision by smoothing the data prior to the FWHM measurement, but this did not result in a different result than shown in Table 1.
Table 1.
Analysis of simulated STEM signal peaks in linescans over gold nanoparticles of diameter d positioned at the top surface (with respect to the electron beam propagation direction) of a 1 μm thick carbon substrate. The signal-to-noise-ratio SNR and full-width at half-maximum FWHM of visible nanoparticles (see text) are given for two different numbers of electrons per pixel N. The error margins were determined from the standard deviations of the analysis of ten line-scans for each data point.
| N | d (nm) | SNR | FWHM (nm) |
|---|---|---|---|
| 62415 | 1 | 5.3 ± 0.8 | 0.8 ± 0.1 |
| 5 | 23.0 ± 0.5 | 4.2 ± 0.1 | |
| 10 | 44.2 ± 0.6 | 8.5 ± 0.1 | |
|
| |||
| 1248 | 1 | 3 | - |
| 5 | 5.4 ± 0.6 | 3.0 ± 0.5 | |
| 10 | 8.3 ± 0.3 | 7.6 ± 0.4 | |
These results demonstrate that the CASINO software can be used to simulate the achievable signal-to-noise-ratio for specific sample and microscope settings. Inversely, the simulations can also be used to find the smallest detectable nanoparticle for a given electron dose, which presents the noise-limited resolution.
Simulation of 3D focal-series
It was recently demonstrated that the STEM can be used to acquire three-dimensional (3D) datasets via focal series (van Benthem, 2005). We have shown that the vertical (axial) resolution δz achievable on nanoparticles in a thin sample is a linear function of the diameter of the nanoparticles (de Jonge, et al., 2010b):
| (14) |
A vertical resolution of the order of 10 nm can be achieved when using a STEM with spherical-aberration correction (van Benthem, 2005). An important question is what axial resolution can be achieved on nanoparticles in thicker samples where beam blurring plays a role. The CASINO software was used to evaluate the axial resolution of STEM imaging of gold nanoparticles of d = 10 nm in a 1 μm thick carbon substrate. The nanoparticles were at three different vertical positions: on the top, in the middle, and at the bottom of the carbon substrate. STEM images were simulated for three different focus positions corresponding to the three positions of the nanoparticles, see Figure 6A for a schematic of the sample geometry. The simulation parameters were: N = 105 (I = 0.8 nA and τ = 20 μs), E = 200 keV, α = 41 mrad, df = 0.1 nm, the pixel size was 1 nm for horizontal scan and 10 nm for vertical scan (focal-series), β1 = 94 mrad, and β2 = 611 mrad. The microscope settings were similar to the ones used previously for 3D imaging of a platinum replica of a biological specimen with an aberration-corrected STEM (de Jonge, et al., 2010b).
Figure 6.
Monte Carlo simulation of 3D STEM datasets of 10 nm diameter gold nanoparticles embedded in a 1 μm thick carbon film and placed at different depths. The beam semi-angle was 41 mrad. (A) Schematic of the sample (not to scale) used for the simulation. (B), (D), and (F) XY images and linescans showing the number of detected electrons N versus horizontal position x, at different focus positions zf: (B) at 0 nm, (D) at 500 nm, and (F) at 1000 nm. (C), (E), and (G) XZ linescans showing the number of detected electrons N versus vertical focal plane zf, across the gold nanoparticles as a function of nanoparticle position: (C) on the top, (E) in the middle, and (G) at the bottom of the carbon substrate. (H) Side view in the XZ plane of the three nanoparticles.
Figure 6B shows a simulated image in the horizontal plane with the focus at the top of the substrate (zf = 0 nm), and a horizontal linescan over the three nanoparticles. The left nanoparticle, placed on top of the carbon, appears in focus, while the other two are out of focus. The figure also shows a horizontal linescan over the middle of the sample. Figure 6D and F present similar images and linescans, but for the focal plane in the middle (zf = 0.5 μm), and at the bottom (zf = 1 μm) of the substrate, respectively. Table 2 summarizes the calculated SNR and FWHM obtained from these three linescans. The SNR of a particular nanoparticle was the highest when the electron beam was focused at that nanoparticle, and nanoparticles imaged with an out-of-focus electron beam were not visible above the noise level. Both the nanoparticles at the top and at the middle exhibited a FWHM = 8 and 9 nm, respectively, when imaged in-focus, consistent with their size within the error margin of the calculation. The FWHM of the bottom nanoparticle was increased to 26 nm, caused by electron probe broadening due to beam-sample interactions. The diameter of an electron probe broadened by beam-sample interactions dblur can be calculated with the following equation (de Jonge, et al., 2010a; Reimer & Kohl, 2008):
| (15) |
with E in eV. For 1 μm thick carbon using a 200 keV electron beam, dblur = 16 nm. This number fits within a factor of two the simulated result, which is a reasonable agreement, considering the accuracy of equation (15).
Table 2.
Analysis of simulated 3D STEM signal peaks in horizontal linescans over 10 nm diameter gold nanoparticles embedded at three different vertical positions z in a 1 μm thick carbon substrate at three different focus positions zf. The analysis results of the vertical linescans are also included.
| zf (nm) | z (nm) | SNR | FWHM (nm) |
|---|---|---|---|
| 0 | 0 | 53 | 8 |
| 500 | 5 | 34 | |
| 1000 | 3 | - | |
|
| |||
| 500 | 0 | 4 | - |
| 500 | 20 | 9 | |
| 1000 | 3 | - | |
|
| |||
| 1000 | 0 | 2 | - |
| 500 | 3 | - | |
| 1000 | 6 | 26 | |
|
| |||
| Vertical Line-scans | 0 | 48 | 2.8×102 |
| 500 | 20 | 3.4×102 | |
| 1000 | 5 | 1.4×103 | |
Figure 6C, E, G and H show the vertical linescans and images in XZ direction for these three nanoparticle positions. To analyze the linescans we have calculated the SNR and FWHM values for the vertical linescans, see Table 2. The highest SNR was obtained for the top nanoparticle, similar as obtained in the horizontal linescan. The lowest SNR was for the bottom nanoparticle at the limit of visibility. The FWHM values of the vertical line scans increased with depth of the nanoparticle in the samples, consistent with the picture of electron probe broadening. The achievable vertical (axial) resolution δz on nanoparticles of a diameter of 10 nm in a thin sample is expected to amount to 244 nm (equation (14)). This calculation agrees within 15% with the result of 2.8×102 nm obtained with the Monte Carlo simulations for the top nanoparticle. Larger differences were obtained with particles deeper in the sample. This difference is likely caused by the effect of beam broadening, which was not taken into account in equation (14).
These results demonstrate that the CASINO software can be used to calculate the achievable axial resolution for specific sample and microscope settings.
Comparison of experimental and simulated liquid STEM images
We have recently introduced a new STEM methodology to image proteins tagged with gold nanoparticles on eukaryotic cells in liquid with nanometer resolution (de Jonge, et al., 2009). The liquid samples were imaged via the use of a microfluidic chamber with electron transparent silicon nitride windows. The achievable resolution on gold nanoparticles in liquid was investigated in a previous study which included Monte Carlo simulations (de Jonge, et al., 2010a). In the following we will compare a liquid STEM image with a simulated image. Figure 7A shows the sample geometry. The sample contained 5 and 28 nm diameter gold nanoparticles on the top surface of a 50 nm thick silicon nitride window on a 7.5 μm water layer and a second 50 nm thick silicon nitride window. The nominal diameters specified by the supplier were 5 and 30 nm. The analysis of signal peaks in line-scans over gold nanoparticles give an average FWHM of about 5 and 28 nm, respectively. Therefore, nanoparticle diameters of 5 and 28 nm were used in the simulations. Figure 7B is a liquid STEM image as obtained from experiment. The microscope settings were, α = 11 mrad, N = 35000 (I = 0.28 nA and τ = 20 μs), E = 200 keV, df = 0.5 nm, the pixel size was 0.84 nm, β1 = 94, mrad and β2 = 611 mrad. The same sample geometry, with the positions of the nanoparticles determined from the liquid STEM image, was modeled with CASINO, and STEM images were simulated for the same conditions as the experiment. Figure 7C shows the simulated image.
Figure 7.
Comparison of experimental and simulated STEM images of nanoparticles on a water layer enclosed between two electron-transparent silicon nitride windows. (A) Schematic of the sample (not to scale) used for the simulation. (B) and (C): experimental and simulated STEM images, respectively. (D) Linescans showing the number of detected electrons N versus horizontal position x, of the smaller nanoparticle (5 nm) extracted from experimental (B) and simulated (C) images and indicated by the white lines L1. (E) Linescans showing the number of detected electrons N versus horizontal position x, of the larger nanoparticle (28 nm) extracted from the experimental (B) and simulated (C) images and indicated by the white lines L2.
Linescans were drawn over several nanoparticles for a quantitative comparison of the experimental- and the simulated image. The grey levels S of the pixels of the STEM image were converted to numbers of detected electrons N. The value of N was calculated by estimating the contrast C and brightness B of the microscope (those numbers were not available) using the linear relation,
| (16) |
We assumed a quantum detection efficiency of unity for the experimental and simulated images (the detection efficiency of the used ADF detector (Fischione Instruments) is known to be high (Kirkland & Thomas, 1996)). As a consequence, the average of the experimental background NB should equal the simulated N′B. This equality was used as a first constraint. The second constraint was obtained by supposing a Poisson distribution of the noise of the background signal, thus:
| (17) |
Others showed that the Poisson characteristics of the noise propagates through the whole imaging chain, from the electron source, the electron beam, via electrons scattered from the sample, and through the detection chain (Frank, 2005). In the worst case, the non-Poisson noise component maximum contribution to the standard deviation is expected to be 10% (Frank, 2005). The grey levels of the simulated image in C were adjusted to those of the experimental data by using the inverse of relation (16) and using the factors B and C determined from the experimental data. By comparing Figure 7B and C it can be seen that the brightness was similar for both images.
Linescans over two nanoparticles in each image were further analyzed. Figure 7D compares experimental- and simulated linescans at the position of a nanoparticle with d = 5 nm (linescan L1). The number of electrons corresponds between the experiment and the simulation, both in the background and at the position of the nanoparticle. This agreement shows that the assumptions of 100% detection efficiency, and Poisson statistics were valid. More importantly, it shows that the method of using Monte Carlo simulations agrees with the experimental data. The experimental SNR was determined to amount to 4.6 (equation (13), with the background level determined from the average signal of the horizontal positions of −30 to −10 nm). The analysis of 6 nanoparticles with different linescan directions revealed an average SNR = 5.3 ± 0.5. Comparatively, a value SNR = 4.9 was found for the simulated linescan. Thus, the signal-to-noise-ratio values agreed between experiment and simulation within the error margin. Note that the values of SNR and FWHM determined for the experiment data are independent of the values of C and B.
Figure 7E shows experimental- and simulated linescans for a nanoparticle with d = 28 nm (linescan L2). For the experimental linescan L2 it was found that SNR = 12, and SNR = 14 ± 1 applied for a total of 6 nanoparticles with different linescan directions. The signal-to-noise-ratio for the simulated linescan L2 amounted to 22, a factor of 1.6 larger than the experimental data. The simulation on nanoparticles thicker than the total mean-free-path-length for elastic scattering apparently leads to inaccuracy. Nevertheless, the agreement of the simulation and the experiment for the background signal, and the signal on the smaller nanoparticles demonstrates that Monte Carlo simulations can be used to predict the achievable resolution and signal-to-noise-ratio for specific sample geometries and microscope settings.
CONCLUSIONS
The STEM imaging modality was included in the CASINO Monte Carlo simulation software. The simulation of Z-contrast images provides a way to calculate the achievable resolution and signal-to-noise-ratio for specific sample geometries and microscope settings. The study of 3D datasets obtained by focal-series showed that beam broadening influenced the axial resolution. The Monte Carlo simulations of STEM imaging of gold nanoparticles on water layers showed agreement with experimental data, from which it can be concluded that Poisson statistics apply, that the quantum efficiency of detection approaches unity, and that the physical models used in the simulations are of sufficient precision. Monte Carlo simulations can be used to optimize microscope settings, e.g., for high resolution, in imaging sessions where a low electron dose is crucial. The simulations can also be used to analyze the image features in a particular experiment for the analysis of the composition of a certain specimen.
Acknowledgments
We thank D.C. Joy, and E.A. Ring for discussions. STEM images were recorded at the Shared Research Equipment user facility at Oak Ridge National Laboratory sponsored by the Division of Scientific User Facilities, U.S. Department of Energy. Research supported by NIH grant R01-GM081801 (to NJ, NPD, and HD).
References
- AOYAMA K, TAKAGI T, HIRASE A, MIYAZAWA A. STEM tomography for thick biological specimens. Ultramicroscopy. 2008;109:70–80. doi: 10.1016/j.ultramic.2008.08.005. [DOI] [PubMed] [Google Scholar]
- BETHE H. Theory of the Passage of Fast Corpuscular Rays Through Matter. Ann Physik. 1930;5(5):325–400. [Google Scholar]
- BETHE HA. In: Handbuch der Physik. Geiger J, Scheel K, editors. Berlin: Springer; 1933. p. 273. [Google Scholar]
- CREWE AV, WALL J, LANGMORE J. Visibility of single atoms. Science. 1970;168:1338–1340. doi: 10.1126/science.168.3937.1338. [DOI] [PubMed] [Google Scholar]
- DE JONGE N, PECKYS DB, KREMERS GJ, PISTON DW. Electron microscopy of whole cells in liquid with nanometer resolution. Proc Natl Acad Sci. 2009;106:2159–2164. doi: 10.1073/pnas.0809567106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE JONGE N, POIRIER-DEMERS N, DEMERS H, PECKYS DB, DROUIN D. Nanometer-resolution electron microscopy through micrometers-thick water layers. Ultramicroscopy. 2010a;110(9):1114–1119. doi: 10.1016/j.ultramic.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE JONGE N, SOUGRAT R, NORTHAN B, PENNYCOOK SJ. Three-dimensional scanning transmission electron microscopy for biological specimen. Microscopy and Microanalysis. 2010b;16(1):54–63. doi: 10.1017/S1431927609991280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE JONGE N, SOUGRAT R, NORTHAN BM, PENNYCOOK SJ. Three-dimensional scanning transmission electron microscopy of biological specimens. Microsc Microanal. 2010c;16(1):54–63. doi: 10.1017/S1431927609991280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DROUIN D, COUTURE AR. Development of a simulation tool for real world SEM applications. Microscopy and Microanalysis. 2002;8(Suppl 2):702–703. [Google Scholar]
- DROUIN D, COUTURE AR, JOLY D, TASTET X, AIMEZ V, GAUVIN R. CASINO V2.42 --- A Fast and Easy-to-use Modeling Tool for Scanning Electron Microscopy and Microanalysis Users. Scanning. 2007;29(3):92–101. doi: 10.1002/sca.20000. [DOI] [PubMed] [Google Scholar]
- FRANK L. Noise in secondary electron emission: the low yield case. J Electron Microsc (Tokyo) 2005;54(4):361–365. doi: 10.1093/jmicro/dfi044. [DOI] [PubMed] [Google Scholar]
- HOHMANN-MARRIOTT MF, SOUSA AA, AZARI A, GLUSHAKOVA S, ZHANG G, ZIMMERBERG J, LEAPMAN RD. Nanoscale 3D cellular imaging by axial scanning transmission electron tomography. Nature Methods. 2009;6:729–732. doi: 10.1038/nmeth.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOVINGTON P, DROUIN D, GAUVIN R. CASINO: A new Monte Carlo code in C language for electron beam interaction - Part I: description of the program. Scanning. 1997;19(1):1–14. [Google Scholar]
- JABLONSKI A, SALVAT F, POWELL CJ. NIST Electron Elastic-Scattering Cross-Section Database - Version 3.1. National Institute of Standards and Technology; 2003. [Google Scholar]
- JOY DC. Monte Carlo Modeling for Electron Microscopy and Microanalysis. New York: Oxford University Press; 1995. [Google Scholar]
- JOY DC, LUO S. An empirical stopping power relationship for low-energy electrons. Scanning. 1989;11:176. [Google Scholar]
- KIRKLAND EJ, THOMAS MG. A high efficiency annular dark field detector for STEM. Ultramicroscopy. 1996;62:79–88. doi: 10.1016/0304-3991(95)00092-5. [DOI] [PubMed] [Google Scholar]
- KYSER DF. In: Introduction to Analytical Electron Microscopy. Hren JJ, Goldstein JI, Joy DC, editors. New York: Plenum Press; 1979. [Google Scholar]
- MUELLER SA, ENGEL A. Biological scanning transmission electron microscopy: imaging and single molecule mass determination. Chimia. 2006;60:749–753. [Google Scholar]
- NELLIST PD, CHISHOLM MF, DELLBY N, KRIVANEK OL, MURFITT MF, SZILAGYI ZS, LUPINI AR, BORISEVICH A, SIDES WH, PENNYCOOK SJ. Direct sub-angstrom imaging of a crystal lattice. Science. 2004;305:1741. doi: 10.1126/science.1100965. [DOI] [PubMed] [Google Scholar]
- NEWBURY DE, MYKLEBUST RL. A Monte Carlo electron trajectory simulation for analytical electron microscopy. In: Geiss RH, editor. Analytical electron microscopy. San Francisco Press; 1981. pp. 91–98. [Google Scholar]
- REIMER L. Scanning Electron Microscopy: Physics of Image Formation and Microanalysis. Springer; 1998. [Google Scholar]
- REIMER L, KOHL H. Transmission Electron Microscopy: Physics of Image Formation and Microanalysis. Springer; 2008. [Google Scholar]
- ROSE A. Television pickup tubes and the problem of noise. Adv Electron. 1948;1:131–166. [Google Scholar]
- SALVAT F, JABLONSKI A, POWELL CJ. ELSEPA -- Dirac partial-wave calculation of elastic scattering of electrons and positrons by atoms, positive ions and molecules. Computer Physics Communications. 2005;165:157–190. [Google Scholar]
- SOUSA AA, HOHMANN-MARRIOTT M, ARONOVA MA, ZHANG G, LEAPMAN RD. Determination of quantitative distributions of heavy-metal stain in biological specimens by annular dark-field STEM. J Struct Biol. 2008;162:14–28. doi: 10.1016/j.jsb.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOUSA AA, HOHMANN-MARRIOTT MF, ZHANG G, LEAPMAN RD. Monte Carlo electron-trajectory simulations in bright-field and dark-field STEM: Implications for tomography of thick biological sections. Ultramicroscopy. 2009;109(3):213–221. doi: 10.1016/j.ultramic.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN BENTHEM K, LUPINI AR, KIM M, BAIK HS, DOH SJ, LEE JH, OXLEY MP, FINDLAY SD, ALLEN LJ, PENNYCOOK SJ. Three-dimensional imaging of individual hafnium atoms inside a semiconductor device. Applied Physics Letters. 2005;87:034104-1–034104-3. [Google Scholar]
- WILLIAMS DB, CARTER CB. Transmission Electron Microscopy: a Textbook for Materials Science. New York: Plenum Press; 1996. [Google Scholar]